Introduction
Activins are members of the transforming growth
factor-β (TGF-β) superfamily and are growth and differentiation
factors that exert effects on a broad range of cells and tissues
(1,2).
Activin signaling plays a role in regulating the normal
proliferation and differentiation in epidermal keratinocytes. A
previous study summarized activin signaling transduction and
interaction between activin and TGF-β signaling during hair
follicle formation, hair growth cycling, skin function, and wound
healing (3). Follistatin is an
activin-binding protein that functions as an antagonist by binding
TGF-β family members such as activin, bone morphogenetic proteins,
myostatin, growth differentiation factor 11, and TGF-β1 to block
interaction with their signaling receptors. Activin and follistatin
play important roles in skin development, inflammatory processes,
angiogenesis, and wound healing. There is information about the
expression of activin subunits and receptors in fibroblasts and
keratinocytes, but there is no report of regulation of follistatin
expression in these cells (4).
The biological activity of activin is regulated by
specific heteromeric complexes consisting of type I and type II
serine/threonine kinase receptors. Activin receptor (ActR) type II
binds activin independently of ActR I are unable to signal without
ActR I. Formation of heteromeric complexes of ActR I and ActR II is
required for mediation of cellular signals (5). The signal transduction pathway is
conserved for the TGF-β superfamily members, involving the
receptor-Smad system. Smad is a primary mediator of TGF-β
signaling. Smad2 and Smad3 heterodimerize with Smad4 and
translocate to the nucleus to participate in transcriptional
regulation of target genes (6). Smad7
functions as an inhibitor of TGF-β signaling, including activin
signaling (7). Transcription of SMAD7
is induced by TGF-β or activin, which indicates the negative
feedback mechanism of TGF-β/activin signaling (8). The role of the activin/Smad pathway in
regulating in vitro-aged normal fibroblasts remains unclear.
Previous studies have shown that number of normal human dermal
fibroblast gradually reduced with increasing passage number, and
expression of aging biomarkers, including procollagen type I and
VII, elastin, fibrillin-1, and SIRT1 and SIRT6 were downregulated
by passaging (9).
We investigated changes of activin, its receptors,
and Smad-signaling gene expression with increasing passage number
in normal human dermal fibroblasts.
Materials and methods
Culture of normal human dermal
fibroblasts
Normal human dermal fibroblasts were isolated from
tissue removed after circumcision of two 13- and 14-year-old males.
The procedures followed in the present study were approved by the
Institutional Review Board Committee of the Kyung Hee University
Medical Center (Seoul, Republic of Korea; KMC IRB no. 0407-01), and
adhered to the recommendations of The Declaration of Helsinki. The
human dermal fibroblasts were cultured in Dulbecco's modified
Eagle's medium containing 10% fetal bovine serum and antibiotics
(Gibco; Thermo Fisher Scientific, Inc.) at 37˚C in a humidified
atmosphere of 5% CO2 and 95% air. Previously, our group
characterized the isolated fibroblasts of different passages for
their proliferative capacity, and the results demonstrated that
their population doubling time was significantly increased with
passage number (9), confirming that
the proliferative capacity of the fibroblasts gradually declined
with serial passaging. The cell population doubling time was
calibrated by a formula of Kuchler (10).
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was purified from cultured cells using the
TRIzol reagent method and following the manufacturer's protocol
(Invitrogen; Thermo Fisher Scientific, Inc.). First-strand cDNA
synthesis was performed with 1 µg of total RNA and
Oligo(dT)15 primers using a reverse transcription system
(Promega Corporation), according to the manufacturer's protocol.
