Dementia is a failure of cognitive abilities
characterized by severe neurodegeneration in selective neural
systems, and the incidence of dementia is predicted to increase
significantly within 20 years (1).
Dementia is one of the most significant health burdens, and, at
present, there are no suitable disease-modifying agents for
treatment of progressive dementia. Alzheimer's disease (AD) is the
most common form of dementia, and is the most significant
health-concern worldwide (2).
Pathologically, AD is a slowly progressing neurodegenerative
disorder categorized by severe damage of neurons and synapses
(3). Aberrations of amyloid-β
peptide may be responsible for the neuro-synaptic malfunctions
leading to cognitive deficits in AD (4). Genetic factors account for ~80% of the
risk contributing to AD, while modifiable factors associated with
lifestyle may also be involved (5).
Epidemiological studies have suggested that nutrition is one of the
most important yet modifiable risk factors of AD (6). Risk factors for vascular dementia, the
second most common cause of dementia, include hypertension and
metabolic syndrome, which are also modifiable lifestyle factors.
Managing these non-genetic risk factors may provide effective
opportunities to prevent the progressive cognitive decline.
Studies have shown that oxidative stress represents
a major risk factor associated with the pathology of dementia
(7,8). Substantial evidence has established
that oxidative stress as an aspect in AD is associated with
neuronal apoptosis and brain dysfunction (9). Particularly, mitochondrial dysfunction
is a conspicuous feature observed during neurodegeneration
(10), which is of importance in the
formation of reactive oxygen species (ROS) and thus DNA damage. ROS
are a group of oxygen-containing molecules which contribute to
increased oxidative stress, and are formed as a result of oxygen
metabolism in cells. Thus, increased oxidative stress may result in
increased DNA damage, and subsequently apoptosis, which contributes
to the degeneration of neuronal tissue. Detoxification of ROS
and/or reduction of ROS levels may protect neurons from DNA damage
and apoptosis. Since metabolic processes physiologically produce
ROS, any means used to reduce ROS levels may assist in decreasing
the prevalence and incidence of dementia. Cells possess machinery
to retain genomic integrity in response to genotoxic damage. There
is increasing evidence which supports the use of different
antioxidants as a treatment for AD (11), including vitamins (12). In addition, dietary and nutritional
approaches are of significant importance in the management of AD.
Improving and altering nutrient intake may reduce the progression
of chronic neurodegenerative diseases, an area which has recently
been gaining increased interest (13), and nutritional plans are
progressively being integrated into public health strategies
(14). In the present review, the
functions of key intra-cellular signaling molecules involved in
oxidative genotoxic stress and DNA repair in neurons are
summarized, offering a clarification on the molecular mechanisms
for the treatment of dementia. Specific attention is placed on the
mechanisms underlying the neuroprotective effects of specific
nutrients against dementia in reducing brain damage, which may be
used as an efficient therapeutic intervention.
In AD, significant molecular and biochemical changes
result in an increase in amyloid-β substance, which is modified by
ROS into a toxic product that promotes apoptosis of neurons
(15), suggesting a link between
progression of AD and oxidative stress. In cells, metabolic
processes physiologically produce ROS that cause oxidative damage
to DNA (16), and this physiological
production of ROS is associated with aging of the brain and
neurodegeneration. Under physiological conditions, ROS may act as a
second messenger in cells (17). ROS
controls several physiological processes, such as the hypoxic
response and inflammation, as well as the regulation of growth
factor signaling (18). Abnormal
accumulation of amyloid-β inhibits long-term potentiation in
neurons which is prevented by treatment with antioxidants (19). Therefore, decreasing oxidative damage
in the brain may inhibit or reduce the damage to neurons. ROS may
exert its effects on cells through the regulation of several target
molecules, including PI3K/AKT/PTEN (20). Interactions of ROS with amyloid-β
have been shown to prevent mitochondrial respiration (21). In addition, increased levels of ROS
within the mitochondria of neurons may disturb synaptic plasticity,
and thus memory formation/retention (22). Therefore, ensuring that ROS levels
are maintained within physiological ranges may improve outcomes in
patients with AD by preventing/reducing damage to neurons. Neurons
exhibit considerably high levels of metabolic activity and use
distinct oxidative damage-repair mechanisms to reverse DNA damage
(23). Therefore, malfunctions of
the DNA repair system in the brain may result in neurological
dysfunction. Damaged DNA can be repaired by the DNA repair
machinery, which consists of ataxia telangiectasia-mutated (ATM)
and ATR, BRCA1, PTEN and others (24). Abnormalities in these molecules are
often observed in patients with neurodegenerative diseases
(25).
