Introduction
Hepatocellular carcinoma (HCC), the most common type
of primary liver cancer, is the third leading cause of
cancer-associated mortality globally (1). It is the fifth most common cancer type
in men and the seventh most common cancer type in women. The
incidence of HCC varies topographically, with the majority of cases
occurring in developing countries. Southeast Asia and Sub-Saharan
Africa host >75% of HCC cases, with incidence rates exceeding 20
per 100,00 individuals (2). Southern
European countries have intermediate incidence rates, whereas North
America, South America and Northern Europe have the lowest
incidence rates (<5 per 100,000 individuals) (2).
A previous report suggests that the incidence of HCC
in areas with high and intermediate incidence may stabilize or even
decrease due to vaccination programs for hepatitis B virus (HBV)
and higher HBV treatment rates in China and Taiwan (3). The decrease in HCC incidence in Japan
and Southern Europe may be associated with an aging cohort of
patients with hepatitis C virus (HCV) infection (4). In contrast, there is a rapid increase in
the incidence of HCC in low-incidence areas, including the United
States (5). The two most important
causes of the rise in incidence in the USA are growing populations
of patients with an advanced HCV infection and non-alcoholic
steatohepatitis (2). HCC also has one
of the fastest growing mortality rates of all solid tumor types.
While the prognosis for most solid cancer types improved between
1994 and 2003, the mortality rate for HCC almost doubled (6). These trends warrant a further search for
an effective treatment. Numerous signaling pathways serve a
function in the development of HCC, but those involving vascular
endothelial growth factor (VEGF) angiogenesis stand out (7,8).
Benja-ummarit (BU) (‘Phaetsat Songkhro’) is a Thai
traditional medicine from the Thai scripture ‘Tadbunjob’ (9). BU extract is used to treat patients with
asthma, cough, short breath, liver abscess and anorexia (9). In Thai traditional medicine, all of
those symptoms are interpreted as signs of a malignant tumor
(10). In addition, BU is also used
as a laxative in Thai traditional medicine (10). It is composed of eight herbs [aloe
vera (Aloe barbadensis), red physic nut (Baliospermum
montanum), kaffir lime (Citrus hystrix), asafoetida
(Ferula assafoetida), gamboge resin (Garcinia
hanburyi), Javanese long pepper (Piper Chaba), black
pepper (Piper nigrum), ginger (Zingiber officinale)]
and epsom salt (magnesium sulfate) (9). A chemical component of gamboge resin,
gambogic acid, inhibits the proliferation of numerous types of
cancer cells in vitro, including HepG2(11) and SMMC-7221(12). In addition, evidence suggests that
when gambogic acid is co-administered with docetaxel, a
chemotherapy drug, an increased inhibitory effect is observed
against the proliferation of gastric and colorectal cancer cell
lines (13). It has also been
revealed that gambogic derivatives inhibit the proliferation of the
HepG2 and A549 cancer cell lines (14). Furthermore, gambogic acid has an
anti-angiogenic effect in numerous cancer types (15-19),
exhibits anti-inflammatory activity (20-22)
and anti-invasion activity against A549 human lung cancer cells
(23) and osteosarcoma cell lines
(24). Furthermore, when used against
human breast carcinoma MCF-7 cells or human chronic myelogenous
leukemia K562 cells, the cells are arrested at the G2/M (25) and G0/G1 transitions of the cell cycle
(26), respectively. It has also been
revealed that the decreased adhesion of human cancer cells is an
effect of gambogic acid (27).
Previously, a crude BU extract has been claimed to exert an
anti-proliferative effect on human lung cancer (A549) and liver
cancer (HepG2) cells by inducing apoptosis via reactive oxygen
species (ROS) generation (10).
To the best of our knowledge, only a single clinical
report has been published about the beneficial effects of BU in
patients with HCC (28). This
prospective descriptive study was performed in patients with
certified HCC. A total of 96 patients were treated with 300-1,200
mg BU twice daily in addition to standard drug treatment. Once BU
had been administered continuously for 2 months, the quality of
life of patients taking BU was significantly better compared with
that of similar patients not receiving BU. The study was undertaken
in 5 public hospitals in Thailand and used the Thai Modified
Function Living Index Cancer Questionnaire Version 2 score.
Subsequent to a follow-up of 1 year, the survival rate of this
patient group was higher compared with the control group. No
serious adverse effects were reported (28).
The successful in vitro studies and the
promising clinical study prompt interest in BU as an (adjuvant)
treatment for HCC. Furthermore, the aforementioned
anti-angiogenesis and anti-cancer effects of gambogic acid, which
is a chemical component of gamboge resin which is present in BU,
suggest that BU may inhibit the proliferation of cancer cells.
However, since the majority of the cited mechanistic studies were
performed in vitro, it is not easy to differentiate general
from specific cytotoxic effects. For all these reasons, a relevant
and reliable animal model is necessary to elucidate the putative
functions of BU in a solid biochemical base. For this reason, the
present study assessed the effects of BU extract in an established
rat liver cancer in vivo. In this model, the putative
anti-angiogenic effect of BU extract was also assessed.
Materials and methods
Preparation of a 95% ethanol extract
of BU
The 95% ethanol extract of BU was provided by Dr.
Arunporn Itharat from the Faculty of Medicine of Thammasat
University (9). The BU formula
consisted of aloe vera (2.48%), red physic nut (9.92%), kaffir lime
(33.06%), asafoetida (2.48%), gamboge resin (4.96%), Javanese long
pepper (2.48%), black pepper (2.48%), ginger (2.48%) and epsom salt
(39.66%). Dried plant material (300 g) in a hot air oven at 50˚C
was macerated in 95% ethanol for 3 days, filtered through a Whatman
No. 1 filter paper and concentrated using an evaporator.
Composition of the ethanolic extract
of BU
Liquid chromatography-mass spectrometry (LC-MS) was
used to determine the compounds in the BU extract. The analysis was
performed on an Agilent HPLC 1260 series consisting of a vacuum
degasser, a binary pump, an autosampler and a column thermostat
equipped with QTOF 6540 UHD accurate mass (Agilent Technologies
GmbH). MassHunter Software B06.0 (Agilent Technologies GmbH) was
used to control the LC-MS.
The separation of the sample solution was performed
on a Luna C18, 150x4.6 mm, 5 µm column (Phenomenex, Torrance). A 10
µl sample of each filtrated extract at a concentration of 20 mg/ml
was injected into the LC system with a solvent flow rate of 500
µl/min. The mobile phase consisted of a gradient elution between
water (solvent A) and acetonitrile (solvent B), each containing
0.1% v/v formic acid. The linear gradient elution was 5-95% for
solvent B starting at 0-35 min with holding for 5 min and post-run
for 5 min. The column temperature was controlled at 35˚C. The mass
analysis was performed using a QTOF 6540 UHD accurate mass
spectrometer. The conditions for the negative electrospray
ionization source were drying gas (N2) at a flow rate of
10 l/min, a drying gas temperature of 350˚C, nebulizer 30 psi,
fragmentor 100 V, capillary voltage 3,500 V and scan spectra from
m/z 100-1,000 amu. The auto MS/MS for the fragmentation was set
with collision energies of 10, 20 and 40 V. The positive mode was
also set up with the same MS conditions as the negative mode.
Animals
All animal experiments were performed according to
the Thai guidelines for the care and use of experimental animals,
subsequent to being approved by the Animal Ethics Committee of the
Faculty of Medicine, Srinakharinwirot University (Bangkok,
Thailand; approval no. 3/2558). A total of 42 male Wistar rats (6-7
weeks old) weighing 200-250 g were obtained from the National
Laboratory Animal Center of Mahidol University (Bangkok, Thailand).
The animals were acclimatized for one week. All animals were
maintained under standardized hygienic conditions throughout the
experimental period, including a temperature of 21-22˚C, humidity
at 55±5%, a standard 12 h light-dark regime and ad libitum
access to standard diet and tap water.
Experimental design
The experimental protocol for HCC induction was
based on El-Ashmawy et al (29). For the induction of HCC, 200 mg/kg
diethylnitrosamine (DEN; Sigma-Aldrich; Merck KGaA) was injected
intraperitoneally (i.p.) in a single dose. Following 14 days, the
rats were subjected to i.p. injections of 300 mg/kg thioacetamide
(TAA) (Sigma-Aldrich; Merck KGaA) 3 times weekly for 4 weeks. Then
the rats were left for 2 further weeks without any treatment. At
the end of the induction period (8 weeks), HCC rats were weighed
and randomly divided into 6 groups: i) No treatment; ii) treatment
with propylene glycol: Tween 80: deionized water (4:1:4), a solvent
of BU; iii) treatment with 30 mg/kg Sorafenib (30-34);
or treatment with iv) 1 mg/kg, v) 10 mg/kg or vi) 50 mg/kg BU.
Doses of BU used in the present study were based on those
previously used in vitro (9)
and demonstrated to be safe in a toxicity test in rats (Intharit
et al, preliminary study). During the time course of the
experimental tumor study (16 weeks), a set of criteria was
developed to follow the rats' condition. These included their
external physical appearance, appearance of any visible lesions,
changes in body weight and behavioral responses to external stimuli
(including light or noise and so on), which reflect pain and
distress in the animals. The humane endpoint in the present study
was based on a weight loss exceeding 20% of the body weight of the
rats in the control group. Subsequent to 16 weeks, experimental
rats (n=7 per group) were anesthetized with an i.p. injection of 45
mg/kg pentobarbital sodium prior to sacrifice by decapitation with
a rodent guillotine. Liver tissues and blood samples were collected
for histological and immunohistochemical analyses, liver function
tests, and reverse transcription-quantitative PCR (RT-qPCR) and
western blot analyses.
Measurement of liver/body weight
ratio
At the end of the treatment period, the body weight
of all animals along with their respective livers were measured in
order to determine the liver-to-body-weight ratio in each
group.
Assay of serum alanine
aminotransferase (ALT) and albumin
Blood samples, collected by cardiac puncture, were
assayed in a standard clinical lab for serum ALT and albumin. The
reagent kits for ALT (cat. no. 7D56-21) and albumin (cat. no.
7D53-23) were used (Abbott Pharmaceutical Co. Ltd.). The signals
were detected by ARCHITEC model Ci16200 (Abbott Pharmaceutical Co.
Ltd.).
Histopathological study
Liver tissues were fixed overnight at 4˚C in 4%
(v/v) formaldehyde solution, dehydrated in an ascending series of
ethanol (50, 70, 80, 90, 95 and 100%), cleared in xylene and
embedded in paraffin. Specimens were sliced into sections that were
5 µm thick. The slides were stained with hematoxylin for 6 min and
eosin for 1 min at room temperature and scanned with a panoramic
digital slide scanner (3DHISTECH Ltd.). For each image, an area of
4,000x2,500 µm (10 mm2) was randomly selected to locate
and calculate the cancer area characterized histopathologically by
the presence of thick-cell cords (35) using the CaseViewer software
(v1.3.0.41885; https://www.3dhistech.com/caseviewer). A total of 21
images of each animal group were sampled, in which three
specialists in liver histopathology identified the thick-cell cords
and located the cancer areas. The mean cancer area in each group
was then calculated.
