Introduction
L-citrulline (Cit) is a free amino acid and
functions as an intermediate in the urea cycle. Cit can be readily
converted to L-arginine in the kidney, vascular endothelium and
other tissues, and is utilized for the production of nitric oxide
via L-arginine (1,2). Thus, Cit has been reported to regulate
blood pressure via nitric oxide production (1,2). It has
been reported that Cit modulates cytokine production by macrophages
(upregulation of IL-6 and downregulation of TNF-α) in diabetic and
obese individuals (3). In addition,
Cit exhibits anti-inflammatory action on ethanol-induced gastric
ulcers and non-alcoholic fatty liver disease by reducing
pro-inflammatory cytokine (IL-6 and TNF-α) production (4,5). Recently,
it was reported that administration of Cit and glucosamine (GlcN)
in a rat model of osteoarthritis (OA) reduced cartilage damage
(6). However, the mechanism
underlying their chondroprotective effects have not been
elucidated.
As a functional food material, GlcN exhibits a
chondroprotective effect on animal models of OA and rheumatoid
arthritis by suppressing production of inflammatory cytokines in
synoviocytes and chondrocytes (7-10).
Furthermore, GlcN improves the symptoms of knee OA in humans; thus,
GlcN is widely used for the prevention and treatment of OA
(11). N-acetylglucosamine (GlcNAc),
a derivative of GlcN, exhibits anti-inflammatory and
chondroprotective effects in a rat model of OA by reducing IL-6
production, cyclooxygenase expression and type II collagen
degradation (12-15).
Furthermore, GlcNAc is reported to improve the symptoms of knee OA
in humans (16,17).
Based on these findings, it is hypothesized that
Cit, GlcN and GlcNAc may cooperatively act and exhibit improved
anti-inflammatory effects in inflammatory joint diseases. However,
to the best of our knowledge, the combined effect of Cit, GlcN and
GlcNAc on inflammatory joint diseases has not been evaluated. Thus,
in the present study, a focus was placed on synovial cells, which
are primarily involved in inflammatory joint diseases, and the
combined effect of Cit, GlcN and GlcNAc on the synovial cell
inflammation was assessed.
Materials and methods
Cell line and reagents
MH7A human synovial cells were purchased from RIKEN
BioResource Center (cat. no. RCB1512). Cit, GlcNAc and GlcN
hydrochloride were supplied by Protein Chemical Co., Ltd. IL-1β and
FBS were purchased from PeproTech, Inc. and Nichirei Biosciences,
Inc., respectively. RPMI-1640 medium, RIPA lysis buffer with
protease inhibitor cocktail, Phosphatase Inhibitor Cocktail™ and
Sample Buffer solution with reducing reagent (6x) for SDS-PAGE were
obtained from Nacalai Tesque, Inc.
Cell culture
MH7A cells were maintained in RPMI-1640 medium
supplemented with 10% FBS and 100-fold diluted
Penicillin-Streptomycin mixed solution (Nacalai Tesque, Inc.), and
maintained at 37˚C with 5% CO2.
Measurement of IL-6
MH7A cells (3x104 cells/well) were seeded
and cultured in 24-well plates overnight. Cells were incubated with
Cit (0.5 mM), GlcNAc (0.5 mM) or GlcN (0.5-1.0 mM) alone, or a
combination of Cit and GlcN, Cit and GlcNAc, or GlcN and GlcNAc for
2 h at 37˚C. Subsequently, cells were stimulated with 50 pg/ml
IL-1β in a total volume of 0.5 ml RPMI-1640 medium for 24 h at
37˚C. IL-6 in the culture supernatants was quantified using the
DuoSet ELISA Development kit (cat. no. DY206-05; R&D Systems,
Inc.). Briefly, a monoclonal antibody specific for IL-6 was
precoated onto microtiter plates (96-well half area flat bottom;
Corning, Inc.). Standards and samples were pipetted into the wells,
and IL-6 was bound to immobilized antibody. After washing with PBS
containing 0.05% Tween-20, an enzyme-linked polyclonal antibody
specific for IL-6 was added to the wells. Following washing with
PBS containing 0.05% Tween-20, a substrate solution
(1x3,3',5,5'-Tetramethylbenzidine substrate Solution; cat. no.
00-4201-56; eBioscinece; Thermo Fisher Scientific, Inc.) was added
to the wells, and the color was developed. The reaction was stopped
by addition of sulfuric acid, and the intensity of developed color
was measured using a microplate reader at 450 nm (Model 680;
Bio-Rad Laboratories, Inc.).