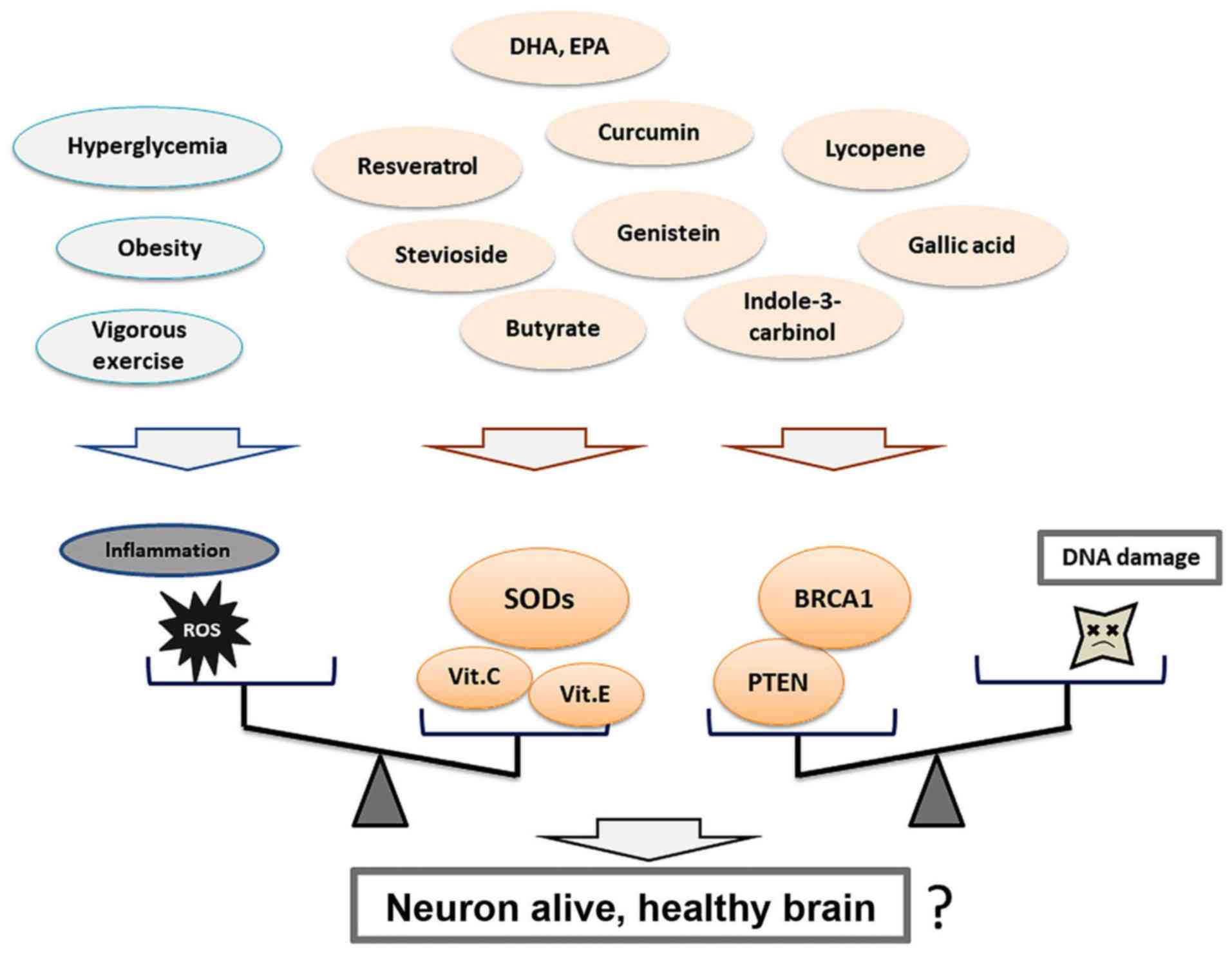
ROS are free radicals under physiological
conditions. Hyperglycemia exacerbates the accumulation of ROS in
neurons leading to increased apoptosis (26). Several environmental and lifestyle
associated factors, including tobacco smoking, alcohol consumption,
exposure to ionizing radiation, infections, inflammation and even
the aging process may result in increased oxidative stress
(27). In addition, in population
studies, obese patients have been shown to possess significantly
higher serum levels of ROS, suggesting that obesity may increase
oxidative stress (28).
High-intensity exercise increases oxidative damage and induces
disruption of the blood-brain barrier (28). Exercising may upregulate the
expression of endogenous antioxidants and thus reduce oxidative
damage; however, vigorous exercise may result in the accumulation
of ROS (29). Consistent exposure to
oxidative stress is an initiator of various chronic diseases
including degenerative disorders, diabetes, cardiovascular diseases
and cancer. In general however, cells are able to correct damage to
DNA as a result of oxidative stress to a certain extent.
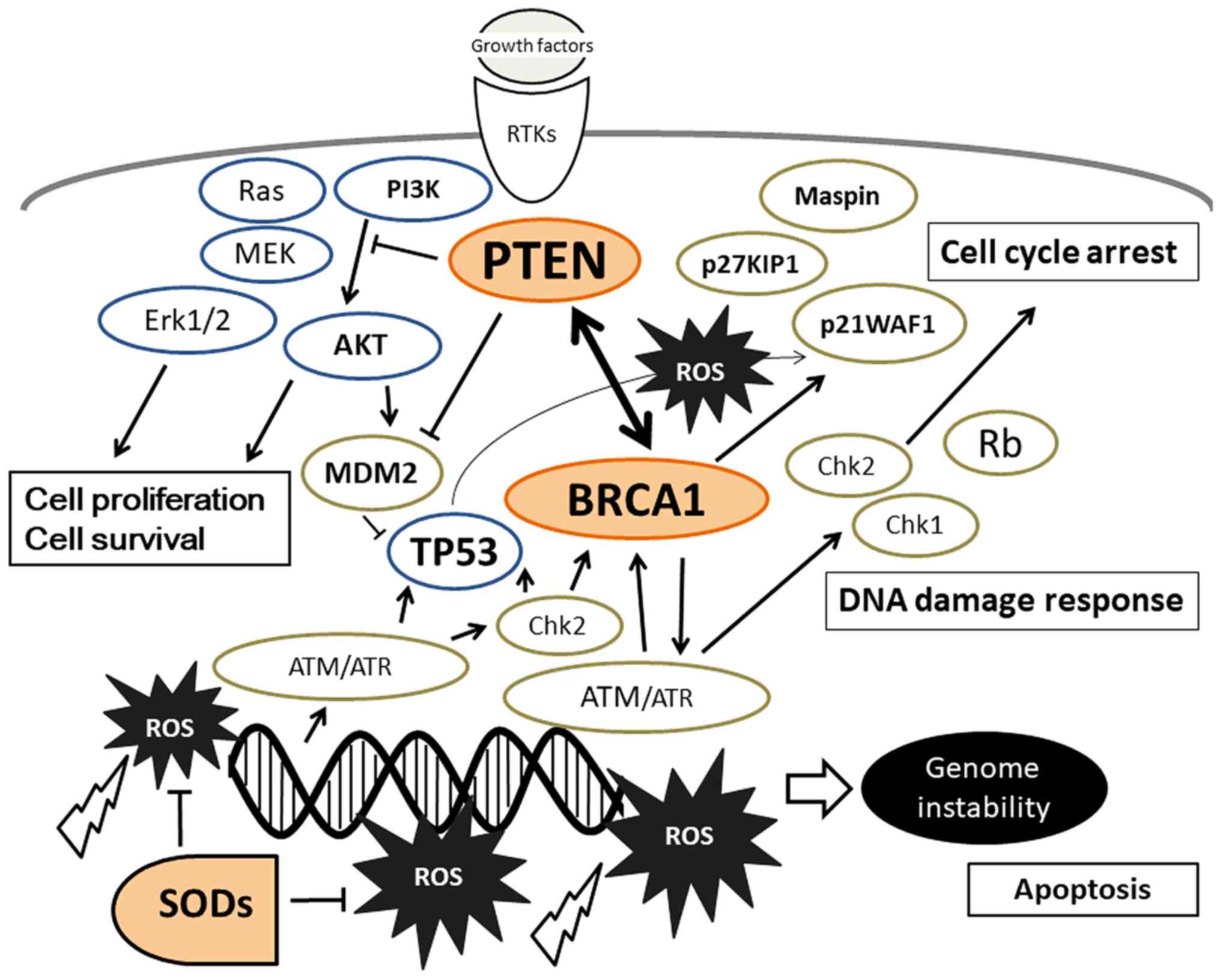
PTEN is a tumor suppressor with dual-specificity
phosphatase activity; protein phosphatase activity and lipid
phosphatase activity, and antagonizes the activity of PI3K
(30). Cells lacking PTEN have
higher levels of PIP3 which activates downstream targets of
PI3K/AKT. The PI3K/AKT signal regulates a variety of cellular
events including proliferation, survival and apoptosis of cells
(Fig. 1). PTEN is associated with
the apoptotic cascade, which may be a result of its effect on
decreasing PI3K/AKT signaling (31).
