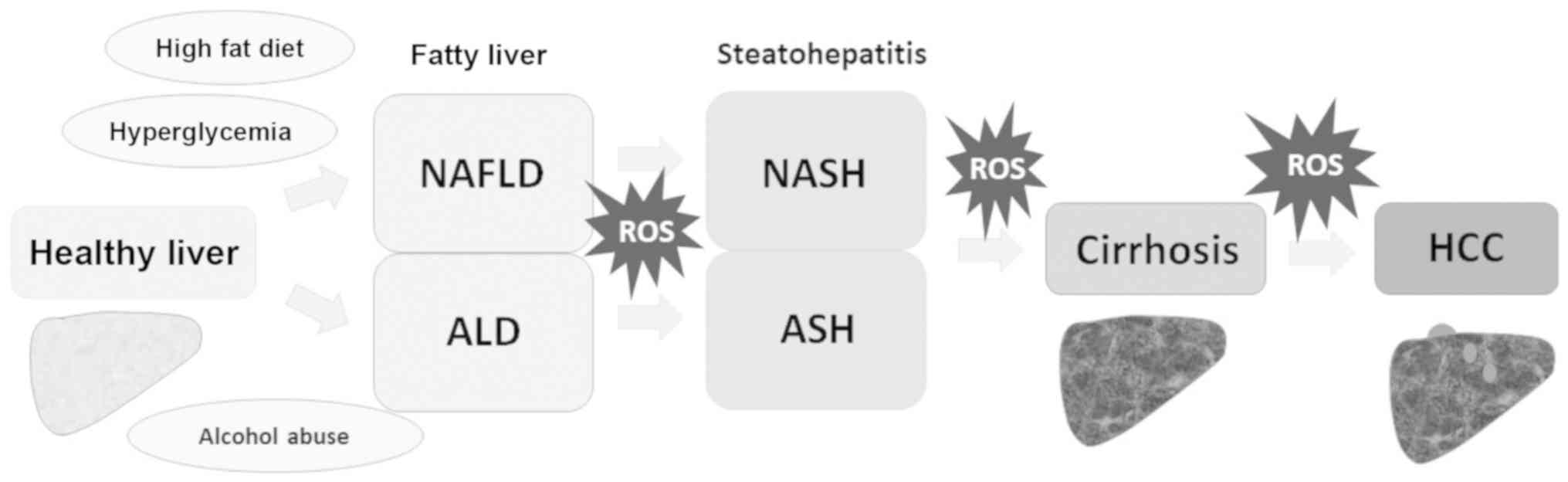
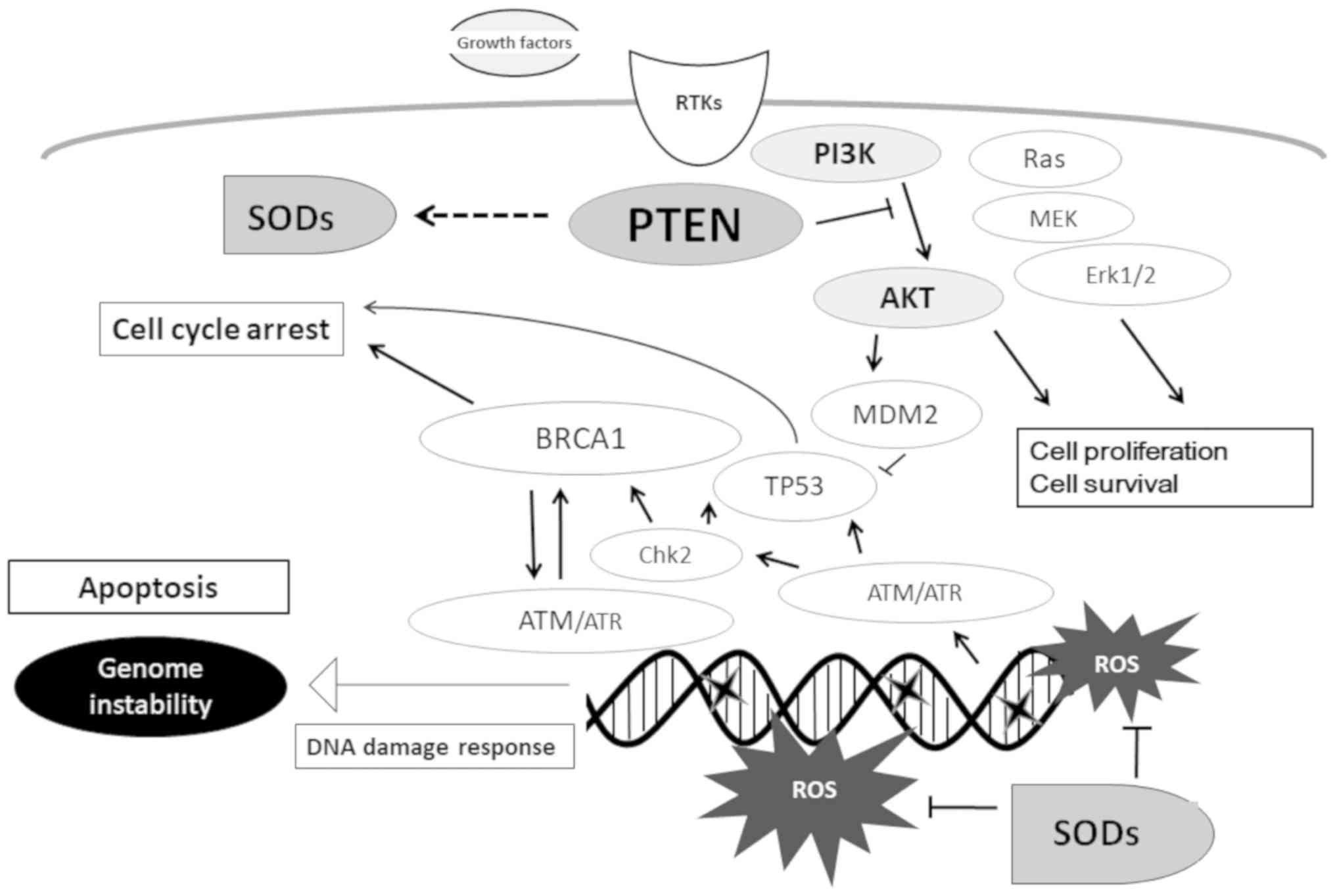
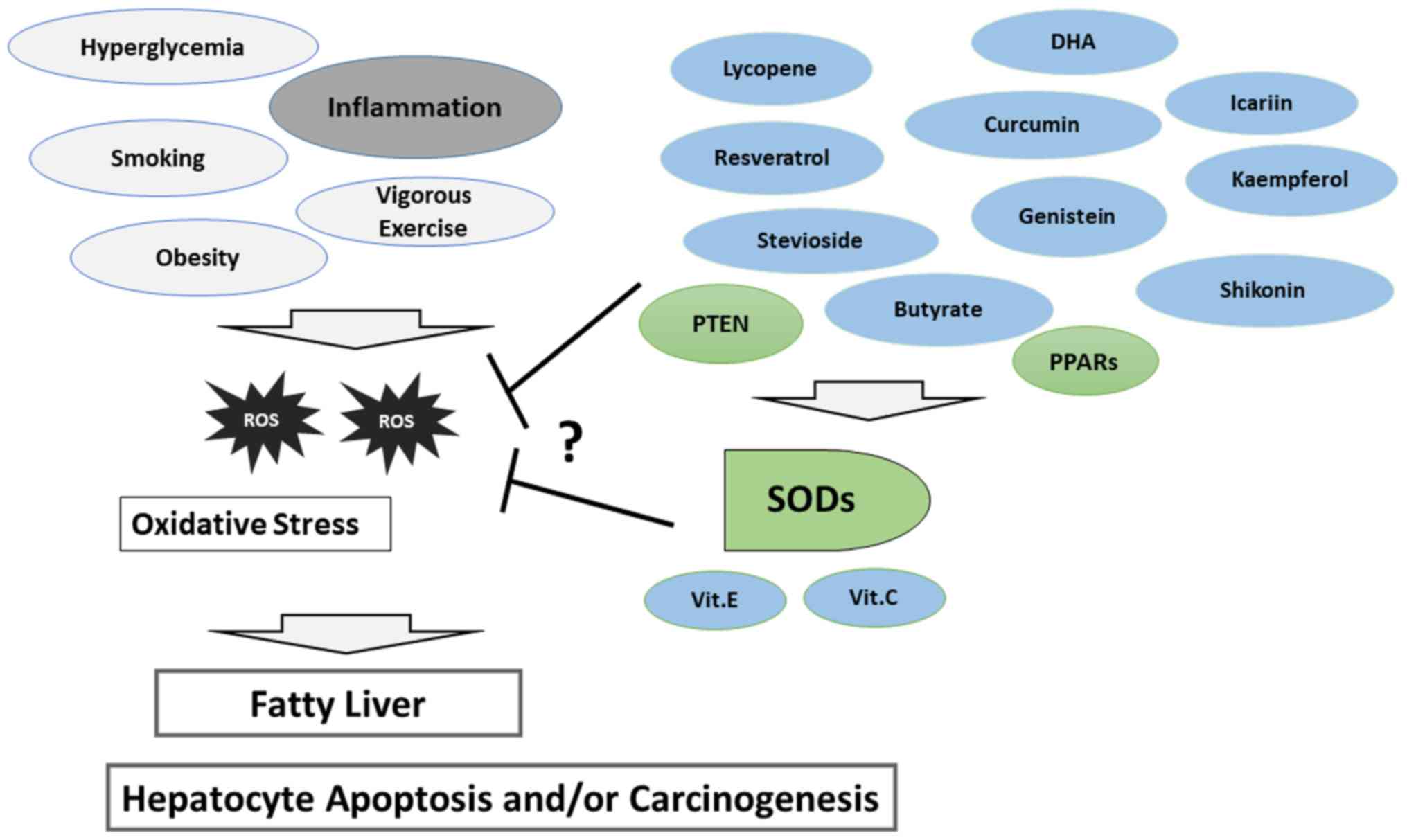
|
1
|
Protzer U, Maini MK and Knolle PA: Living
in the liver: Hepatic infections. Nat Rev Immunol. 12:201–213.
2012.PubMed/NCBI View
Article : Google Scholar
|
|
2
|
Liu A, Galoosian A, Kaswala D, Li AA,
Gadiparthi C, Cholankeril G, Kim D and Ahmed A: Nonalcoholic fatty
liver disease: Epidemiology, liver transplantation trends and
outcomes, and risk of recurrent disease in the graft. J Clin Transl
Hepatol. 6:420–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Sundaram V and Morgan TR: Will studies in
nonalcoholic steatohepatitis help manage alcoholic steatohepatitis?
Clin Liver Dis. 23:157–165. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Eslam M and George J: Genetic insights for
drug development in NAFLD. Trends Pharmacol Sci. 40:506–516.
2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Chalasani N, Younossi Z, Lavine JE, Diehl
