Introduction
Infertility is a complex disease and is defined as a
couple's inability to have children with unprotected regular
intercourse after one year; ~15% of couples suffer from infertility
worldwide, amounting to 48.5 million couples (1). Several causes contribute to infertility,
and male infertility is responsible for 50% of the total number of
cases (2). Despite there being
several causes of male infertility discussed by previous studies
(3,4),
the cause of 50% of cases of abnormal spermatogenesis are still
unknown. Previous studies have shown that 30-80% of male
infertility cases are caused by the damaging effects of oxidative
stress (5,6).
In vitro experiments have shown that normal
quantities of reactive oxygen species (ROS) are sufficient to
induce mutations, capacitation and ultimately fertilization
(6-10).
Oxidative stress occurs when there is an imbalance between the
production of ROS and the natural antioxidant defense mechanisms
(11-13).
Several studies have also shown that the levels of ROS in infertile
sperm samples are significantly higher compared with the levels
from fertile samples (14). The
damaging effects of oxidative stress cause DNA damage and lead to
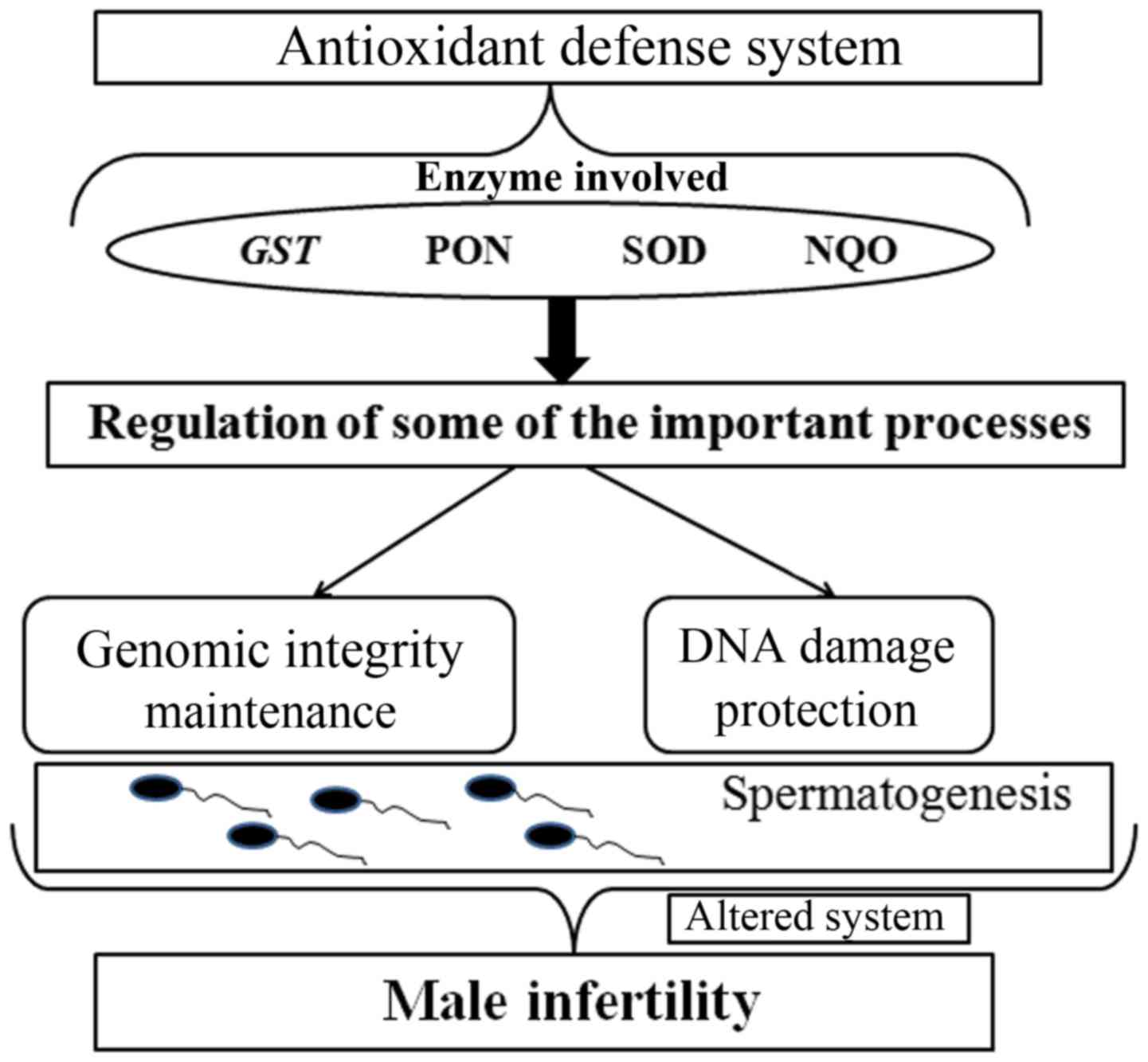
the loss of sperm integrity and function (Fig. 1) (11).