In part, neuronal cell death may be attributed to the differences
in PTEN expression (32). Inhibition
of PTEN protects synaptic function and thus cognition in animal
models of AD (33). Suppressing PTEN
and/or increasing the activity of AKT reduces the levels of
oxidative stress, and thus decreases cell death, suggesting that
AKT activation may be required for neuroprotection. Thus, PTEN
contributes to the defense mechanisms against severe oxidative
damage in the brain. It has been shown that PTEN insufficiency
results in an increase in mitochondrial activity, consistent with
the activation of the AKT signaling pathway (34), and thus may increase the levels of
ROS.
In addition to PTEN, BRCA1 serves an important role
in the response to DNA damage (35).
The PI3K/AKT signaling pathway has been shown to be constitutively
activated in BRCA1-defective cells (35). BRCA1, is one of the best studied and
prominent suppressors of breast cancer, mutations of which are
associated with breast and/or ovarian cancer risk in addition to
genetic susceptibility (36). BRCA1
exerts several effects on the DNA repair system (37). BRCA1-related hereditary cancer is a
type of cancer with functional defects in the DNA repair mechanisms
(38). Mutations of the BRCA1 gene
are associated with increased genomic instability (30,31),
which may increase the rate of accumulation of genetic mutations.
The primary recognition molecule for DNA damage is ATM, which is
the checkpoint kinase that phosphorylates a number of proteins
including BRCA1 in response to DNA damage (39). Reduced levels of BRCA1 expression
have been observed in the brains of patients with AD (40). Knocking down neuronal expression of
BRCA1 results in an increased rate of DNA double-stranded breaks,
synaptic plasticity impairments and memory deficits (40). Therefore, BRCA1 may support neuronal
integrity and cognitive function by protecting the neuronal genome,
and depletion of neuronal BRCA1 may result in cognitive deficits.
Activation of the DNA repair system to protect neurons may occur
during the early stages of neurodegeneration, as the impairment of
BRCA1 accelerates disease progression (41). Oxidative damage to the DNA of neurons
has been demonstrated to be a significant contributing factor in
the development of dementia. BRCA1 reduces the production of ROS
(42), which in-turn, results in
decreased oxidative damage to the DNA (35). BRCA1 also supports the telomere,
alterations of which may result in neurodegeneration (43). BRCA1 serves an important role in
telomere maintenance, although the exact mechanisms remain unknown
(43). Telomere length insufficiency
is a typical feature of degenerating neurons in the brains of
patients with dementia (44).
Additionally, there may be an indirect association between PTEN and
BRCA1 gene function (45). PTEN
inhibition represses nuclear translocation of BRCA1, which impairs
DNA repair resulting in an accumulation of DNA damage (46).
Due to a lack of reliable treatment options, brain
dysfunction and/or dementia is an increasing public health concern.
A number of disease-protective factors, such as physical activity,
sleep and dietary patterns, have been proposed by epidemiological
research (47). Among these factors,
dietary choices may exhibit certain neuroprotective effects. In
particular, dietary choices may result in alterations to AKT/PTEN
as well as BRCA1 signaling, and may prevent neurodegenerative
diseases or reduce progression. Several plants and fruits are
promising candidates for reducing the progression or risk of
dementia diseases. An ingredient derived from the root of
Curcuma longa, curcumin, present in culinary turmeric, may
reverse the effects of dementia on memory (48). The neuroprotective effects of
curcumin may be mediated through modification of the PI3K/AKT
signaling pathway (49). Kaempferol
is a flavanol present in several plants, including grapefruit and
edible berries, which has been shown to demonstrate neuroprotective
effects in a rat animal model (50),
and Kaempferol protects neurons through activation of AKT signaling
(51). A neuroprotective ingredient
of a Chinese medicinal herb, Herba epimedii, Icariin,
reduces PTEN expression following activation of PI3K/AKT signaling
(52). Furthermore, certain
components of rosemary herb prevent the expression of PTEN in K562
myeloid cells (53). In contrast to
this, the expression levels of PTEN are increased following
treatment with Ginsenoside (54).
Fat soluble lycopene is a carotenoid with a red
pigment found in several fruits and vegetables such as tomatoes.
Treatment with the lycopene increased the mRNA expression levels of
BRCA1(55). In addition, lycopene
increases phosphorylation of BRCA1 in breast cancer cells (56). Treatment with phytoestrogens result
in higher expression levels of BRCA1 by reversing DNA
hypermethylation (57), and
individuals with phytoestrogen-rich diets possessed increased
expression levels of BRCA1 mRNA (58). Rats exposed to genistein showed
higher expression levels of BRCA1 in the mammary glands (59). Genistein and indole-3-carbinol are
biochemicals derived from soy and green vegetables, respectively.
These phytoestrogens have been shown to reduce the risk of AD
progression (60). Furthermore,
gallic acid, a phenolic compound present in natural plants,
increases the phosphorylation of BRCA1(61).
