Introduction
Endometriosis is defined as the presence of
oestrogen-dependent endometrial tissue outside the uterus and
affects 5-10% of women of reproductive age (1-3).
Although endometriosis is a benign, chronic inflammatory disease,
its pathophysiology remains unclear (1-4).
Diagnosis of endometriosis is performed by laparoscopy and
confirmed by histopathological examination of sample biopsies
(5). This procedure in combination
with the presence of non-specific symptoms, such as dysmenorrhea,
pelvic pain, dyspareunia, gastrointestinal (GI) symptoms and
infertility, lead to diagnostic delays of 6-7 years (3,4).
Specifically, GI symptoms can easily be misinterpreted for
irritable bowel syndrome (IBS), a common GI disorder affecting ~10%
of adults (4). At present, there are
no independent validated biomarkers for diagnosis of endometriosis
or to explain the GI symptoms, despite the promising ongoing
research in this field (2,6).
A recent study described increased levels of the
cytoplasmic protein AXIN1 in patients with laparoscopically
confirmed endometriosis compared with those of the control subjects
(7). AXIN1 downregulates activity of
the Wnt signalling pathway, which serves a role in a number of
biological processes, including cellular proliferation, tissue
homeostasis, development of the immune system and other systemic
effects (8-10).
Further investigation of AXIN1, and on its associations with
several subtypes of endometriosis may allow for a deeper
understanding of this disease. In addition, investigation of the
potential correlation of AXIN1 expression with the expression of
other readily measurable inflammatory markers, such as
high-sensitivity c-reactive protein (hs-CRP) and
faecal-calprotectin (F-calprotectin), may potentially offer
additional diagnostic information, as these proteins have been
demonstrated to be useful biomarkers in detecting inflammation
(11,12). These factors may be altered in chronic
inflammation and are performed as routine analyses in daily
clinical practice (6). Although
hs-CRP is irrelevant for the diagnosis of endometriosis (13), it has been used as a marker with high
sensitivity to diagnose diseases with GI symptoms, such as IBS
(11). Therefore, it could
potentially be used to explain GI symptoms in patients with
endometriosis. The plasma levels of calprotectin have been
hypothesized to serve an important role in a number of
gynaecological conditions, including cervical inflammation
(14,15). F-calprotectin is the biomarker with
the highest sensitivity for local inflammation in the GI tract
(16,17). To the best of our knowledge,
calprotectin has not been previously measured in faecal samples of
patients with endometriosis, although intestinal involvement is
often noted in this disease (3,4).
The present study measured the expression levels of
inflammatory biomarkers, and examined whether they were associated
with clinical signs of endometriosis, such as duration,
localisation of disease and GI symptoms. To address this
hypothesis, the correlations between plasma levels of AXIN1 and
hs-CRP were examined in association with F-calprotectin and
haemoglobin levels, as well as the number of erythrocytes,
leukocytes and platelets in the blood of 64 patients with
endometriosis. Furthermore, the correlations and the differences in
the levels of these biomarkers were calculated in association with
clinical signs and GI symptoms. In a larger cohort of 172 patients,
the associations were examined between AXIN1 and hs-CRP plasma
levels and disease characteristics, clinical symptoms and medical
treatment. The aim of the present study was to investigate the
potential relevance of AXIN1 expression and of inflammatory
biomarkers, including hs-CRP and F-calprotectin, in patients with
endometriosis. The study further aimed to evaluate the correlations
between these inflammatory biomarkers and the clinical signs and GI
symptoms of the patients with endometriosis.
Materials and methods
Ethics approval and consent to
participate
The present study was performed according to the
Declaration of Helsinki (18).
Ethical approval was obtained from the Ethics Review Board of Lund
University [approval nos. 2012/564 (09/10/2012) and 2016/56
(03/05/2016)]. All participants provided written informed consent
prior to participation.
Study participants
Patients with laparoscopy-verified endometriosis
were recruited to the present cross-sectional study at the
Department of Gynaecology, Skane University Hospital, Malmo,
Sweden. The first cohort was recruited between March 2013 and July
2014 and included 100 women, and the second recruitment phase was
between September 2016 and March 2017, with 72 women. Therefore, in
total 172 women, median age 38 year (age range 19-50 years), were
recruited for the present study. The details of patient
recruitment, and the inclusion and exclusion criteria are described
in detail elsewhere, along with the basal characteristics of the
cohort (7,19).
Study design
All participants were interviewed and completed a
questionnaire regarding their sociodemographic factors, lifestyle
habits and medical history, as well as the validated visual
analogue scale for irritable bowel syndrome (VAS-IBS) (20). The blood samples were collected and
centrifuged at 1,000 x g for 10 min, at 4˚C. Whole blood, plasma
and serum were frozen at -20˚C and/or at -80˚C, and analysis of the
plasma levels of AXIN1 and hs-CRP was performed.
In the second cohort of 72 patients, faecal samples
were collected from 64 patients, frozen at -80˚C, and analysed for
F-calprotectin according to the clinical routines at the Department
of Clinical Chemistry (21).
Therefore, the inflammatory markers investigated in blood, such as
haemoglobin, erythrocytes, leukocytes and platelets, were only
measured for those 64 patients, according to the clinical routines
at the Department of clinical Chemistry (21).
Gastrointestinal symptoms
The VAS-IBS questionnaire assesses the severity of
several GI symptoms, such as abdominal pain, diarrhoea,
constipation, bloating and flatulence, vomiting and nausea,
psychological well-being and the effect of intestinal symptoms on
the quality of the patients' life over the last 2 weeks. The
severity of these symptoms is measured on a continuous scale from
0-100 mm, where 100 represents severe symptoms and 0 no symptoms.
The scales were inverted from the original version, prior to the
analysis. In addition, two dichotomous questions probed the
experience of defecation urgency and the sensation of incomplete
evacuation. The replies were determined by ‘yes’ or ‘no’ (20). The reference values from 52 healthy
women, recruited from hospital staff (median age 44 years, range
22-77 years), who had not undergone prior abdominal surgery, were
used as controls as previously published (22).
Biological sample analysis
Haemoglobin, erythrocytes, leukocytes and platelets
in blood (B), and hs-CRP levels in plasma (P) were analysed
according to clinical routine procedures at the Department of
Clinical Chemistry. The reference values for healthy individuals
were obtained from the same department using the same methods as
described above. F-calprotectin levels were analysed by ELISA
[CalproLab™ ELISA TEST (ALP/HRP); Calpro AS] at the Department of
Clinical Chemistry. The lowest detection levels were 25 mg/kg.
Values <50 mg/kg were considered normal, 50-100 mg/kg was
considered a grey zone and those >100 mg/kg were considered
pathological (21). Values in the
grey zone may occur also be observed in the healthy subjects, and
the clinical significance of values in this range is unclear.
Plasma AXIN1 levels were analysed using sandwich ELISA (cat. no.
MBS762601; MyBioSource, Inc.; lot no. H2497C119) according to the
manufacturer's protocol (7).
Data categorization
Body mass index (BMI) was divided into normal-weight
(<25 kg/m2), overweight (25-29.9 kg/m2) or
obese (≥30 kg/m2) according to the WHO classification
(23). Smoking habits were divided
into non-smokers (at examination) and smokers. Physical activity
was divided into <1 h or ≥h per week. Alcohol consumption was
divided into drinking >1 or at least 1 standard glass of alcohol
per week. The localisation of endometriosis was divided into
isolated or non-isolated ovarian lesions, independent of the
localisation of the spread lesions, and to subjects with or without
bowel involvement. Medical treatment was divided into current use
or not currently using (at examination), independent of the history
of use. Age, duration of disease and GI symptoms as well as VAS-IBS
scales were divided into quartiles for the calculations with
logistic regression.