The primer sequences and product size were as follows: activin A
(5'-GGACATCGGCTGGAATGACT-3' and 5'-GGCACT CACCCTCGCAGTAG-3', 71
bp), follistatin (5'-CAGTAAGTC GGATGAGCCTGTCT-3' and
5'-CAGCTTCCTTCATGG CACACT-3', 74 bp), ActR IA (5'-AGGCTGCTTCCAGGT
TTATGAG-3' and 5'-TGGCAGCACTCCACAGCTT-3', 81 bp), ActR IB
(5'-TACTCTGTGTCTGGCAGGCTACTC-3' and 5'-GCTTTGGTTCCACAGTCTGAGAT-3',
82 bp), ActR IIA (5'-CCTGTTTTAAGAGATTATTGGCAGAA-3' and
5'-TGCGTCGTGATCCCAACAT-3', 84 bp), ActR IIB
(5'-TTCGATTTGAGCCAGGGAAA-3' and 5'-GAGCAC CTCAGGAGCCATGT-3', 80
bp), and β-actin (5'-GCGAGA AGATGACCCAGATC-3',
5'-GGATAGCACAGCCTGGAT AG-3'; 77 bp). qPCR was performed on the
StepOneplus Real-Time PCR system using Power SYBR-Green PCR Master
Mix (Applied Biosystems; Thermo Fisher Scientific, Inc.). PCR was
performed with 1 µl of cDNA in 20 µl reaction mixtures, consisting
of 10 µl of Power SYBR-Green PCR Master Mix, 2 µl of primers, and 7
µl of PCR-grade water. The reactions were performed with a
denaturation step at 95˚C for 10 min, followed by 40 cycles of 95˚C
for 15 sec and 60˚C for 1 min. The relationship between a target
gene and β-actin was determined using the formula 2-(target
gene - β-actin), and the relative quantities of transcripts
were measured.
Immunoblot analysis
Cells were collected, washed with cold
phosphate-buffered saline, and then treated with lysis buffer
containing 1 mM PMSF (Cell Signaling Technology, Inc.). Protein
concentrations were determined using BCA protein assays (Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocol.
Ten micrograms of protein were fractionated on 10 or 12% sodium
dodecyl sulfate polyacrylamide gel electrophoresis gels and
transferred onto a nitrocellulose membrane (Amersham Pharmacia
Biotech). The membranes were blocked with 1% bovine serum albumin
(BSA) for 1 h at room temperature and then incubated overnight at
4˚C with antibodies against Smad2, phosph-Smad2, Smad3,
phosphor-Smad3, Smad4 (Cell Signaling Technology, Inc.), Smad7
(Santa Cruz Biotechnology, Inc.), and β-actin (Sigma-Aldrich; Merck
KGaA). The antibodies were diluted (1:1,000 for Smad2, 3 and 4;
1:250 for Smad7; 1:5,000 for β-actin) using Tris-buffered saline
containing 0.05% Tween-20 (TBS-T). After washing with TBS-T for 1
h, the membranes were incubated for 1 h at room temperature with
anti-rabbit and anti-mouse horseradish peroxidase-conjugated
secondary antibodies diluted 1:3,000 (1:10,000 for β-actin) in
TBS-T. The membranes were subsequently washed with TBS-T for 1 h,
and proteins were detected using Amersham ECL Prime Western
Blotting Detection Reagent (GE Healthcare Life Sciences). Protein
expression was analyzed using an Amersham Imager 600 (GE Healthcare
Life Sciences). Protein band densities were measured using ImageJ
analysis software (ImageJ, version 1.44; National Institutes of
Health).
Statistical analysis
Data are expressed as the mean ± standard error.
Data were compared by one-way analysis of variance and Tukey's post
hoc test. Statistical analyses were performed using the GraphPad
Prism 5 software (GraphPad Software Inc.). P<0.05 and P<0.01
were considered to indicate statistical significance.