Immunohistochemistry
The deparaffinized tissue sections were treated with
xylene and rehydrated in a descending series of ethanol (100, 95,
90, 80, 70 and 50%), followed by antigen retrieval in 10 mM sodium
citrate buffer (pH 6.0) for 10 min at 120˚C using an autoclave. The
blocking solution TENG-T (10 mM Tris, 5 mM EDTA, 150 mM NaCl, 0.25%
gelatin and 0.05% Tween 20; pH 8.0) containing 10% goat serum was
applied for 30 min at room temperature to the slides to block any
non-specific binding, and the slides were incubated with mouse
anti-VEGF immunoglobulin G (IgG; cat. no. sc-53462; Santa Cruz
Biotechnology, Inc., Dallas; 1:50) at 4˚C overnight. The slides
were then incubated for 2 h at room temperature with alkaline
phosphatase-conjugated goat anti-mouse IgG (cat. no. 11569520,
Sigma-Aldrich; Merck KGaA; 1:100). Subsequent to washing in
phosphate buffered saline (PBS), the sections were incubated in
substrate containing nitroblue tetrazolium
chloride/5-bromo-4-chloro-3-indolyl phosphate (toluidine salt;
Dako; Agilent Technologies GmbH) diluted in 100 mM Tris (pH 9.5),
100 mM NaCl and 50 mM MgCl2 at room temperature for
30-120 min to visualize the immunopositive areas in the tissues.
The staining reaction was stopped by washing with distilled water.
The sections were dehydrated with ethanol, cleared in xylene and
covered with Permount® prior to being examined and
photographed under a light microscope (Olympus Corporation).
RNA isolation and RT-qPCR assays
The livers were homogenized with a sonicator (Sonics
& Materials, Inc.) followed by total RNA isolation using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). Total RNA
with 2 µg of each RNA sample was reverse transcribed with the High
Capacity cDNA Reverse Transcriptase kit (Applied Biosystems; Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocol
(25˚C for 10 min, 37˚C for 120 min, 85˚C for 5 min and hold at
4˚C). RT-qPCR was set up using the SsoAdvanced™
SYBR® Green Supermix (Bio-Rad Laboratories, Inc.), along
with cDNA and commercial PrimePCR™ primers (Bio-Rad
Laboratories, Inc.). The primer used was rat Vegfa (unique
assay ID: qRnoCED0002159). The differences in sample RNA content
were normalized to rat β-actin (Actb) expression (unique
assay ID: qRnoCID0056984). The conditions of the reactions were as
follows: 95˚C for 2 min of polymerase activation, 40 cycles of 95˚C
for 5 sec for denaturation and 60˚C for 30 sec for primer annealing
and extension. Each sample's mRNA expression was measured in
triplicate to ensure the fidelity and accuracy of the results using
a CFX96 Real-Time PCR Detection System and Bio-Rad
manager™ software version 1.3.1 (Bio-Rad Laboratories,
Inc.). The quantification of relative mRNA expression was
calculated using the 2-∆∆Cq method
(36).
Protein extraction and western blot
analysis
A total of 50 mg frozen liver was homogenized in 500
µl radioimmunoprecipitation lysis buffer (Santa Cruz Biotechnology,
Inc.,). The lysate was centrifuged at 4˚C and 12,000 x g for 15 min
to collect the supernatant fraction. Protein concentration was
determined using a Bradford protein assay (Bio-Rad Laboratories,
Inc.). Total protein samples (40 µg) were separated on 12%
SDS-polyacrylamide gels (Bio-Rad Laboratories, Inc.) and
transferred onto a 0.2 µm polyvinylidene difluoride membrane. The
membrane was blocked at room temperature for 1 h with 5% non-fat
milk in 1X PBS-0.1%Tween 20 (PBS-T), then washed with 1X PBS-T, and
incubated overnight at 4˚C with a primary mouse anti-VEGF antibody
(cat. no. sc-53462; Santa Cruz Biotechnology; diluted 1:500).
Subsequent to washing thrice in 1X PBS-T for 5 min, the membrane
was incubated at room temperature for 90 min with horseradish
peroxidase-conjugated goat anti-mouse secondary antibody (cat. no.
7076S lot 32; Santa Cruz Biotechnology; diluted 1:10,000) followed
by 3x10 min washes with 1X PBS-T. Protein bands were developed
using enhanced chemiluminescence (Bio-Rad Laboratories, Inc.) and
quantified using densitometry with Scion Image software version
Beta 4.0.3 (Meyer Instruments, Inc.). β-actin was used as a loading
control.
Statistical analysis
Data were analyzed using a one-way analysis of
variance with a Tukey's post-hoc test (PSPP 0.10.4; http://gnu.org). All results were represented as the
mean ± standard deviation. P<0.05 was considered to indicate a
statistically significant difference.
Results
Composition of the ethanolic extract
of BU
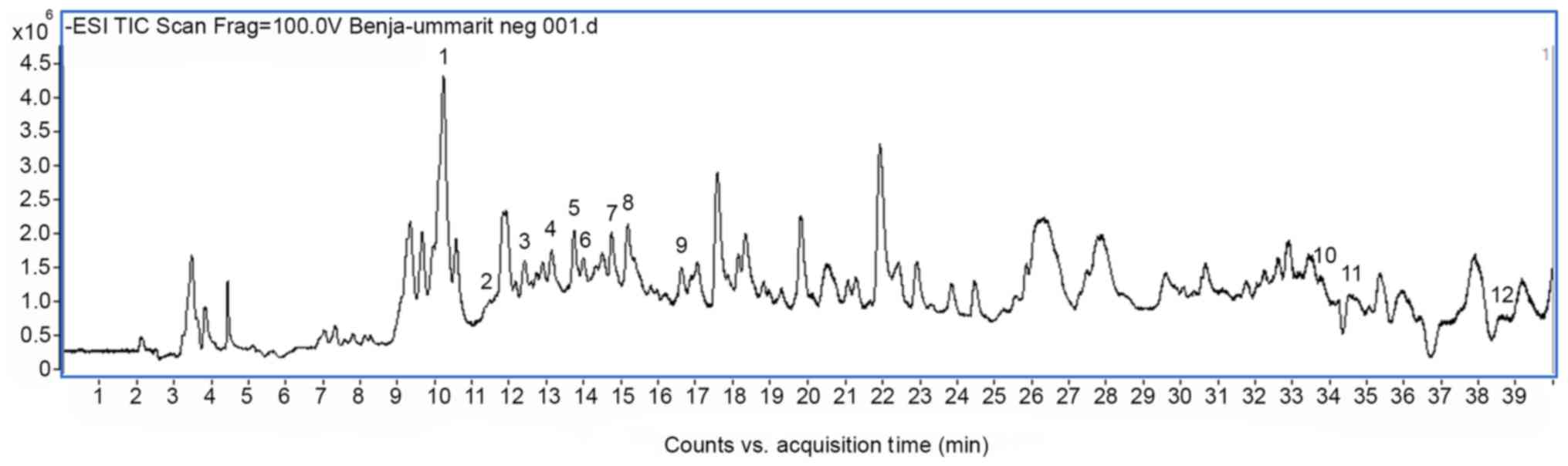
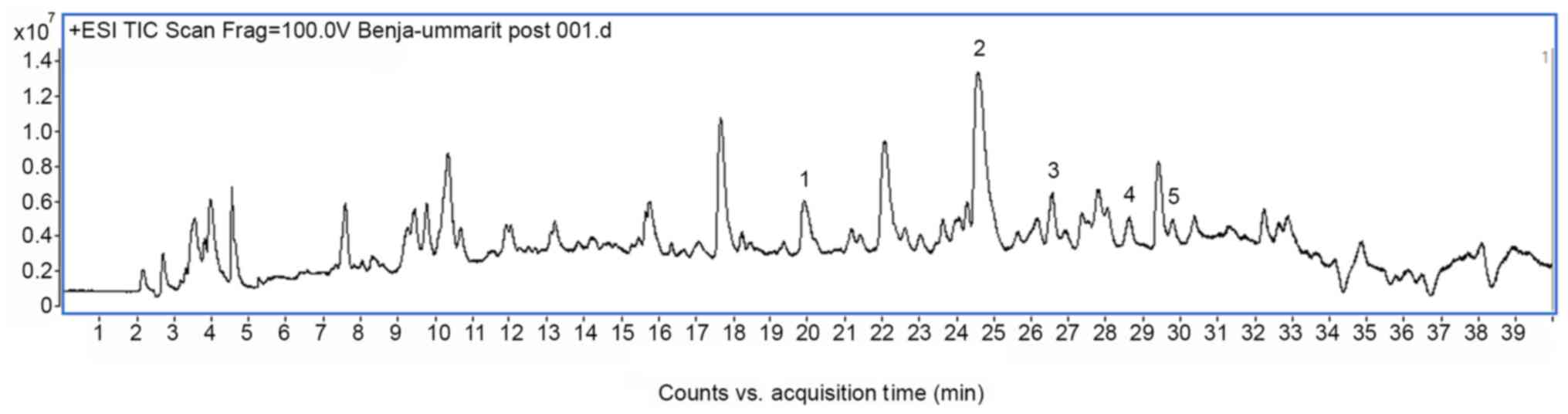
In the negative mode, 12 compounds were tentatively
identified based on their molecular mass and fragmentation pattern
(Fig. 1 and Table I). Aloin, aloesin and their
derivatives together with dihydrogambogic acid and gambogic acid
were indicated to be present in the extract. These compounds were
identified in aloe vera and gamboge resin. In addition, five
alkaloids from black pepper were identified in the positive mode
(Fig. 2 and Table II). The chromatograms, mass spectra
data and compounds proposal were presented in Figs. 1 and 2
and Tables I and II. This analysis confirms the previously
published results for the original extract (9).
 | Table IResults of liquid chromatography-mass
spectrometry and the identification of putative active components
in the ethanolic Benja-ummarit extract monitored in the
electrospray ionization negative mode. |
Table I
Results of liquid chromatography-mass
spectrometry and the identification of putative active components
in the ethanolic Benja-ummarit extract monitored in the
electrospray ionization negative mode.