Statistical analysis
In the present study, two hypotheses were examined:
i) Different inflammatory biomarkers were correlated with each
other; and ii) the levels of inflammatory biomarkers were affected
by disease characteristics, clinical signs, GI symptoms and medical
treatment. The expression levels of hs-CRP and F-calprotectin that
were below the limit of detection were set to the lowest detectable
level (0.6 mg/l and 25 mg/kg, respectively). All variables were
found to be non-normally distributed and analysis was performed
using a Fisher's exact test, Spearman's correlation test or a Mann
Whitney U test. To further assess the association between the
expression levels of AXIN1 and hs-CRP, binary logistic regression
was performed with the biomarkers as the dependent variables and
stratified according to the median value into lower or higher
levels, and sociodemographic factors, medication and GI symptoms
were the independent variables. The lowest category was set as the
reference. P-values for trend and for log-transformed, continuous
variables were calculated. The values are presented as the number
(percentage), median (interquartile range), or odds ratio (OR) and
95% confidence interval (CI). The data were analysed using SPSS
version 25.0 (IBM, Corp.). P<0.05 was considered to indicate a
statistically significant difference.
Results
Basal characteristics
The majority of the 64 patients from the second
cohort (55 patients, 85.9%) had suffered from GI complaints during
the 2 weeks prior to their inclusion in the study. A limited
percentage of the patients (10 patients, 15.6%) were smokers and 38
patients (59.4%) had a low physical activity. A total of 40
patients (62.5%), were treated with hormonal therapy, in some cases
with more than one drug. A total of 20 women (31.3%) were treated
with oestrogen or combined oral contraception, whereas 19 women
(29.7%) were treated with progestin and 7 women (10.9%) were
treated with gonadotropin-releasing hormone (GnRH) analogues. In
addition, 11 women (17.2%) were treated with opioids (Table I).
 | Table IClinicopathological characteristics
of the 64 patients in the second cohort with available faecal
samples. |
Table I
Clinicopathological characteristics
of the 64 patients in the second cohort with available faecal