AM, Brunt EM, Cusi K, Charlton M and Sanyal AJ: The diagnosis and
management of non-alcoholic fatty liver disease: Practice Guideline
by the American Association for the Study of Liver Diseases,
American College of Gastroenterology, and the American
Gastroenterological Association. Hepatology. 55:2005–2023.
2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Anstee QM, Reeves HL, Kotsiliti E, Govaere
O and Heikenwalder M: From NASH to HCC: Current concepts and future
challenges. Nat Rev Gastroenterol Hepatol. 16:411–428.
2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Karaman H, Karaman A, Donmez-Altuntas H,
Bitgen N, Hamurcu Z, Oguz A and Karakukcu C: Investigation of
genome instability in patients with non-alcoholic steatohepatitis.
World J Gastroenterol. 19:5295–5301. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ma Z, Zhang Y, Li Q, Xu M, Bai J and Wu S:
Resveratrol improves alcoholic fatty liver disease by
downregulating HIF-1α expression and mitochondrial ROS production.
PLoS One. 12(e0183426)2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Simões ICM, Fontes A, Pinton P, Zischka H
and Wieckowski MR: Mitochondria in non-alcoholic fatty liver
disease. Int J Biochem Cell Biol. 95:93–99. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Schattenberg JM and Schuppan D:
Nonalcoholic steatohepatitis: The therapeutic challenge of a global
epidemic. Curr Opin Lipidol. 22:479–488. 2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
García-Ruiz C and Fernández-Checa JC:
Mitochondrial oxidative stress and antioxidants balance in fatty
liver disease. Hepatol Commun. 2:1425–1439. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Poljsak B: Strategies for reducing or
preventing the generation of oxidative stress. Oxid Med Cell
Longev. 2011(194586)2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Michelotti GA, Machado MV and Diehl AM:
NAFLD, NASH and liver cancer. Nat Rev Gastroenterol Hepatol.