If balance between ROS production and antioxidant mechanisms is
disturbed, the emergent oxidative stress results in DNA damage and
affects spermatogenesis (15).
Generally, the antioxidant defense system is able to
effectively neutralize ROS in the body (16,17). This
precise system is regulated by several enzymes, such as glutathione
S-transferase (GST), paraoxonase, superoxide dismutase (SOD)
and NAD(P)H dehydrogenase quinone (12,18). When
the genes encoding these enzymes are mutated or abnormally
expressed, the antioxidant system may be disrupted, resulting in
damage to sperm DNA. Therefore, polymorphisms in these antioxidant
genes and their gene-gene interactions have been studied to
investigate a possible association with an increased risk of male
infertility (19-23).
However, there may be several antioxidant genes or polymorphisms
that may be potential risk factors for male infertility that have
not been discovered. To fill this gap, the association between
seven potential functional polymorphisms in five enzyme genes
(NQO1 rs1800566, SOD2 rs4880, GSTM3 rs1571858,
rs3814309, rs7483, GSTM5 rs11807 and GSTP1 rs1695) in
the oxidative stress pathway and the risk of male infertility in
248 cases and 310 controls were assessed.
GSTM3 is a member of the GST family and is
located on chromosome 1p13. Polimanti et al (24) showed that GST SNPs may be associated
with complex diseases including male infertility and
embryotoxicity. Reactive oxygen metabolites can damage the DNA of
sperm, and GSTM3 may function to protect sperm through its
molecular mechanism of inactivating cytotoxic substances. Thus,
three SNPs of GSTM3 (rs1571858, rs3814309, rs7483) were
selected and tested for any association with male infertility.
NAD(P)H: quinone oxidoreductase1 (NQO1) serves an
important role in protecting against oxidative stress by
functioning as a cytoplasmic2-electron reductase (25,26).
Several studies have reported that polymorphisms in NQO1 are
associated with male infertility (12,22,27,28);
thus one polymorphism of NQO1 was chosen and investigated
for association with male infertility.
Ji et al (22)
found a significant association between SOD2 rs4880 and male
infertility. Furthermore, Yan et al (23) also found that the SOD2 rs4880
variant genotype was associated with a low level of SOD
activity.
Materials and methods
Subjects and population
The present study was performed in accordance with
the Declaration of Helsinki (29),
and was approved by the Ethics Committee of Jinling Hospital
(Nanjing, China). Written informed consent was obtained from all
participants. Samples were collected from a total of 636 patients
of Han-Chinese ethnicity that had been diagnosed with unexplained
male factor infertility by the Laboratory Medicine, Jinling
Hospital, Nanjing University School of Medicine, between April 2013
and July 2015. At least two semen analyses were performed for all
patients, and those that were found to have genetic factors
(chromosomal anomalies), AZF microdeletions of the Y chromosome,
clinical factors (varicocele, cryptorchidism or orchitis) or
infections were excluded from the present study. In the final
analysis, 248 men with idiopathic infertility were included,
including 146 men with azoospermia or severe oligozoospermia (0<
sperm concentration <5x106/ml; mean age, 28.5±4.3
years; range, 19-39 years) and 102 men with oligozoospermia (sperm
concentration: 5-15x106/ml; mean age, 28.8±4.9 years;
range, 19-38 years).
A total of 310 fertile men (mean age, 28.3±4.3
years; range, 19-40 years) were enrolled in the control group.
These men had at least 1 child as reported by direct survey and
lacked any history of requiring assisted reproduction technology.