| Peak | Retention time,
min | m/z [M-H]- | MS/MS
fragmentation | Tentative
identification | Formula | Error (ppm) |
|---|
| 1 | 10.091 | 393.1197 | 273.0850, 203.0769,
59.0376 | Aloesin or
aloeresin B derivative |
C19H22O9 | -1.51 |
| 2 | 11.67 | 393.1173 | | Aloesin or
aloeresin B derivative |
C19H22O9 | 4.59 |
| 3 | 12.356 | 393.1171 | 273.0820, 203.0753,
125.0274, 59.0161 | Aloesin or
aloeresin B derivative |
C19H22O9 | 5.1 |
| 4 | 12.711 | 393.117 | 273.0829, 203.0757,
59.0175 | Aloesin or
aloeresin B derivative |
C19H21O9 | 5.36 |
| 5 | 13.699 |
479.1164a | 433.1248, 270.0606,
187.9765 | 5-Hydroxyaloin
A |
C21H22O10 | 6.47 |
| 6 | 14.108 | 539.1537 | 375.1186, 273.0837,
163.0445, 119.0541 |
2'-o-p-Coumaroylaloesin |
C28H28O11 | 4.05 |
| 7 | 14.731 | 417.1172 | 297.0841 | Aloin A |
C21H22O9 | 4.57 |
| 8 | 15.165 | 417.1169 | 297.0854,
205.0195 | Aloin B |
C21H22O9 | 5.29 |
| 9 | 16.859 | 553.1659 | 443.1397,
279.0694 |
2'-p-Methoxycoumaroylaloeresin |
C29H30O11 | 10.19 |
| 10 | 33.791 | 629.3115 | 461.1940, 392.1234,
337.0694 | Dihydrogambogic
acid derivative |
C38H46O8 | 0.78 |
| 11 | 34.499 | 629.3106 | 541.3295, 461.1939,
392.1246 | Dihydrogambogic
acid derivative |
C38H46O8 | 2.21 |
| 12 | 38.64 | 627.2951 | | Gambogic acid |
C38H44O8 | 1.27 |
 | Table IIResults of liquid chromatography-mass
spectrometry and the identification of putative active components
in the ethanolic Benja-ummarit extract monitored in the
electrospray ionization positive mode. |
Table II
Results of liquid chromatography-mass
spectrometry and the identification of putative active components
in the ethanolic Benja-ummarit extract monitored in the
electrospray ionization positive mode.
| Peak | Retention time,
min | m/z [M+H]+ | MS/MS
fragmentation | Tentative
identification | Formula | Error (ppm) |
|---|
| 1 | 19.8 | 336.3314 | 290.2888,
81.0712 | Pipericine |
C22H41NO | -15.78 |
| 2 | 24.4 | 286.149 | 201.0579,