Results
Regulation of activin, follistatin and
ActR mRNA with increasing passages
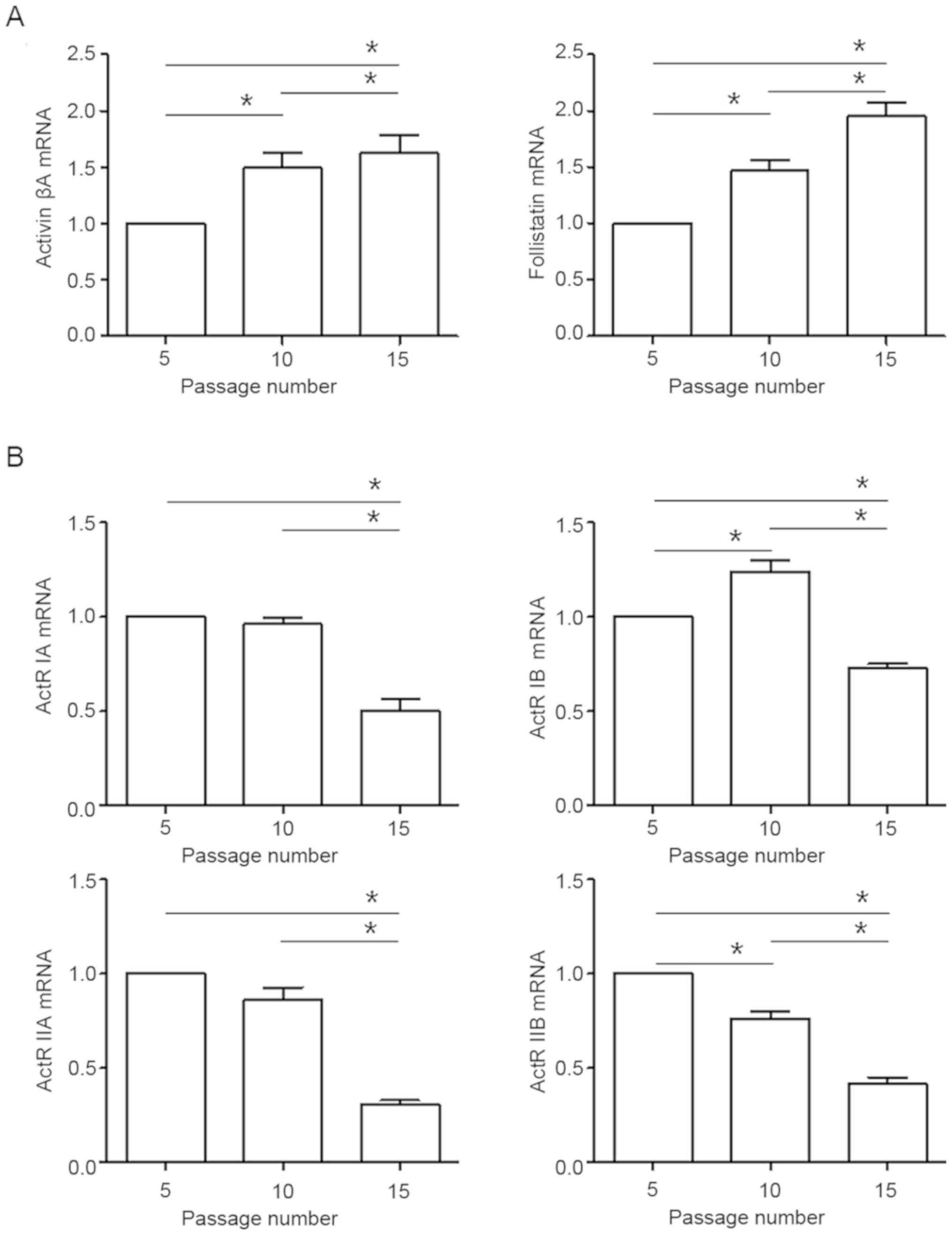
Cells were cultured 5-15 passages in vitro,
and expression of activin signal gene mRNA was measured by qPCR.
The activin A and follistatin transcripts were significantly
enhanced with increasing passage number (Fig. 1A). The ActR IB mRNA level was
increased at passage 10 compared to passage 5 but then decreased at
passage 15. The ActR IA, IIA and IIB transcripts were significantly
reduced with increasing passage number (Fig. 1B).