All of the controls were selected from the same hospital. The semen
analysis for sperm concentration, motility and morphology was
performed according to the World Health Organization criteria
(2010) (30).
SNP selection
SNP selection as performed through extensive mining
of the International HapMap Project and dbSNP. HapMap is a catalog
of common genetic polymorphisms in the human genome, which
describes the forms of these mutations, their location in the DNA,
and their distribution within the same population and between
different populations (ncbi.nlm.nih.gov/variation/tools/1000genomes).
dbSNP is world's largest database for nucleotide variations, which
also contains human single nucleotide variations, microsatellites,
and small-scale insertions and deletions along with the original
publications (ncbi.nlm.nih.gov/SNP/). A total of 7 potential
functional polymorphisms were identified in the genes of five
enzyme involved in oxidative stress response.
Genomic DNA extraction and
genotyping
Genomic DNA was extracted from the leukocytes in
venous blood from each patient and controls using a blood DNA
extraction kit (Tiangen Biotech Co., Ltd.). The DNA was purified
using a Genomic DNA Purification kit (DU530UV/VIS
spectrophotometer; Beckman Coulter, Inc.). Genotyping was performed
using the Mass ARRAY platform. Briefly, SNPs were detected using a
Sequenom Mass ARRAY RS1000 according to the manufacturer's
protocol. The multiplexed SNP Mass EXTENDED assay was designed by
Sequenom Mass ARRAY Assay Design software version 3.0 (Sequenom).
Data management and analysis were performed using a Sequenom Mass
ARRAY Analyzer 4.0 system (Sequenom).
Statistical analysis
The expected frequencies of genotypes in the control
group were tested for Hardy-Weinberg equilibrium (HWE) using the
exact test. The allele and genotype frequencies of the controls and
patients were directly calculated by counting. To examine the
associations between genetic polymorphisms and male infertility,
the odds ratio (OR) and 95% confidence intervals (CI) were
calculated using a logistic regression model with SPSS version 11.0
(SPSS, Inc.). Two-tailed P<0.05 was considered to indicate a
statistically significant difference. Haplotype analysis was
performed using SHEsis (analysis.bio-x.cn/myAnalysis.php).
Results
Clinical characteristics of the study
population
The present case-controlled study contained 248
infertility patients and 310 fertile controls. The position and
minor allele frequency of the seven functional SNPs found in the
Chinese population in the HapMap database are presented in Table I. The genotypes of seven polymorphisms
in antioxidant genes were determined in the control group and case
groups (azoospermia or severe oligozoospermia and oligozoospermia;
Table II). The genotype frequencies
of all investigated polymorphisms were found to be in HWE in all of
the control groups.
 | Table IPrimary information on the seven
assessed SNPs in antioxidant genes. |
Table I
Primary information on the seven
assessed SNPs in antioxidant genes.
| Gene: SNP | Location or amino
acid change | MAF in Chinese
populationa |
|---|
| NQO1: rs1800566
C-T | nsSNP/P187S | 0.5 |
| SOD2: rs4880
T-C | nsSNP/V16A | 0.117 |
| GSTM3: rs1571858
A-G | Intronic | 0.252 |
| GSTM3: rs3814309
C-T | 3'-UTR | 0.243 |
| GSTM3: rs7483
A-G | nsSNP/V224I | 0.243 |
| GSTM5 rs11807
A-G | 3'-UTR | 0.146 |
| GSTP1: rs1695
A-G | nsSNP/I105V | 0.185 |
 | Table IIDistribution of the control and case
groups by genotype. |
Table II
Distribution of the control and case
groups by genotype.