Phosphorylation of p38 MAPK, NF-κB and
ERK1/2
MH7A cells (3x104/well) were seeded and
cultured in 24-well plates overnight. Cells were incubated with Cit
(0.5 mM), GlcNAc (0.5 mM) or GlcN (0.5 mM) alone, or a combination
of Cit and GlcN, Cit and GlcNAc, or GlcN and GlcNAc for 2 h, and
stimulated with 200 pg/ml IL-1β for 30 min at 37˚C. Following
washing with ice-cold PBS, the cells were harvested in 100
µl RIPA lysis buffer containing 1/100 v/v Phosphatase
Inhibitor Cocktail™. Following sonication, the lysates were
centrifuged at 4˚C at 12,000 x g for 10 min, and the protein
concentrations of the supernatants were determined using a
Bicinchoninic Acid protein assay kit (Pierce; Thermo Fisher
Scientific, Inc.) using bovine serum albumin as the standard. The
supernatants were mixed with 6x SDS-PAGE sample buffer and boiled
for 3 min. The mixtures (containing 10 µg protein/lane) were
separated by 10% SDS-PAGE and electrophoretically transferred to an
Immobilon-P PVDF membrane (EMD Millipore). To detect phosphorylated
p38 MAPK, NF-κB and ERK1/2, the membranes were blocked at room
temperature for 30 min in Blocking One solution (Nacalai Tesque,
Inc.), and probed with a mouse anti-phospho-(p)-p38 MAPK
(pT180/pY182) monoclonal antibody (1:1,000; cat. no. 612168; BD
Pharmingen, BD Biosciences), anti-p-NF-κB p65 (Ser536) rabbit
monoclonal antibody (1:1,000; cat. no. 3033; Cell Signaling
Technology, Inc.) or anti-p-ERK1/2 MAPK (Thr202/Tyr204) rabbit
antibody (1:1,000; cat. no. 9101; Cell Signaling Technology, Inc.)
at 4˚C overnight. Horseradish peroxidase (HRP)-conjugated goat
anti-mouse immunoglobulin (Ig)G/IgM (1:5,000; cat. no. 115-035-044;
Jackson ImmunoResearch Laboratories, Inc.) or HRP-conjugated goat
anti-rabbit IgG (1:5,000; cat. no. 12-348; Chemicon International;
Thermo Fisher Scientific, Inc.) were used as the secondary
antibodies, and incubated with the membranes at room temperature
for 1 h.
The membranes were stripped by incubating in Restore
Western Stripping Buffer (Pierce; Thermo Fisher Scientific, Inc.)
at 37˚C for 30 min. Total p38 MAPK, NF-κB and ERK1/2 expression in
each sample was detected by reprobing with mouse anti-p38 MAPK
antibody (1:2,000; SAPK2a; cat. no. 612168; BD Pharmingen, BD
Biosciences), anti-NF-κB p65 rabbit monoclonal antibody (1:2,000;
cat. no. 8242; Cell Signaling Technology, Inc.) or anti-ERK1/2 MAPK
rabbit antibody (1:2,000; cat. no. 9102; Cell Signaling Technology,
Inc.) at 4˚C overnight, followed by incubating with HRP-conjugated
goat anti-mouse (1:5,000) or anti-rabbit (1:5,000) IgG secondary
antibody at room temperature for 1 h. GAPDH expression was detected
by incubating with a mouse anti-GAPDH monoclonal antibody at 4˚C
overnight (1:50,000; cat. no. MAB374; Chemicon International;
Thermo Fisher Scientific, Inc.) and subsequently HRP-conjugated
goat anti-mouse IgG/IgM at room temperature for 1 h (1:5,000).
GAPDH was used as the loading control.
Signals were detected using SuperSignal West Dura
Chemiluminescent Substrate (Pierce; Thermo Fisher Scientific,
Inc.), and quantified using a LAS-3000 luminescent image analyzer
and Multi Gauge version 3.0 (both from Fujifilm).
Statistical analysis
Data are expressed as the mean ± standard deviation,
and were analyzed using a one-way ANOVA with a post-hoc Tukey's
test using GraphPad Prism version 6 (GraphPad Software, Inc.).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effects of Cit, GlcNAc and GlcN on
IL-1β-stimulated IL-6 production by MH7A cells
GlcN has been reported to significantly reduce the
production of inflammatory mediators and inflammatory cytokines
such as NO, PGE2 and IL-8 by synovial cells in
vitro at 1 mM (9). Therefore, to
evaluate the combined effects of Cit with GlcNAc and GlcN on
IL-1β-stimulated IL-6 production by MH7A cells, the concentration
of GlcN was reduced to 0.5 mM, a concentration at which GlcN is
unlikely to suppress cytokine production. In fact, when MH7A was
stimulated with IL-1β in the presence of GlcN (0.5 and 1.0 mM), 0.5
mM GlcN did not significantly reduce IL-6 production, whereas 1 mM
GlcN significantly suppressed production (data not shown). Thus, in
the present study, the concentrations of Cit and GlcNAc were
adjusted to 0.5 mM (the same concentration as GlcN), and their
combined effect was determined. IL-1β stimulation markedly
increased the production of IL-6 by MH7A cells (Fig. 1). GlcN alone did not affect
IL-1β-stimulated IL-6 production by MH7A cells at 0.5 mM. However,
Cit significantly suppressed IL-6 production (P<0.001).