Regulation of Smad signaling protein
with increasing passages
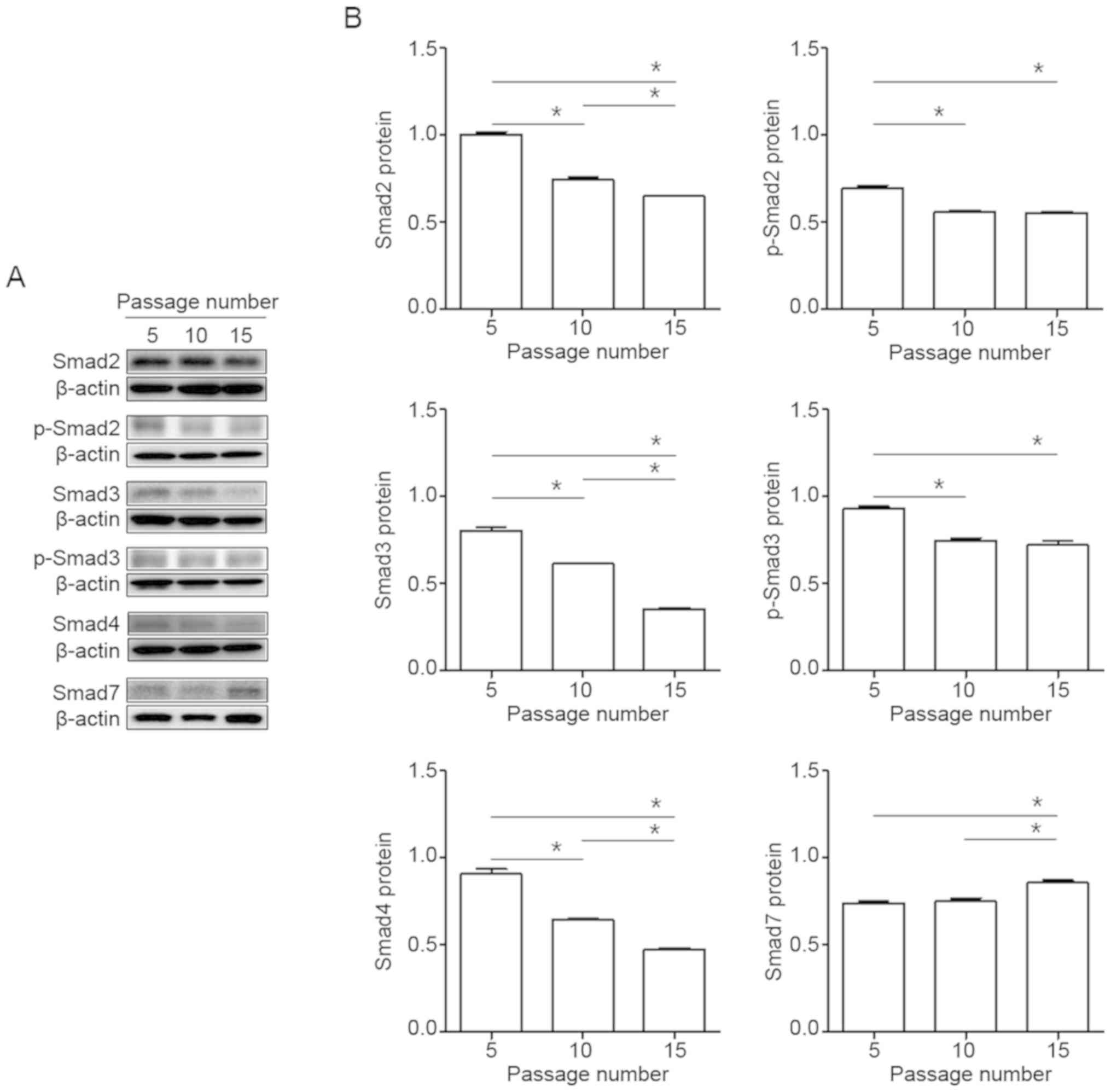
To determine a potential role of Smad signaling in
normal human dermal fibroblasts, cells were cultured 5-15 passages
in vitro, and expression of Smad signal genes protein was
measured by immunoblot analysis. The phosphorylation of Smad2 and 3
proteins was significantly decreased with increasing passage
number, and the level of Smad4 protein was reduced. The Smad7 of
Smad inhibitor was enhanced with increasing passages number
(Fig. 2A and B).
Discussion
Skin aging involves a variety of processes that are
influenced by physiological phenomena from chronologic or natural
aging, as well as environmental factors of photoaging (11). Activin, its receptors, and follistatin
(activin binding proteins) are important regulators of cell
proliferation, differentiation and apoptosis in hair follicle
initiation, hair cycling, normal skin homeostasis, and wound
healing (3). Activins are involved in
wound repair through regulation of skin and immune cell migration
and differentiation, as well as re-epithelialization and formation
of granulation tissue. Activins also affect the expression of
collagens through fibroblasts and modulate scar formation (12). We found that the activin A and
follistatin transcripts were significantly increased with
increasing passage number. These results suggest that activin A and
follistatin signals are enhanced in aging fibroblasts during
senescence. Activins have shown an important role in wound repair
as well as in keratinocyte differentiation, in dermal fibrosis, and
possibly also in human skin disease (13). An increase in the level of activin A
mRNA in human basal cell carcinomas and squamous cell carcinomas
was noted compared with normal skin, whereas the level of
follistatin mRNA was similar in normal and tumorigenic skin
(14). Activin and follistatin are an
important regulatory system modulating developmental and
regeneration processes (15). Activin
A and follistatin are produced by normal and abnormal hepatocytes
and help regulate hepatocyte regeneration in the healthy liver.