| Genotype | All cases | Azoospermia |
Oligozoospermia | Control |
PHWE |
|---|
| rs4880 | | | | | 0.086 |
|
TT:TC:CC | 188:55:05 | 112:32:02 | 76:23:03 | 240:69:1 | |
|
MAF | 13% | 22% | 25% | 11.50% | |
| rs1571858 | | | | | 0.576 |
|
AA:AG:GG | 139:92:17 | 81:53:12 | 58:39:05 | 174:114:22 | |
|
MAF | 26% | 26% | 24% | 26% | |
| rs3814309 | | | | | 0.733 |
|
CC:CT:TT | 139:92:17 | 81:53:12 | 58:39:05 | 180:111:19 | |
|
MAF | 26% | 26% | 24% | 24% | |
| rs7483 | | | | | 0.798 |
|
AA:AG:GG | 142:89:17 | 82:52:12 | 60:37:05 | 82:110:18 | |
|
MAF | 25% | 26% | 23% | 24% | |
| rs11807 | | | | | 0.642 |
|
AA:AG:GG | 176:66:6 | 103:41:02 | 73:25:04 | 0.97302083 | |
|
MAF | 16% | 15% | 16% | 16% | |
| rs1695 | | | | | 0.729 |
|
AA:AG:GG | 147:95:6 | 85:58:03 | 62:37:03 | 197:99:14 | |
|
MAF | 22% | 22% | 21% | 21% | |
| rs1800566 | | | | | 0.43 |
|
TT:CT:CC | 54:124:70 | 33:76:37 | 33:48:21 | 84:148:78 | |
|
MAF | 53% | 37% | 44% | 49% | |
Association between polymorphisms in
oxidative stress genes and risk of male infertility
The associations of polymorphisms in oxidative
stress genes and risk of male infertility are shown in Table III. There were no significant
associations found between the seven polymorphisms and risk of male
infertility using a logistic regression model. Haplotype analysis
for the three GSTM3 SNPs was performed; there were no
significant associations between these three SNPs and male
infertility. It was hypothesized that gene-gene interactions may
contribute to male infertility. To test this hypothesis,
statistical analysis of gene-gene interactions between NQO1,
SOD2, GSTM3, GSTM5 and GSTP1 was
performed. There was a decreased risk in male infertility with a
gene-gene interaction between GSTM3 rs3814309 and
NQO1 rs1800566 (CC x CT/TT; OR=0.55, 95% CI=0.34-0.92;
P=0.022; Table IV). There was also a
significant association between gene-gene interactions of
GSTM3 rs1571858 and NQO1 rs1800566 and azoospermia
(AG/GG x CC; OR=0.42, 95% CI=0.18-0.97; P=0.043).
 | Table IIIAssociation between SNPs in
antioxidant genes and risk of male infertility in subjects. |
Table III
Association between SNPs in
antioxidant genes and risk of male infertility in subjects.
| | All subjects,
n=558 | Azoospermia,
n=456 | Oligozoospermia,
n=412 |
|---|
| SNP | n | P-value | OR (95% CI) | n | P-value | OR (95% CI) | n | P-value | OR (95% CI) |
|---|
| GSTP1 rs1695 |
|
AA | 344 | 0.161 | Ref | 282 | 0.155 | Ref | 259 | 0.611 | Ref |
|
AG | 194 | 0.163 | 1.29
(0.92-1.83) | 157 | 0.146 | 1.36
(0.90-2.05) | 136 | 0.477 | 1.19
(0.74-1.91) |
|
GG | 20 | 0.267 | 0.57
(0.22-1.53) | 17 | 0.281 | 0.50
(0.14-1.77) | 17 | 0.556 | 0.68
(0.19-2.45) |
|
AG/GG | 214 | 0.302 | 1.20
(0.85-1.69) | 174 | 0.275 | 1.25
(0.84-1.87) | 153 | 0.616 | 1.13
(0.71-1.78) |
| GSTM3
rs1571858 |
|
AA | 313 | 0.992 | Ref | 255 | 0.914 | Ref | 232 | 0.738 | Ref |
|
AG | 206 | 0.955 | 1.01
(0.71-1.44) | 167 | 0.995 | 0.99
(0.66-1.52) | 153 | 0.914 | 1.03
(0.64-1.64) |
|
GG | 39 | 0.923 | 0.97