Combination of Cit and GlcN did not further suppress IL-6
production, although the combination significantly suppressed IL-6
production compared with IL-1β alone or GlcN + IL-1β (Fig. 1A; P<0.05).
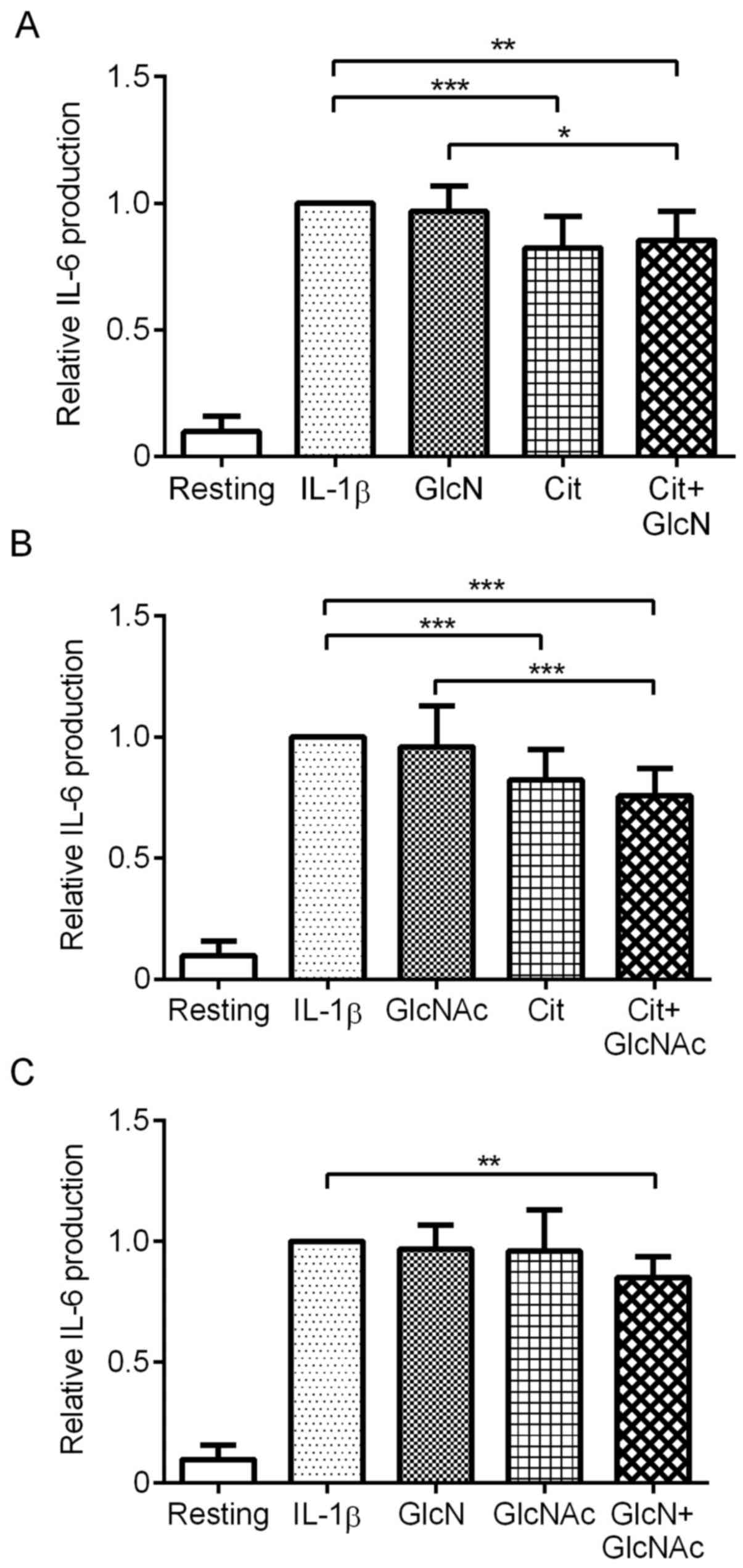 | Figure 1Effect of Cit, GlcNAc or GlcN, and
their combinations on IL-1β-stimulated IL-6 production by MH7A
cells. (A) MH7A cells were incubated with or without IL-1β in the
presence of 0.5 mM GlcN, 0.5 mM Cit or Cit + GlcN for 24 h. (B)
MH7A cells were incubated with or without IL-1β in the presence of
0.5 mM GlcNAc, 0.5 mM Cit or Cit + GlcNAc for 24 h. (C) MH7A cells
were incubated with or without IL-1β in the presence of 0.5 mM
GlcN, 0.5 mN GlcNAc, or GlcN + GlcNAc for 24 h. IL-6 production was
quantified in the supernatant using ELISA. Data are presented as
the mean ± standard deviation of 10-16 separate experiments.