The aforementioned potential compounds found in
foodstuffs which may exhibit neuroprotective effects, predominantly
do so by exerting some sort of influence on tumor suppressor
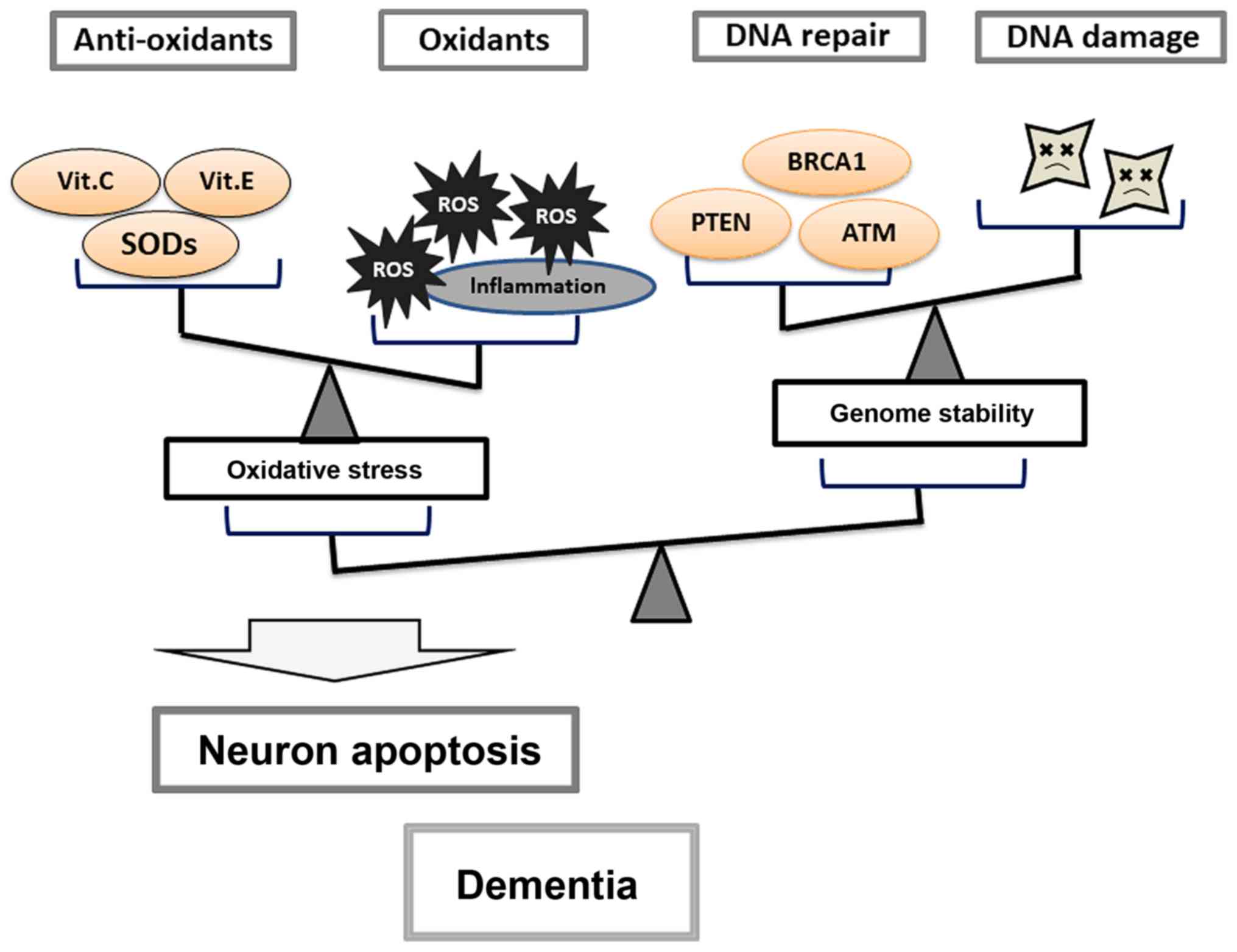
molecules, such as PTEN and BRCA1. Thus, PTEN and BRCA1 functions
may be important for individual brain health (Fig. 2). As mentioned above, imbalances in
the activity between PTEN and AKT may contribute to the
pathogenesis of dementia. Therefore, appropriate activation and/or
inhibition for maintaining the balance of kinases may be important
(31). Certain food and/or dietary
components may aid in maintaining the balance of these signaling
molecules by modulating their functional activities (Fig. 3). Thus, future studies should focus
on determining the most appropriate method of using these
neuroprotective compounds identified in in vitro studies and
animal models, and translating them to bedside therapeutics.
Superoxide dismutases (SODs) exhibit robust
antioxidant activity characterized by their ability to scavenge ROS
(61). SODs catalyze the reaction of
superoxide to hydrogen peroxide (62). As aberrantly increased ROS levels
results in extensive oxidative DNA damage, SODs have been suggested
to serve as a principal defense system against oxidative stress.
There are three types of SODs that have been identified in humans,
SOD1-3. Cytosolic SOD1 may serve a role in conjunction with
Cu2+/Zn2+ ions in the prevention of central
nervous system damage (63). Several
mutations in the SOD1 gene are responsible for mitochondrial
impairments, leading to progressive neurodegenerative diseases
including familial amyotrophic lateral sclerosis (64). SOD1-null animals also develop other
seemingly unrelated diseases, such as muscle atrophy (65). SOD2 is a functional tumor suppressor,
and SOD2 expression has been reported to be significantly reduced
in several tumors (65). SOD2 and
Mn2+ ions are present in the matrix of the mitochondria,
the primary site of free radical formation from the electron
transport chain. ATP production in mitochondria is impaired in
patients with AD (66). Therefore,
the primary function of SOD2 may be to protect the mitochondria
against oxidative damage. SOD3 and Cu2+/Zn2+
are secreted into the extracellular matrix and contribute to
metabolic regulation of neurons by altering the rate of blood flow
(67), and may be induced by
chemical antioxidants such as vitamin C (68). Dietary intake of Cu2+
stabilizes SOD activity, indicating a potential therapeutic benefit
(69). It has been suggested that
inhibition of ROS by SODs decreases neuronal cell apoptosis,
microglial cell activation, and disruption of the blood brain
barrier, thus maintaining brain health (70). Active aglycone-genistein inhibits ROS
production by activating SODs (71).
Lycopene also inhibits neuronal apoptosis by reducing ROS levels,
and by improving mitochondrial function (72). Expression of SODs is dependent on the
activity of peroxisome proliferator-activated receptors (73). Therefore, resveratrol analogs, which
activates peroxisome proliferator-activated receptors, may increase
the mRNA and protein expression levels of SODs (74). Furthermore, increased expression of
SOD2 has been observed following administration of grape juice
(75). The polyphenolic antioxidant,
resveratrol, and calorie restriction may promote human longevity.
Stevioside, a natural sweetener, may also increase the expression
of SOD1-3(76). In addition,
butyrate, a short-chain fatty acid, increases the expression of
SODs (77).
Antioxidant supplements may reduce cognitive
decline. Vitamin C, vitamin E and the vitamin-like substance
coenzyme Q10 have been used to treat patients with dementia with
some efficacy (78), and the plasma
levels of vitamin C have been found to be considerably lower in
patients with AD (79). Decreased
levels of plasma vitamin E are associated with an increased risk of
neurodegenerative disorders (80).