135.0458 | Piperine |
C17H19NO3 | -18.28 |
| 3 | 26.5 | 312.1637 | 227.0735, 169.0669,
112.0772 | Piperettine |
C19H21NO3 | -13.71 |
| 4 | 28.5 | 340.1959 | 179.1329, 112.0771,
103.0556 |
Dehydropipernoline |
C21H25NO3 | -15.23 |
| 5 | 29.7 | 356.2274 | 255.1415,
135.0457 | Pipercide |
C22H29NO3 | -15.10 |
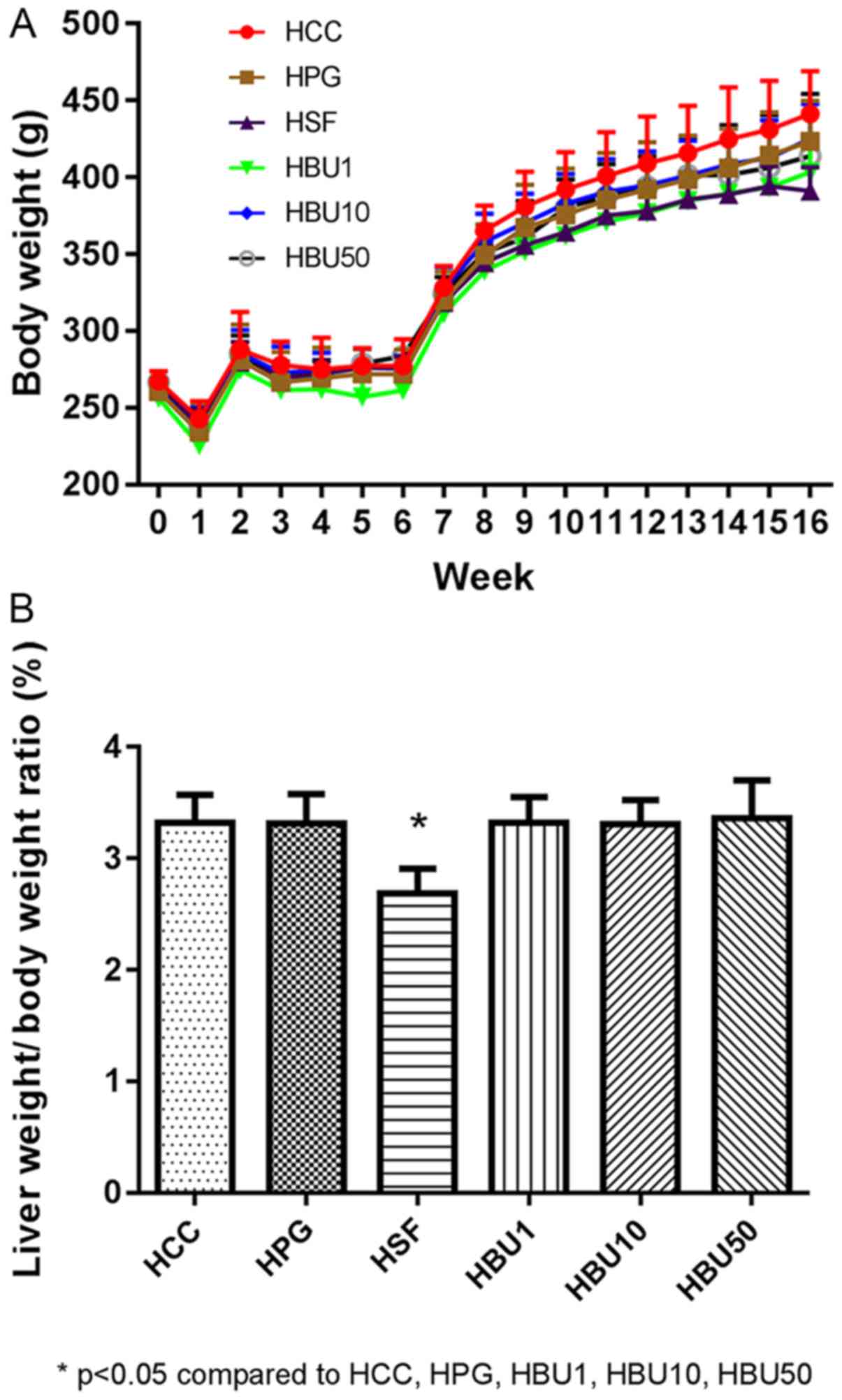
Changes in body weight and liver/body
weight ratio
Fig. 3A presents the
changes in the body weight in the respective groups of rats. Apart
from the anticipated adverse effects of DEN administration at the
beginning of the experiment and of thioacetamide during weeks 2-6,
the experimental animals gained weight at the same rate as the
control animals. To identify the potentially harmful general effect
of the treatments on the liver, the liver/body weight ratio was
determined (Fig. 3B). Rats treated
with Sorafenib had a significantly reduced liver/body weight ratio
compared with all other groups (P<0.05). There were no
significant effects in the BU-treated groups when compared with the
non-treated and vehicle-treated groups (Fig. 3).
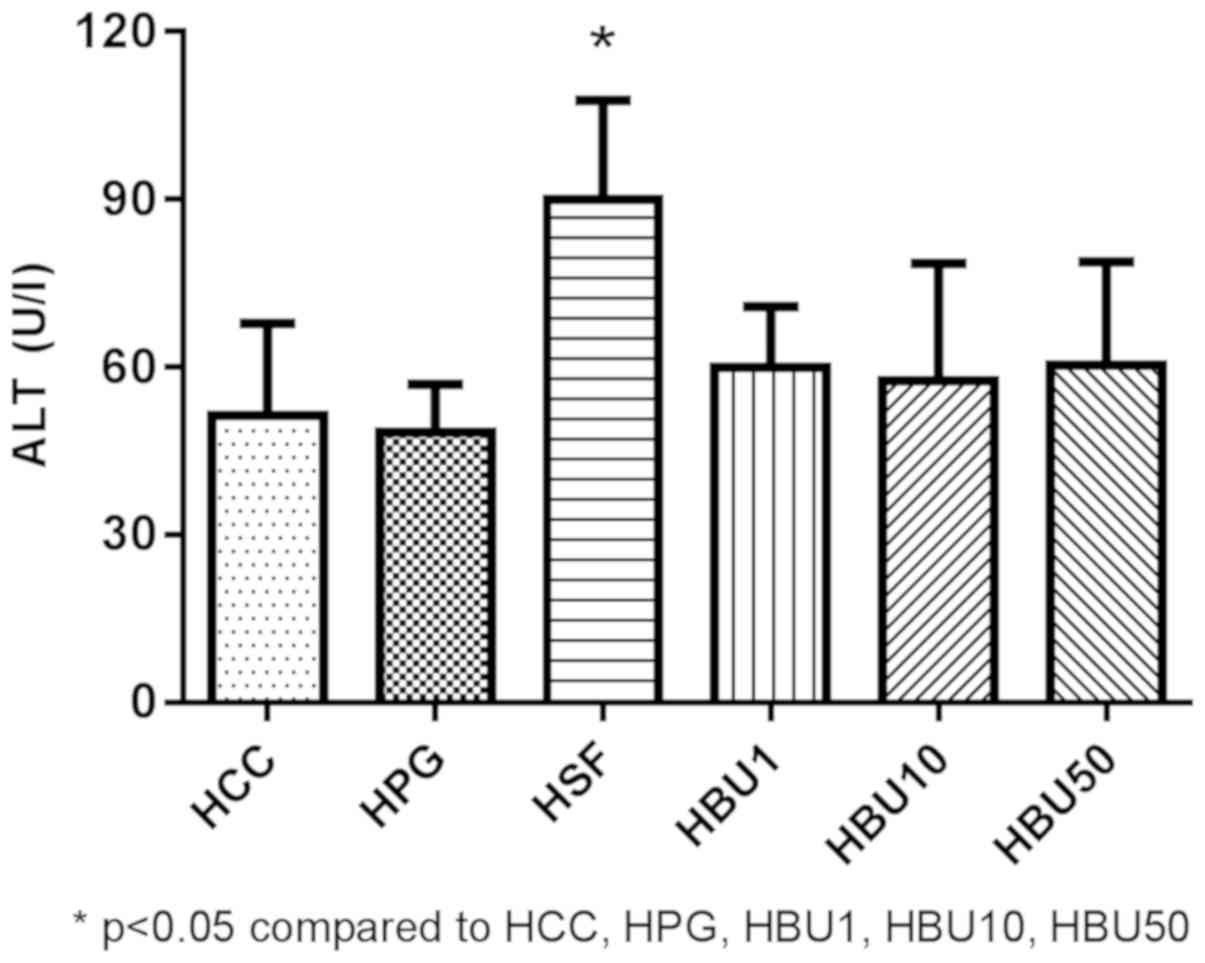
Biochemical markers
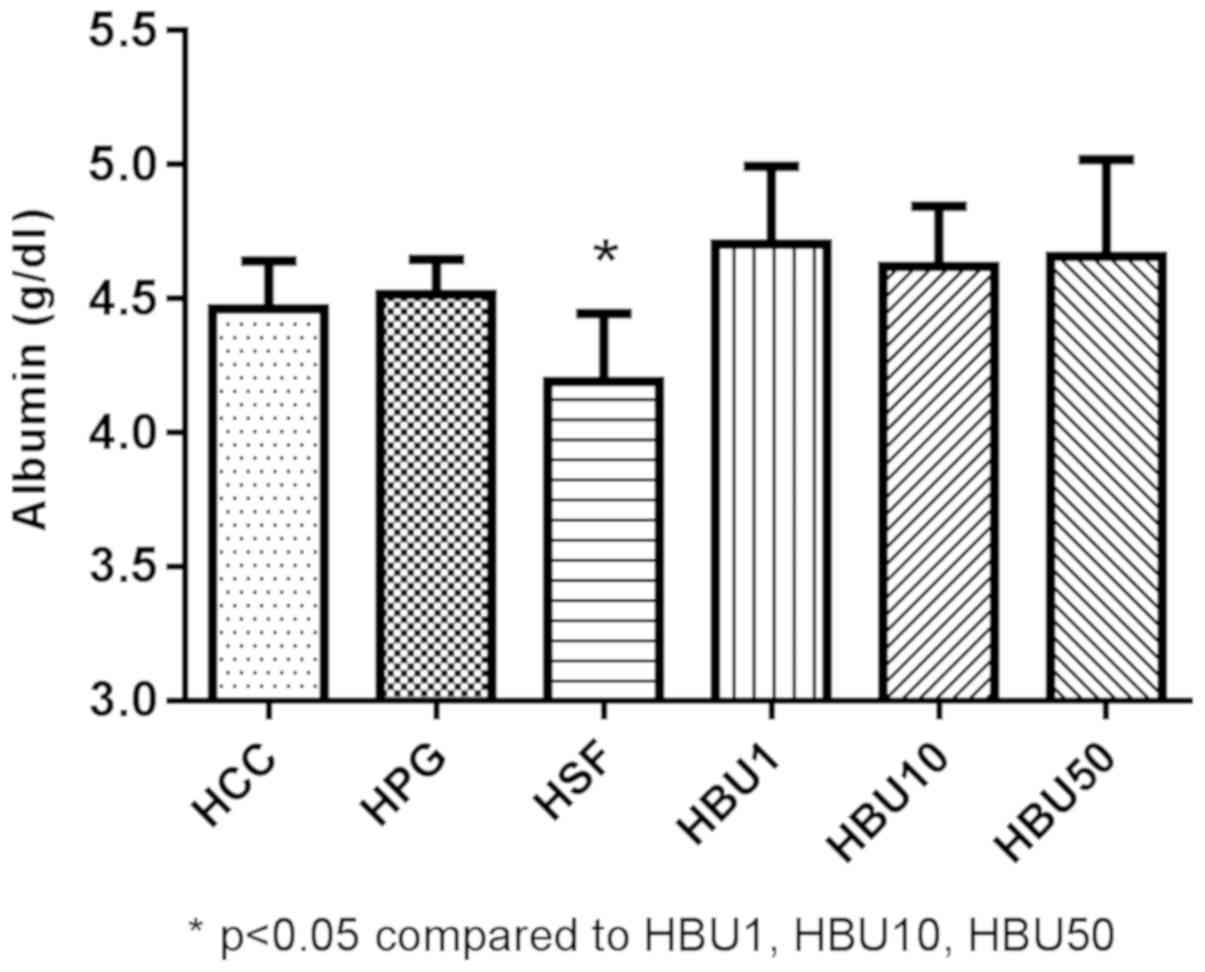
Figs. 4 and 5 revealed the effects of BU on liver
function tests (serum ALT activity and albumin content) in the
experimental animals. The serum ALT level was significantly
increased in the Sorafenib-treated group compared with all other
groups (P<0.05; Fig. 4). On the
other hand, the serum albumin concentration was significantly lower
in the Sorafenib-treated group compared with all other groups
(P<0.05; Fig. 5). The results
demonstrate that Sorafenib causes liver injury.
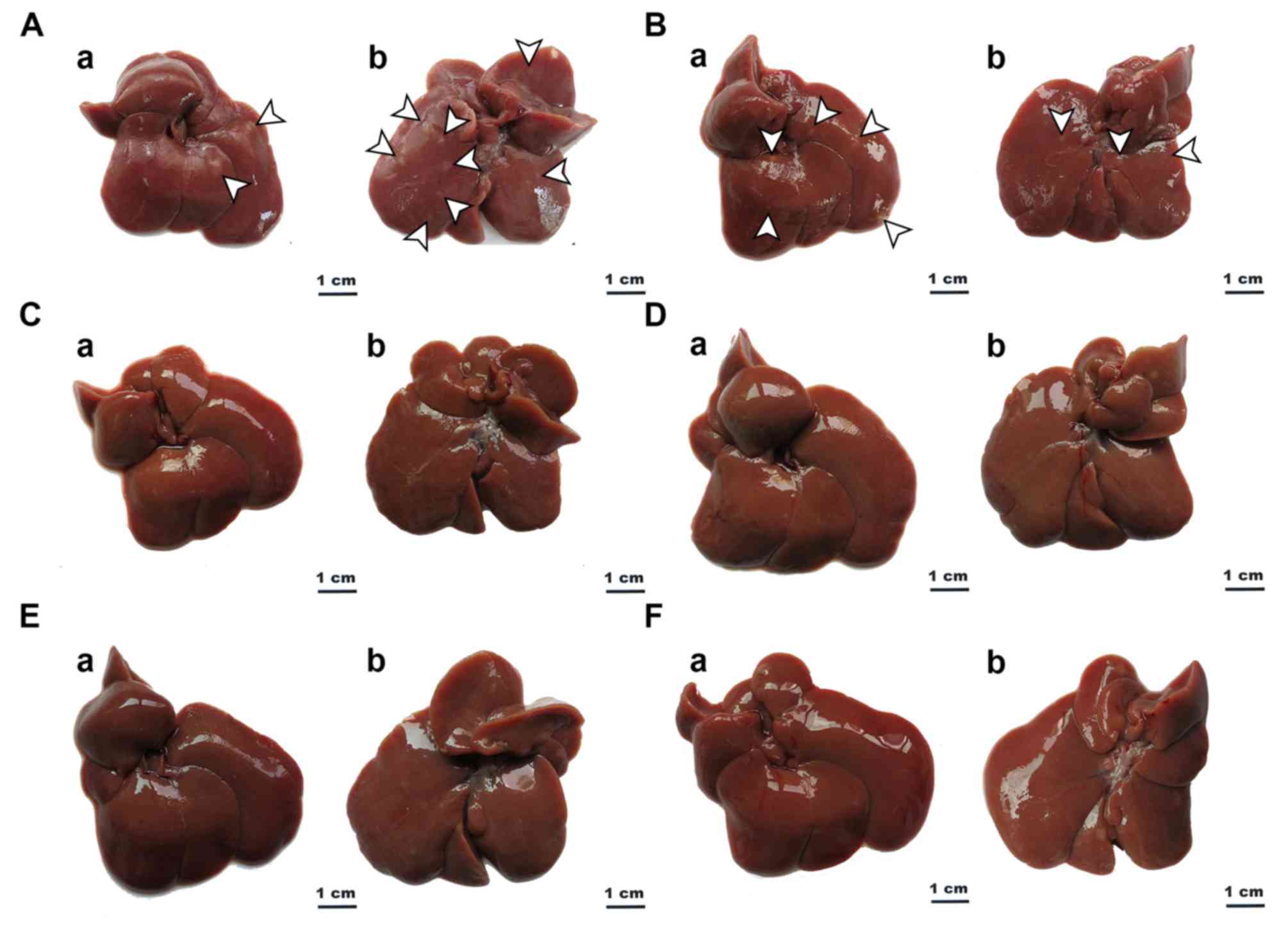
Gross anatomy and histopathology
Fig. 6 revealed that
Sorafenib (Fig. 6C) and BU (Fig. 6D-F) suppressed nodule growth compared
with untreated and vehicle-treated rats with HCC (Fig. 6A and B).