samples.
| Clinicopathological
characteristics | Values |
|---|
| Age, year
(IQR) | 38.00
(32.25-42.75) |
| BMI,
kg/m2 (IQR) | 24.78
(21.92-28.00) |
| Smoking, n (%) | 10 (15.6) |
| Alcohol consumption
≥1 standard glass/week, n (%) | 24 (37.5) |
| Physical activity
≥1 h/week, n (%) | 26 (40.6) |
| Duration of
endometriosis diagnosis, years (IQR) | 12.00
(5.00-19.25) |
| Duration of GI
symptoms, year (IQR) | 16.50
(7.00-21.00) |
| Isolated ovarian
endometriosis, n (%) | 26 (40.6) |
| Bowel endometriosis
affecting the GI tract alone or along with other locations, n
(%) | 18 (28.1) |
| Current hormonal
treatment, n (%) | 40 (62.5) |
| Current opioid
treatment, n (%) | 11 (17.2) |
| Abdominal pain,
mm |
|
Experimental
values | 43 (13-72) |
|
Reference
values | 5 (1-15) |
| Diarrhoea (mm) |
|
Experimental
values | 17 (2-60) |
|
Reference
values | 3 (0-10) |
| Constipation
(mm) |
|
Experimental
values | 26 (2-56) |
|
Reference
values | 9 (1-22) |
| Bloating and
flatulence (mm) |
|
Experimental
values | 61 (19-76) |
|
Reference
values | 14 (1-29) |
| Vomiting and nausea
(mm) |
|
Experimental
values | 15 (2-50) |
|
Reference
values | 2 (0-3) |
| Psychological
well-being (mm) |
|
Experimental
values | 32 (12-62) |
|
Reference
values | 4 (0-16) |
| Intestinal
symptoms' affected quality of life (mm) |
|
Experimental
values | 51 (16-78) |
|
Reference
values | 2 (0-18) |
| Defecation urgency,
n (%) | 22 (34.4) |
| Incomplete
evacuation when defecating, n (%) | 37 (57.8) |
Inflammatory biomarkers
The majority of the women in the present study
exhibited biomarker levels within the normal reference range
(Table II). P-AXIN1 expression
levels were negatively correlated with F-calprotectin levels
(Table II) and haemoglobin levels
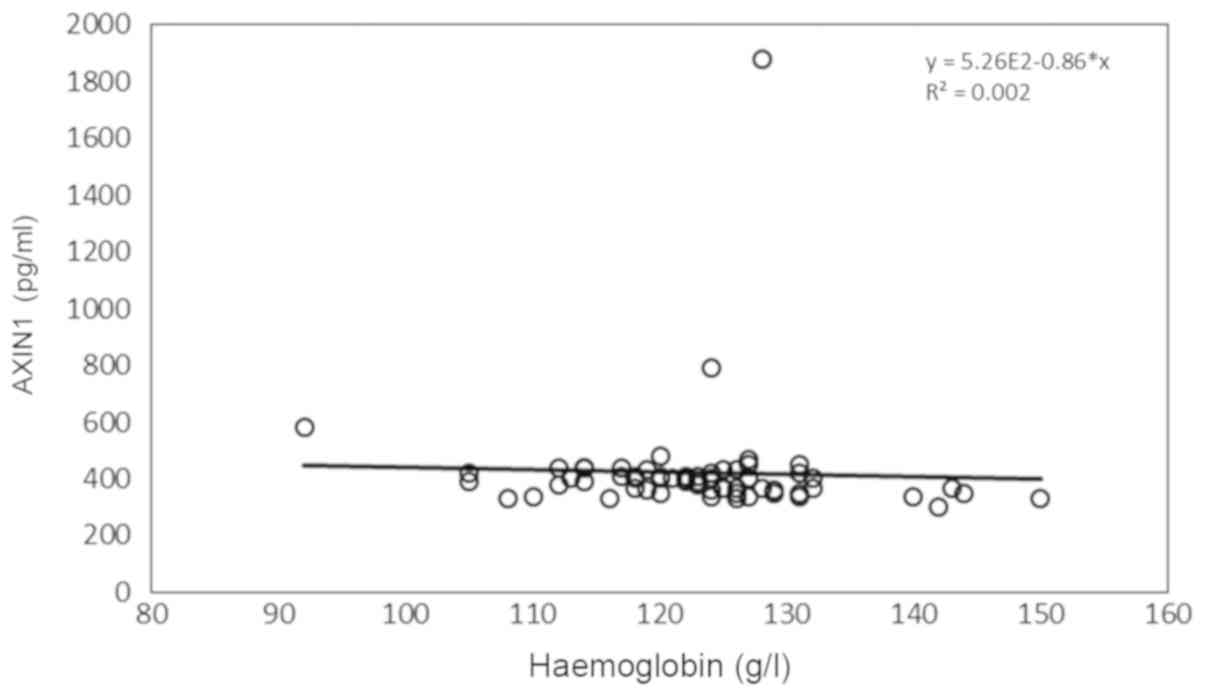
(Fig. 1), as well as the number of
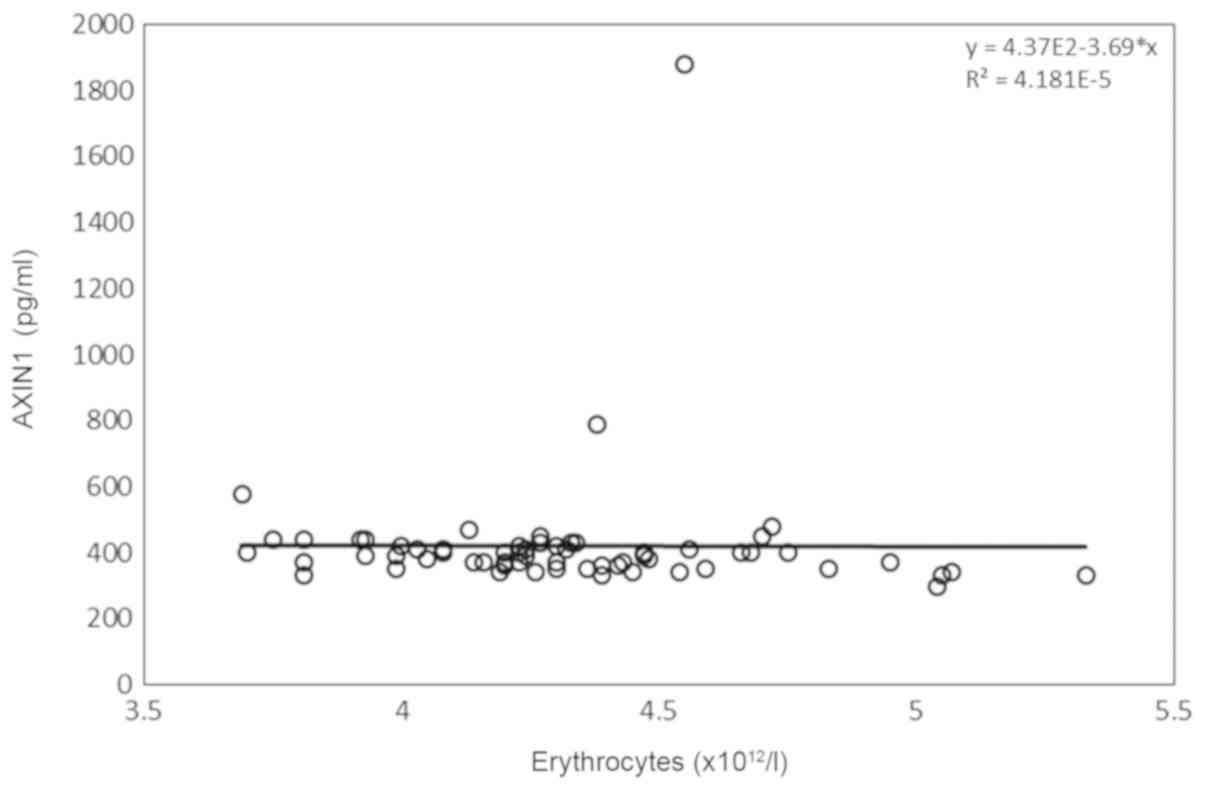
erythrocytes (Fig. 2) and platelets
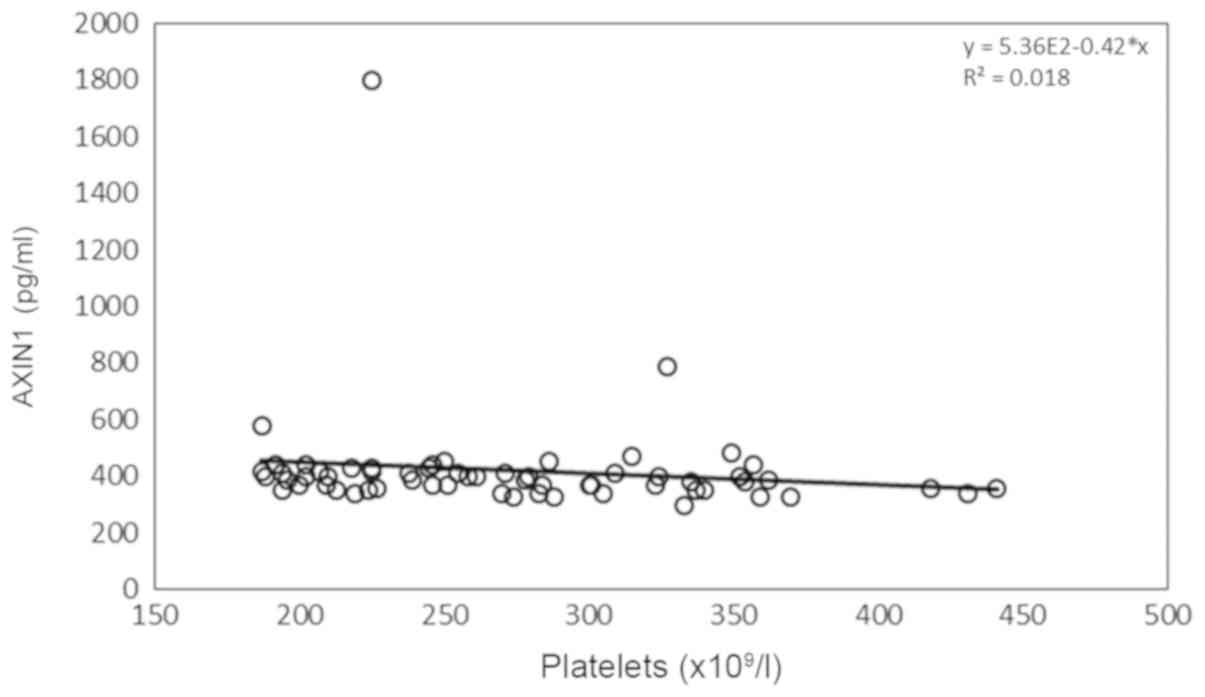
(Fig. 3). P-hs-CRP levels were
positively correlated with F-calprotectin levels and the number of
B-leukocytes (Table II).
 | Table IIInflammatory biomarkers and their
correlations with AXIN1 and hs-CRP levels in plasma. |
Table II
Inflammatory biomarkers and their
correlations with AXIN1 and hs-CRP levels in plasma.