*P<0.05, **P<0.01 and
***P<0.001. Cit, L-citrulline; GlcNAc,
N-acetylglucosamine; GlcN, glucosamine. |
Next, the combined effect of Cit and GlcNAc on
IL-1β-stimulated IL-6 production was evaluated. GlcNAc alone did
not affect IL-6 production by MH7A cells at 0.5 mM, whereas Cit
significantly suppressed the IL-6 production (P<0.001), as
described above. Notably, the combination of Cit and GlcNAc further
reduced IL-6 production, compared with Cit alone, although the
reduction was not significant. Furthermore, combination of Cit and
GlcNAc significantly suppressed IL-6 production, compared with
IL-1β alone and GlcNAc + IL-1β (Fig.
1B; P<0.001).
Subsequently, the combined effect of GlcN and GlcNAc
on IL-1β-stimulated IL-6 production was evaluated. GlcN and GlcNAc
alone did not significantly affect IL-6 production by MH7A cells.
However, combination of GlcN and GlcNAc significantly suppressed
IL-6 production, compared with IL-1β alone (Fig. 1C; P<0.01).
Morphological analysis of the cytotoxic effects of
Cit, GlcN or GlcNAc on IL-1β-stimulated human synovial MH7A cells
was assessed. None of these substances induced cell death (such as
apoptosis and necrosis) in IL-1β-stimulated MH7A cells when
incubated with 0.5 or 1.0 mM of the compound (data not shown).
Effects of Cit, GlcNAc and GlcN on
phosphorylation of ERK1/2
It has been shown that GlcN exerts an
anti-inflammatory effect by suppressing pro-inflammatory cytokine
production at >1 mM on mouse macrophage-like cells (RAW 264.7),
human umbilical vein endothelial cells (HUVECs) and human colonic
epithelial cells (HT-29) due to the suppression of p38 MAPK and
NF-κB signaling (18-20).
Thus, to determine whether the suppressive action of Cit + GlcN,
Cit + GlcNAc, and GlcN + GlcNAc on IL-1β-stimulated IL-6 production
was also mediated by the suppression of p38 MAPK and NF-κB
signaling, the effects of these substances on the phosphorylation
of p38 MAPK and NF-κB p65 was investigated. IL-1β stimulation
markedly increased the phosphorylation of p38 MAPK and NF-κB p65
(Fig. 2A and B). However, none of the treatments or their
combinations (0.5 mM each) suppressed the p38 MAPK and NF-κB
activation in synovial cells (Fig. 2A
and B). It has also been shown that
ERK1/2 signaling is involved in the IL-1β-mediated activation of
chondrocytes and osteoblasts (21).
Thus, the combination of Cit + GlcN, Cit + GlcNAc, and GlcN +
GlcNAc on suppression of IL-1β-stimulated phosphorylation of ERK1/2
in synovial cells was determined. IL-1β stimulation only slightly
but substantially increased the phosphorylation of ERK (Fig. 3). GlcN (0.5 mM) alone did not affect
the phosphorylation of ERK1/2 (Fig.
3A). Notably, Cit substantially suppressed the phosphorylation
of ERK1/2, although the suppression was not statistically
significant. Interestingly, the combination of Cit + GlcN further
reduced the phosphorylation of ERK1/2, compared with Cit alone,
although the reduction was not significant. Combination of Cit and
GlcN significantly suppressed the phosphorylation of ERK1/2
compared with IL-1β alone and GlcN + IL-1β (P<0.05).