(0.49-1.89) | 34 | 0.679 | 1.17
(0.55-2.48) | 27 | 0.46 | 0.68
(0.24-1.88) |
|
AG/GG | 245 | 0.985 | 1.00
(0.72-1.41) | 201 | 0.896 | 1.03
(0.69-1.53) | 180 | 0.897 | 0.97
(0.62-1.53) |
| SOD2 rs4880 |
|
TT | 428 | 0.242 | Ref | 352 | 0.495 | Ref | 316 | 0.153 | Ref |
|
TC | 124 | 0.932 | 1.02
(0.68-1.52) | 101 | 0.98 | 0.99
(0.62-1.60) | 92 | 0.743 | 0.93
(0.60-1.43) |
|
CC | 6 | 0.92 | 6.84
(0.74-55.10) | 3 | 0.237 | 4.29
(0.39-47.76) | 4 | 0.147 | 5.37
(0.55-52.11) |
|
TC/CC | 130 | 0.654 | 1.09
(0.74-1.62) | 104 | 0.867 | 1.04
(0.65-1.66) | 96 | 0.952 | 0.99
(0.65-1.51) |
| GSTM5 rs11807 |
|
AA | 396 | 0.936 | Ref | 323 | 0.587 | Ref | 293 | 0.447 | Ref |
|
AG | 147 | 0.925 | 1.02
(0.70-1.49) | 122 | 0.73 | 1.08
(0.70-1.68) | 106 | 0.211 | 0.76
(0.50-1.17) |
|
GG | 15 | 0.734 | 0.83
(0.29-2.39) | 11 | 0.346 | 0.48
(0.10-2.24) | 13 | 0.75 | 0.84
(0.28-2.50) |
|
AG/GG | 162 | 1 | 1.00
(0.69-1.45) | 133 | 0.927 | 1.02
(0.66-1.57) | 119 | 0.208 | 0.77
(0.51-1.16) |
| GSTM3 rs7483 |
|
AA | 324 | 0.862 | Ref | 264 | 0.612 | Ref | 242 | 0.618 | Ref |
|
AG | 199 | 0.841 | 1.04
(0.73-1.48) | 162 | 0.823 | 1.05
(0.69-1.60) | 147 | 0.991 | 0.99
(0.68-1.47) |
|
GG | 35 | 0.592 | 1.21
(0.60-2.43) | 30 | 0.322 | 1.48
(0.68-3.21) | 23 | 0.339 | 1.40
(0.70-2.80) |
|
AG/GG | 234 | 0.73 | 1.06
(0.76-1.49) | 192 | 0.608 | 1.11
(0.75-1.65) | 170 | 0.758 | 1.06
(0.74-1.52) |
| GSTM3
rs3814309 |
|
CC | 319 | 0.872 | Ref | 261 | 0.685 | Ref | 238 | 0.7 | Ref |
|
CT | 203 | 0.695 | 1.07
(0.75-1.53) | 164 | 0.782 | 1.06
(0.70-1.61) | 150 | 0.984 | 1.00
(0.68-1.48) |
|
TT | 36 | 0.676 | 1.16
(0.58-2.31) | 31 | 0.387 | 1.40
(0.65-3.03) | 24 | 0.407 | 1.34
(0.67-2.66) |
|
CT/TT | 239 | 0.633 | 1.09
(0.78-1.52) | 195 | 0.603 | 1.11
(0.75-1.65) | 174 | 0.768 | 1.06
(0.74-1.52) |
| NQO1 rs1800566 |
|
CC | 148 | 0.332 | Ref | 115 | 0.561 | Ref | 111 | 0.699 | Ref |
|
CT | 272 | 0.737 | 0.93
(0.63-1.40) | 224 | 0.746 | 1.08
(0.67-1.74) | 196 | 0.293 | 0.94
(0.62-1.45) |
|
TT | 138 | 0.164 | 0.72
(0.45-1.15) | 117 | 0.51 | 0.83
(0.47-1.45) | 105 | 0.413 | 0.88
(0.49-1.34) |
|
CT/TT | 410 | 0.415 | 0.86
(0.59-1.25) | 341 | 0.967 | 0.99
(0.63-1.58) | 301 | 0.598 | 0.90
(0.60-1.34) |
 | Table IVAssociation between SNPs in
antioxidant gene-gene interactions and male infertility risk. |
Table IV
Association between SNPs in
antioxidant gene-gene interactions and male infertility risk.
| Groups | Gene-Gene | Genotypes | n | OR (95% CI) | P-value |
|---|
| All subjects,
n=558 |
rs3814309/rs1800566 | CC | CC | 87 | Ref | 0.058 |
| | | CC | CT/TT | 232 | 0.55
(0.34-0.92) | 0.022 |
| | | CT/TT | CT/TT | 178 | 0.80
(0.48-1.33) | 0.085 |
| | | CT/TT | CC | 61 | 0.52
(0.26-1.00) | 0.052 |
| Azoospermia,
n=456 |
rs1571858/rs1800566 | AA | CC | 65 | Ref | 0.116 |
| | | AA | CT/TT | 190 | 0.61
(0.34-1.01) | 0.1 |
| | | AG/GG | CT/TT | 161 | 0.84
(0.46-1.52) | 0.554 |
| | | AG/GG | CC | 50 | 0.42
(0.18-0.97) | 0.043 |
Discussion
Male infertility is a complex disease that is caused
by several factors (31-34).