| | | | | AXIN1 | hs-CRP |
|---|
| Biomarkers | Normal range | Pathological
values, n (%) | Median (IQR) | Correlation
coefficient | P-value | Correlation
coefficient | P-value |
|---|
| P-AXIN1 | N/A | - | 390.00
(357.50-420.00) | 1.00 | - | -0.053 | 0.682 |
| F-calprotectin | <50 mg/kg | 11 (17.2) | 25.00
(25.00-29.50) | -0.368 | 0.003b | 0.280 | 0.028a |
| B-haemoglobin | 117-153 g/l | 12 (18.8) | 124.00
(118.00-127.25) | -0.276 | 0.030a | -0.041 | 0.752 |
| B-erythrocytes |
3.9-5.2x1012/l | 7 (11.0) | 4.28
(4.08-4.50) | -0.271 | 0.033a | 0.019 | 0.885 |
| B-leukocytes |
3.5-8.8x109/l | 18 (28.2) | 7.75
(6.50-9.12) | -0.177 | 0.168 | 0.288 | 0.023a |
| B-platelets |
165-387x109/l | 3 (4.7) | 265.50
(218.75-324.75) | -0.302 | 0.017a | 0.208 | 0.104 |
| P-hs-CRP | <3.0 mg/l | 16 (25.0) | 1.10
(0.60-3.22) | -0.050 | 0.758 | 1.00 | - |
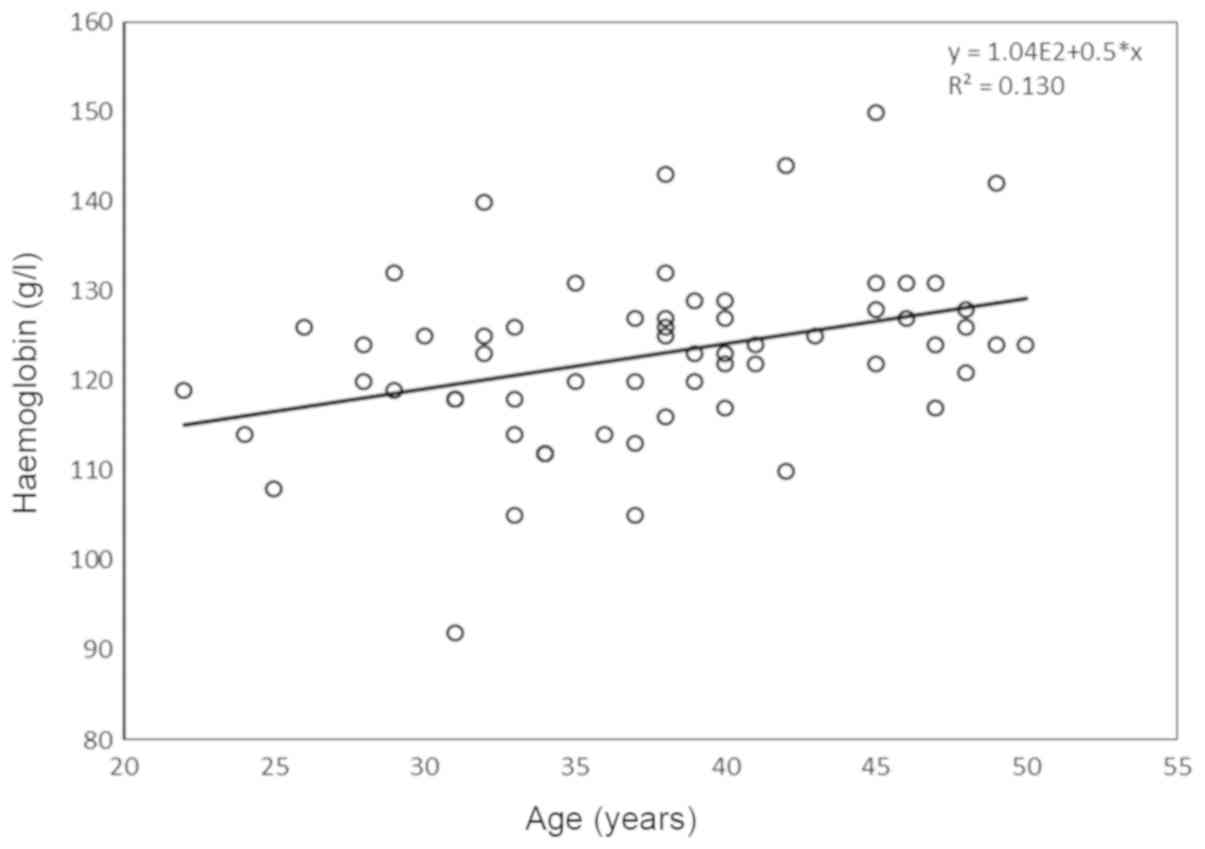
B-haemoglobin levels were significantly correlated
with age (Fig. 4), whereas patients
with physical activity ≥1 h/week had a lower number of
B-erythrocytes compared with women with less physical activity
[4.20 (4.04-4.32)x1012/l vs. 4.38
(4.19-4.64)x1012/l, P=0.040], without affecting
haemoglobin concentration (data not shown). The expression levels
of B-haemoglobin and the numbers of B-erythrocytes, B-leukocytes
and B-platelets were not associated with BMI, smoking, alcohol
consumption, duration of endometriosis (based on the date when the
diagnosis was performed), duration or degree of GI symptoms,
treatment provided or localisation of endometriosis (data not
shown).
Of the 64 women examined from the second cohort, 18
women (28.1%) presented with F-calprotectin levels >25 mg/kg, 6
women (9.4%) exhibited grey zone levels, and 5 women (7.8%) had
high F-calprotectin levels. The patients with F-calprotectin values
≤25 mg/kg (n=46, 71.9%) exhibited higher AXIN1 levels [400
(370-430) pg/ml vs. 350 (340-380) pg/ml; P=0.002] and lower hs-CRP
values [1 (0.6-1.8) mg/l vs. 2.7 (0.69-6.35) mg/l; P=0.029],
compared with the patients with measurable F-calprotectin levels.
Women with F-calprotectin levels >25 mg/kg experienced higher
degrees of constipation [50.50 (11.00-71.50) mm vs. 16.50
(2.00-45.50) mm; P=0.048]. These subjects experienced more frequent
incomplete evacuation when defecating [n=14 (87.5%) vs. n=23
(50.0%); P=0.009], compared to women with F-calprotectin levels ≤25
mg/kg. The localisation and duration of endometriosis were not
associated with the levels of F-calprotectin (data not shown).
Correlations between AXIN1 and hs-CRP
levels in the plasma of the patients
To further assess the correlations between AXIN1 and
hs-CRP levels in the plasma, and their association with the
clinical signs and the GI symptoms of endometriosis, the total
cohort of 172 patients with a median age of 38 years (age range
19-50 years) and a median BMI of 24.34 (21.80-27.10)
kg/m2 was used (19). The
median levels of AXIN1 were 300 (170-380) pg/ml, and for hs-CRP
levels 1.10 (0.60-3.25) mg/l. A total of 80 patients (46.5%) were
treated with hormonal treatment. A total of 42 women (24.4%) were
treated with oestrogen or combined oral contraception, whereas 30
women (17.4%) were treated with progestin and 15 women (8.7%) were
treated with GnRH analogues. A total of 30 women (17.4%) were
treated with opioids (Table SI). A
total of 92 patients (53.5%) could distinguish between symptoms
from the GI tract and their endometriosis symptoms; whereas for 49
patients (28.5%), the origin of the GI symptoms could not be
determined.
There was no correlation between AXIN1 and hs-CRP
levels (r=-0.018; P=0.818). BMI was correlated with hs-CRP levels
(r=0.288; P<0.001). AXIN1 levels were not correlated with BMI
(P=0.700), but were correlated with the duration of endometriosis
(r=0.172; P=0.047) and the duration of GI symptoms (r=0.244;
P=0.009). Furthermore, significant correlations were identified
between AXIN1 levels and the degree of vomiting and nausea
(r=0.176; P=0.024) as well as with the intestinal symptoms' effect
on the quality of life (r=0.222; P=0.004). Hs-CRP levels were not
correlated with specific GI symptoms (data not shown). Women that
were treated with hormonal therapy exhibited higher levels of AXIN1
and hs-CRP compared with those without treatment (P=0.036 and
P<0.001, respectively). Treatment with oestrogen, combined oral
contraception (P=0.048 and P<0.001, respectively) and progestin
(P=0.010 and P=0.036, respectively) also affected the levels of the
aforementioned markers (Table III).