10:656–665. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Jacome-Sosa MM and Parks EJ: Fatty acid
sources and their fluxes as they contribute to plasma triglyceride
concentrations and fatty liver in humans. Curr Opin Lipidol.
25:213–220. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Keating SE, Hackett DA, George J and
Johnson NA: Exercise and non-alcoholic fatty liver disease: A
systematic review and meta-analysis. J Hepatol. 57:157–166.
2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Gruszewska E, Gudowska M, Wojtowicz E,
Cylwik B, Szmitkowski M and Chrostek L: The higher prevalence of
non-alcoholic versus alcoholic steatohepatitis in alcoholics. Clin
Lab. 61:1769–1774. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wang Z, Li N, Wang B and Lin J:
Nonalcoholic fatty liver disease progression in rats is accelerated
by splenic regulation of liver PTEN/AKT. Saudi J Gastroenterol.
21:232–238. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Flisiak-Jackiewicz M and Lebensztejn DM:
Update on pathogenesis, diagnostics and therapy of nonalcoholic
fatty liver disease in children. Clin Exp Hepatol. 5:11–21.
2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Knapik DM, Perera P, Nam J, Blazek AD,
Rath B, Leblebicioglu B, Das H, Wu LC, Hewett TE, Agarwal SK Jr, et
al: Mechanosignaling in bone health, trauma and inflammation.
Antioxid Redox Signal. 20:970–995. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Smiles WJ, Parr EB, Coffey VG,
Lacham-Kaplan O, Hawley JA and Camera DM: Protein coingestion with
alcohol following strenuous exercise attenuates alcohol-induced
intramyocellular apoptosis and inhibition of autophagy. Am J
Physiol Endocrinol Metab. 311:E836–E849. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Uzelac L, Škalamera Đ, Mlinarić-Majerski
K, Basarić N and Kralj M: Selective photocytotoxicity of anthrols
on cancer stem-like cells: The effect of quinone methides or
reactive oxygen species. Eur J Med Chem. 137:558–574.
2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Caputo F, Vegliante R and Ghibelli L:
Redox modulation of the DNA damage response. Biochem Pharmacol.
84:1292–1306. 2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Barnes JL, Zubair M, John K, Poirier MC
and Martin FL: Carcinogens and DNA damage. Biochem Soc Trans.
46:1213–1224. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Mansouri A, Gattolliat CH and Asselah T:
Mitochondrial dysfunction and signaling in chronic liver diseases.
Gastroenterology. 155:629–647. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Medina-Navarro R, Duran-Reyes G,
Diaz-Flores M, Hicks JJ and Kumate J: Glucose-stimulated acrolein
production from unsaturated fatty acids. Hum Exp Toxicol.
23:101–105. 2004.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Futosi K, Fodor S and Mócsai A: Neutrophil
cell surface receptors and their intracellular signal transduction
pathways. Int Immunopharmacol. 17:638–650. 2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Banoth B and Cassel SL: Mitochondria in
innate immune signaling. Transl Res. 202:52–68. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Mena S, Ortega A and Estrela JM: Oxidative
stress in environmental-induced carcinogenesis. Mutat Res.
674:36–44. 2009.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Venza I, Visalli M, Oteri R, Teti D and
Venza M: Combined effects of cigarette smoking and alcohol
consumption on antioxidant/oxidant balance in age-related macular
degeneration. Aging Clin Exp Res. 24:530–536. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
30
|
Rains JL and Jain SK: Oxidative stress,
insulin signaling, and diabetes. Free Radic Biol Med. 50:567–575.
2011.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhu W, Jia Q, Wang Y, Zhang Y and Xia M:
The anthocyanin cyanidin-3-O-β-glucoside, a flavonoid, increases
hepatic glutathione synthesis and protects hepatocytes against
reactive oxygen species during hyperglycemia: Involvement of a
cAMP-PKA-dependent signaling pathway. Free Radic Biol Med.
52:314–327. 2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Roh HT, Cho SY and So WY: Obesity promotes
oxidative stress and exacerbates blood-brain barrier disruption
after high-intensity exercise. J Sport Health Sci. 6:225–230.
2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
He F, Li J, Liu Z, Chuang CC, Yang W and
Zuo L: Redox mechanism of reactive oxygen species in exercise.
Front Physiol. 7(486)2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Smith Sonneborn J: Alternative strategy
for Alzheimer's disease: Stress response triggers. Int J Alzheimers
Dis. 2012(684283)2012.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Farombi EO, Shyntum YY and Emerole GO:
Influence of chloroquine treatment and Plasmodium falciparum
malaria infection on some enzymatic and non-enzymatic antioxidant
defense indices in humans. Drug Chem Toxicol. 26:59–71.