Sperm DNA integrity and expression is regulated by a precise system
in body, and any damage to sperm DNA may result in spermatogenesis
failure and thus male infertility. Previously, several studies have
shown that oxidative stress is associated with male infertility
through damaging sperm DNA (7,35). SNPs
have been shown to be associated with the activities of antioxidant
defense system enzymes. In the present study, a case-controlled
study to investigate the association of seven SNPs (NQO1
rs1800566, SOD2 rs4880, GSTM3 rs1571858, rs3814309,
rs7483, GSTM5 rs11807 and GSTP1 rs1695) in
antioxidant genes with male infertility in Chinese individuals was
performed. The results showed there were no associations between
the seven SNPs and male infertility. Haplotype analysis of three
GSTM3 SNPs and gene-gene interaction analysis of the seven
SNPs was also performed. There were no significant associations
identified using haplotype analysis; however there was a decreased
risk for male infertility with a gene-gene interaction between
GSTM3 rs3814309 and NQO1 rs1800566 (CC x CT/TT) in
all subjects and a significant interaction between GSTM3
rs1571858 and NQO1 rs1800566 (AG/GG x CC) in
azoospermia.
GSTM3 rs1571858 is located in the intronic
region, and rs3814309 is located on the 3'-untranslated region of
GSTM3. However, no previous studies have investigated the
effect of rs3814309 and rs1571858 on GSTM3 function, to the
best of our knowledge. rs7483 is a missense mutation, which results
in a substitution of the expected Val amino acid with Ile. There
was no association between the three SNPs (rs1571858, rs3814309 and
rs7483) of GSTM3 and a risk of male infertility. However, a
gene-gene interaction between GSTM3 rs3814309 and
NQO1 rs1800566 (CC x CT/TT) was associated with a decreased
risk for male infertility in all subjects. In the present study,
the genotype RR (homozygous mutation) was of low abundance in the
case group, which may have affected the true result. Therefore, a
larger sample size of patients with the three SNPs (rs3814309,
rs1800566 and rs7483) who suffer from male infertility is required
to confirm these results.
NQO1 rs1800566 is a missense mutation
(P187S), which is hypothesized to influence the enzymatic activity
and concentration of NQO1. However, there was no association
between NQO1 rs1800566 and risk of male infertility. This
result is consistent with a previous report by Ji et al
(22), who also found no significant
association between this polymorphism and male infertility,
suggesting that NQO1 rs1800566 is not a risk factor for male
infertility. A gene-gene interaction between GSTM3 rs1571858
and NQO1 rs1800566 (AG/GG x CC) was a significant factor for
male infertility in patients with azoospermia. Although the
rs1571858 SNP is located in the intronic region of GSTM3, it
was hypothesized that rs1571858 may affect GSTM3 function
via an unknown mechanism.
The present study has some limitations.
Gene-environment interaction analysis for male infertility was not
performed. The lifestyles of patients including smoking, drinking
or other potentially detrimental habits contribute to male
infertility. Additionally, the demographic characteristics of
enrolled participants, including clinical data, profession, health
status index are not presented. As in all case-controlled studies,
a selection bias may exist, which may influence the discovery of
real associations. Finally, the association of polymorphisms in
antioxidant genes and male infertility in other ethnicities were
not assessed. Therefore, any associations, or lack thereof,
demonstrated in the present study, should be confirm with a larger
more diverse cohort.
Acknowledgements
We would like to thank Dr Bangshun He, Nanjing First
Hospital for their expert advice on data analysis.