Pathological increase of both activin and follistatin in hepatocyte
is shown with several liver diseases including fibrosis, cirrhosis,
and hepatocellular carcinoma (16).
Activin A has shown an increase in serum from older women (43-47
years old) relative to younger women (19-38 years old) (17,18). The
expression of follistatin is altered in the tissue during fibrosis,
suggesting a role of endogenous follistatin in regulating the
fibrotic effect of activin (19). We
could not perform ELISA of activin A and follistatin using
supernatant. This is because cells must be cultured for several
periods of time, so after 3 days of incubation, the medium must be
exchanged with a new medium and as the number of passages
increases, the growth rate of the cells is delayed and incubation
time is not constant. In addition each time the medium was
exchanged to maintain cell growth, various factors were lost from
the supernatant and new materials were created. The signal
transduction pathway is highly conserved for the TGF-β superfamily
members, involving the receptor-Smad system. Similar to TGF-β, bone
morphogenic protein (BMP), and activin needs two type of the cell
surface receptors (Type I and II receptor) for its signal
transduction (20). However, the
mechanisms through which activin signaling pathways cause aging in
dermal fibroblasts are not well understood. In this study, we used
passaged fibroblasts as a model for skin aging, focusing on the
signal pathway as the cause of intrinsic senescence. We found that
ActR IA, IB, IIA and IIB transcript levels were all reduced in
late-passage fibroblasts. These results showed that passaged human
fibroblasts undergo decreased ActR I and II transcription during
replicative senesces, suggesting repressed in aging fibroblasts as
a results of downregulated ActR binding capacity. When human skin
ages, decreases in the size and rigidity of fibroblasts enhance
TGF-β receptor type II (TGFβRII) mRNA and protein levels. TGFβRII
promoter activity is also decreased, suggesting that downregulation
of TGFβRII expression may result, at least in part, from reduced
gene transcription (21). Aging rat
prostate showed significantly decreased TGFβRI and TGFβRII mRNA in
older rat tissue recombinants compared with 4-, 12- and
17-month-old recombinant tissues. In contrast, normal rat prostates
of different age groups showed no effect on the levels of TGFβRI
and TGFβRII mRNA (22). The levels of
TGFβRII mRNA and protein in the epidermis of the forearm
(sun-exposed) of the elderly were significantly lower than those of
upper-inner arm (sun-protected) skin of the same individual
(23). Activin and its receptor are
expressed primarily in mouse skin and are primarily localized to
fibroblasts, and increased expression of activin β-subunits in the
skin after injury suggests a role in skin repair (24).
Smads constitute a structurally similar family of
proteins that are key signal transducers for receptors in the TGF-β
superfamily and are critical for regulation of cell development and
growth (25). Phosphorylated Smad2
and Smad3 associated with Smad4 induce signal transduction
generated by TGF-β and activin, while Smad7 inhibits this
intracellular signaling (26). We did
find significant decrease in Smad2, 3 and 4 protein levels with
increasing passage number, and phosphorylation of Smad2 and 3 was
reduced. However, Smad7 was increased at late-passage number. These
results suggest that Smad genes suppress signaling activity during
replicative senescence in normal human dermal fibroblasts, and that
Smad7 acts as feedback regulation of the activin signaling pathway.