2003.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Johnson F and Giulivi C: Superoxide
dismutases and their impact upon human health. Mol Aspects Med.
26:340–352. 2005.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Smith MR, Fernandes J, Go YM and Jones DP:
Redox dynamics of manganese as a mitochondrial life-death switch.
Biochem Biophys Res Commun. 482:388–398. 2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Turgeon MO, Perry NJS and Poulogiannis G:
DNA damage, repair, and cancer metabolism. Front Oncol.
8(15)2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Nakanishi A, Wada Y, Kitagishi Y and
Matsuda S: Link between PI3K/AKT/PTEN pathway and NOX proteinin
diseases. Aging Dis. 5:203–211. 2014.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Bankoglu EE, Tschopp O, Schmitt J, Burkard
P, Jahn D, Geier A and Stopper H: Role of PTEN in oxidative stress
and DNA damage in the liver of Whole-Body Pten Haplodeficient Mice.
PLoS One. 11(e0166956)2016.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Lyu J, Yu X, He L, Cheng T, Zhou J, Cheng
C, Chen Z, Cheng G, Qiu Z and Zhou W: The protein phosphatase
activity of PTEN is essential for regulating neural stem cell
differentiation. Mol Brain. 8(26)2015.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Oganesian A, Poot M, Daum G, Coats SA,
Wright MB, Seifert RA and Bowen-Pope DF: Protein tyrosine
phosphatase RQ is a phosphatidylinositol phosphatase that can
regulate cell survival and proliferation. Proc Natl Acad Sci USA.
100:7563–7568. 2003.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Carnero A and Paramio JM: The
PTEN/PI3K/AKT pathway in vivo, cancer mouse models. Front Oncol.
4(252)2014.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Chen CY, Chen J, He L and Stiles BL: PTEN:
Tumor suppressor and metabolic regulator. Front Endocrinol
(Lausanne). 9(338)2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Kitagishi Y and Matsuda S: Redox
regulation of tumor suppressor PTEN in cancer and aging (Review).
Int J Mol Med. 31:511–515. 2013.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Liu Y, Yan J, Sun C, Li G, Li S, Zhang L,
Di C, Gan L, Wang Y, Zhou R, et al: Ameliorating mitochondrial
dysfunction restores carbon ion-induced cognitive deficits via
co-activation of NRF2 and PINK1 signaling pathway. Redox Biol.
17:143–157. 2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Liu Y, Cao Y, Sun S, Zhu J, Gao S, Pang J,
Zhu D and Sun Z: Transforming growth factor-beta1 upregulation
triggers pulmonary artery smooth muscle cell proliferation and
apoptosis imbalance in rats with hypoxic pulmonary hypertension via
the PTEN/AKT pathways. Int J Biochem Cell Biol. 77:141–154.
2016.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Luo X, Liao R, Hanley KL, Zhu HH, Malo KN,
Hernandez C, Wei X, Varki NM, Alderson N, Chu C, et al: Dual Shp2
and Pten deficiencies promote Non-alcoholic Steatohepatitis and
genesis of liver tumor-initiating cells. Cell Rep. 17:2979–2993.
2016.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Piguet AC, Saran U, Simillion C, Keller I,
Terracciano L, Reeves HL and Dufour JF: Regular exercise decreases
liver tumors development in hepatocyte-specific PTEN-deficient mice
independently of steatosis. J Hepatol. 62:1296–1303.
2015.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Gao M and Liu D: CRISPR/Cas9-based Pten
knock-out and Sleeping Beauty Transposon-mediated Nras knock-in
induces hepatocellular carcinoma and hepatic lipid accumulation in
mice. Cancer Biol Ther. 18:505–512. 2017.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Eritja N, Arjó G, Santacana M, Gatius S,
Ramírez-Núñez O, Arcal L, Serrano JCE, Pamplona R, Dolcet X, Piñol
C, et al: Oral intake of genetically engineered high-carotenoid
corn ameliorates hepatomegaly and hepatic steatosis in PTEN
haploinsufficient mice. Biochim Biophys Acta. 1862:526–535.
