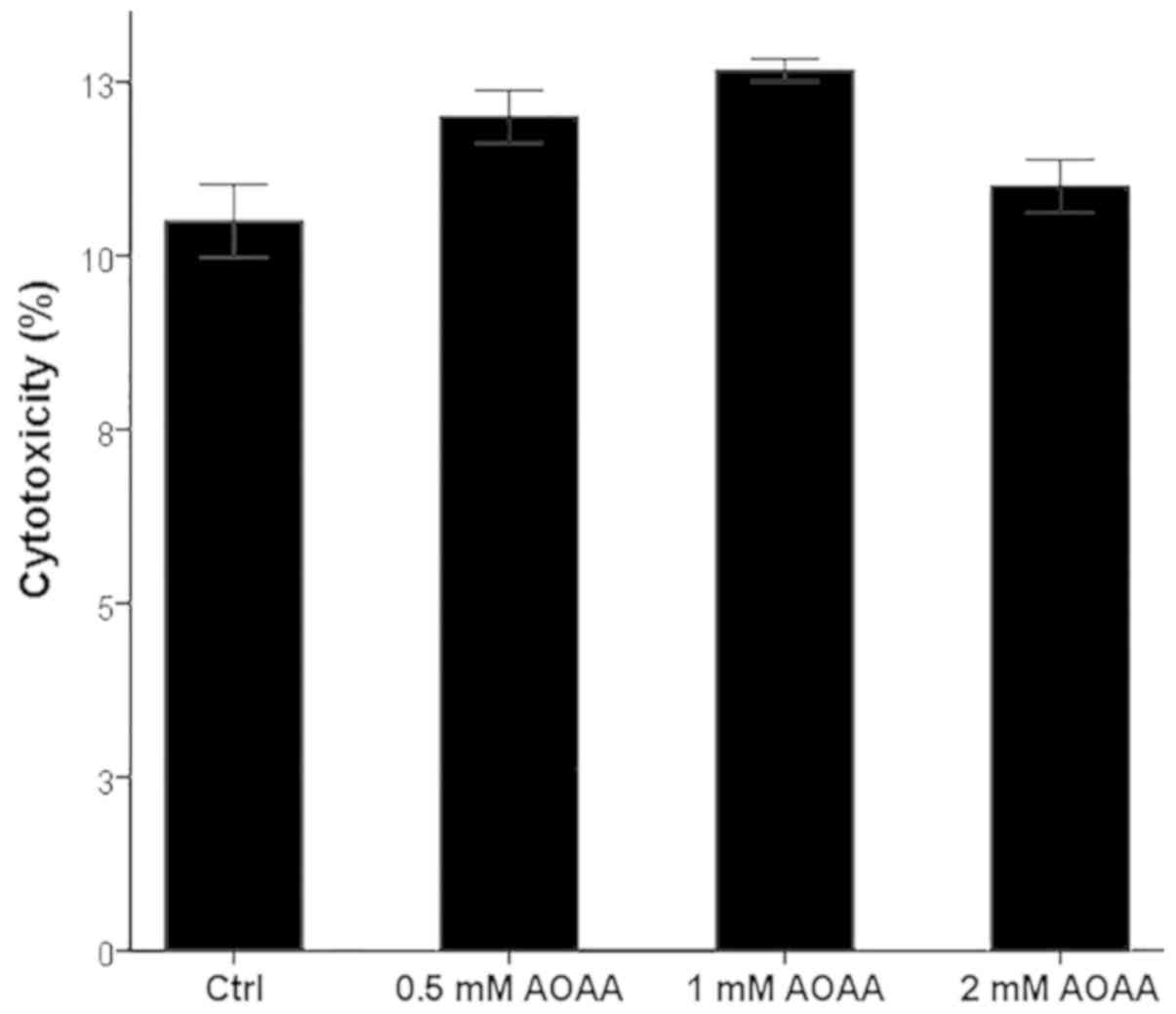
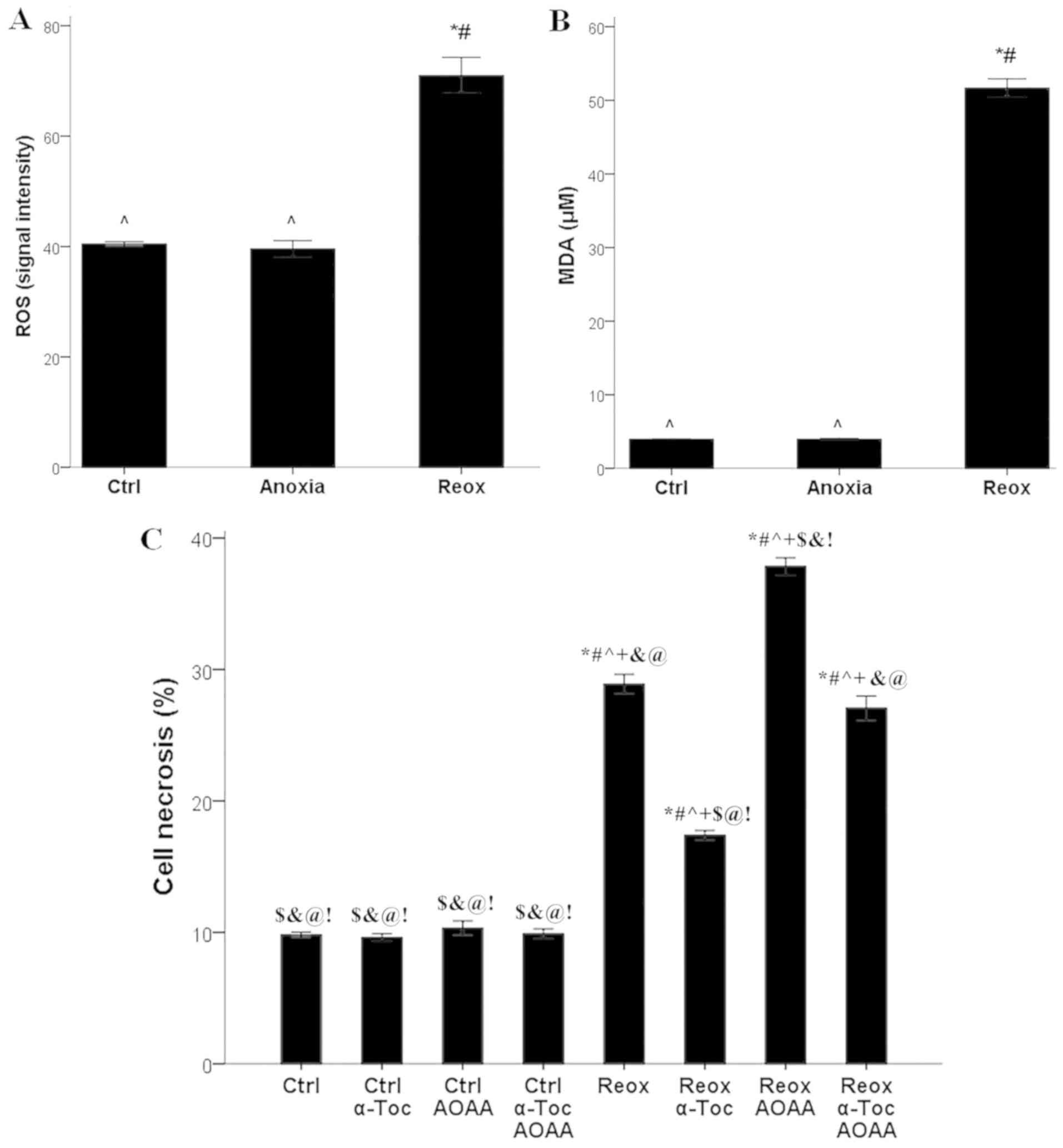
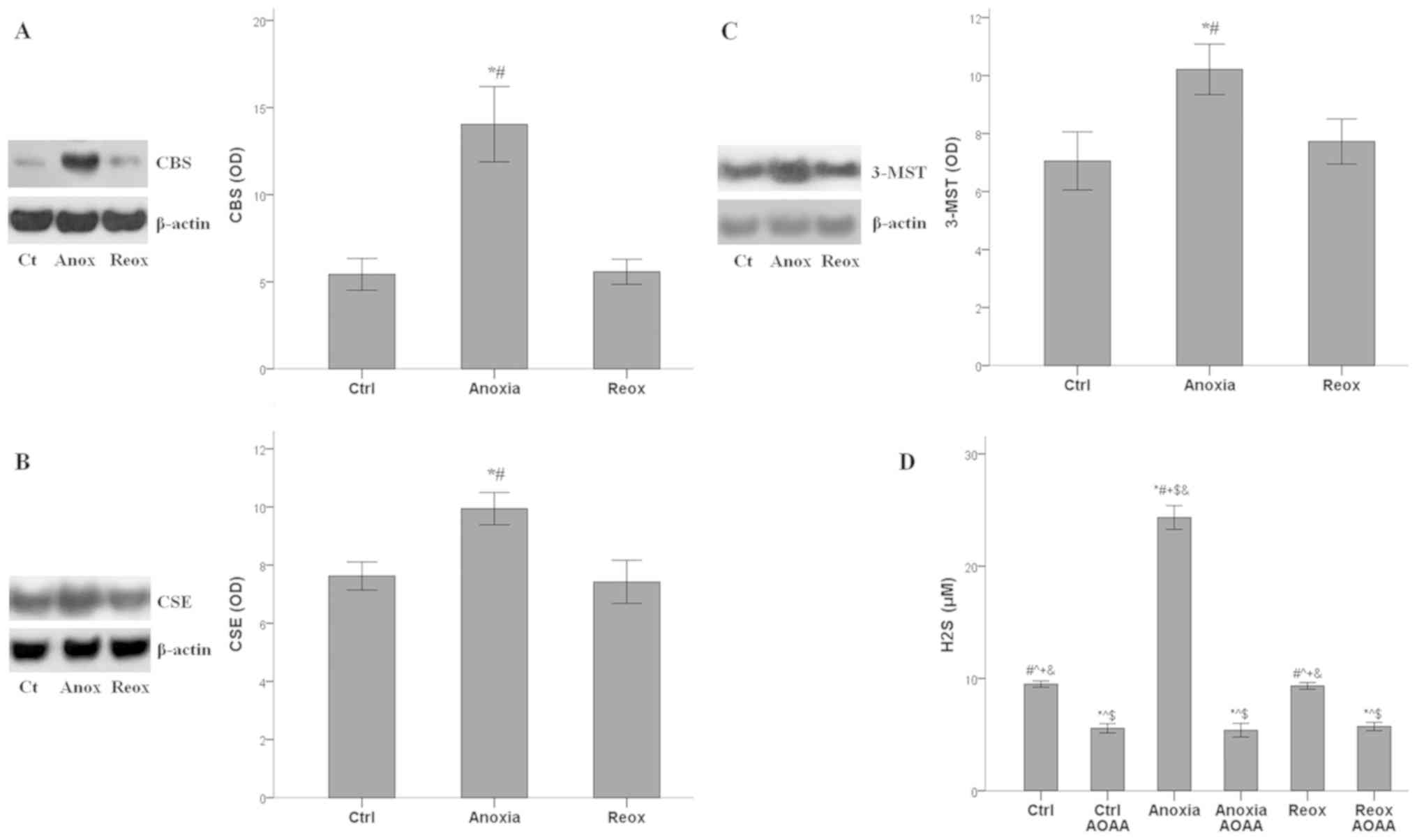
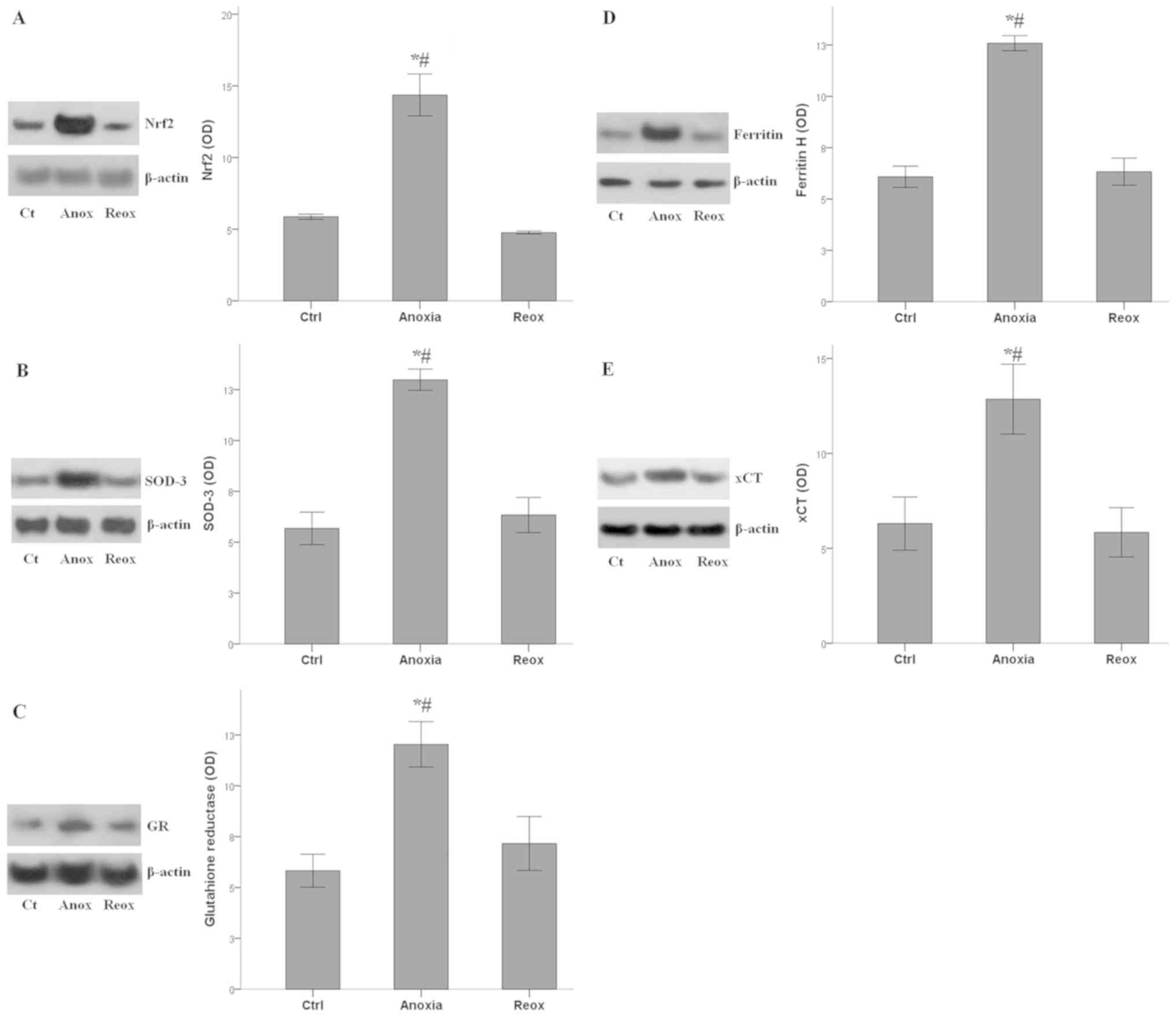
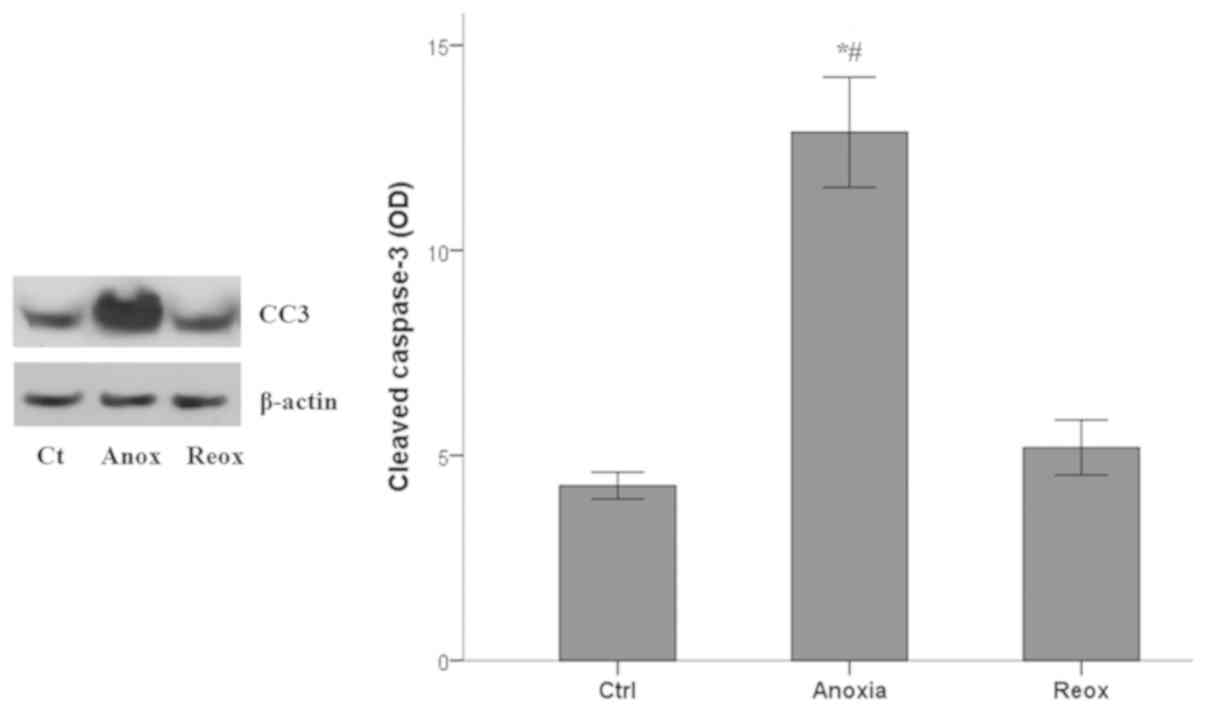
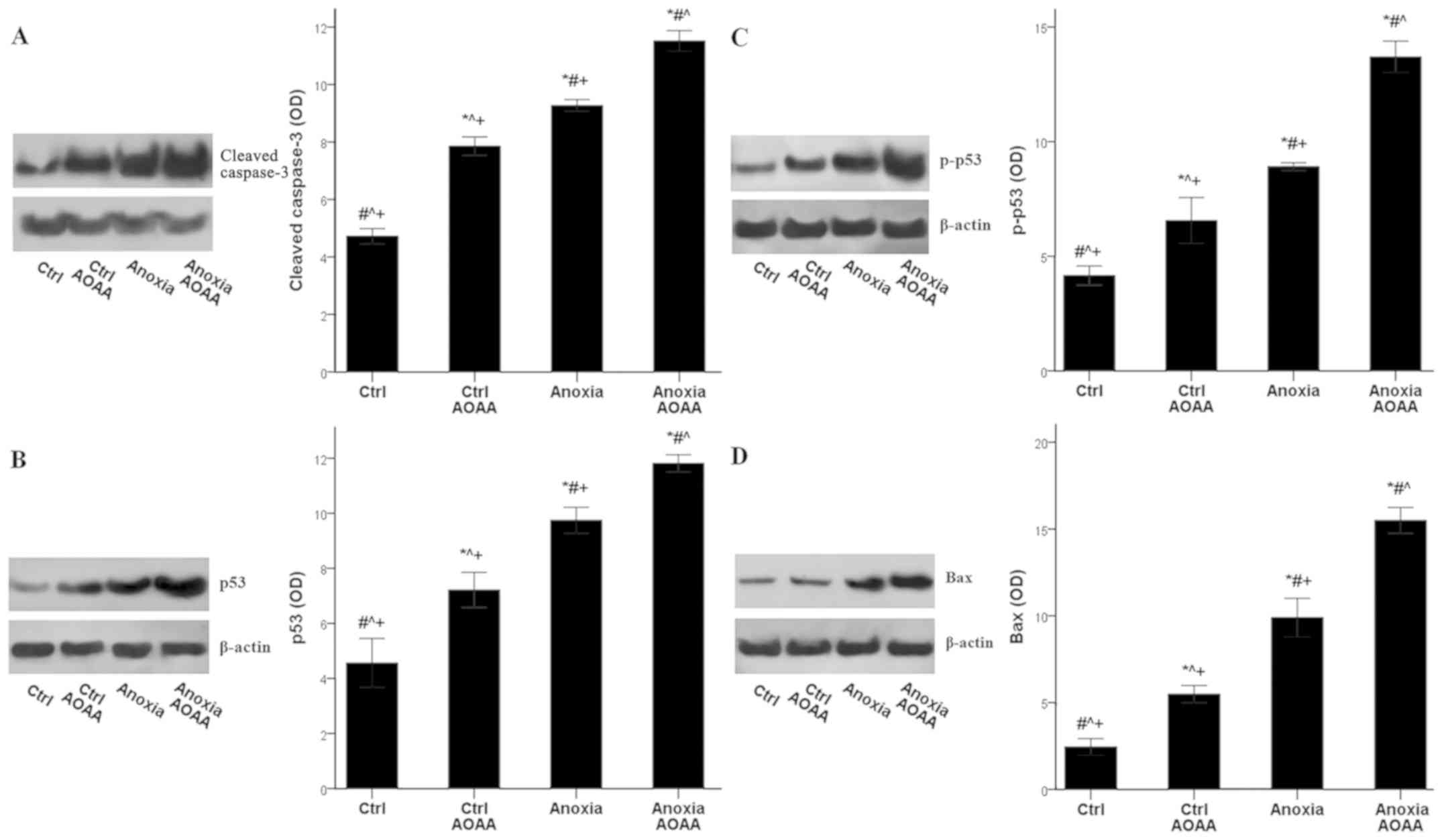
|
1
|
Neri M, Riezzo I, Pascale N, Pomara C and
Turillazzi E: Ischemia/reperfusion injury following acute
myocardial infarction: A critical issue for clinicians and forensic
pathologists. Mediators Inflamm. 2017(7018393)2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bakthavachalam P and Shanmugam PST:
Mitochondrial dysfunction - Silent killer in cerebral ischemia. J