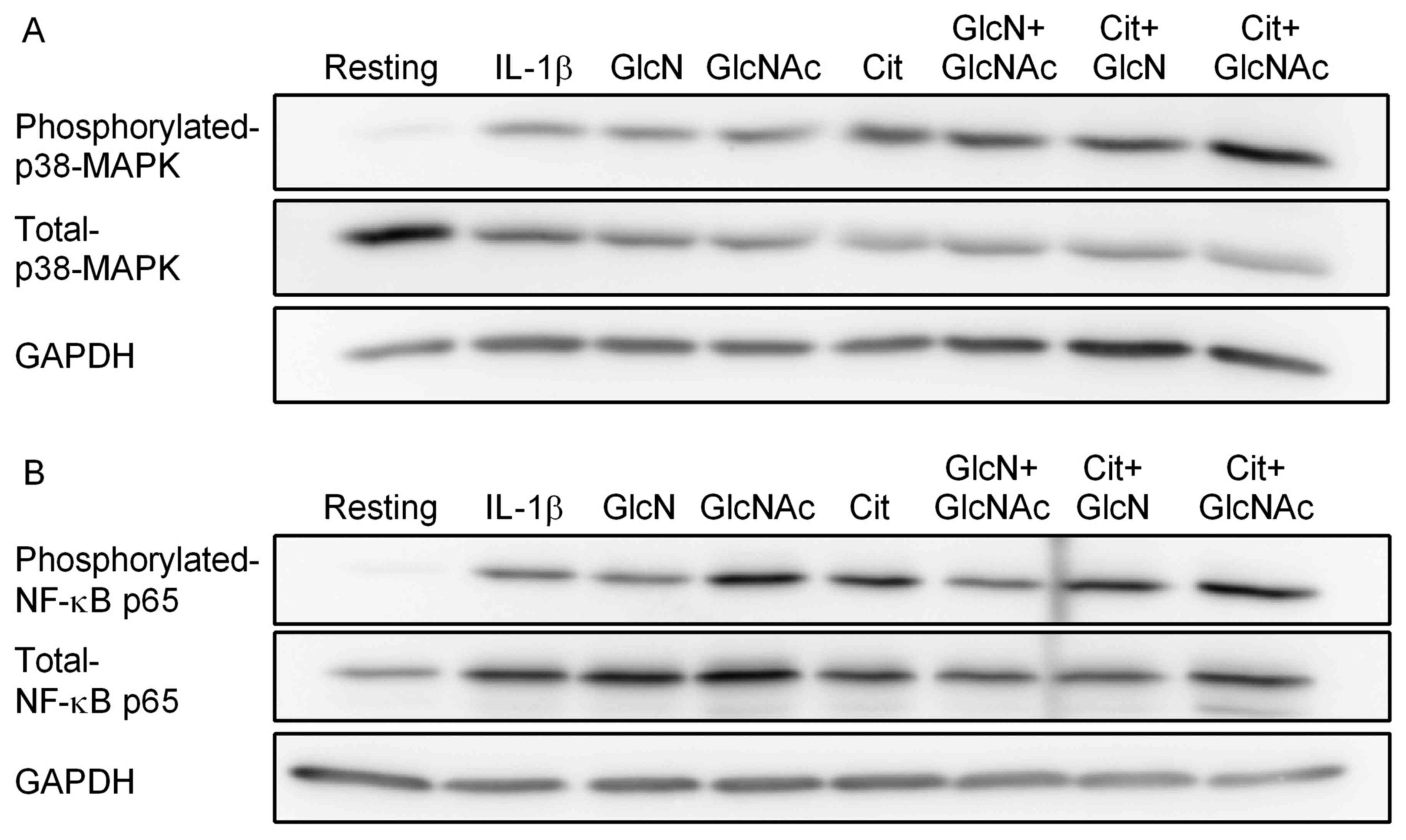 | Figure 2Effect of Cit, GlcNAc or GlcN, and
their combinations on IL-1β-stimulated phosphorylation of p38 MAPK
and NF-κB p65 by MH7A cells. MH7A cells were incubated with or
without IL-1β in the presence of 0.5 mM GlcN, 0.5 mM GlcNAc or 0.5
mM Cit alone for 30 min. Alternatively, MH7A cells were incubated
with IL-1β in the presence of a combination of GlcN + GlcNAC, Cit +
GlcN, or Cit + GlcNAc for 30 min. Western blotting was performed to
assess the expression of phosphorylated and total (A) p38 MAPK, and
(B) NF-κB p65. GAPDH was used as the loading control. The images
are representative of three separate experiments. The vertical
lines between GlcN + GlcNAc and Cit + GlcN in the NF-κB p65 blots
are non-specific binding. Cit, L-citrulline; GlcNAc,
N-acetylglucosamine; GlcN, glucosamine; NF-κB, nuclear
factor-κB. |
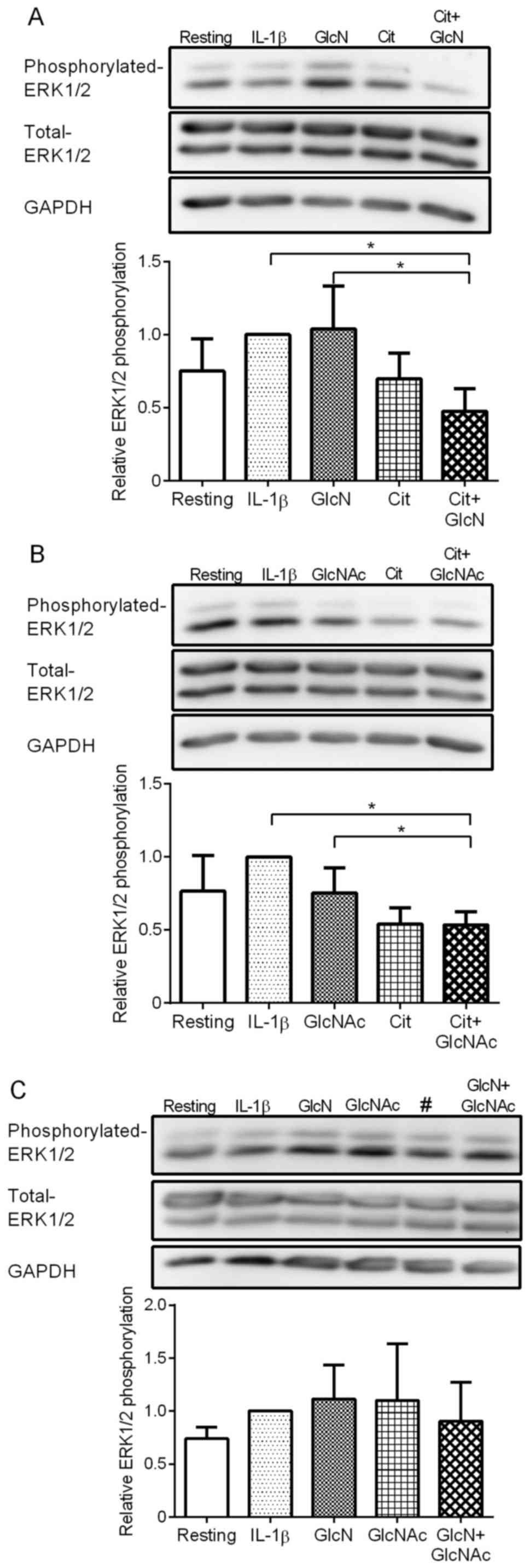 | Figure 3Effect of Cit, GlcNAc or GlcN, and
their combinations on IL-1β-stimulated phosphorylation of ERK1/2 by
MH7A cells. (A) MH7A cells were incubated with or without IL-1β in
the presence of 0.5 mM GlcN, 0.5 mM Cit or Cit + GlcN for 30
min.(B) MH7A cells were incubated with or without IL-1β in the
presence of 0.5 mM GlcNAc, 0.5 mM Cit or Cit + GlcNAc for 30 min.
(C) MH7A cells were incubated with or without IL-1β in the presence
of 0.5 mM GlcN, 0.5 mN GlcNAc, or GlcN + GlcNAc for 30 min. Western
blotting was performed to assess the expression of phosphorylated
and total ERK1/2. GAPDH was used as the loading control. The blots
are representative of three separate experiments. Data are
presented as the mean ± standard deviation of three separate
experiments. *P<0.05. #, MH7A cells incubated with
IL-1β and 0.5 mM Cit, which was excluded from calculation of the
data (graph) shown in (C) Cit, L-citrulline; GlcNAc,
N-acetylglucosamine; GlcN, glucosamine. |
The combined effect of Cit and GlcNAc on the
IL-1β-stimulated phosphorylation of ERK1/2 was evaluated. GlcNAc
(0.5 mM) alone did not notably affect the phosphorylation of ERK1/2
by MH7A cells (Fig. 3B), whereas Cit
substantially suppressed the phosphorylation of ERK1/2 (Fig. 3A). Notably, the combination of Cit and
GlcNAc suppressed the phosphorylation of ERK1/2 compared with IL-1β