Funding
This work was supported by Nanjing Science and
Technology Planning Project (grant no. 201605004), Jangsu Science
and Technology Planning Project (grant no. BE2018713), The Central
Military Commission Logistics Support Department, The Military
Family Planning Special Research Task Plan Key Projects (grant no.
18JS005), and The Subject Of Nanjing General Hospital, IHH new
pathogenic locus identification and pathogenesis research (grant
no. 2017046).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XX conceived and designed the present study. YY and
PZ analyzed the data and wrote the manuscript. TL collected the
samples. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Jinling Hospital (Nanjing, China). All patients
provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Agarwal A, Mulgund A, Hamada A and Chyatte
MR: A unique view on male infertility around the globe. Reprod Biol
Endocrinol. 13(37)2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Sato Y, Tajima A, Tsunematsu K, Nozawa S,
Yoshiike M, Koh E, Kanaya J, Namiki M, Matsumiya K, Tsujimura A, et
al: An association study of four candidate loci for human male
fertility traits with male infertility. Hum Reprod. 30:1510–1514.
2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Esteves SC, Miyaoka R and Agarwal A: An
update on the clinical assessment of the infertile male
[corrected]. Clinics (Sao Paulo). 66:691–700. 2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Hamada AJ, Esteves SC and Agarwal A: A
comprehensive review of genetics and genetic testing in
azoospermia. Clinics (Sao Paulo). 68(Suppl 1):39–60.
2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Tremellen K: Oxidative stress and male
infertility-a clinical perspective. Hum Reprod update. 14:243–258.
2008.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ko EY, Sabanegh ES Jr and Agarwal A: Male
infertility testing: Reactive oxygen species and antioxidant
capacity. Fertil Steril. 102:1518–1527. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wright C, Milne S and Leeson H: Sperm DNA
damage caused by oxidative stress: Modifiable clinical, lifestyle
and nutritional factors in male infertility. Reprod Biomed Online.
28:684–703. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Agarwal A and Allamaneni SS: Role of free
radicals in female reproductive diseases and assisted reproduction.
Reprod Biomed Online. 9:338–347. 2004.PubMed/NCBI View Article : Google Scholar
|
|
9
|
De Lamirande E and Cagnon C: Human sperm
hyperactivation and capacitation as parts of an oxidative process.
Free Radic Biol Med. 14:157–166. 1993.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Tremellen K, Miari G, Froiland D and
Thompson J: A randomised control trial examining the effect of an
antioxidant (Menevit) on pregnancy outcome during IVF-ICSI
treatment. Aust N Z J Obstet Gynaecol. 47:216–221. 2007.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Said TM, Agarwal A, Sharma RK, Thomas AJ
Jr and Sikka SC: Impact of sperm morphology on DNA damage caused by
oxidative stress induced by beta-nicotinamide adenine dinucleotide
phosphate. Fertil Steril. 83:95–103. 2005.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ichioka K, Nagahama K, Okubo K, Soda T,
Ogawa O and Nishiyama H: Genetic polymorphisms in glutathione
S-transferase T1 affect the surgical outcome of varicocelectomies
in infertile patients. Asian J Androl. 11:333–341. 2009.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Pisoschi AM and Pop A: The role of
antioxidants in the chemistry of oxidative stress: A review. Eur J
Med Chem. 97:55–74. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Guz J, Gackowski D, Foksinski M, Rozalski
R, Zarakowska E, Siomek A, Szpila A, Kotzbach M, Kotzbach R and
Olinski R: Comparison of oxidative stress/DNA damage in semen and
blood of fertile and infertile men. PLoS One.
8(e68490)2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Shen H and Ong C: Detection of oxidative
DNA damage in human sperm and its association with sperm function
and male infertility. Free Radic Biol Med. 28:529–536.
2000.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Amir Aslani B and Ghobadi S: Studies on
oxidants and antioxidants with a brief glance at their relevance to
the immune system. Life Sci. 146:163–173. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Reszka E and Wasowicz W: Significance of
genetic polymorphisms in glutathione S-transferase multigene family
and lung cancer risk. Int J Occup Med Environ Health. 14:99–113.