Neurol Sci. 375:417–423. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Tsukamoto T, Chanthaphavong RS and Pape
HC: Current theories on the pathophysiology of multiple organ
failure after trauma. Injury. 41:21–26. 2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Bonventre JV and Yang L: Cellular
pathophysiology of ischemic acute kidney injury. J Clin Invest.
121:4210–4221. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
5
|
Eleftheriadis T, Pissas G, Antoniadi G,
Liakopoulos V and Stefanidis I: Cell death patterns due to warm
ischemia or reperfusion in renal tubular epithelial cells
originating from human, mouse, or the native hibernator hamster.
Biology (Basel). 7(E48)2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ma Q: Role of Nrf2 in oxidative stress and
toxicity. Annu Rev Pharmacol Toxicol. 53:401–426. 2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Corsello T, Komaravelli N and Casola A:
Role of hydrogen sulfide in NRF2- and sirtuin-dependent maintenance
of cellular redox balance. Antioxidants (Basel).
7(E129)2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Eleftheriadis T, Pissas G, Nikolaou E,
Liakopoulos V and Stefanidis I: The H2S-Nrf2-antioxidant proteins
axis protects renal tubular epithelial cells of the native
hibernator syrian hamster from reoxygenation-induced cell death.
Biology (Basel). 8(74)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Eleftheriadis T, Pissas G, Liakopoulos V
and Stefanidis I: Factors that may protect the native hibernator
syrian hamster renal tubular epithelial cells from ferroptosis due
to warm anoxia-reoxygenation. Biology (Basel).
8(E22)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Carey HV, Andrews MT and Martin SL:
Mammalian hibernation: Cellular and molecular responses to
depressed metabolism and low temperature. Physiol Rev.
83:1153–1181. 2003.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Storey KB and Storey JM: Metabolic rate
depression: The biochemistry of mammalian hibernation. Adv Clin
Chem. 52:77–108. 2010.PubMed/NCBI
|
|
12
|