2016.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Trohatou O, Zagoura D, Orfanos NK, Pappa
KI, Marinos E, Anagnou NP and Roubelakis MG: miR-26a Mediates
Adipogenesis of amniotic fluid mesenchymal Stem/Stromal cells via
PTEN, Cyclin E1, and CDK6. Stem Cells Dev. 26:482–494.
2017.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Qiu W, Fderico L, Naples M, Avramoglu RK,
Meshkani R, Zhang J, Tsai J, Hussain M, Dai K, Iqbal J, et al:
Phosphatase and tensin homolog (PTEN) regulates hepatic
lipogenesis, microsomal triglyceride transfer protein, and the
secretion of apolipoprotein B-containing lipoproteins. Hepatology.
48:1799–1809. 2008.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Iida S, Ono A, Sayama K, Hamaguchi T,
Fujii H, Nakajima H, Namba M, Hanafusa T, Matsuzawa Y and Moriwaki
K: Accelerated decline of blood glucose after intravenous glucose
injection in a patient with Cowden disease having a heterozygous
germline mutation of the PTEN/MMAC1 gene. Anticancer Res.
20:1901–1904. 2000.PubMed/NCBI
|
|
55
|
Zeng T, Zhang CL, Zhao N, Guan MJ, Xiao M,
Yang R, Zhao XL, Yu LH, Zhu ZP and Xie KQ: Impairment of Akt
activity by CYP2E1 mediated oxidative stress is involved in chronic
ethanol-induced fatty liver. Redox Biol. 14:295–304.
2018.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Shearn CT and Petersen DR: Understanding
the tumor suppressor PTEN in chronic alcoholism and hepatocellular
carcinoma. Adv Exp Med Biol. 815:173–184. 2015.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Peyrou M, Bourgoin L and Foti M: PTEN in
non-alcoholic fatty liver disease/non-alcoholic steatohepatitis and
cancer. Dig Dis. 28:236–246. 2010.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Slavin J, Jacobs D and Marquart L:
Whole-grain consumption and chronic disease: Protective mechanisms.
Nutr Cancer. 27:14–21. 1997.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Nourhashemi F, Gillette-Guyonnet S,
Andrieu S, Ghisolfi A, Ousset PJ, Grandjean H, Grand A, Pous J,
Vellas B and Albarede JL: Alzheimer disease: Protective factors. Am
J Clin Nutr. 71 (Suppl):643S–649S. 2000.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Tokuhira N, Kitagishi Y, Suzuki M, Minami
A, Nakanishi A, Ono Y, Kobayashi K, Matsuda S and Ogura Y:
PI3K/AKT/PTEN pathway as a target for Crohn's disease therapy
(Review). Int J Mol Med. 35:10–16. 2015.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Barreto R, Kawakita S, Tsuchiya J, Minelli
E, Pavasuthipaisit K, Helmy A and Marotta F: Metal-induced
oxidative damage in cultured hepatocytes and hepatic lysosomal
fraction: Beneficial effect of a Curcumin/Absinthium compound. Chin
J Dig Dis. 6:31–36. 2005.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Shehzad A, Qureshi M, Anwar MN and Lee YS:
Multifunctional curcumin mediate multitherapeutic effects. J Food
Sci. 82:2006–2015. 2017.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Qiang Z, Meng L, Yi C, Yu L, Chen W and
Sha W: Curcumin regulates the miR-21/PTEN/Akt pathway and acts in
synergy with PD98059 to induce apoptosis of human gastric cancer
MGC-803 cells. J Int Med Res. 47:1288–1297. 2019.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Andújar I, Ríos JL, Giner RM and Recio MC:
Pharmacological properties of shikonin-a review of literature since
2002. Planta Med. 79:1685–1697. 2013.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Xie L, Li M, Liu D, Wang X, Wang P, Dai H,
Yang W, Liu W, Hu X and Zhao M: Secalonic Acid-F, a Novel
Mycotoxin, represses the progression of hepatocellular carcinoma
via MARCH1 regulation of the PI3K/AKT/β-catenin signaling pathway.
Molecules. 24(pii: E393)2019.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Wang H, Chen L, Zhang X, Xu L, Xie B, Shi
H, Duan Z, Zhang H and Ren F: Kaempferol protects mice from
d-GalN/LPS-induced acute liver failure by regulating the ER
stress-Grp78-CHOP signaling pathway. Biomed Pharmacother.