Neither AXIN1, nor hs-CRP levels, were affected by treatment with
GnRH analogues (P=1.000 and P=0.657, respectively) or opioid
treatment (P=0.375 and P=0.423, respectively).
 | Table IIIAXIN1 and hs-CRP levels in patients
treated with different hormonal treatments. |
Table III
AXIN1 and hs-CRP levels in patients
treated with different hormonal treatments.
| | AXIN1, pg/ml | hs-CRP, mg/l |
|---|
| Treatment | Median (IQR) | P-value | Median (IQR) | P-value |
|---|
| Current hormonal
treatment | | 0.036 | |
<0.001b |
|
Yes,
n=80 | 340.00
(187.50-400.00) | | 1.65
(0.74-4.68) | |
|
No,
n=92 | 270.00
(160.00-370.00) | | 0.77
(0.60-2.20) | |
| Current oestrogen
or combined oral contraception | | 0.048 | |
<0.001b |
|
Yes,
n=42 | 360.00
(210.00-400.00) | | 2.70
(0.99-4.80) | |
|
No,
n=92 | 270.00
(160.00-370.00) | | 0.77
(0.60-2.20) | |
| Current progestin
treatment | | 0.010 | | 0.036a |
|
Yes,
n=30 | 350.00
(260.00-420.00) | | 1.50
(0.74-4.10) | |
|
No,
n=92 | 270.00
(160.00-370.00) | | 0.77
(0.60-2.20) | |
In the women whom did not undergo hormonal treatment
(n=92), there was a significant correlation between their AXIN1
levels and the duration of GI symptoms (r=0.270, P=0.044) or the
effect of intestinal symptoms on quality of life (r=0.301;
P=0.005), whereas, there was no significant correlation between
AXIN1 levels, the duration of endometriosis symptoms and the degree
of vomiting and nausea (P=0.128 and P=0.337, respectively). Hs-CRP
levels were correlated with BMI (r=0.229, P=0.036), following
exclusion of the patients who underwent hormonal treatment.
Following logistic regression analysis, no
associations were noted between AXIN1 levels and sociodemographic
factors or other characteristics. However, there was a clear
tendency towards an association with hormonal treatment (P=0.061;
Table IV). The duration of GI
symptoms and the degree of diarrhoea, constipation, vomiting and
nausea, and the effect of intestinal symptoms on quality of life
were all associated with higher levels of AXIN1 (Table V). Hs-CRP levels were associated with
higher BMI values and with hormonal treatment. These conclusions
remained following adjustment for both variables [OR, 11.704 (CI,
2.486-55.108), P=0.002 for obese individuals compared with
normal-weight individuals and OR, 2.361 (CI, 1.215-4.588), P=0.011
for hormonal treatment compared with no hormonal treatment,
respectively). The localisation of endometriosis did not affect
plasma levels of AXIN1 (Table IV) or
hs-CRP (data not shown).
 | Table IVAssociations between sociodemographic
factors and medical history with AXIN1 levels. |
Table IV
Associations between sociodemographic
factors and medical history with AXIN1 levels.
|
Characteristics | AXIN1 <300
pg/ml, n=82 | AXIN1 ≥300 pg/ml,
n=86 | Odds ratio | 95% Confidence
intervals | P-value |
|---|
| Age, years, n
(%) |
|
<33 | 21 (25.6) | 22 (25.6) | 1 | | |
|
33-37 | 24 (29.3) | 20 (23.3) | 0.795 | 0.343-1.847 | 0.594 |
|
38-42 | 18 (22.0) | 22 (25.6) | 1.167 | 0.492-2.767 | 0.726 |
|
≥43 | 19 (23.2) | 22 (25.6) | 1.105 | 0.469-2.604 | 0.819 |
| BMI,
kg/m2, n (%) |
|
<25 | 50 (61.0) | 47 (54.7) | 1 | | |
|
25-29.9 | 25 (30.5) | 26 (30.2) | 1.106 | 0.562-2.180 | 0.770 |
|
≥30 | 6 (7.3) | 11 (12.8) | 1.950 | 0.668-5.694 | 0.222 |
|
Missing | 1 (1.2) | 2 (2.3) | - | - | - |
| Smoking, n (%) |
|
No
smoking | 68 (82.9) | 74 (86.0) | 1 | | |
|
Smoking | 14 (17.1) | 11 (12.8) | 0.722 | 0.307-1.699 | 0.456 |
|
Missing | | 1 (1.2) | - | - | - |
| Alcohol
consumption, n (%) |
|
<1
glass/week | 53 (64.6) | 51 (59.3) | 1 | | |
|
≥1
glass/week | 29 (35.4) | 34 (39.5) | 1.218 | 0.651-2.281 | 0.537 |
|
Missing | | 1 (1.2) | - | - | - |
| Physical activity,
n (%) |
|
<1
h/week | 37 (45.1) | 48 (55.8) | 1 | | |
|
≥1
h/week | 45 (54.9) | 37 (43.0) | 0.634 | 0.344-1.167 | 0.143 |
|
Missing | | 1 (1.2) | - | - | - |
| Duration of
endometriosis, year, n (%) |
|
<5 | 21 (25.6) | 10 (11.6) | 1 | | |
|
5-10 | 17 (20.7) | 18 (20.9) | 2.224 | 0.815-6.064 | 0.118 |
|
11-17 | 17 (20.7) | 10 (20.9) | 2.224 | 0.815-6.064 | 0.118 |
|
≥18 | 15 (18.3) | 17 (19.8) | 2.380 | 0.855-6.628 | 0.097 |
|
Missing | 12 (14.6) | 23 (26.7) | - | - | - |
| Localisation of
endometriosis, n (%) |
|
Ovarian | 31 (37.8) | 34 (39.5) | 1 | | |
|
Outside
ovarian | 47 (57.3) | 50 (58.1) | 0.970 | 0.517-1.819 | 0.924 |
|
Missing | 4 (4.9) | 2 (2.3) | - | - | - |
| Treatment, n
(%) |
|
No hormonal
treatment | 50 (61.0) | 40 (46.5) | 1 | | |
|
Hormonal
treatment | 32 (39.0) | 46 (53.5) | 1.797 | 0.973-3.319 | 0.061 |
 | Table VAssociations between GI symptoms and
lower or higher AXIN1 levels. |
Table V
Associations between GI symptoms and
lower or higher AXIN1 levels.