Powell CR, Dillon KM and Matson JB: A
review of hydrogen sulfide (H2S) donors: Chemistry and potential
therapeutic applications. Biochem Pharmacol. 149:110–123.
2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yang G, Zhao K, Ju Y, Mani S, Cao Q,
Puukila S, Khaper N, Wu L and Wang R: Hydrogen sulfide protects
against cellular senescence via S-sulfhydration of keap1 and
activation of Nrf2. Antioxid Redox Signal. 18:1906–1919.
2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hourihan JM, Kenna JG and Hayes JD: The
gasotransmitter hydrogen sulfide induces Nrf2-target genes by
inactivating the keap1 ubiquitin ligase substrate adaptor through
formation of a disulfide bond between Cys-226 and Cys-613. Antioxid
Redox Signal. 19:465–481. 2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Niture SK and Jaiswal AK: Nrf2 protein
up-regulates antiapoptotic protein Bcl-2 and prevents cellular
apoptosis. J Biol Chem. 287:9873–9886. 2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Niture SK and Jaiswal AK: Nrf2-induced
antiapoptotic Bcl-xL protein enhances cell survival and drug
resistance. Free Radic Biol Med. 57:119–131. 2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wang TX, Shi XY and Liu YH: Endogenous
cystathionine-gamma-lyase/hydrogen sulfide pathway regulates
apoptosis of HepG2 cells. Yao Xue Xue Bao. 48:1233–1240.