111:468–475. 2019.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Xu B, Jiang C, Han H, Liu H, Tang M, Liu
L, Ji W, Lu X, Yang X, Zhang Y and Liu Y: Icaritin inhibits the
invasion and epithelial-to-mesenchymal transition of glioblastoma
cells by targeting EMMPRIN via PTEN/AKt/HIF-1α signalling. Clin Exp
Pharmacol Physiol. 42:1296–1307. 2015.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Lee MK, Choi YJ, Sung SH, Shin DI, Kim JW
and Kim YC: Antihepatotoxic activity of icariin, a major
constituent of Epimedium koreanum. Planta Med. 61:523–526.
1995.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Lou K, Yang M, Duan E, Zhao J, Yu C, Zhang
R, Zhang L, Zhang M, Xiao Z, Hu W and He Z: Rosmarinic acid
stimulates liver regeneration through the mTOR pathway.
Phytomedicine. 23:1574–1582. 2016.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Yoshida H, Okumura N, Kitagishi Y,
Nishimura Y and Matsuda S: Ethanol extract of Rosemary repressed
PTEN expression in K562 culture cells. Int J Appl Biol Pharm
Technol. 2:316–322. 2011.
|
|
71
|
Wang Z, Hu JN, Yan MH, Xing JJ, Liu WC and
Li W: Caspase-mediated anti-apoptotic effect of ginsenoside Rg5, a
main rare ginsenoside, on acetaminophen-induced hepatotoxicity in
mice. J Agric Food Chem. 65:9226–9236. 2017.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Sarady JK, Zuckerbraun BS, Bilban M,
Wagner O, Usheva A, Liu F, Ifedigbo E, Zamora R, Choi AM and
Otterbein LE: Carbon monoxide protection against endotoxic shock
involves reciprocal effects on iNOS in the lung and liver. FASEB J.
18:854–856. 2004.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Huo Y, Win S, Than TA, Yin S, Ye M, Hu H
and Kaplowitz N: Antcin H Protects against acute liver injury
through disruption of the interaction of c-Jun-N-terminal kinase
with mitochondria. Antioxid Redox Signal. 26:207–220.
2017.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Qian Y, Guan T, Huang M, Cao L, Li Y,
Cheng H, Jin H and Yu D: Neuroprotection by the soy isoflavone,
genistein, via inhibition of mitochondria-dependent apoptosis
pathways and reactive oxygen induced-NF-κB activation in a cerebral
ischemia mouse model. Neurochem Int. 60:759–767. 2012.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Fan YJ, Rong Y, Li PF, Dong WL, Zhang DY,
Zhang L and Cui MJ: Genistein protection against
acetaminophen-induced liver injury via its potential impact on the
activation of UDP-glucuronosyltransferase and antioxidant enzymes.
Food Chem Toxicol. 55:172–181. 2013.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Stice CP, Xia H and Wang XD: Tomato
lycopene prevention of alcoholic fatty liver disease and
hepatocellular carcinoma development. Chronic Dis Transl Med.
4:211–224. 2018.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Robinett NG, Peterson RL and Culotta VC:
Eukaryotic copper-only superoxide dismutases (SODs): A new class of
SOD enzymes and SOD-like protein domains. J Biol Chem.
293:4636–4643. 2018.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Desjardins F, Sekkali B, Verreth W, Pelat
M, De Keyzer D, Mertens A, Smith G, Herregods MC, Holvoet P and
Balligand JL: Rosuvastatin increases vascular endothelial PPARgamma
expression and corrects blood pressure variability in obese
dyslipidaemic mice. Eur Heart J. 29:128–137. 2008.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Chatterjee A, Ronghe A, Padhye SB, Spade
DA, Bhat NK and Bhat HK: Antioxidant activities of novel
resveratrol analogs in breast cancer. J Biochem Mol Toxicol: 32,
2018 doi: 10.1002/jbt.21925.
|
|
80
|
Ribeiro CCD, Silva RM, Campanholo VMLP,
Ribeiro DA, Ribeiro Paiott AP and Forones NM: Effects of grape
juice in superoxide dismutase and catalase in colorectal cancer
carcinogenesis induced by azoxymethane. Asian Pac J Cancer Prev.