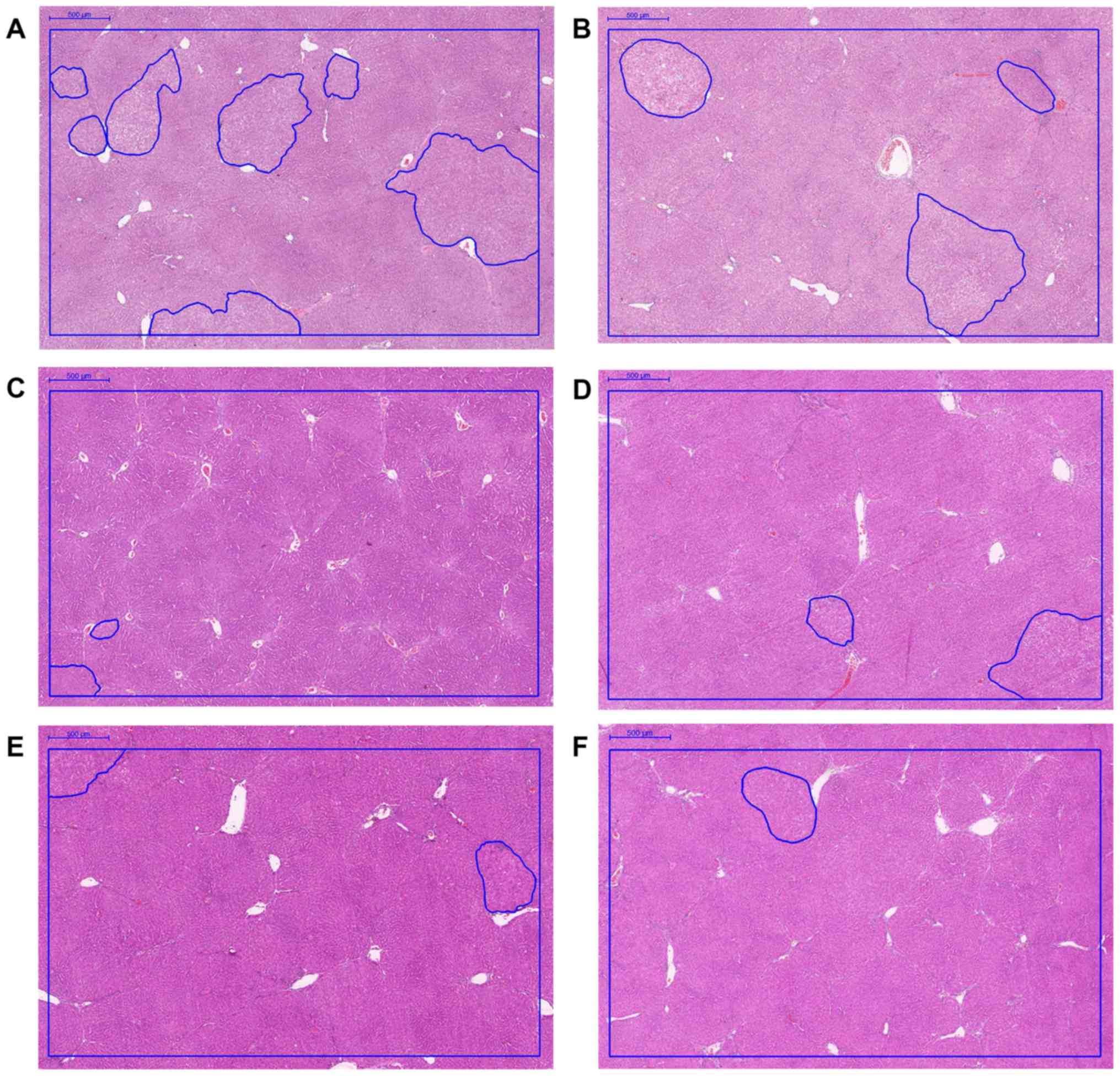
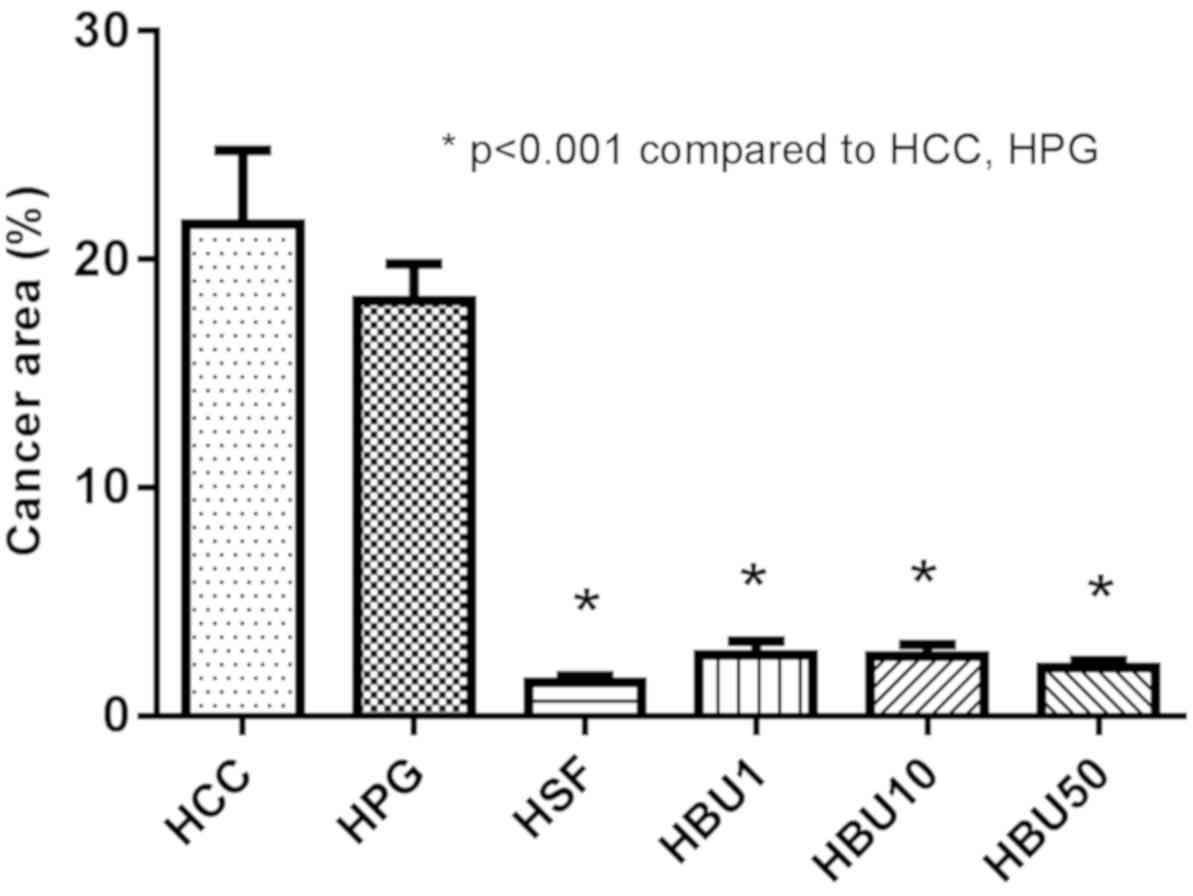
Sections revealed the characteristic histopathological thick-cell
cord changes in the HCC nodules of non-treated (Figs. 7A and 8,
22±9%) and vehicle-treated rats (Figs.
7B and 8, 18±4%). The percentage
of the cancer area was lowest in the Sorafenib-treated group
compared with all other groups (Figs.
7C and 8, 1.5±0.7%). The
percentage of the cancer area in the BU-treated group decreased
dose-dependently from 2.7±1.6% (Figs.
7D and 8; 1 mg), to 2.6±1.4%
(Figs. 7E and 8; 10 mg) to 2.1±0.8% (Figs. 7F and 8;
50 mg), and was significantly reduced when compared with the
vehicle-treated group (P<0.05). These data reveal that BU
reduces cancer growth in vivo.
BU inhibits VEGF expression
Next, the mechanisms by which BU exerts its
antitumor effect in vivo were investigated.
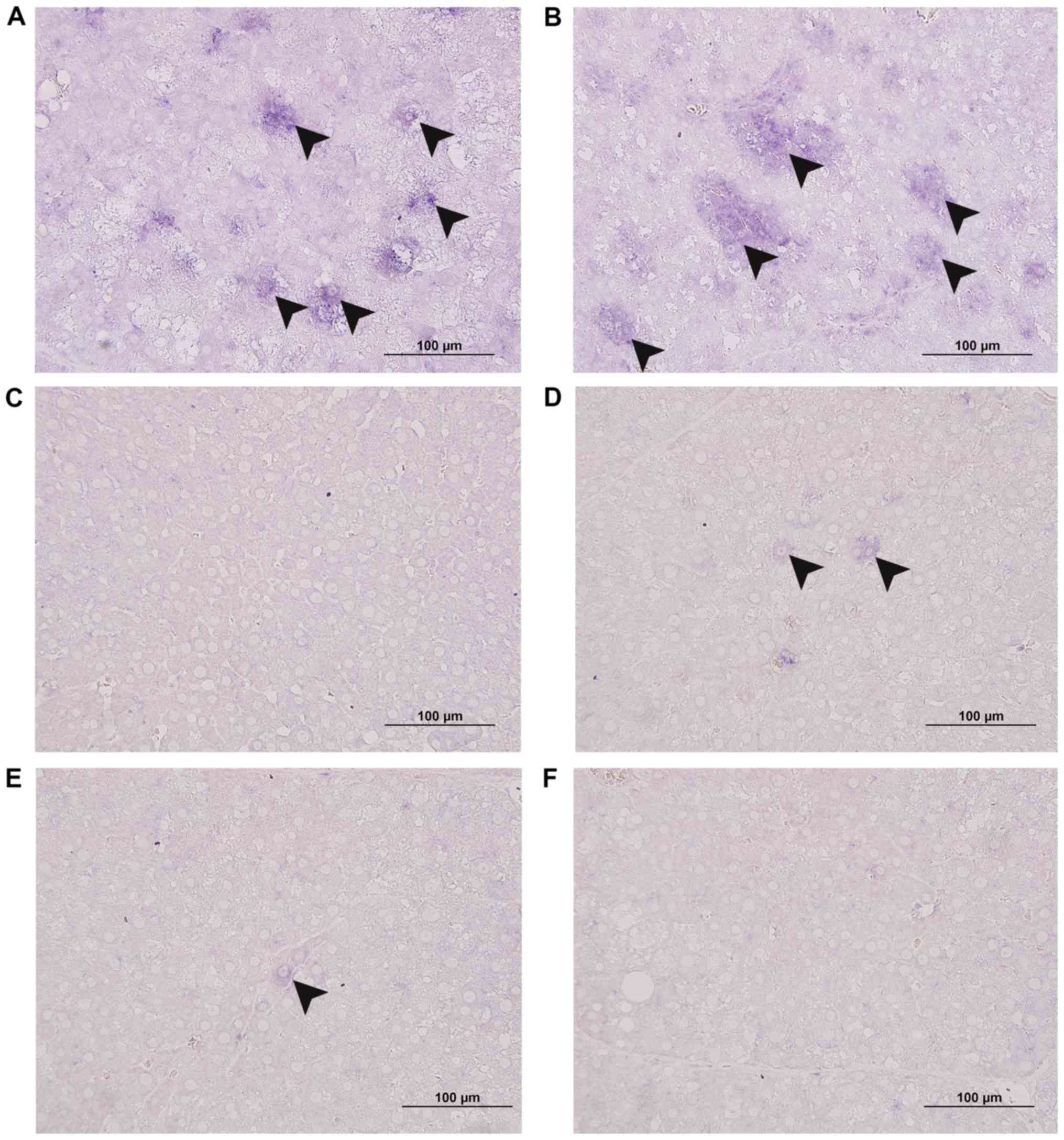
Immunohistochemical analysis revealed that the cytoplasmic VEGF
concentration was markedly increased in cancerous areas (Fig. 9A; arrowheads) and that BU solvent
alone did not change that result (Fig.
9B). In contrast, Sorafenib (Fig.