2013.PubMed/NCBI
|
|
18
|
Yang G, Sun X and Wang R: Hydrogen
sulfide-induced apoptosis of human aorta smooth muscle cells via
the activation of mitogen-activated protein kinases and caspase-3.
FASEB J. 18:1782–1784. 2004.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Breza J Jr, Soltysova A, Hudecova S,
Penesova A, Szadvari I, Babula P, Chovancova B, Lencesova L, Pos O,
Breza J, et al: Endogenous H2S producing enzymes are involved in
apoptosis induction in clear cell renal cell carcinoma. BMC Cancer.
18(591)2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ma K, Liu Y, Zhu Q, Liu CH, Duan JL, Tan
BK and Zhu YZ: H2S Donor, S-propargyl-cysteine, increases CSE in
SGC-7901 and cancer-induced mice: Evidence for a novel anti-cancer
effect of endogenous H2S? Plos One. 6(e20525)2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Peake BF, Nicholson CK, Lambert JP, Hood
RL, Amin H, Amin S and Calvert JW: Hydrogen sulfide preconditions
the db/db diabetic mouse heart against ischemia-reperfusion injury
by activating Nrf2 signaling in an Erk-dependent manner. Am J
Physiol Heart Circ Physiol. 304:H1215–H1224. 2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ji K, Xue L, Cheng J and Bai Y:
Preconditioning of H 2 S inhalation protects against cerebral
ischemia/reperfusion injury by induction of HSP70 through
PI3K/Akt/Nrf2 pathway. Brain Res Bull. 121:68–74. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ganster F, Burban M, de la Bourdonnaye M,
Fizanne L, Douay O, Loufrani L, Mercat A, Calès P, Radermacher P,
Henrion D, et al: Effects of hydrogen sulfide on hemodynamics,
inflammatory response and oxidative stress during resuscitated
hemorrhagic shock in rats. Crit Care. 14(R165)2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Satterly SA, Salgar S, Hoffer Z, Hempel J,
DeHart MJ, Wingerd M, Raywin H, Stallings JD and Martin M: Hydrogen
sulfide improves resuscitation via non-hibernatory mechanisms in a
porcine shock model. J Surg Res. 199:197–210. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Han SJ, Kim JI, Park JW and Park KM:
Hydrogen sulfide accelerates the recovery of kidney tubules after
renal ischemia/reperfusion injury. Nephrol Dial Transplant.
30:1497–1506. 2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Sekijima M, Sahara H, Miki K, Villani V,
Ariyoshi Y, Iwanaga T, Tomita Y and Yamada K: Hydrogen sulfide
prevents renal ischemia-reperfusion injury in CLAWN miniature
swine. J Surg Res. 219:165–172. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Xu Z, Prathapasinghe G, Wu N, Hwang SY,
Siow YL and O K: Ischemia-reperfusion reduces
cystathionine-beta-synthase-mediated hydrogen sulfide generation in
the kidney. Am J Physiol Renal Physiol. 297:F27–F35.
2009.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Asimakopoulou A, Panopoulos P, Chasapis
CT, Coletta C, Zhou Z, Cirino G, Giannis A, Szabo C, Spyroulias GA
and Papapetropoulos A: Selectivity of commonly used pharmacological
inhibitors for cystathionine β synthase (CBS) and cystathionine γ
lyase (CSE). Br J Pharmacol. 169:922–932. 2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Miyamoto R, Otsuguro K, Yamaguchi S and
Ito S: Contribution of cysteine aminotransferase and
mercaptopyruvate sulfurtransferase to hydrogen sulfide production
in peripheral neurons. J Neurochem. 130:29–40. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Stockwell BR, Friedmann Angeli JP, Bayir
H, Bush AI, Conrad M, Dixon SJ, Fulda S, Gascón S, Hatzios SK,
Kagan VE, et al: Ferroptosis: A regulated cell death nexus linking
metabolism, redox biology, and disease. Cell. 171:273–285.