|
Characteristics | AXIN1 <300
pg/ml, n=82 | AXIN1 ≥300 pg/ml,
n=86 | Odds ratio | 95% Confidence
intervals | P-value |
|---|
| Duration of GI
symptoms, years, n (%) |
|
<5 | 12 (14.6) | 14 (16.3) | 1 | | |
|
5-9 | 15 (18.3) | 15 (17.4) | 1.429 | 0.499-4.091 | 0.774 |
|
10-19 | 15 (18.3) | 14 (16.3) | 1.538 | 0.532-4.449 | 0.680 |
|
≥20 | 14 (17.1) | 14 (16.3) | 3.889 | 1.348-11.216 | 0.778 |
|
Missing | 26 (31.7) | 29 (33.7) | - | - | - |
|
P for
trend | | | | | 0.763 |
|
P for log
value | | | | | 0.036a |
| Abdominal pain, mm,
n (%) |
|
<10 | 23 (28.0) | 18 (20.9) | 1 | | |
|
10-39 | 24 (29.3) | 23 (26.7) | 1.225 | 0.528-2.840 | 0.637 |
|
40-71 | 18 (22.0) | 18 (20.9) | 1.278 | 0.520-3.138 | 0.593 |
|
≥72 | 16 (19.5) | 25 (29.1) | 1.997 | 0.828-4.813 | 0.124 |
|
P for
trend | | | | | 0.133 |
|
P for log
value | | | | | 0.156 |
| Diarrhoea, mm, n
(%) |
|
0 | 31 (37.8) | 22 (25.6) | 1 | | |
|
2-14 | 9 (11.0) | 19 (22.1) | 2.975 | 1.135-7.793 | 0.027a |
|
15-54 | 21 (25.6) | 20 (23.3) | 1.342 | 0.591-3.049 | 0.482 |
|
≥55 | 20 (24.4) | 23 (26.7) | 1.620 | 0.720-3.646 | 0.243 |
|
P for
trend | | | | | 0.407 |
|
P for log
value | | | | | 0.285 |
| Constipation, mm, n
(%) |
|
0 | 30 (36.6) | 16 (18.6) | 1 | | |
|
2-27 | 12 (14.6) | 25 (29.1) | 3.906 | 1.561-9.778 | 0.004b |
|
28-69.4 | 20 (24.4) | 21 (24.4) | 1.969 | 0.831-4.662 | 0.124 |
|
≥69.5 | 19 (23.2) | 21 (24.4) | 2.072 | 0.870-4.936 | 1.000 |
|
P for
trend | | | | | 0.216 |
|
P for log
value | | | | | 1.00 |
| Bloating and
flatulence, mm, n (%) |
|
<17.5 | 22 (26.8) | 20 (23.3) | 1 | | |
|
17.5-54 | 19 (23.2) | 23 (26.7) | 1.332 | 0.565-3.140 | 0.513 |
|
55-79 | 14 (17.1) | 23 (26.7) | 1.807 | 0.736-4.440 | 0.197 |
|
≥80 | 26 (31.7) | 18 (20.9) | 0.762 | 0.324-1.787 | 0.531 |
|
P for
trend | | | | | 0.656 |
|
P for log
value | | | | | 0.905 |
| Vomiting and
nausea, mm, n (%) |
|
0 | 39 (47.6) | 22 (25.6) | 1 | | |
|
2-8 | 5 (6.1) | 19 (22.1) | 6.736 | 2.209-20.546 | 0.001b |
|
9-44 | 19 (23.2) | 21 (24.4) | 1.959 | 0.870-4.410 | 0.104 |
|
≥45 | 18 (22.0) | 22 (25.6) | 2.167 | 0.961-4.886 | 0.062 |
|
P for
trend | | | | | 0.095 |
|
P for log
value | | | | | 0.040a |
| Psychological
well-being, mm, n (%) |
|
<8 | 22 (26.8) | 19 (22.1) | 1 | | |
|
8-29 | 22 (26.8) | 21 (24.4) | 1.105 | 0.469-2.604 | 0.819 |
|
30-63.4 | 18 (22.0) | 23 (26.7) | 1.480 | 0.620-3.532 | 0.378 |
|
≥63.5 | 19 (23.2) | 21 (24.4) | 1.280 | 0.535-3.063 | 0.580 |
|
P for
trend | | | | | 0.460 |
|
P for log
value | | | | | 0.434 |
| Intestinal
symptoms' effect (mm) |
|
<8.5 | 27 (32.9) | 15 (17.4) | 1 | | |
|
8.5-39 | 20 (24.4) | 21 (24.4) | 1.890 | 0.784-4.554 | 0.156 |
|
40-74 | 22 (26.8) | 20 (23.3) | 1.636 | 0.682-3.924 | 0.270 |
|
≥75 | 12 (14.6) | 28 (32.6) | 4.200 | 1.665-10.592 | 0.002b |
|
P for
trend | | | | | 0.005b |
|
P for log
value | | | | | 0.007b |
| Incomplete
evacuation |
|
No
symptom | 36 (43.9) | 31 (36.0) | 1 | | |
|
Symptom | 44 (53.7) | 49 (57.0) | 1.293 | 0.689-2.427 | 0.888 |
| Defecation
urgency |
|
No
urgency | 49 (59.8) | 48 (55.8) | 1 | | |
|
Urgency | 31 (37.8) | 29 (33.7) | 0.955 | 0.502-1.818 | 0.423 |
Discussion
The primary findings of the present study were that
there was a negative correlation between P-AXIN1 levels and the
levels of F-calprotectin, B-haemoglobin as well as the number of
B-erythrocytes and B-platelets. Furthermore, there was a positive
correlation between P-AXIN1 levels and the duration of
endometriosis, duration of GI symptoms as well as the degree of
certain GI symptoms. In a logistic regression model, significant
associations were identified between higher AXIN1 levels and the
duration of GI symptoms, the degree of diarrhoea, constipation,
vomiting and nausea and the intestinal symptoms' effect on quality
of life. Furthermore, P-AXIN1 levels were increased in patients
with hormonal treatment. Higher hs-CRP levels were associated with
obesity and hormonal treatment.
AXIN1 acts as a repressor of the Wnt signalling
pathway (8). Additionally, AXIN1
expression is increased in women with endometriosis compared with
the expression in control subjects (7). The Wnt signalling pathway is responsible
for a number of biological processes including growth and
proliferation of cells in various organs (9,10).
Previous studies suggested that this pathway enabled tissue
regeneration, which could improve wound repair (9,24).
Dysregulation of the Wnt signalling pathway is attributed to a
number of diseases, such as ovarian cancer, intestinal
inflammation, bacterial infection and autoimmune disorders
(9,10,24-29).
The mechanisms of action of the Wnt signalling pathway have been
previously described (8,9,24,26,28,30-32).
AXIN1 acts as a scaffolding protein, which interacts with other
proteins, such as GSK3, APC, CK1α and β-catenin (9). By building a ‘destruction complex’,
AXIN1 negatively regulates the Wnt signalling pathway via
phosphorylation of β-catenin (9,24). It has
been suggested that AXIN1 is the rate limiting protein of the
destruction complex, which suggests that the increase in AXIN1
expression represents the increased destruction of β-catenin and
the inhibition of the Wnt signalling pathway (9,26).
However, the quantitative ratios of other proteins involved in the
Wnt signalling pathway or in β-catenin degradation have to be
established to confirm this hypothesis (9). The findings of the present study showed
there was a negative correlation between AXIN1 levels and certain
inflammatory markers, which reflects the complex and intimate
regulation between several inflammatory pathways and the Wnt
signalling pathway (9,24,30-32).