Vitamin E acts as a scavenger of free radicals (81), and thus, may exhibit a
neuroprotective effect by scavenging ROS. In addition, ingestion of
vitamin E is associated with an increase in the levels of SODs
(80). Dietary omega-3
polyunsaturated fatty acid (PUFA) has been demonstrated to improve
memory and learning processes (82).
Long-term diets rich in omega-3 PUFA may lead to lower levels of
DNA damage caused by oxidative stress (83). Perilla frutescens is a good
source of the omega-3 PUFA. The perilla-diet promotes neuronal
signaling and alters synaptic plasticity, improving learning and
memory (84), possibly by enhancing
intracellular SOD activity (85).
Together, these studies support the hypothesis that SODs, as well
as antioxidant vitamins, offer a certain degree of neural
protection against dementia progression. However, the association
between neuroprotection and nutrient consumption is a complex
matter of study. Difficulties in the variabilities of human-diets
makes this a challenging subject to research.
To maintain physiological cellular function, cells
prevent against oxidative damage through the use of antioxidants.
In neurons, excess oxidative stress may result in neuronal cell
death and potentially dementia. In dementia, genomic DNA damage is
a feature of the pathogenesis of neurodegeneration; however, DNA
damage may be additionally explained by a lack of or improper DNA
repair mechanisms. Therefore, increased production of ROS and/or
alterations in BRCA1 and PTEN function concurrently suggest a
neurodegenerative stimulus present in dementia. Several compounds
in naturally occurring foodstuffs may exhibit neuroprotective
effects, which may facilitate DNA repair or reduce ROS-production,
and some of these neuroprotective compounds may form the basis of
future potential therapeutic options for preventing or limiting the
progression of dementia. Future therapeutic strategies should
utilize the observation that defects in the key processes required
for neuronal homeostasis, which results in unfavorable neuronal
conditions, and this should represent a basis for the development
of dietary treatments for dementia. One aspect to consider is the
difference between psychiatric illnesses and dementia. For
treatment of psychiatric illnesses, it is important to maintain the
levels of key intracellular molecules balanced (31). For dementia, it is also imperative to
limit or prevent neuronal apoptosis (Fig. 3). However, both these aspects are
important for keeping the brain functioning healthily.
Numerous neuroprotective factors have been suggested
as potential targets for preventing or limiting neuronal apoptosis.
For example, phytoestrogens may rescue neurons and glial cells from
cell apoptosis by preventing oxidative stress. However, despite
experimental interpretations based on in vitro and in
vivo studies, the translational value of the neuroprotective
compounds in the clinical setting remains to be determined. The
potential therapeutic effects for preventing dementia should be
more cautiously considered in clinical research (86). It may also be possible to use these
compounds found in natural foodstuffs as an adjuvant alongside
established treatment modalities. Further mechanistic studies are
required to understand the detailed molecular mechanisms underlying
the neuroprotective effects of the compounds highlighted in the
present review. Additionally, clinical studies are required to
determine their efficacy in humans.
In conclusion, ROS as well as PTEN and BRCA1 tumor
suppressors may be involved in the pathogenesis of dementia and
neuroprotective compounds found in certain diets may reduce or
prevent dementia by reducing oxidative DNA damage.
Not applicable.
This work was supported in part by JSPS KAKENHI
(grant no. JP18K17964) and a grant from Nara Women's University in
Japan.
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
MM and SM conceived the subject of the review. MM,
YI, YN, AT, YK and SM participated in writing and revising the
manuscript. All authors read and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Kivipelto M, Ngandu T, Laatikainen T,
Winblad B, Soininen H and Tuomilehto J: Risk score for the
prediction of dementia risk in 20 years among middle aged people: A
longitudinal, population-based study. Lancet Neurol. 5:735–741.
2006.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Finneran DJ and Nash KR: Neuroinflammation
and fractalkine signaling in Alzheimer's disease. J
Neuroinflammation. 16(30)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Trujillo-Estrada L, Dávila JC,
Sánchez-Mejias E, Sánchez-Varo R, Gomez-Arboledas A, Vizuete M,
Vitorica J and Gutiérrez A: Early neuronal loss and
axonal/presynaptic damage is associated with accelerated amyloid-β
accumulation in AβPP/PS1 Alzheimer's disease mice subiculum. J
Alzheimers Dis. 42:521–541. 2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zhao J, Gao W, Yang Z, Li H and Gao Z:
Nitration of amyloid-β peptide (1-42) as a protective mechanism for
the amyloid-β peptide (1-42) against copper ion toxicity. J Inorg
Biochem. 190:15–23. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Tanzi RE: The genetics of Alzheimer
disease. Cold Spring Harb Perspect Med. 2(a006296)2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ravi SK, Narasingappa RB and Vincent B:
Neuro-nutrients as anti-Alzheimer's disease agents: A critical
review. Crit Rev Food Sci Nutr. 59:2999–3018. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Barus R, Béné J, Deguil J, Gautier S and
Bordet R: Drug interactions with dementia-related
pathophysiological pathways worsen or prevent dementia. Br J
Pharmacol. 176:3413–3434. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Butterfield DA and Halliwell B: Oxidative
stress, dysfunctional glucose metabolism and Alzheimer disease. Nat
Rev Neurosci. 20:148–160. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Tian JS, Zhai QJ, Zhao Y, Chen R and Zhao
LD: 2-(2-benzofuranyl)-2-imidazoline (2-BFI) improved the
impairments in AD rat models by inhibiting oxidative stress,
inflammation and apoptosis. J Integr Neurosci. 16:385–400.
2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Perez Ortiz JM and Swerdlow RH:
Mitochondrial dysfunction in Alzheimer's disease: Role in
pathogenesis and novel therapeutic opportunities. Br J Pharmacol.
176:3489–3507. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Chang KH, Cheng ML, Chiang MC and Chen CM:
Lipophilic antioxidants in neurodegenerative diseases. Clin Chim
Acta. 485:79–87. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Gugliandolo A, Bramanti P and Mazzon E:
Role of Vitamin E in the treatment of Alzheimer's disease: Evidence
from animal models. Int J Mol Sci. 18(E2504)2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yu J, Zhu H, Taheri S, Mondy W, Perry S
and Kindy MS: Impact of nutrition on inflammation, tauopathy, and
behavioral outcomes from chronic traumatic encephalopathy. J
Neuroinflammation. 15(277)2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Tako E, Bar H and Glahn RP: The combined
application of the Caco-2 cell bioassay coupled with in vivo
(Gallus gallus) feeding trial represents an effective
approach to predicting Fe bioavailability in humans. Nutrients.