2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Stipanuk MH and Beck PW: Characterization
of the enzymic capacity for cysteine desulphhydration in liver and
kidney of the rat. Biochem J. 206:267–277. 1982.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Talaei F, Bouma HR, Van der Graaf AC,
Strijkstra AM, Schmidt M and Henning RH: Serotonin and dopamine
protect from hypothermia/rewarming damage through the CBS/H2S
pathway. PLoS One. 6(e22568)2011.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Fadeel B and Orrenius S: Apoptosis: A
basic biological phenomenon with wide-ranging implications in human
disease. J Intern Med. 258:479–517. 2005.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Yang WS and Stockwell BR: Ferroptosis:
Death by lipid peroxidation. Trends Cell Biol. 26:165–176.
2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Linkermann A, Skouta R, Himmerkus N, Mulay
SR, Dewitz C, De Zen F, Prokai A, Zuchtriegel G, Krombach F, Welz
PS, et al: Synchronized renal tubular cell death involves
ferroptosis. Proc Natl Acad Sci USA. 111:16836–16841.
2014.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Fang YZ, Yang S and Wu G: Free radicals,
antioxidants, and nutrition. Nutrition. 18:872–879. 2002.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Takano N, Peng YJ, Kumar GK, Luo W, Hu H,
Shimoda LA, Suematsu M, Prabhakar NR and Semenza GL:
Hypoxia-inducible factors regulate human and rat cystathionine
β-synthase gene expression. Biochem J. 458:203–211. 2014.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Wang M, Guo Z and Wang S: Regulation of
cystathionine γ-lyase in mammalian cells by hypoxia. Biochem Genet.
52:29–37. 2013.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Li M, Nie L, Hu Y, Yan X, Xue L, Chen L,
Zhou H and Zheng Y: Chronic intermittent hypoxia promotes
expression of 3-mercaptopyruvate sulfurtransferase in adult rat
medulla oblongata. Auton Neurosci. 179:84–89. 2013.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Yamawaki K, Kanda H and Shimazaki R: Nrf2
activator for the treatment of kidney diseases. Toxicol Appl
Pharmacol. 360:30–37. 2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Wu QQ, Wang Y, Senitko M, Meyer C, Wigley
WC, Ferguson DA, Grossman E, Chen J, Zhou XJ, Hartono J, et al:
Bardoxolone methyl (BARD) ameliorates ischemic AKI and increases
expression of protective genes Nrf2, PPARγ, and HO-1. Am J Physiol
Renal Physiol. 300:F1180–F1192. 2011.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Liu M, Reddy NM, Higbee EM, Potteti HR,
Noel S, Racusen L, Kensler TW, Sporn MB, Reddy SP and Rabb H: The
Nrf2 triterpenoid activator, CDDO-imidazolide, protects kidneys
from ischemia-reperfusion injury in mice. Kidney Int. 85:134–141.
2014.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Han P, Qin Z, Tang J, Xu Z, Li R, Jiang X,
Yang C, Xing Q, Qi X, Tang M, et al: RTA-408 protects kidney from
ischemia-reperfusion injury in mice via activating Nrf2 and
downstream GSH biosynthesis gene. Oxid Med Cell Longev.
2017(7612182)2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Liu F, Ni W, Zhang J, Wang G, Li F and Ren
W: Administration of curcumin protects kidney tubules against renal
ischemia-reperfusion injury (RIRI) by modulating nitric oxide (NO)
signaling pathway. Cell Physiol Biochem. 44:401–411.
2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Zhao L, Xu L, Tao X, Han X, Yin L, Qi Y
and Peng J: Protective effect of the total flavonoids from rosa
laevigata michx fruit on renal ischemia-reperfusion injury through
suppression of oxidative stress and inflammation. Molecules.
21(E952)2016.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Brady CA and Attardi LD: p53 at a glance.
J Cell Sci. 123:2527–2532. 2010.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Molitoris BA, Dagher PC, Sandoval RM,
Campos SB, Ashush H, Fridman E, Brafman A, Faerman A, Atkinson SJ,
Thompson JD, et al: siRNA targeted to p53 attenuates ischemic and
cisplatin-induced acute kidney injury. J Am Soc Nephrol.
20:1754–1764. 2009.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Wakabayashi N, Slocum SL, Skoko JJ, Shin S
and Kensler TW: When NRF2 talks, who's listening? Antioxid Redox
Signal. 13:1649–1663. 2010.PubMed/NCBI View Article : Google Scholar
|