alone and GlcNAc + IL-1β (P<0.05).
Finally, the combination of GlcN and GlcNAc on
IL-1β-stimulated phosphorylation of ERK1/2 was assessed. GlcN and
GlcNAc alone did not significantly affect the phosphorylation of
ERK1/2 in MH7A cells (Fig. 3C).
However, the combination of GlcN and GlcNAc slightly suppressed the
phosphorylation of ERK1/2 compared with GlcN and GlcNAc alone,
although the suppression was not statistically significant.
Discussion
The aim of the present study was to evaluate the
anti-inflammatory effects of Cit, GlcN and GlcNAc on synovial
cells, and determine the combined effect of Cit, GlcN and GlcNAc on
synovial cell inflammation, as assessed by the IL-1β-induced IL-6
production. GlcN has been reported to significantly inhibit the
production of inflammatory mediators and inflammatory cytokines
such as NO, PGE2 and IL-8 by synovial cells in
vitro at a concentration of 1 mM (9). Therefore, to evaluate the combined
effects of Cit and GlcNAc with GlcN on IL-1β-stimulated IL-6
production by MH7A cells, the concentration of GlcN was reduced to
0.5 mM, a concentration at which GlcN did not significantly reduce
IL-6 production (data not shown), and the concentrations of Cit and
GlcNAc were also adjusted to 0.5 mM (the same concentration as
GlcN). The results showed that 0.5 mM GlcN did not suppress IL-6
production (Fig. 1). Similarly, 0.5
mM GlcNAc did not suppress IL-6 production, although 0.5 mM Cit
alone significantly suppressed IL-6 production. The combined effect
of Cit, GlcNAc and GlcN was examined, and the results showed that
Cit + GlcN and Cit + GlcNAc significantly suppressed not only IL-6
production but also the phosphorylation of ERK1/2. Combination of
GlcN + GlcNAc also significantly suppressed IL-6 production and
reduced phosphorylation of ERK1/2. These results suggest that among
Cit, GlcNAc and GlcN, combination of Cit + GlcN and Cit + GlcNAc
more potently exerts an anti-inflammatory effect on synovial cells
when combined, and may therefore possibly be used to alleviate
inflammatory joint diseases. However, whether the combination of
Cit + GlcN and Cit + GlcNAc exhibits anti-inflammatory and
chondroprotective effects in vivo using animal OA models
should be determined.