Smads signaling is repressed in aging fibroblasts as a result of
decreased ActR binding capacity. Taken together, these results
indicate that expression of ActR is decreased, and Smad binding
force are reduced. However, inactivation of Smad2 and Smad3
phosphorylation decreased binding of Smad4, Therefore, it affects
the acitivn/Smad signaling in replication senescence. The
immunoreactivity of pSmad2 was observed to decrease in the
epidermis of photoaged forearm skin versus matched intrinsically
aged skin. This decrease was also found in the epidermis of
upper-inner arm skin of the elderly versus the young. The
UV-induced downregulation of TGFβRII and the concerted
over-expression of Smad7 cause inhibition of the TGF-β-induced
phosphorylation of Smad2(23). In
thyroid cells, the expression of Smad and the presence of pSmad2/3
and Smad4 proteins in the nucleus of tumor cells indicate
propagation of TGF-β/activin signaling. However, high expression of
Smad7 in most malignant tumors may contribute to reduction of Smad
antiproliferative signaling in thyroid carcinomas (27). Overexpression of Smad7 blocked
TGF-β1-induced Smad3 phosphorylation and nuclear accumulation of
Smad2/3 in fibroblasts derived from human Peyronie's disease
(28). Recently we have demonstrated
that the TGF-β signaling pathway is controlled primarily by
downregulation of receptors at the transcriptional level, and
suppression of the TGF-β/Smad pathway is associated with reduced
MMP, TIMP, and collagen type I and III expression in aging human
dermal fibroblasts, suggesting that activation of NF-κB, Akt-JNK
and MAPK reduce TGF-β/Smad transcription in passaged normal human
dermal fibroblasts (29). We assumed
that decrease of TGF-β/activin/Smad pathways in aging human dermal
fibroblasts is reduced receptor binding capacity as well as
activity of Smad phosphorylation. Moreover, aging of fibroblasts
exerted downregulation of expression effects on target gene by
decreasing TGF-β/Activin/Smad signal pathway.
In conclusion, we demonstrated that increasing
passage number in normal human dermal fibroblasts enhanced activin
A and follistatin, whereas activin signaling pathway is controlled
primarily through downregulation of ActR at the transcriptional
level, and suppressed the expression of Smad signaling.
Acknowledgements
Not applicable.
Funding
This research was supported by Basic Science
Research Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Education
(NRF-2017R1D1A1B03027993).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YIK performed most of the experiments, analyzed data
and wrote the manuscript. MKS contributed to the study concept and
design of the project. CL analyzed the data. All authors have read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
DePaolo LV, Bicsak TA, Erickson GF,
Shimasaki S and Ling N: Follistatin and activin: A potential
intrinsic regulatory system within diverse tissues. Proc Soc Exp
Biol Med. 198:500–512. 1991.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Xia Y and Schneyer AL: The biology of
activin: Recent advances in structure, regulation and function. J
Endocrinol. 202:1–12. 2009.PubMed/NCBI View Article : Google Scholar
|
|
3
|
McDowall M, Edwards NM, Jahoda CA and Hynd
PI: The role of activins and follistatins in skin and hair follicle
development and function. Cytokine Growth Factor Rev. 19:415–426.
2008.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kawakami S, Fujii Y and Winters SJ:
Follistatin production by skin fibroblasts and its regulation by
dexamethasone. Mol Cell Endocrinol. 172:157–167. 2001.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Attisano L, Wrana JL, Montalvo E and
Massagué J: Activation of signalling by the activin receptor
complex. Mol Cell Biol. 16:1066–1073. 1996.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Moustakas A, Souchelnytskyi S and Heldin
CH: Smad regulation in TGF-β signal transduction. J Cell Sci.
114:4359–4369. 2001.PubMed/NCBI
|
|
7
|
Nakao A, Afrakhte M, Morén A, Nakayama T,
Christian JL, Heuchel R, Itoh S, Kawabata M, Heldin NE, Heldin CH,
et al: Identification of Smad7, a TGFbeta-inducible antagonist of
TGF-beta signalling. Nature. 389:631–635. 1997.PubMed/NCBI View
Article : Google Scholar
|
|
8
|
Afrakhte M, Morén A, Jossan S, Itoh S,
Sampath K, Westermark B, Heldin CH, Heldin NE and ten Dijke P:
Induction of inhibitory Smad6 and Smad7 mRNA by TGF-β family
members. Biochem Biophys Res Commun. 249:505–511. 1998.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kim KS, Park HK, Lee JW, Kim YI and Shin
MK: Investigate correlation between mechanical property and aging
biomarker in passaged human dermal fibroblasts. Microsc Res Tech.
78:277–282. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kuchler RJ: Development of animal cell
populations in vitro. In: Biochemical Methods in Cell Culture and
Virology. Kuchler RJ (ed). Dowden, Hutchinson & Ross, Inc.,
Stroudsburg PA, pp90-113. 1977.
|
|
11
|
Sjerobabski-Masnec I and Situm M: Skin
aging. Acta Clin Croat. 49:515–518. 2010.PubMed/NCBI
|
|
12
|
Moura J, da Silva L, Cruz MT and Carvalho
E: Molecular and cellular mechanisms of bone morphogenetic proteins
and activins in the skin: Potential benefits for wound healing.