8(E732)2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Jazvinšćak Jembrek M, Hof PR and Šimić G:
Ceramides in Alzheimer's disease: Key mediators of neuronal
apoptosis induced by oxidative stress and Aβ accumulation. Oxid Med
Cell Longev. 2015(346783)2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Mitra J, Guerrero EN, Hegde PM, Wang H,
Boldogh I, Rao KS, Mitra S and Hegde ML: New perspectives on
oxidized genome damage and repair inhibition by pro-oxidant metals
in neurological diseases. Biomolecules. 4:678–703. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhou DR, Eid R, Miller KA, Boucher E,
Mandato CA and Greenwood MT: Intracellular second messengers
mediate stress inducible hormesis and programmed cell death: A
review. Biochim Biophys Acta Mol Cell Res. 1866:773–792.
2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Görlach A, Dimova EY, Petry A,
Martínez-Ruiz A, Hernansanz-Agustín P, Rolo AP, Palmeira CM and
Kietzmann T: Reactive oxygen species, nutrition, hypoxia and
diseases: Problems solved? Redox Biol. 6:372–385. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Freund RK, Gibson ES, Potter H and
Dell'Acqua ML: Inhibition of the Motor protein Eg5/Kinesin-5 in
amyloid β-mediated impairment of hippocampal long-term potentiation
and dendritic spine loss. Mol Pharmacol. 89:552–559.
2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ikeda Y, Murakami M, Nakagawa Y, Tsuji A,
Kitagishi Y and Matsuda S: Diet induces hepatocyte protection in
fatty liver disease via modulation of PTEN signaling. Biomed Rep.
12:295–302. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Schaefer PM, von Einem B, Walther P,
Calzia E and von Arnim CA: Metabolic characterization of intact
cells reveals intracellular amyloid beta but not its precursor
protein to reduce mitochondrial respiration. PLoS One.
11(e0168157)2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Richetin K, Moulis M, Millet A, Arràzola
MS, Andraini T, Hua J, Davezac N, Roybon L, Belenguer P, Miquel MC
and Rampon C: Amplifying mitochondrial function rescues adult
neurogenesis in a mouse model of Alzheimer's disease. Neurobiol
Dis. 102:113–124. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Canugovi C, Misiak M, Ferrarelli LK,
Croteau DL and Bohr VA: The role of DNA repair in brain related
disease pathology. DNA Repair (Amst). 12:578–587. 2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ali R, Rakha EA, Madhusudan S and Bryant
HE: DNA damage repair in breast cancer and its therapeutic
implications. Pathology. 49:156–165. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Tokarz P, Kaarniranta K and Blasiak J:
Role of the cell cycle Re-initiation in DNA damage response of
post-mitotic cells and its implication in the pathogenesis of
neurodegenerative diseases. Rejuvenation Res. 19:131–139.
2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Li WA, Moore-Langston S, Chakraborty T,
Rafols JA, Conti AC and Ding Y: Hyperglycemia in stroke and
possible treatments. Neurol Res. 35:479–491. 2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Mena S, Ortega A and Estrela JM: Oxidative
stress in environmental-induced carcinogenesis. Mutat Res.
674:36–44. 2009.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Roh HT, Cho SY and So WY: Obesity promotes
oxidative stress and exacerbates blood-brain barrier disruption
after high-intensity exercise. J Sport Health Sci. 6:225–230.
2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Thirupathi A and Pinho RA: Effects of
reactive oxygen species and interplay of antioxidants during
physical exercise in skeletal muscles. J Physiol Biochem.
74:359–367. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Tsai CY, Wu JCC, Fang C and Chang AYW:
PTEN, a negative regulator of PI3K/Akt signaling, sustains brain
stem cardiovascular regulation during mevinphos intoxication.
Neuropharmacology. 123:175–185. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Matsuda S, Ikeda Y, Murakami M, Nakagawa
Y, Tsuji A and Kitagishi Y: Roles of PI3K/AKT/GSK3 pathway involved
in psychiatric Illnesses. Diseases. 7: pii(E22)2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ogino M, Ichimura M, Nakano N, Minami A,
Kitagishi Y and Matsuda S: Roles of PTEN with DNA repair in
Parkinson's disease. Int J Mol Sci. 17: pii(E954)2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Knafo S, Sánchez-Puelles C, Palomer E,
Delgado I, Draffin JE, Mingo J, Wahle T, Kaleka K, Mou L,
Pereda-Perez I, et al: PTEN recruitment controls synaptic and
cognitive function in Alzheimer's models. Nat Neurosci. 19:443–453.
2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Chen CY, Chen J, He L and Stiles BL: PTEN:
Tumor suppressor and metabolic regulator. Front Endocrinol
(Lausanne). 9(338)2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Yi YW, Kang HJ, Kim HJ, Hwang JS, Wang A
and Bae I: Inhibition of constitutively activated phosphoinositide
3-kinase/AKT pathway enhances antitumor activity of
chemotherapeutic agents in breast cancer susceptibility gene
1-defective breast cancer cells. Mol Carcinog. 52:667–675.
2013.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Krivokuca A, Boljevic I, Jovandic S, Magic
Z, Mandic A, Tomasevic Z and Brankovic-Magic M: Germline mutations
in cancer susceptibility genes in high grade serous ovarian cancer
in Serbia. J Hum Genet. 64:281–290. 2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Yoshino Y, Endo S, Chen Z, Qi H, Watanabe
G and Chiba N: Evaluation of site-specific homologous recombination
activity of BRCA1 by direct quantitation of gene editing
efficiency. Sci Rep. 9(1644)2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Savage KI and Harkin DP: BRCA1, a
‘complex’ protein involved in the maintenance of genomic stability.
FEBS J. 282:630–646. 2015.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Fernandes N, Sun Y, Chen S, Paul P, Shaw
RJ, Cantley LC and Price BD: DNA damage-induced association of ATM
with its target proteins requires a protein interaction domain in
the N terminus of ATM. J Biol Chem. 280:15158–15164.
2005.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Suberbielle E, Djukic B, Evans M, Kim DH,
Taneja P, Wang X, Finucane M, Knox J, Ho K, Devidze N, et al: DNA
repair factor BRCA1 depletion occurs in Alzheimer brains and
impairs cognitive function in mice. Nat Commun.