It has been shown that production of inflammatory
cytokines and mediators following IL-1β-stimulation is mediated by
signaling pathways involving NF-κB, p38 MAPK, ERK1/2 and c-Jun
N-terminal kinase (JNK)1/2 in chondrocytes (21). GlcN has been reported to exert its
anti-inflammatory effects by inhibiting the production of
inflammatory mediators and cytokines via suppression of the p38
MAPK and NF-κB signaling pathways in macrophages (murine macrophage
cell line RAW264.7), endothelial cells (HUVECs) and colonic
epithelial cells (human colon cancer cell line HT-29) (18-20).
Furthermore, GlcN has been reported to suppress the
IL-1β-stimulated phosphorylation of p38 MAPK but not the
phosphorylation of ERK1/2 and JNK1/2 in human chondrosarcoma cells
(22), and GlcN suppressed
IL-1β-induced production of IL-6 and cyclooxygenase-2 expression in
IL-1β-stimulated chondrocytes without affecting ERK, JNK and
p38MAPK signaling (14). Previously,
the chondroprotective effect of GlcN was evaluated using
chondrosarcoma SW1353 cells (22). In
the present study, the protein expression of MMP-3 was
significantly increased by IL-1β stimulation; however, the
phosphorylation of ERK was only slightly increased by IL-1β
stimulation. Thus, it is possible that the slight increase of
phosphorylated ERK observed in the present study may also be
involved in increased production of IL-6 following IL-1β
stimulation.
Combination of Cit + GlcN and Cit + GlcNAc
suppressed activity of the ERK1/2 signaling pathway, but not the
p38 MAPK and NF-κB signaling pathways. Thus, the combination of Cit
+ GlcN and Cit + GlcNAc may suppress the ERK1/2 signaling pathway,
which is different from the pathway involved in GlcN or GlcNAc
mediated suppression. Furthermore, the combination of Cit + GlcN
and Cit + GlcNAc potently suppressed IL-1β-stimulated IL-6
production as well, compared with either GlcN or GlcNAc alone.
Thus, it is likely that the combination of Cit + GlcN and Cit +
GlcNAc synergistically exerts its anti-inflammatory action
(suppression of ERK1/2 signaling and IL-6 production) on synovial
MH7A cells via different signaling pathway (ERK1/2) from those (p38
MAPK and NF-κB signaling pathways) involved in suppression mediated
by GlcN or GlcNAc.
In conclusion, the combination of Cit with GlcN and
GlcNAc synergistically exerted an anti-inflammatory effect on
synovial cells, thereby possibly exhibiting chondroprotective
effects and alleviating inflammatory joint disease. The enhanced
effect of Cit was likely mediated via a different signaling pathway
from those regulated by GlcN or GlcNAc.
Acknowledgements
The authors would like to thank Dr Taisuke Murakami
and Dr Mamoru Igarashi (Department of Host Defense and Biochemical
Research, Juntendo University, Graduate School of Medicine), for
technical assistance and helpful discussions and Mr. Kiminori Iida
(Protein Chemical Co., Ltd.) for supplying materials utilized in
this study.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YY and AS conceived and designed the study. YY and
IN interpreted the results, and prepared the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Solomonson LP, Flam BR, Pendleton LC,
Goodwin BL and Eichler DC: The caveolar nitric oxide
synthase/arginine regeneration system for NO production in
endothelial cells. J Exp Biol. 206:2083–2087. 2003.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Khalaf D, Krüger M, Wehland M, Infanger M
and Grimm D: The effects of oral L-arginine and L-citrulline
supplementation on blood pressure. Nutrients.
11(E1679)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Breuillard C, Bonhomme S, Couderc R,
Cynober L and De Bandt JP: In vitro anti-inflammatory effects of
citrulline on peritoneal macrophages in Zucker diabetic fatty rats.
Br J Nutr. 113:120–124. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Liu Y, Tian X, Gou L, Fu X, Li S, Lan N
and Yin X: Protective effect of L-citrulline against
ethanol-induced gastric ulcer in rats. Environ Toxicol Pharmacol.