It has been suggested that aberrant activation of
the Wnt signalling pathway may promote the development of
endometriosis through increased cell migration and invasion
(33,34). It remains to be determined whether the
increased levels of AXIN1 and the downregulation of the Wnt
signalling pathway serve as an initiating step in the development
and establishment of endometriosis, or whether the elevated AXIN1
levels are a secondary compensatory mechanism in response to
increased β-catenin levels. It is also unclear whether AXIN1 is a
pro- or an anti-inflammatory factor. An anti-inflammatory effect of
AXIN1 would explain the inverse correlation with other inflammatory
biomarkers. Further research is required in order to exclude the
effects of hormonal treatment on AXIN1 levels, and to establish the
causal link between increased AXIN1 levels and decreased levels of
other inflammatory biomarkers. Higher AXIN1 and hs-CRP levels were
observed in the treated patients of the present cross-sectional
study. These levels may hypothetically indicate that hormonal
treatment is given to the patients with the highest levels of
inflammation. Furthermore, the therapeutic effect of hormonal
therapy may be mediated by increased AXIN1 levels. Blood samples
should be collected before commencing treatment for suspected
endometriosis, and the patients should be re-examined
regularly.
AXIN1 levels were positively associated with several
GI symptoms. Approximately half of the patients could distinguish
whether the symptoms originated from the GI tract or whether they
were of gynaecological origin. Nevertheless, it is unclear whether
the patients who could distinguish their symptoms had only referred
to symptoms from the GI tract in their answers, or if the symptoms
represented complaints from both sites. Therefore, it is unclear
what the symptoms mean, and the only evidence presented was that
the patient suffered from GI symptoms, as described previously
(3,4).
However, independent of whether the patient can distinguish their
symptoms from different organs, the GI symptoms may have the same
or similar underlying pathophysiology. Low-grade inflammation and
visceral hypersensitivity are considered important factors in the
aetiology of the GI symptoms in both IBS and endometriosis
(4,35). The association between GI symptoms and
AXIN1 levels seemed to be unique for endometriosis, since AXIN1
levels in patients with MC (7) and
IBS (unpublished data) did not exhibit an association with GI
symptoms. However, the associations may be influenced by
psychological factors that in turn affect both nausea and the
influence of symptoms on the quality of life (36). The lower number of patients may
explain the fact that some correlations disappeared following
exclusion of patients with hormonal treatment. Therefore, the
reduced correlation and loss of significance do not necessarily
indicate the absence of correlations.
Hs-CRP levels were not correlated with AXIN1 levels,
or associated with disease duration or the degree of GI symptoms.
These findings support previous studies stating that hs-CRP levels
are irrelevant in the diagnosis of endometriosis (13). The association between hs-CRP levels
with higher BMI levels may reflect low-grade inflammation due to
obesity (37).
F-calprotectin has been reported as a marker for the
differentiation between IBS and inflammatory bowel diseases
(16,17), and as a potential biomarker for the
severity of IBS (12). Measurable
F-calprotectin levels were most often noted in participants who
experienced incomplete evacuation when defecating, and in
participants with a higher degree of constipation. These conditions
are characterized by increased straining, which may lead to
invasion of leukocytes in the mucosa and measurable calprotectin
levels in faeces (38). Therefore,
F-calprotectin may reflect straining, rather than an association
with endometriosis, since bowel involvement of endometriosis did
not affect F-calprotectin levels. However, F-calprotectin levels in
the grey zone, and even high levels, may be found in some healthy
subjects as well. This effect could possibly be explained by drug
intake (39,40). The low number of patients with
measurable F-calprotectin levels in the present study suggest that
it is not of major importance in endometriosis.
Overall, no correlations between the localisation of
endometriosis and the levels of inflammatory biomarkers were found.
Therefore, the ability to classify the stage and localisation of
endometriosis is a continuous concern for research and is
considered important in creating a common classification when
comparing case reports and clinical studies.
Several other biomarkers, such as CA-125 and
CA-19-9, have also been analysed in patients with endometriosis
(6). However, no biomarkers were
considered to be useful for the diagnosis of endometriosis
(6,41). The usefulness of AXIN1 as a biomarker
has to be further examined, independently of other biomarkers.
Furthermore, even if AXIN1 is not a useful biomarker, it may be an
interesting factor to study to learn more regarding the
pathophysiology underlying disease initiation and/or development
and clinical manifestations of the disease. Thus, the aim of the
present study was to not only to identify biomarkers, but to also
understand the mechanisms underlying endometriosis.
The primary strength of the present study was the
large sample size of the patients with measurable AXIN1 levels. The
primary limitations are the cross-sectional design without the
prospective follow-up and the inability to describe the causality
of the findings.
In conclusion, in patients with endometriosis,
P-AXIN1 levels were negatively correlated with F-calprotectin and
humoral inflammatory biomarkers, with the exception of hs-CRP. In
addition, they were positively correlated with the duration of
endometriosis and the GI symptoms, as well as with vomiting and
nausea and the symptoms' effect on quality of life. Increased AXIN1
levels were associated with duration of GI symptoms and the degree
of specific GI symptoms. AXIN1 levels were increased in patients
with hormonal treatment. The measurement of hs-CRP, F-calprotectin
and other humoral biomarkers was determined to be of no value in
endometriosis. Future studies are required to determine the effect
of hormonal treatment on P-AXIN1, as well as the potential role of
AXIN1 in the development of endometriosis and associated symptoms,
and its role as a potential biomarker for the disease.
Supplementary Material
Clinicopathological characteristics of
the entire cohort (n=172).
Acknowledgements
We would like to thank Dr Per Ekström, Professor Lil
Valentin and Dr Johanna Nordengren from the Department of
Gynecology, Skane University Hospital, Malmö, for identifying and
helping with the recruitments of patients.
Funding
The present study was funded by grants from Bengt
Ihre Foundation (2017), Dir Albert Påhlsson's Foundation (2016) and
Development Foundation of Region Skane (2018).
Availability of data and materials
The datasets used and/or analysed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
The present study was designed by KD, ME, BR and BO.
KD, ME, BR and BO performed the experiments. KD, ME and BO analysed
the data. KD wrote the original draft of the manuscript. ME, BR and
BO reviewed and revised the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was performed in accordance with
the Declaration of Helsinki. Ethical approval was obtained from the
Ethics Review Board of Lund University [approval nos. 2012/564
(09/10/2012) and 2016/56 (03/05/2016)]. All participants provided
written informed consent prior to participation.
Patient consent for publication
All participants provided written informed consent
for publication of their data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Eskenazi B and Warner ML: Epidemiology of
endometriosis. Obstet Gynecol Clin North Am. 24:235–258.
1997.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Creed J, Maggrah A, Reguly B and Harbottle
A: Mitochondrial DNA deletions accurately detect endometriosis in
symptomatic females of child-bearing age. Biomark Med. 13:291–306.
2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bulun SE, Yilmaz BD, Sison C, Miyazaki K,
Bernardi L, Liu S, Kohlmeier A, Yin P, Milad M and Wei J:
Endometriosis. Endocr Soc. 40:1048–1079. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Viganò D, Zara F and Usai P: Irritable
bowel syndrome and endometriosis: New insights for old diseases.
Dig Liver Dis. 50:213–219. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Giudice LC and Kao LC: Endometriosis.
Lancet. 364:1789–1799. 2004.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Fassbender A, Burney RO, O DF, D'Hooghe T
and Giudice L: Update on biomarkers for the detection of
endometriosis. Biomed Res Int. 2015(130854)2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ek M, Roth B, Engström G and Ohlsson B:
AXIN1 in plasma or serum is a potential new biomarker for
endometriosis. Int J Mol Sci. 20(189)2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kikuchi A: Roles of axin in the Wnt
signalling pathway. Cell Signal. 11:777–788. 1999.PubMed/NCBI View Article : Google Scholar
|
|
9
|
MacDonald BT, Tamai K and He X:
Wnt/beta-catenin signaling: Components, mechanisms, and diseases.