2001.PubMed/NCBI
|
|
18
|
Forsberg L, de Faire U and Morgenstern R:
Oxidative stress, human genetic variation, and disease. Arch
Biochem Biophys. 389:84–93. 2001.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Jun S, Pierce A and Dory L: Extracellular
superoxide dismutase polymorphism in mice: Allele-specific effects
on phenotype. Free Radic Biol Med. 48:590–596. 2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Gallagher CJ, Ahn K, Knipe AL, Dyer AM,
Richie JP Jr, Lazarus P and Muscat JE: Association between
haplotypes of manganese superoxide dismutase (SOD2), smoking, and
lung cancer risk. Free Radic Biol Med. 46:20–24. 2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Nejatizadeh A, Kumar R, Stobdan T, Goyal
AK, Sikdar S, Gupta M, Javed S and Pasha MA: Endothelial nitric
oxide synthase gene haplotypes and circulating nitric oxide levels
significantly associate with risk of essential hypertension. Free
Radic Biol Med. 44:1912–1918. 2008.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ji G, Gu A, Wang Y, Huang C, Hu F, Zhou Y,
Song L and Wang X: Genetic variants in antioxidant genes are
associated with sperm DNA damage and risk of male infertility in a
Chinese population. Free Radic Biol Med. 52:775–780.
2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yan L, Liu J, Wu S, Zhang S, Ji G and Gu
A: Seminal superoxide dismutase activity and its relationship with
semen quality and SOD gene polymorphism. J Assist Reprod Genet.
31:549–554. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Polimanti R, Piacentini S and Fuciarelli
M: HapMap-based study of human soluble glutathione S-transferase
enzymes: The role of natural selection in shaping the single
nucleotide polymorphism diversity of xenobiotic-metabolizing genes.
Pharmacogenet Genomics. 21:665–672. 2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Yang FY, Guan QK, Cui YH, Zhao ZQ, Rao W
and Xi Z: NAD(P)H quinone oxidoreductase 1 (NQO1) genetic C609T
polymorphism is associated with the risk of digestive tract cancer:
A meta-analysis based on 21 case-control studies. Eur J Cancer
Prev. 21:432–441. 2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Han FF, Guo CL, Gong LL, Jin Z and Liu LH:
Effects of the NQO1 609C>T polymorphism on leukemia
susceptibility: Evidence from a meta-analysis. Asian Pac J Cancer
Prev. 14:5311–5316. 2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Corrales J, Fang X, Thornton C, Mei W,
Barbazuk WB, Duke M, Scheffler BE and Willett KL: Effects on
specific promoter DNA methylation in zebrafish embryos and larvae
following benzo[a]pyrene exposure. Comp Biochem Physiol C Toxicol
Pharmacol. 163:37–46. 2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zappa F, Ward T, Butler J, Pedrinis E and
Mcgown A: Overexpression of NAD(P)H:quinone oxidoreductase 1 in
human reproductive system. J Histochem Cytochem. 49:1187–1188.
2001.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Goodyear MD, Krleza-Jeric K and Lemmens T:
The declaration of Helsinki. BMJ. 335:624–625. 2007.PubMed/NCBI View Article : Google Scholar
|
|
30
|
World Health Organization (WHO): World
Health Statistics 2010. Fact sheet N°290. WHO, Geneva, 2010.
|
|
31
|
Miyamoto T, Minase G, Okabe K, Ueda H and
Sengoku K: Male infertility and its genetic causes. J Obstet
Gynaecol Res. 41:1501–1505. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Oliva A, Spira A and Multigner L:
Contribution of environmental factors to the risk of male
infertility. Hum Reprod. 16:1768–1776. 2001.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhang S, Tang Q, Wu W, Yuan B, Lu C, Xia
Y, Ding H, Hu L, Chen D, Sha J and Wang X: Association between DAZL
polymorphisms and susceptibility to male infertility: Systematic
review with meta-analysis and trial sequential analysis. Sci Rep.
4(4642)2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Jiang W, Sun H, Zhang J, Zhou Q, Wu Q, Li
T, Zhang C, Li W, Zhang M and Xia X: Polymorphisms in Protamine 1
and Protamine 2 predict the risk of male infertility: A
meta-analysis. Sci Rep. 5(153000)2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Hosen MB, Islam MR, Begum F, Kabir Y and
Howlader MZH: Oxidative stress induced sperm DNA damage, a possible
reason for male infertility. Iran J Reprod Biomed. 13:525–532.
2015.PubMed/NCBI
|