34:280–287. 2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Darabi Z, Darand M, Yari Z, Hedayati M,
Faghihi A, Agah S and Hekmatdoost A: Inflammatory markers response
to citrulline supplementation in patients with non-alcoholic fatty
liver disease: A randomized, double blind, placebo-controlled,
clinical trial. BMC Res Notes. 12(89)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Jintake C, Azuma K and Okamoto Y:
Combination of glucosamine hydrochloride and citrulline for
experimental osteoarthritis model. Chitin Chitosan Res.
25(180)2019.(In Japanese).
|
|
7
|
Largo R, Alvarez-Soria MA, Díez-Ortego I,
Calvo E, Sánchez-Pernaute O, Egido J and Herrero-Beaumont G:
Glucosamine inhibits IL-1beta-induced NFkappaB activation in human
osteoarthritic chondrocytes. Osteoarthritis Cartilage. 11:290–298.
2003.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Naito K, Watari T, Furuhata A, Yomogida S,
Sakamoto K, Kurosawa H, Kaneko K and Nagaoka I: Evaluation of the
effect of glucosamine on an experimental rat osteoarthritis model.
Life Sci. 86:538–543. 2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hua J, Sakamoto K, Kikukawa T, Abe C,
Kurosawa H and Nagaoka I: Evaluation of the suppressive actions of
glucosamine on the interleukin-1beta-mediated activation of
synoviocytes. Inflamm Res. 56:432–438. 2007.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Nagaoka I: Recent aspects of the
chondroprotective and anti-inflammatory actions of glucosamine, a
functional food. Juntendo Med J. 60:580–587. 2014.
|
|
11
|
Ogata T, Ideno Y, Akai M, Seichi A, Hagino
H, Iwaya T, Doi T, Yamada K, Chen ZA, Li Y and Hayashi K: Effects
of glucosamine in patients with osteoarthritis of the knee: A
systematic review and meta-analysis. Clin Rheumatol. 37:2479–2487.
2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kubomura D, Ueno T, Yamada M and Nagaoka
I: Evaluation of the chondroprotective action of
N-acetylglucosamine in a rat experimental osteoarthritis model. Exp
Ther Med. 14:3137–3144. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Shikhman AR, Brinson DC, Valbracht J and
Lotz MK: Differential metabolic effects of glucosamine and
N-acetylglucosamine in human articular chondrocytes. Osteoarthritis
Cartilage. 17:1022–1028. 2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Shikhman AR, Kuhn K, Alaaeddine N and Lotz
M: N-acetylglucosamine prevents IL-1beta-mediated activation of
human chondrocytes. J Immunol. 166:5155–5160. 2001.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Shikhman AR, Amiel D, D'Lima D, Hwang SB,
Hu C, Xu A, Hashimoto S, Kobayashi K, Sasho T and Lotz MK:
Chondroprotective activity of N-acetylglucosamine in rabbits with
experimental osteoarthritis. Ann Rheum Dis. 64:89–94.
2005.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Katsuno S, Eguchi C, Yoshimura K, Yamamoto
T, Tomonaga A and Nagaoka I: Effects and safety of milk beverage
contaning N-acetyl glucosamine on knee joint pain and biomarkers of
type II collagen metabolism: Preliminary study on open design. Jpn
Pharmacol Ther. 38:435–445. 2010.
|
|
17
|
Naraoka Y, Harada H, Katagiri M, Yamamura
H and Shirasawa T: N-acetyl glucosamine and proteoglycan containing
supplement improves the locomotor functions of subjects with knee
pain. Drug Discov Ther. 11:140–145. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Rafi MM, Yadav PN and Rossi AO:
Glucosamine inhibits LPS-induced COX-2 and iNOS expression in mouse
macrophage cells (RAW 264.7) by inhibition of p38-MAP kinase and
transcription factor NF-kappaB. Mol Nutr Food Res. 51:587–593.
2007.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ju Y, Hua J, Sakamoto K, Ogawa H and
Nagaoka I: Modulation of TNF-alpha-induced endothelial cell
activation by glucosamine, a naturally occurring amino
monosaccharide. Int J Mol Med. 22:809–815. 2008.PubMed/NCBI
|
|
20
|
Yomogida S, Hua J, Sakamoto K and Nagaoka
I: Glucosamine suppresses interleukin-8 production and ICAM-1
expression by TNF-alpha-stimulated human colonic epithelial HT-29
cells. Int J Mol Med. 22:205–211. 2008.PubMed/NCBI
|
|
21
|
Jenei-Lanzl Z, Meurer A and Zaucke F:
Interleukin-1β signaling in osteoarthritis-chondrocytes in focus.
Cell Signal. 53:212–223. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lin YC, Liang YC, Sheu MT, Lin YC, Hsieh
MS, Chen TF and Chen CH: Chondroprotective effects of glucosamine
involving the p38 MAPK and Akt signaling pathways. Rheumatol Int.
28:1009–1016. 2008.PubMed/NCBI View Article : Google Scholar
|

