Arch Dermatol Res. 305:557–569. 2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Beer HD, Gassmann MG, Munz B, Steiling H,
Engelhardt F, Bleuel K and Werner S: Expression and function of
keratinocyte growth factor and activin in skin morphogenesis and
cutaneous wound repair. J Investig Dermatol Symp Proc. 5:34–39.
2000.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Antsiferova M, Huber M, Meyer M,
Piwko-Czuchra A, Ramadan T, MacLeod AS, Havran WL, Dummer R, Hohl D
and Werner S: Activin enhances skin tumourigenesis and malignant
progression by inducing a pro-tumourigenic immune cell response.
Nat Commun. 2(576)2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kojima I, Maeshima A and Zhang YQ: Role of
the activin-follistatin system in the morphogenesis and
regeneration of the renal tubules. Mol Cell Endocrinol.
180:179–182. 2001.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Refaat B, Ashshi AM, El-Shemi AG and Azhar
E: Activins and Follistatin in Chronic Hepatitis C and Its
Treatment with Pegylated-Interferon-α Based Therapy. Mediators
Inflamm. 2015(287640)2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Santoro N, Adel T and Skurnick JH:
Decreased inhibin tone and increased activin A secretion
characterize reproductive aging in women. Fertil Steril.
71:658–662. 1999.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Barrios-Silva LV, Parnell M, Shinwari ZB,
Chaudhary GA, Xenofontos T, van Bekhoven A, McArthur S and Elliott
BT: Activin subfamily peptides predict chronological age in humans.
Physiol Rep. 6(e13823)2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hedger MP and de Kretser DM: The activins
and their binding protein, follistatin-Diagnostic and therapeutic
targets in inflammatory disease and fibrosis. Cytokine Growth
Factor Rev. 24:285–295. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Massagué J: TGF-β signal transduction.
Annu Rev Biochem. 67:753–791. 1998.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Fisher GJ, Shao Y, He T, Qin Z, Perry D,
Voorhees JJ and Quan T: Reduction of fibroblast size/mechanical
force down-regulates TGF-β type II receptor: Implications for human
skin aging. Aging Cell. 15:67–76. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhao H, Patra A, Tanaka Y, Li LC and
Dahiya R: Transforming growth factor-β(s) and their receptors in
aging rat prostate. Biochem Biophys Res Commun. 294:464–469.
2002.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Han KH, Choi HR, Won CH, Chung JH, Cho KH,
Eun HC and Kim KH: Alteration of the TGF-β/SMAD pathway in
intrinsically and UV-induced skin aging. Mech Ageing Dev.
126:560–567. 2005.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Hübner G, Hu Q, Smola H and Werner S:
Strong induction of activin expression after injury suggests an
important role of activin in wound repair. Dev Biol. 173:490–498.
1996.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Hata A and Chen YG: TGF-β Signaling from
Receptors to Smads. Cold Spring Harb Perspect Biol.
8(a022061)2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Massagué J, Seoane J and Wotton D: Smad
transcription factors. Genes Dev. 19:2783–2810. 2005.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Matsuo SE, Fiore AP, Siguematu SM, Ebina
KN, Friguglietti CU, Ferro MC, Kulcsar MA and Kimura ET: Expression
of SMAD proteins, TGF-beta/activin signaling mediators, in human
thyroid tissues. Arq Bras Endocrinol Metabol. 54:406–412.
2010.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Choi MJ, Song KM, Park JM, Kwon MH, Kwon
KD, Park SH, Ryu DS, Ryu JK and Suh JK: Effect of SMAD7 gene
overexpression on TGF-β1-induced profibrotic responses in
fibroblasts derived from Peyronie's plaque. Asian J Androl.
17:487–492. 2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kim YI, Kim KS, Ahn HJ, Kang IH and Shin
MK: Reduced matrix metalloproteinase and collagen transcription
mediated by the TGF-β/Smad pathway in passaged normal human dermal
fibroblasts. J Cosmet Dermatol. Sep 11. 2019.(Epub ahead of print).
PubMed/NCBI View Article : Google Scholar
|