9C) and BU treatment prevented the formation of VEGF-positive
cancer areas (Fig. 9D-F) in rats with
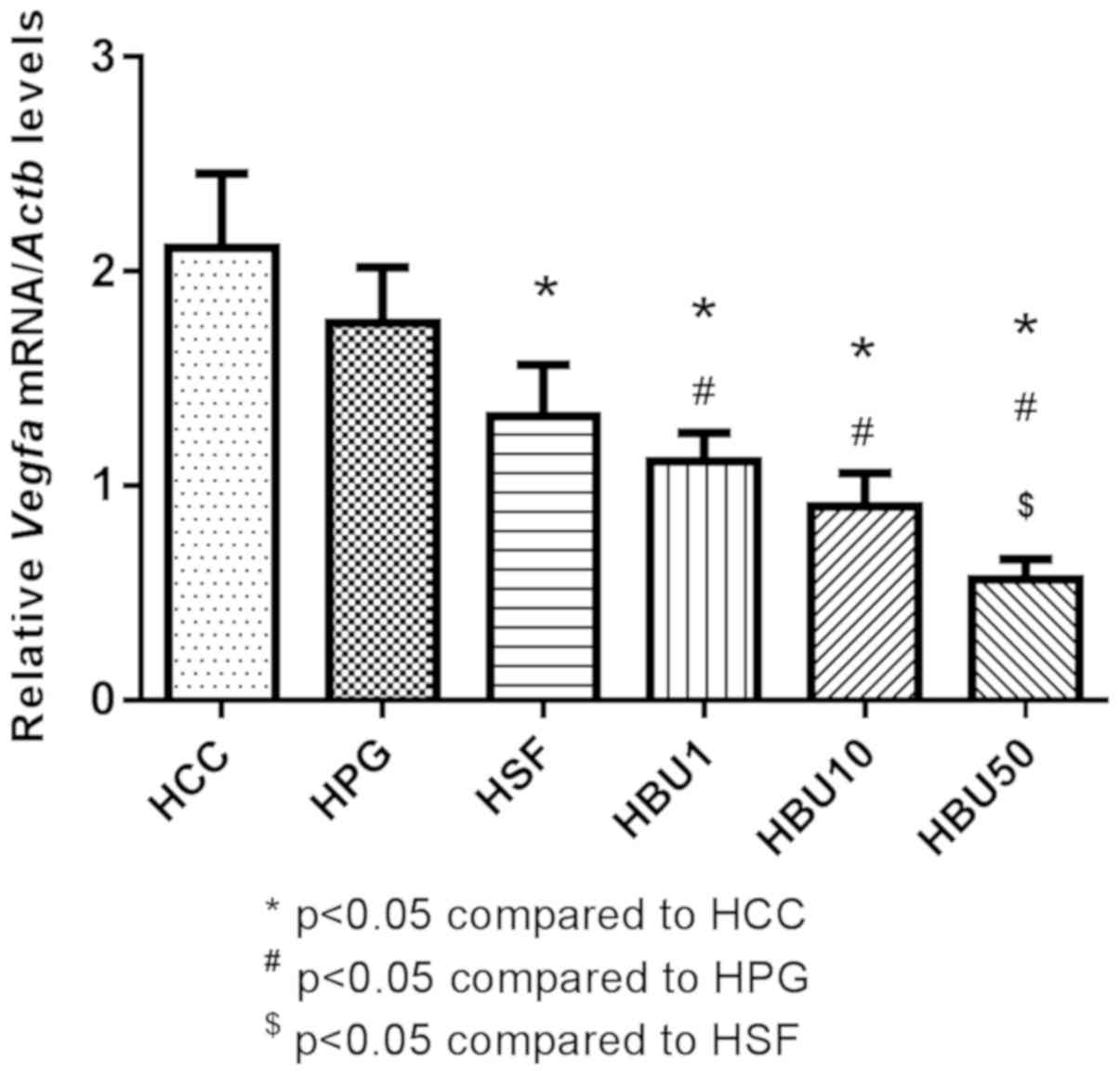
HCC. In agreement with these results, VEGF mRNA expression was
revealed to be significantly downregulated by Sorafenib compared
with the control group (P<0.05) and an even stronger and
dose-dependent downregulation by increasing doses of BU compared
with the control groups (P<0.05; Fig.
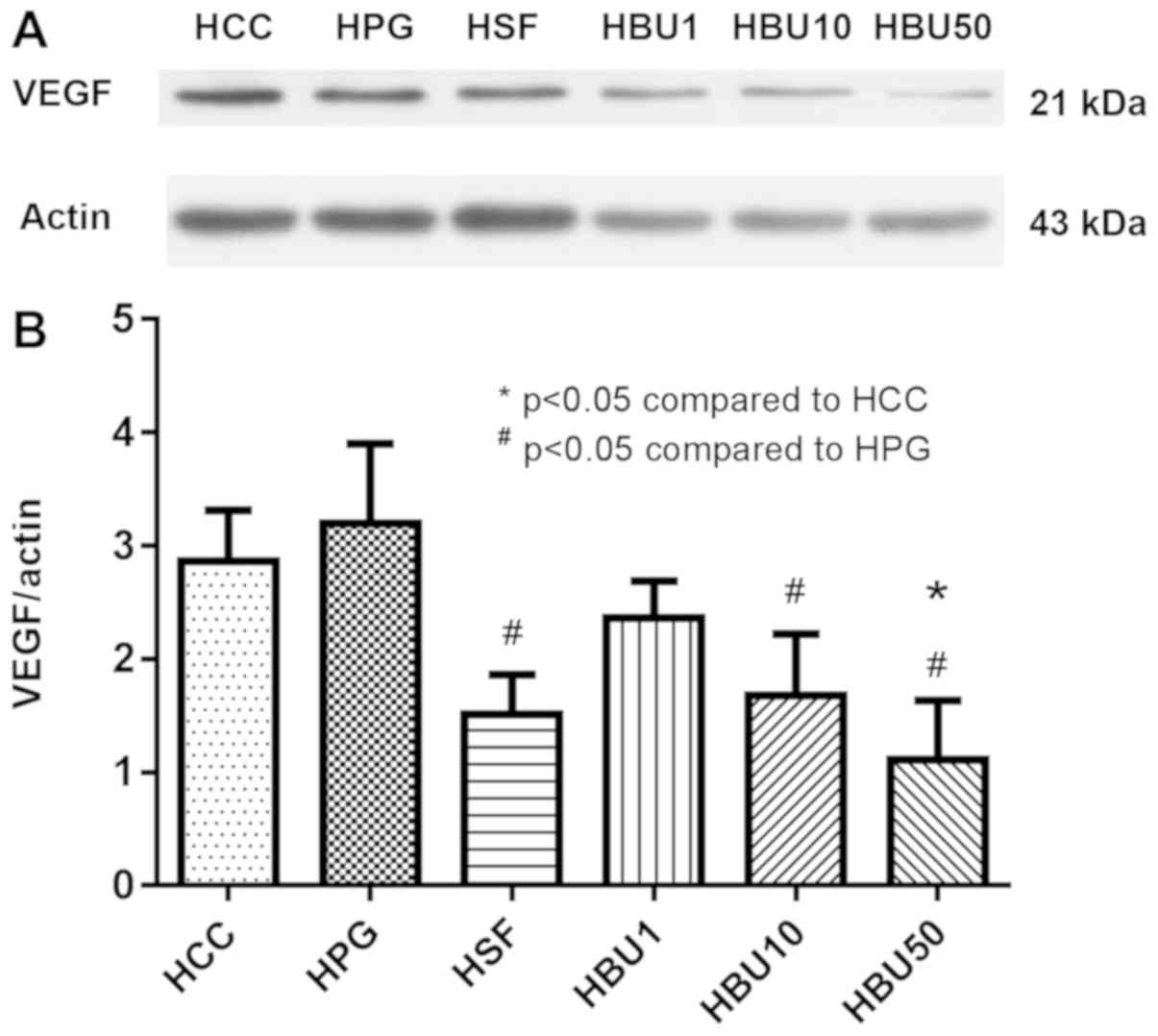
10). Similarly, western blot analysis of the liver revealed
that VEGF protein content was, compared with untreated and
vehicle-treated rats with HCC, decreased significantly by Sorafenib
treatment and treatment with the two highest doses (10 and 50 mg)
of BU (P<0.05; Fig. 11). These
results suggest that the anticancer activity of BU is mediated at
least in part by the inhibition of VEGF expression in rats with
HCC.
Discussion
BU is a traditional Thai herbal medicine containing
a crude extract of eight plants and thus consists of numerous
ingredients. The word ‘Benja-ummarit’ is a combination of the Thai
words ‘Benja’ (five) and ‘Ummarit’ (holy compound or nectar).
Although BU typically contains the extracts of eight plants and
epsom salts (MgSO4), its main active ingredients appear
to be derived from only four plants (gamboge resin, aloe vera,
asafoetida and red physic nut) and MgSO4 (9). The other four plant extracts (kaffir
lime, ginger, Javanese long pepper and black pepper) are added
during the preparation of BU to reduce its toxicity, to eliminate
the accumulation of gas in the alimentary canal and to increase
appetite (9).
The present study performed experiments with the
crude extract of BU as opposed to admixing highly purified
components of BU, as the aim was to initially demonstrate that a
crude BU extract exerts a similar effect in vivo as was
demonstrated in vitro previously in a HepG2 HCC cell line
(9,10). Furthermore, the ingredients of BU may
have additive or even synergistic effects, as the cytotoxicity of
crude extracts of BU was higher compared with that of each of its
ingredients separately in the HepG2 cell line (9). In this respect, it is encouraging that
the in vitro may be extrapolated in vivo. Following
the same experimental protocol as used in the previous study and
comparing treatment with 1 mg/kg crude BU extract with 1 mg/kg
gamboge resin revealed that the cancer areas in the liver of rats
with HCC were ~1.5-fold larger in the gamboge resin-treated group
compared with the BU-treated animals (preliminary data not
shown).
The anti-angiogenic effect of BU has been ascribed
to numerous components of the BU extract, including gambogic acid
in the gamboge resin (17,19,22),
aloe-emodin and aloin in aloe vera (37,38),
galbanic acid in asafetida (39),
6-gingerol in ginger (40-42),
piplartine in Javanese long pepper (43) and piperine in black pepper (44). Among these substances, gambogic acid
appears to have the strongest anti-angiogenic activity (16,17,19,22)
and was present in easily detectable amounts in the extract used in
the present study. Gambogic acid is claimed to function via the
inhibition of the VEGF receptor 2(22) and its downstream protein kinases SRC
proto-oncogene, non-receptor tyrosine kinase, protein tyrosine
kinase 2, extracellular signal-regulated kinase (ERK), p38 and
protein kinase B (AKT) (16,19) or by inhibiting the egl-9 family
hypoxia inducible factor 1-von Hippel-Lindau tumor
suppressor-hypoxia inducible factor-1α pathway (17).
The anti-proliferative property of a crude extract
of BU has been ascribed to much the same components as its
anti-angiogenic effects, including gambogic acid in gamboge resin
(13,16,45),
aloe-emodin in aloe vera (46,47),
galbanic acid in asafetida (39),
6-gingerol or zingerone in ginger (40,41,48,49),
piplartine in Javanese long pepper (50) and piperine in black pepper (51-55).
6-Gingerol or zingerone in ginger reportedly inhibits cell
proliferation via cell cycle arrest at the G1 phase (40) via the downregulation of cyclin D1
expression (48,49) or inhibition of nuclear factor-κβ
activation (41). Similarly, piperine
in black pepper may induce cell cycle arrest via the downregulation
of cyclin D1 (51,52,54).
Piperine from black pepper stops cell proliferation in breast
cancer stem cells by inhibiting Wnt/β-catenin signaling (51). In contrast, aloe-emodin in aloe vera
appears to inhibit cell proliferation via the phosphorylation of
AKT and ERK (46). Piplartine in
Javanese long pepper inhibits cell-cycle progression in various
tumor cells by inactivating cyclin-dependent kinase 2 and
destabilizing cyclin D1(50).
In addition to the anti-angiogenic and
anti-proliferative properties of BU, each ingredient may have
further effects. Aloe-emodin in aloe vera, 6-gingerol in ginger and
piperine in black pepper are all reported to inhibit tumor invasion
and metastasis through the suppression of expression of matrix
metalloproteinase-2/9 (38,42). All ingredients of BU, except epsom
salts, have been revealed to exert apoptotic activity in various
cancer cell lines, including SMMC-7721(56), a human glioblastoma cell line (U87MG)
(57), prostate cancer (58), H460 non-small cell lung carcinoma
(59), adriamycin-resistant human
leukemia (K562/ADR) (60),
MCF-7(61), IOMM-Lee and CH157MN
(62) via the overexpression of BCL2
associated X, apoptosis regulator (60-62),
caspase 3 (57,62), caspase 8(57), caspase 9(59), induced ROS accumulation (56,61) or via
the AKT/mitogen-activated protein kinase pathway (60).