Dev Cell. 17:9–26. 2009.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Shi J, Chi S, Xue J, Yang J, Li F and Liu
X: Emerging role and therapeutic implication of Wnt signaling
pathways in autoimmune diseases. J Immunol Res.
2016(9392132)2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hod K, Dickman R, Sperber A, Melamed S,
Dekel R, Ron Y, Halpern Z, Berliner S and Maharshak N: Assessment
of high-sensitivity CRP as a marker of micro-inflammation in
irritable bowel syndrome. Neurogastroenterol Motil. 23:1105–1110.
2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Pletikosic S, Plavsic I, Hauser G and
Tkalcic M: Fecal calprotectin and serum chromogranin A as potential
biomarkers of irritable bowel syndrome symptom severity. Med
Hypotheses. 85:339–342. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Thubert T, Santulli P, Marcellin L, Menard
S, M'Baye M, Streuli I, Borghese B, de Ziegler D and Chapron C:
Measurement of hs-CRP is irrelevant to diagnose and stage
endometriosis: Prospective study of 834 patients. Am J Obstet
Gynecol. 210:533.e1–533.e10. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ni Bhriain H, Trovik J, Wik E, Stefansson
IM, Akslen LA, Salvesen HB and Staff AC: Plasma calprotectin
concentrations in women with endometrial carcinoma. Gynecol Oncol.
114:491–495. 2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kostakis ID, Cholidou KG, Kallianidis K,
Perrea D and Antsaklis A: The role of calprotectin in obstetrics
and gynecology. Eur J Obstet Gynecol Reprod Biol. 151:3–9.
2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yang Z, Clark N and Park KT: Effectiveness
and cost-effectiveness of measuring fecal calprotectin in diagnosis
of inflammatory bowel disease in adults and children. Clin
Gastroenterol Hepatol. 12:253–262, e2. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Mumolo MG, Bertani L, Ceccarelli L, Laino
G, Di Fluri G, Albano E, Tapete G and Costa F: From bench to
bedside: Fecal calprotectin in inflammatory bowel diseases clinical
setting. World J Gastroenterol. 24:3681–3694. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
World Medical Association: World medical
association declaration of Helsinki: Ethical principles for medical
research involving human subjects. JAMA 310: 2191-2194, 2013.
|
|
19
|
Ek M, Roth B, Nilsson PM and Ohlsson B:
Characteristics of endometriosis: A case-cohort study showing
elevated IgG titers against the TSH receptor (TRAb) and mental
comorbidity. Eur J Obstet Gynecol Reprod Biol. 231:8–14.
2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Bengtsson M, Ohlsson B and Ulander K:
Development and psychometric testing of the visual analogue scale
for irritable bowel syndrome (VAS-IBS). BMC Gastroenterol.
7(16)2007.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Labmedicin Skåne Analysportalen. Available
online: http://www.analysportalen-labmedicin.skane.se/.
|
|
22
|
Bengtsson M, Hammar O, Mandl T and Ohlsson
B: Evaluation of gastrointestinal symptoms In different patient
groups using the visual analogue scale for irritable bowel syndrome
(VAS-IBS). BMC Gastroenterol. 11(122)2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
World Health Organization. Global databae
on body mass index. Available from: http://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi.
|
|
24
|
Moparthi L and Koch S: Wnt signaling in
intestinal inflammation. Differentiation. 108:24–32.
2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Clevers H and Nusse R: Wnt/β-catenin
signaling and disease. Cell. 149:1192–1205. 2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Li VS, Ng SS, Boersema PJ, Low TY,
Karthaus WR, Gerlach JP, Mohammed S, Heck AJ, Maurice MM, Mahmoudi
T and Clevers H: Wnt signaling through inhibition of β-catenin
degradation in an intact Axin1 complex. Cell. 149:1245–1256.
2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Arend RC, Londoño-Joshi AI, Straughn JM Jr
and Buchsbaum DJ: The Wnt/β-catenin pathway in ovarian cancer: A
review. Gynecol Oncol. 131:772–779. 2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Silva-García O, Valdez-Alarcón JJ and
Baizabal-Aguirre VM: The Wnt/β-catenin signaling pathway controls
the inflammatory response in infections caused by pathogenic
bacteria. Mediators Inflamm. 2014(310183)2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Katoh M: Multi-layered prevention and
treatment of chronic inflammation, organ fibrosis and cancer
associated with canonical WNT/β-catenin signaling activation
(Review). Int J Mol Med. 42:713–725. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ikeda S, Kishida S, Yamamoto H, Murai H,
Koyama S and Kikuchi A: Axin, a negative regulator of the Wnt
signaling pathway, forms a complex with GSK-3beta and beta-catenin
and promotes GSK-3beta-dependent phosphorylation of beta-catenin.
EMBOJ. 17:1371–1384. 1998.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Song X, Wang S and Li L: New insights into
the regulation of Axin function in canonical Wnt signaling pathway.
Protein Cell. 5:186–193. 2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Mukherjee A, Dhar N, Stathos M, Schaffer
DV and Kane RS: Understanding how Wnt influences destruction
complex activity and β-catenin dynamics. iScience. 6:13–21.
2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Matsuzaki S, Botchorishvili R, Pouly JL
and Canis M: Targeting the Wnt/beta-pathway in endometriosis: A
potentially effective approach for treatment and prevention. Mol
Cell Ther. 2(36)2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhang L, Xiong W, Xiong Y, Liu H and Liu
Y: 17 β-Estradiol promotes vascular endothelial growth factor
expression via the Wnt/β-catenin pathway during the pathogenesis of
endometriosis. Mol Hum Reprod. 22:526–535. 2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Issa B, Onon TS, Agrawal A, Shekhar C,
Morrs J, Hamdy S and Whorwell PJ: Visceral hypersensitivity in
endometriosis: A new target for treatment? Gut. 61:3667–3672.
2012.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Nilholm C, Roth B and Ohlson B: A dietary
intervention with reduction of starch and sucrose leads to reduced
gastrointestinal and extra-intestinal symptoms in IBS patients.
Nutrients. 11: pii(E1662)2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Daryabor G, Kabelitz D and Kalantar K: An
update on immune dysregulation in obesity-related insulin
resistance. Scand J Immunol. 89(e12747)2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Røseth AG, Schmidt PN and Fagerhol MK:
Correlation between faecal excretion of indium-111-labelled
granulocytes and calprotectin, a granulocyte marker protein, in
patients with inflammatory bowel disease. Scand J Gastroenterol.
34:50–54. 1999.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Andréasson K, Scheja A, Saxne T, Ohlsson B
and Hesselstrand R: Faecal calprotectin: A biomarker of
gastrointestinal disease in systemic sclerosis. J Intern Med.
270:50–57. 2011.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Ek M, Roth B, Valentin L, Nordengren J and
Ohlsson B: Autoantibodies common in patients with gastrointestinal
diseases are not found in patients with endometriosis: A
cross-sectional study. Eur J Obstet Gynecol Reprod Biol.
240:370–374. 2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Hirsch M, Begum MR, Paniz É, Barker C and
Davis CJ: Diagnosis and management of endometriosis: A systematic
review of international and national guidelines. BJOG. 125:556–564.
2018.PubMed/NCBI View Article : Google Scholar
|