6(8897)2015.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Moruno-Manchon JF, Koellhoffer EC,
Gopakumar J, Hambarde S, Kim N, McCullough LD and Tsvetkov AS: The
G-quadruplex DNA stabilizing drug pyridostatin promotes DNA damage
and downregulates transcription of Brca1 in neurons. Aging (Albany
NY). 9:1957–1970. 2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
So EY and Ouchi T: BRAT1 deficiency causes
increased glucose metabolism and mitochondrial malfunction. BMC
Cancer. 14(548)2014.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Tanaka H, Phipps EA, Wei T, Wu X, Goswami
C, Liu Y, Sledge GW Jr, Mina L and Herbert BS: Altered expression
of telomere-associated genes in leukocytes among BRCA1 and BRCA2
carriers. Mol Carcinog. 57:567–575. 2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Zhan Y and Hägg S: Telomere length
dhortening in Alzheimer's disease: Procedures for a causal
investigation using single nucleotide polymorphisms in a mendelian
randomization study. Methods Mol Biol. 1750:293–306.
2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Minami A, Nakanishi A, Ogura Y, Kitagishi
Y and Matsuda S: Connection between Tumor Suppressor BRCA1 and PTEN
in damaged DNA repair. Front Oncol. 4(318)2014.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Maidarti M, Clarkson YL, McLaughlin M,
Anderson RA and Telfer EE: Inhibition of PTEN activates bovine
non-growing follicles in vitro but increases DNA damage and reduces
DNA repair response. Hum Reprod. 34:297–307. 2019.PubMed/NCBI View Article : Google Scholar
|
|
47
|
A. Zhao C, Noble JM, Marder K, Hartman JS,
Gu Y and Scarmeas N: Dietary patterns, physical activity, sleep,
and risk for dementia and cognitive decline. Curr Nutr Rep.
7:335–345. 2018.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Liu D, Wang Z, Gao Z, Xie K, Zhang Q,
Jiang H and Pang Q: Effects of curcumin on learning and memory
deficits, BDNF, and ERK protein expression in rats exposed to
chronic unpredictable stress. Behav Brain Res. 271:116–121.
2014.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Sang Q, Sun D, Chen Z and Zhao W: GF and
PI3K/Akt signaling participate in the ventral motor neuronal
protection of curcumin in sciatic nerve injury rat models. Biomed
Pharmacother. 103:1146–1153. 2018.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Hussein RM, Mohamed WR and Omar HA: A
neuroprotective role of kaempferol against chlorpyrifos-induced
oxidative stress and memory deficits in rats via GSK3β-Nrf2
signaling pathway. Pestic Biochem Physiol. 152:29–37.
2018.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Wu B, Luo H, Zhou X, Cheng CY, Lin L, Liu
BL, Liu K, Li P and Yang H: Succinate-induced neuronal
mitochondrial fission and hexokinase II malfunction in ischemic
stroke: Therapeutical effects of kaempferol. Biochim Biophys Acta
Mol Basis Dis. 1863:2307–2318. 2017.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Shen R and Wang JH: The effect of Icariin
on immunity and its potential application. Am J Clin Exp Immunol.
7:50–56. 2018.PubMed/NCBI
|
|
53
|
Yoshida H, Okumura N, Kitagishi Y,
Nishimura Y and Matsuda S: Ethanol extract of Rosemary repressed
PTEN expression in K562 culture cells. Int J App Biol
Pharmaceutical. 2:316–322. 2011.
|
|
54
|
Lu M, Fei Z and Zhang G: Synergistic
anticancer activity of 20(S)-Ginsenoside Rg3 and Sorafenib in
hepatocellular carcinoma by modulating PTEN/Akt signaling pathway.
Biomed Pharmacother. 97:1282–1288. 2018.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Chalabi N, Le Corre L, Maurizis JC, Bignon
YJ and Bernard-Gallon DJ: The effects of lycopene on the
proliferation of human breast cells and BRCA1 and BRCA2 gene
expression. Eur J Cancer. 40:1768–1775. 2004.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Chalabi N, Maurizis JC, Le Corre L, Delort
L, Bignon YJ and Bernard-Gallon DJ: Quantification by affinity
perfusion chromatography of phosphorylated BRCAl and BRCA2 proteins
from tumor cells after lycopene treatment. J Chromatogr B Analyt
Technol Biomed Life Sci. 821:188–193. 2005.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Bosviel R, Dumollard E, Déchelotte P,
Bignon YJ and Bernard-Gallon D: Can soy phytoestrogens decrease DNA
methylation in BRCA1 and BRCA2 oncosuppressor genes in breast
cancer? OMICS. 16:235–244. 2012.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Vissac-Sabatier C, Coxam V, Déchelotte P,
Picherit C, Horcajada MN, Davicco MJ, Lebecque P, Bignon YJ and
Bernard-Gallon D: Phytoestrogen-rich diets modulate expression of
Brca1 and Brca2 tumor suppressor genes in mammary glands of female
Wistar rats. Cancer Res. 63:6607–6612. 2003.PubMed/NCBI
|
|
59
|
Cabanes A, Wang M, Olivo S, DeAssis S,
Gustafsson JA, Khan G and Hilakivi-Clarke L: Prepubertal estradiol
and genistein exposures up-regulate BRCA1 mRNA and reduce mammary
tumorigenesis. Carcinogenesis. 25:741–748. 2004.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Soni M, Rahardjo TB, Soekardi R,
Sulistyowati Y, Lestariningsih Yesufu-Udechuku A, Irsan A and
Hogervorst E: Phytoestrogens and cognitive function: A review.
Maturitas. 77:209–220. 2014.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Weng SW, Hsu SC, Liu HC, Ji BC, Lien JC,
Yu FS, Liu KC, Lai KC, Lin JP and Chung JG: Gallic acid induces DNA
damage and inhibits DNA repair-associated protein expression in
human oral cancer SCC-4 cells. Anticancer Res. 35:2077–2084.
2015.PubMed/NCBI
|
|
62
|
Taysi S, Tascan AS, Ugur MG and Demir M:
Radicals, oxidative/nitrosative stress and preeclampsia. Mini Rev
Med Chem. 19:178–193. 2019.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Vehviläinen P, Koistinaho J and Gundars G:
Mechanisms of mutant SOD1 induced mitochondrial toxicity in
amyotrophic lateral sclerosis. Front Cell Neurosci.