A note of caution with respect to the validity of
all these reported effects of the components of BU is required, as
nearly all these experiments have been performed in vitro.
It is well known that general and specific effects may be difficult
to separate in vitro unless careful dose-response
associations have been established. Dose-response association of
mixtures are, however, problematic, as non-effective compounds may
have a low median lethal dose. The alternative approach is to
perform experiments in vivo, as in the present study
(63,64). In the present study, it was revealed
that the effects of BU extract are selective (only liver cancer
cells are inhibited in their growth, as demonstrated by the normal
liver-body weight ratio, while normal liver cells are not affected
as revealed by the unaltered ALT and albumin concentrations in
serum). Furthermore, the present study attributed a selective
mechanism of action to the BU extract, as the beneficial effect of
BU extract corresponded with a decreased VEGF expression in the
cancerous areas. This indicates that the respective components of
BU must, therefore, contain active ingredients and should allow
their isolation and characterization by established reductionist
schemes, including testing the effectivity of mixtures which have
one component removed.
Sorafenib is an oral multikinase inhibitor that is
used globally for the treatment of advanced or metastatic HCC
(65). The dose of Sorafenib used in
the present study (30 mg/kg/day) was based on previous studies
(30-34).
This dose of Sorafenib produces complete tumor growth inhibition in
mice (30) and reduced tumor
angiogenesis in a mouse HCC xenograft model (34), with a skin rash at the beginning of
the treatment as a minor side-effect in mice (31). The present study revealed that
Sorafenib at the dose administered decreased the liver-to-body
weight ratio and serum albumin concentration, and increased serum
ALT activity more than the BU extract at any of the three
concentrations used. These results indicate that Sorafenib caused
hepatocyte injury in rats. Accordingly, Kuroda et al
(66) reported that patients with HCC
who were treated with 400 mg Sorafenib twice daily for 2 months had
increased serum concentrations of transaminases and bilirubin.
Histologically, these livers exhibited hepatocyte degeneration,
necrosis, lymphocyte infiltration and cholestasis (66). The comparable effectivity of reducing
tumor growth and the lesser degree of cytotoxicity, even at the
highest concentration used, make BU extract or its active principle
an attractive candidate to support or even replace Sorafenib in the
treatment of HCC.
An alcoholic extract of the traditional Thai remedy
BU decreased HCC growth and cancerous VEGF expression in
vivo, without exhibiting a measurable degree of hepatotoxicity.
The present study, to the best of our knowledge, for the first
time, reveals a selective anti-neoplastic effect and, therefore,
qualifies as a promising medical herb for further evaluation as a
form of treatment of HCC.
Acknowledgements
The authors would like to thank Dr. Nitra
Neungchamnong for liquid chromatography-mass spectrometry analysis,
Mr. Thana Chaeyklinthes for laboratory support and Prof. Dr. Wouter
H Lamers for valuable comments and manuscript correction.
Funding
The present study was supported by the Faculty of
Medicine of Srinakharinwirot University (grant no. 252/2558) and
the Thai Traditional Medical Knowledge Fund (grant no. KPT
16/2557).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article excluding raw data, which are
available from the corresponding author on reasonable request.
Authors' contributions
NK designed the experiments, treated the rats with
drugs, conducted reverse transcription-quantitative PCR and western
blot analyses, and analyzed and interpreted the data. AI performed
the Benja-ummarit extraction. SP identified the tumor portions and
performed the histopathological examination. CN collected,
processed and stained the liver biopsies. VK collected specimens,
processed and immunostained the liver samples. WP designed the
experiments, identified the tumor portions, supervised the research
project, interpreted the data and edited the manuscript. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Animal Ethics
Committee of the Faculty of Medicine of Srinakharinwirot University
(Bangkok, Thailand; approval no. 3/2558).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Forner A, Reig M and Bruix J:
Hepatocellular carcinoma. Lancet. 391:1301–1314. 2018. View Article : Google Scholar
|
|
2
|
El-Serag HB: Epidemiology of viral
hepatitis and hepatocellular carcinoma. Gastroenterology.
142:1264–1273.e1. 2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Tajiri H, Tanaka H, Brooks S and Takano T:
Reduction of hepatocellular carcinoma in childhood after
introduction of selective vaccination against hepatitis B virus for
infants born to HBV carrier mothers. Cancer Causes Control.
22:523–527. 2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Tanaka Y, Hanada K, Mizokami M, Yeo AE,
Shih JW, Gojobori T and Alter HJ: A comparison of the molecular
clock of hepatitis C virus in the United States and Japan predicts
that hepatocellular carcinoma incidence in the United States will
increase over the next two decades. Proc Natl Acad Sci USA.
99:15584–15589. 2002.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Altekruse SF, McGlynn KA and Reichman ME:
Hepatocellular carcinoma incidence, mortality, and survival trends
in the United States from 1975 to 2005. J Clin Oncol. 27:1485–1491.
2009.PubMed/NCBI View Article : Google Scholar
|
|
6
|
El-Serag HB: Hepatocellular carcinoma:
Recent trends in the United States. Gastroenterology. 127 (5 Suppl
1):S27–S34. 2004.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Li T, Zhu Y, Qin CY, Yang Z, Fang A, Xu S
and Ren W: Expression and prognostic significance of vascular
endothelial growth factor receptor 1 in hepatocellular carcinoma. J
Clin Pathol. 65:808–814. 2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Vilchez V, Turcios L, Marti F and Gedaly
R: Targeting Wnt/β-catenin pathway in hepatocellular carcinoma
treatment. World J Gastroenterol. 22:823–832. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Intharit N: Cytotoxic activity of
benjaummarit remedy against cancer cells and its biological
activities. Patumthani. Thammasat University Press, 2012.
|
|
10
|
Yaprasert R, Sripanidkulchai B,
Teerachaisakul M, Bancheon K and Banjerdpongchai R: A Thai herbal
formula benja amarit efficiently suppresses lung and liver cancer
cells proliferation through inducing ROS-mediated apoptosis. In:
International conference on liver and lung cancer: Current and
future research, Bangkok, Thailand, January 8-10: 93, 2019.
|
|
11
|
Yan F, Wang M, Li J, Cheng H, Su J, Wang
X, Wu H, Xia L, Li X, Chang HC and Li Q: Gambogenic acid induced
mitochondrial-dependent apoptosis and referred to phospho-Erk1/2
and phospho-p38 MAPK in human hepatoma HepG2 cells. Environ Toxicol
Pharmacol. 33:181–190. 2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Xie H, Qin YX, Zhou YL, Tong LJ, Lin LP,
Geng MY, Duan WH and Ding J: GA3, a new gambogic acid derivative,
exhibits potent antitumor activities in vitro via
apoptosis-involved mechanisms. Acta Pharmacol Sin. 30:346–354.
2009.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zou Z, Xie L, Wei J, Yu L, Qian X, Chen J,
Wang T and Liu B: Synergistic anti-proliferative effects of
gambogic acid with docetaxel in gastrointestinal cancer cell lines.
BMC Complement Altern Med. 12(58)2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Guo XK, Sun HP, Shen S, Sun Y, Xie FL, Tao
L, Guo QL, Jiang C and You QD: Synthesis and evaluation of gambogic
acid derivatives as antitumor agents. Part III. Chem Biodivers.
10:73–85. 2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Abdel-Raheem IT and Abdel-Ghany AA:
Hesperidin alleviates doxorubicin-induced cardiotoxicity in rats. J
Egypt Natl Canc Inst. 21:175–184. 2009.PubMed/NCBI
|
|
16
|
Lu N, Yang Y, You QD, Ling Y, Gao Y, Gu
HY, Zhao L, Wang XT and Guo QL: Gambogic acid inhibits angiogenesis
through suppressing vascular endothelial growth factor-induced
tyrosine phosphorylation of KDR/Flk-1. Cancer Lett. 258:80–89.
2007.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lu N, Hui H, Yang H, Zhao K, Chen Y, You
QD and Guo QL: Gambogic acid inhibits angiogenesis through
inhibiting PHD2-VHL-HIF-1α pathway. Eur J Pharm Sci. 49:220–226.
2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Qiang L, Yang Y, You QD, Ma YJ, Yang L,
Nie FF, Gu HY, Zhao L, Lua N, Qi Q, et al: Inhibition of
glioblastoma growth and angiogenesis by gambogic acid: An in vitro
and in vivo study. Biochem Pharmacol. 75:1083–1092. 2008.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yi T, Yi Z, Cho SG, Luo J, Pandey MK,
Aggarwal BB and Liu M: Gambogic acid inhibits angiogenesis and
prostate tumor growth by suppressing vascular endothelial growth
factor receptor 2 signaling. Cancer Res. 68:1843–1850.
2008.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Cascão R, Vidal B, Raquel H, Neves-Costa
A, Figueiredo N, Gupta V, Fonseca JE and Moita LF: Potent
anti-inflammatory and antiproliferative effects of gambogic acid in
a rat model of antigen-induced arthritis. Mediators Inflamm.
2014(195327)2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Panthong A, Norkaew P, Kanjanapothi D,
Taesotikul T, Anantachoke N and Reutrakul V: Anti-inflammatory,
analgesic and antipyretic activities of the extract of gamboge from
Garcinia hanburyi Hook f. J Ethnopharmacol. 111:335–340.
2007.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wen J, Pei H, Wang X, Xie C, Li S, Huang
L, Qiu N, Wang W, Cheng X and Chen L: Gambogic acid exhibits
anti-psoriatic efficacy through inhibition of angiogenesis and
inflammation. J Dermatol Sci. 74:242–250. 2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Qi Q, Lu N, Li C, Zhao J, Liu W, You Q and
Guo Q: Involvement of RECK in gambogic acid induced anti-invasive
effect in A549 human lung carcinoma cells. Mol Carcinog. 54 (Suppl
1):E13–E25. 2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Xin ZF, Shen CC, Tao LJ, Yan SG and Wu HB:
Gambogic acid inhibits invasion of osteosarcoma via upregulation of
TIMP-1. Int J Mol Med. 31:105–112. 2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Chen J, Gu HY, Lu N, Yang Y, Liu W, Qi Q,
Rong JJ, Wang XT, You QD and Guo QL: Microtubule depolymerization
and phosphorylation of c-Jun N-terminal kinase-1 and p38 were
involved in gambogic acid induced cell cycle arrest and apoptosis
in human breast carcinoma MCF-7 cells. Life Sci. 83:103–109.
2008.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Li R, Chen Y, Zeng LL, Shu WX, Zhao F, Wen
L and Liu Y: Gambogic acid induces G0/G1 arrest and apoptosis
involving inhibition of SRC-3 and inactivation of Akt pathway in
K562 leukemia cells. Toxicology. 262:98–105. 2009.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Li C, Lu N, Qi Q, Li F, Ling Y, Chen Y,
Qin Y, Li Z, Zhang H, You Q and Guo Q: Gambogic acid inhibits tumor
cell adhesion by suppressing integrin β1 and membrane lipid
rafts-associated integrin signaling pathway. Biochem Pharmacol.