19:2839–2844. 2018.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Rodrigues AD, Scheffel TB, Scola G, Dos
Santos MT, Fank B, Dani C, Vanderlinde R, Henriques JA, Coitinho AS
and Salvador M: Purple grape juices prevent
pentylenetetrazol-induced oxidative damage in the liver and serum
of Wistar rats. Nutr Res. 33:120–125. 2013.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Geeraert B, Crombé F, Hulsmans M,
Benhabilès N, Geuns JM and Holvoet P: Stevioside inhibits
atherosclerosis by improving insulin signaling and antioxidant
defense in obese insulin-resistant mice. Int J Obes (Lond).
34:569–577. 2010.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Moselhy SS, Ghoneim MA and Khan JA: In
vitro and in vivo evaluation of antimicrobial and antioxidant
potential of stevia extract. Afr J Tradit Complement Altern Med.
13:18–21. 2016.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Ma N, Abaker JA, Bilal MS, Dai H and Shen
X: Sodium butyrate improves antioxidant stability in sub-acute
ruminal acidosis in dairy goats. BMC Vet Res.
14(275)2018.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Jin CJ, Engstler AJ, Sellmann C,
Ziegenhardt D, Landmann M, Kanuri G, Lounis H, Schröder M, Vetter W
and Bergheim I: Sodium butyrate protects mice from the development
of the early signs of non-alcoholic fatty liver disease: Role of
melatonin and lipid peroxidation. Br J Nutr: 1-12, Nov 23, (Epub
ahead of print).
|
|
86
|
Ren T, Zhu L, Shen Y, Mou Q, Lin T and
Feng H: Protection of hepatocyte mitochondrial function by
blueberry juice and probiotics via SIRT1 regulation in
non-alcoholic fatty liver disease. Food Funct. 10:1540–1551.
2019.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Abdulkhaleq FM, Alhussainy TM, Badr MM,
Khalil AAA, Gammoh O, Ghanim BY and Qinna NA: Antioxidative stress
effects of vitamins C, E, and B12, and their combination
can protect the liver against acetaminophen-induced hepatotoxicity
in rats. Drug Des Devel Ther. 12:3525–3533. 2018.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Bello RI, Gómez-Díaz C, Burón MI, Alcaín
FJ, Navas P and Villalba JM: Enhanced anti-oxidant protection of
liver membranes in long-lived rats fed on a coenzyme
Q10-supplemented diet. Exp Gerontol. 40:694–706. 2005.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Musalmah M, Nizrana MY, Fairuz AH,
NoorAini AH, Azian AL, Gapor MT and Wan Ngah WZ: Comparative
effects of palm vitamin E and alpha-tocopherol on healing and wound
tissue antioxidant enzyme levels in diabetic rats. Lipids.
40:575–580. 2005.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Aliche-Djoudi F, Podechard N, Collin A,
Chevanne M, Provost E, Poul M, Le Hégarat L, Catheline D, Legrand
P, Dimanche-Boitrel MT, Lagadic-Gossmann D and Sergent O: A role
for lipid rafts in the protection afforded by docosahexaenoic acid
against ethanol toxicity in primary rat hepatocytes. Food Chem
Toxicol. 60:286–296. 2013.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Ide T, Kobayashi H, Ashakumary L, Rouyer
IA, Takahashi Y, Aoyama T, Hashimoto T and Mizugaki M: Comparative
effects of perilla and fish oils on the activity and gene
expression of fatty acid oxidation enzymes in rat liver. Biochim
Biophys Acta. 1485:23–35. 2000.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Manley S and Ding W: Role of farnesoid X
receptor and bile acids in alcoholic liver disease. Acta Pharm Sin
B. 5:158–167. 2015.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Forsyth CB, Farhadi A, Jakate SM, Tang Y,
Shaikh M and Keshavarzian A: Lactobacillus GG treatment
ameliorates alcohol-induced intestinal oxidative stress, gut
leakiness, and liver injury in a rat model of alcoholic
steatohepatitis. Alcohol. 43:163–172. 2009.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Bastian WP, Hasan I, Lesmana CRA, Rinaldi
I and Gani RA: Gut Microbiota profiles in nonalcoholic fatty liver
disease and its possible impact on disease progression evaluated
with transient elastography: Lesson learnt from 60 cases. Case Rep
Gastroenterol. 13:125–133. 2019.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Sharpton SR, Maraj B, Harding-Theobald E,
Vittinghoff E and Terrault NA: Gut microbiome-targeted therapies in
nonalcoholic fatty liver disease: A systematic review,
meta-analysis, and meta-regression. Am J Clin Nutr. 110:139–149.
2019.PubMed/NCBI View Article : Google Scholar
|