8(126)2014.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Sakellariou GK, Davis CS, Shi Y, Ivannikov
MV, Zhang Y, Vasilaki A, Macleod GT, Richardson A, Van Remmen H,
Jackson MJ, et al: Neuron-specific expression of CuZnSOD prevents
the loss of muscle mass and function that occurs in homozygous
CuZnSOD-knockout mice. FASEB J. 28:1666–1681. 2014.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Bravard A, Sabatier L, Hoffschir F, Ricoul
M, Luccioni C and Dutrillaux B: SOD2: A new type of
tumor-suppressor gene? Int J Cancer. 51:476–480. 1992.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Dixit S, Fessel JP and Harrison FE:
Mitochondrial dysfunction in the APP/PSEN1 mouse model of
Alzheimer's disease and a novel protective role for ascorbate. Free
Radic Biol Med. 112:515–523. 2017.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Demchenko IT, Gutsaeva DR, Moskvin AN and
Zhilyaev SY: Involvement of extracellular superoxide dismutase in
regulating brain blood flow. Neurosci Behav Physiol. 40:173–178.
2010.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Singh B and Bhat HK: Superoxide dismutase
3 is induced by antioxidants, inhibits oxidative DNA damage and is
associated with inhibition of estrogen-induced breast cancer.
Carcinogenesis. 33:2601–2610. 2012.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Natarajan G, Perriotte-Olson C, Casey CA,
Donohue TM Jr, Talmon GA, Harris EN, Kabanov AV and Saraswathi V:
Effect of nanoformulated copper/zinc superoxide dismutase on
chronic ethanol-induced alterations in liver and adipose tissue.
Alcohol. 79:71–79. 2019.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Janyou A, Wicha P, Jittiwat J, Suksamrarn
A, Tocharus C and Tocharus J: Dihydrocapsaicin attenuates blood
brain barrier and cerebral damage in focal cerebral
ischemia/reperfusion via oxidative stress and inflammatory. Sci
Rep. 7(10556)2017.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Lee SH, Kim JK and Jang HD: Genistein
inhibits osteoclastic differentiation of RAW 264.7 cells via
regulation of ROS production and scavenging. Int J Mol Sci.
15:10605–10621. 2014.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Sheriff SA, Shaik Ibrahim S, Devaki T,
Chakraborty S, Agarwal S and Pérez-Sánchez H: Lycopene prevents
mitochondrial dysfunction during
d-galactosamine/lipopolysaccharide-induced fulminant hepatic
failure in albino rats. J Proteome Res. 16:3190–3199.
2017.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Desjardins F, Sekkali B, Verreth W, Pelat
M, De Keyzer D, Mertens A, Smith G, Herregods MC, Holvoet P and
Balligand JL: Rosuvastatin increases vascular endothelial PPARgamma
expression and corrects blood pressure variability in obese
dyslipidaemic mice. Eur Heart J. 29:128–137. 2008.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Chatterjee A, Ronghe A, Padhye SB, Spade
DA, Bhat NK and Bhat HK: Antioxidant activities of novel
resveratrol analogs in breast cancer. J Biochem Mol Toxicol.
32(e21925)2018.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Ribeiro CCD, Silva RM, Campanholo VMLP,
Ribeiro DA, Ribeiro Paiotti AP and Forones NM: Effects of Grape
Juice in superoxide dismutase and catalase in colorectal cancer
carcinogenesis induced by Azoxymethane. Asian Pac J Cancer Prev.
19:2839–2844. 2018.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Geeraert B, Crombé F, Hulsmans M,
Benhabilès N, Geuns JM and Holvoet P: Stevioside inhibits
atherosclerosis by improving insulin signaling and antioxidant
defense in obese insulin-resistant mice. Int J Obes (Lond).
34:569–577. 2010.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Ma N, Abaker JA, Bilal MS, Dai H and Shen
X: Sodium butyrate improves antioxidant stability in sub-acute
ruminal acidosis in dairy goats. BMC Vet Res.
14(275)2018.PubMed/NCBI View Article : Google Scholar
|
|
78
|
von Arnim CA, Herbolsheimer F, Nikolaus T,
Peter R, Biesalski HK, Ludolph AC, Riepe M and Nagel G: ActiFE Ulm
Study Group. Dietary antioxidants and dementia in a
population-based case-control study among older people in South
Germany. J Alzheimers Dis. 31:717–724. 2012.PubMed/NCBI View Article : Google Scholar
|
|
79
|
de Oliveira BF, Veloso CA,
Nogueira-Machado JA, de Moraes EN, dos Santos RR, Cintra MT and
Chaves MM: Ascorbic acid, alpha-tocopherol, and beta-carotene
reduce oxidative stress and proinflammatory cytokines in
mononuclear cells of Alzheimer's disease patients. Nutr Neurosci.
15:244–251. 2012.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Ghanbari AA, Shabani K and Mohammad Nejad
D: Protective effects of Vitamin E consumption against 3MT
electromagnetic field effects on oxidative parameters in Substantia
Nigra in Rats. Basic Clin Neurosci. 7:315–322. 2016.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Thiriot C, Durand P, Jasseron MP, Kergonou
JF and Ducousso R: Radiosensitive antioxidant membrane-bound
factors in rat liver microsomes: I. The roles of glutathione and
vitamin E. Biochem Int. 14:1–8. 1987.PubMed/NCBI
|
|
82
|
Vinot N, Jouin M, Lhomme-Duchadeuil A,
Guesnet P, Alessandri JM, Aujard F and Pifferi F: Omega-3 fatty
acids from fish oil lower anxiety, improve cognitive functions and
reduce spontaneous locomotor activity in a non-human primate. PLoS
One. 6(e20491)2011.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Ray SD, Parmar M, Syed I, Rathod J,
Zinkovsky D, Bulku E, Gigliotti J, Hackman RM and Stohs SJ: Long
term exposure effect of a unique metabolic nutrition system
containing a diverse group of phytochemicals on serum chemistry and
genomic and non-genomic changes in the liver of female B6C3F1 mice.
Phytother Res. 22:458–71. 2008.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Lee J, Park S, Lee JY, Yeo YK, Kim JS and
Lim J: Improved spatial learning and memory by perilla diet is
correlated with immunoreactivities to neurofilament and α-synuclein
in hilus of dentate gyrus. Proteome Sci. 10(72)2012.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Byun EB, Cho EJ, Kim YE, Kim WS and Byun
EH: Neuroprotective effect of polysaccharide separated from Perilla
frutescens Britton var acuta Kudo against
H2O2-induced oxidative stress in HT22
hippocampus cells. Biosci Biotechnol Biochem. 82:1344–1358.
2018.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Vallés SL, Borrás C, Gambini J, Furriol J,
Ortega A, Sastre J, Pallardó FV and Viña J: Oestradiol or genistein
rescues neurons from amyloid beta-induced cell death by inhibiting
activation of p38. Aging Cell. 7:112–118. 2008.PubMed/NCBI View Article : Google Scholar
|