82:1873–1883. 2011.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yossathera K, Worakunphanich W,
Teerachaisakul M and Stienrut P: Traditional Thai medicine formula
‘Benja Amarit’ in liver cancer patients: A safety and quality of
life. J Thai Tradit Alterna Med. 15:301–311. 2017.
|
|
29
|
El-Ashmawy NE, El-Bahrawy HA, Shamloula MM
and El-Feky OA: Biochemical/metabolic changes associated with
hepatocellular carcinoma development in mice. Tumour Biol.
35:5459–5466. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Liu L, Cao Y, Chen C, Zhang X, McNabola A,
Wilkie D, Wilhelm S, Lynch M and Carter C: Sorafenib blocks the
RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor
cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer
Res. 66:11851–11858. 2006.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kuczynski EA, Lee CR, Man S, Chen E and
Kerbel RS: Effects of sorafenib dose on acquired reversible
resistance and toxicity in hepatocellular carcinoma. Cancer Res.
75:2510–2519. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kiroplastis K, Fouzas I, Katsiki E,
Patsiaoura K, Daoudaki M, Komninou A, Xolongitas E, Katsika E,
Kaidoglou K and Papanikolaou V: The effect of sorafenib on liver
regeneration and angiogenesis after partial hepatectomy in rats.
Hippokratia. 19:249–255. 2015.PubMed/NCBI
|
|
33
|
Kissel M, Berndt S, Fiebig L, Kling S, Ji
Q, Gu Q, Lang T, Hafner FT, Teufel M and Zopf D: Antitumor effects
of regorafenib and sorafenib in preclinical models of
hepatocellular carcinoma. Oncotarget. 8:107096–107108.
2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Lee Y, Lee SS, Cheong H, Lee CK, Kim N,
Son WC and Hong SM: Intravoxel incoherent motion MRI for monitoring
the therapeutic response of hepatocellular carcinoma to sorafenib
treatment in mouse xenograft tumor models. Acta Radiol.
58:1045–1053. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Schlageter M, Terracciano LM, D'Angelo S
and Sorrentino P: Histopathology of hepatocellular carcinoma. World
J Gastroenterol. 20:15955–15964. 2014.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-ΔΔCT method. Methods. 25:402–408. 2001.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Pan Q, Pan H, Lou H, Xu Y and Tian L:
Inhibition of the angiogenesis and growth of Aloin in human
colorectal cancer in vitro and in vivo. Cancer Cell Int.
13(69)2013.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Suboj P, Babykutty S, Valiyaparambil Gopi
DR, Nair RS, Srinivas P and Gopala S: Aloe emodin inhibits colon
cancer cell migration/angiogenesis by downregulating MMP-2/9, RhoB
and VEGF via reduced DNA binding activity of NF-κB. Eur J Pharm
Sci. 45:581–591. 2012.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Kim KH, Lee HJ, Jeong SJ, Lee HJ, Lee EO,
Kim HS, Zhang Y, Ryu SY, Lee MH, Lü J and Kim S: Galbanic acid
isolated from Ferula assafoetida exerts in vivo anti-tumor activity
in association with anti-angiogenesis and anti-proliferation. Pharm
Res. 28:597–609. 2011.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Kim EC, Min JK, Kim TY, Lee SJ, Yang HO,
Han S, Kim YM and Kwon YG: [6]-Gingerol, a pungent ingredient of
ginger, inhibits angiogenesis in vitro and in vivo. Biochem Biophys
Res Commun. 335:300–308. 2005.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Rhode J, Fogoros S, Zick S, Wahl H,
Griffith KA, Huang J and Liu JR: Ginger inhibits cell growth and
modulates angiogenic factors in ovarian cancer cells. BMC
Complement Altern Med. 7(44)2007.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Weng CJ, Chou CP, Ho CT and Yen GC:
Molecular mechanism inhibiting human hepatocarcinoma cell invasion
by 6-shogaol and 6-gingerol. Mol Nutr Food Res. 56:1304–1314.
2012.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Raj L, Ide T, Gurkar AU, Foley M, Schenone
M, Li X, Tolliday NJ, Golub TR, Carr SA, Shamji AF, et al:
Selective killing of cancer cells by a small molecule targeting the
stress response to ROS. Nature. 475:231–234. 2011.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Doucette CD, Hilchie AL, Liwski R and
Hoskin DW: Piperine, a dietary phytochemical, inhibits
angiogenesis. J Nutr Biochem. 24:231–239. 2013.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Guo QL, You QD, Wu ZQ, Yuan ST and Zhao L:
General gambogic acids inhibited growth of human hepatoma SMMC-7721
cells in vitro and in nude mice. Acta Pharmacol Sin. 25:769–774.
2004.PubMed/NCBI
|
|
46
|
Chang X, Zhao J, Tian F, Jiang Y, Lu J, Ma
J, Zhang X, Jin J, Huang Y, Dong Z, et al: Aloe-emodin suppresses
esophageal cancer cell TE1 proliferation by inhibiting AKT and ERK
phosphorylation. Oncol Lett. 12:2232–2238. 2016.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Chen HC, Hsieh WT, Chang WC and Chung JG:
Aloe-emodin induced in vitro G2/M arrest of cell cycle in human
promyelocytic leukemia HL-60 cells. Food Chem Toxicol.
42:1251–1257. 2004.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Choi JS, Ryu J, Bae WY, Park A, Nam S, Kim
JE and Jeong JW: Zingerone suppresses tumor development through
decreasing cyclin D1 expression and inducing mitotic arrest. Int J
Mol Sci. 19(pii: E2832)2018.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Lee SH, Cekanova M and Baek SJ: Multiple
mechanisms are involved in 6-gingerol-induced cell growth arrest
and apoptosis in human colorectal cancer cells. Mol Carcinog.
47:197–208. 2008.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Jyothi D, Vanathi P, Mangala Gowri P, Rama
Subba Rao V, Madhusudana Rao J and Sreedhar AS: Diferuloylmethane
augments the cytotoxic effects of piplartine isolated from Piper
chaba. Toxicol In Vitro. 23:1085–1091. 2009.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Fofaria NM, Kim SH and Srivastava SK:
Piperine causes G1 phase cell cycle arrest and apoptosis in
melanoma cells through checkpoint kinase-1 activation. PLoS One.
9(e94298)2014.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Kakarala M, Brenner DE, Korkaya H, Cheng
C, Tazi K, Ginestier C, Liu S, Dontu G and Wicha MS: Targeting
breast stem cells with the cancer preventive compounds curcumin and
piperine. Breast Cancer Res Treat. 122:777–785. 2010.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Lai LH, Fu QH, Liu Y, Jiang K, Guo QM,
Chen QY, Yan B, Wan QQ and Shen JG: Piperine suppresses tumor
growth and metastasis in vitro and in vivo in a 4T1 murine breast
cancer model. Acta Pharmacol Sin. 33:523–530. 2012.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Ouyang DY, Zeng LH, Pan H, Xu LH, Wang Y,
Liu KP and He XH: Piperine inhibits the proliferation of human
prostate cancer cells via induction of cell cycle arrest and
autophagy. Food Chem Toxicol. 60:424–430. 2013.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Yaffe PB, Power Coombs MR, Doucette CD,
Walsh M and Hoskin DW: Piperine, an alkaloid from black pepper,
inhibits growth of human colon cancer cells via G1 arrest and
apoptosis triggered by endoplasmic reticulum stress. Mol Carcinog.
54:1070–1085. 2015.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Nie F, Zhang X, Qi Q, Yang L, Yang Y, Liu
W, Lu N, Wu Z, You Q and Guo Q: Reactive oxygen species
accumulation contributes to gambogic acid-induced apoptosis in
human hepatoma SMMC-7721 cells. Toxicology. 260:60–67.
2009.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Arcella A, Oliva MA, Staffier S, Sanchez
M, Madonna M, Riozzi B, Esposito V, Giangaspero F and Frati L:
Effects of aloe emodin on U87MG glioblastoma cell growth: In vitro
and in vivo study. Environ Toxicol. 33:1160–1167. 2018.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Cherian AM, Snima KS, Kamath CR, Nair SV
and Lakshmanan VK: Effect of Baliospermum montanum nanomedicine
apoptosis induction and anti-migration of prostate cancer cells.
Biomed Pharmacother. 71:201–209. 2015.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Oh BS, Shin EA, Jung JH, Jung DB, Kim B,
Shim BS, Yazdi MC, Iranshahi M and Kim SH: Apoptotic effect of
galbanic acid via activation of caspases and inhibition of Mcl-1 in
H460 non-small lung carcinoma cells. Phytother Res. 29:844–849.
2015.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Ren J, Xu Y, Huang Q, Yang J, Yang M, Hu
K, Hu K and Wei K: Chabamide induces cell cycle arrest and
apoptosis by the Akt/MAPK pathway and inhibition of P-glycoprotein
in K562/ADR cells. Anticancer Drugs. 26:498–507. 2015.PubMed/NCBI View Article : Google Scholar
|
|
61
|
de Souza Grinevicius VM, Kviecinski MR,
Santos Mota NS, Ourique F, Porfirio Will Castro LS, Andreguetti RR,
Gomes Correia JF, Filho DW, Pich CT and Pedrosa RC: Piper nigrum
ethanolic extract rich in piperamides causes ROS overproduction,
oxidative damage in DNA leading to cell cycle arrest and apoptosis
in cancer cells. J Ethnopharmacol. 189:139–147. 2016.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Das A, Miller R, Lee P, Holden CA,
Lindhorst SM, Jaboin J, Vandergrift WA III, Banik NL, Giglio P,
Varma AK, et al: A novel component from citrus, ginger, and
mushroom family exhibits antitumor activity on human meningioma
cells through suppressing the Wnt/β-catenin signaling pathway.
Tumour Biol. 36:7027–7034. 2015.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Kirkland DJ, Aardema M, Banduhn N,
Carmichael P, Fautz R, Meunier JR and Pfuhler S: In vitro
approaches to develop weight of evidence (WoE) and mode of action
(MoA) discussions with positive in vitro genotoxicity results.
Mutagenesis. 22:161–175. 2007.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Walmsley RM and Billinton N: How accurate
is in vitro prediction of carcinogenicity? Br J Pharmacol.
162:1250–1258. 2011.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Nakano M, Tanaka M, Kuromatsu R, Nagamatsu
H, Tajiri N, Satani M, Niizeki T, Aino H, Okamura S, Iwamoto H, et
al: Sorafenib for the treatment of advanced hepatocellular
carcinoma with extrahepatic metastasis: A prospective multicenter
cohort study. Cancer Med. 4:1836–1843. 2015.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Kuroda D, Hayashi H, Nitta H, Imai K, Abe
S, Hashimoto D, Chikamoto A, Ishiko T, Beppu T and Baba H:
Successful treatment for sorafenib-induced liver dysfunction: A
report of case with liver biopsy. Surg Case Rep.
2(4)2016.PubMed/NCBI View Article : Google Scholar
|