Introduction
Proteolytic enzymes (proteases, proteinases and
peptidases), belong to Enzyme Classification (EC) number: 3.4. and
are a subgroup of hydrolases (EC 3.). Peptidases hydrolyze peptide
bonds in fundamentally irreversible reactions (1). They serve a key role in numerous
biological processes, including tissue remodeling, wound healing,
blood coagulation, angiogenesis, neurogenesis, ovulation,
fertilization, hemostasis, immunity, inflammation, senescence,
necrosis and apoptosis (1,2). Proteolytic enzymes constitute ~2% of
the mammalian genome and significantly contribute to the enzymatic
content and activity of the gastrointestinal tract (3,4). During
early pregnancy, secretion of embryo-endometrial proteases is
responsible for the hatching of blastocysts and invasion of
implanting embryos into the uterine endometrium (5). This physiological uterine modification
is largely controlled by proteases and/or their inhibitors, and
disorders of the feto-placental homeostasis results in maternal
hypertension, preterm delivery or preeclampsia (6-10),
which are common causes for performing a cesarean delivery.
Aminopeptidases (EC 3.4.11.) are an important subset
of proteases which hydrolyze peptide bonds at the N-terminus of
proteins and peptides (11). They
are generally zinc-dependent metalloenzymes, are responsible for
the limited proteolysis and function as posttranslational modifiers
of enzymes, inactivators of signal peptides and hormones, and
digestive enzymes (11,12).
Meconium is a specific physiological product
produced in the gastrointestinal tract of a fetus, obtained
noninvasively within 2-4 days after birth and, under normal
conditions, is not excreted until/after delivery. Fetal meconium
starts forming at 13 weeks of gestation from cycles of swallowed or
inhaled amniotic fluid, shed epithelial cells, intestinal
secretions and urine (13).
The role of proteolytic enzymes in vital biological
processes, including embryogenesis and intrauterine fetal
development, may be associated with the mode of delivery. This
hypothesis is based on the results of studies which indicate that
fecal protease activity is associated with compositional
alterations in the intestinal microbiota (4), and that the diversity of the meconium
microbiome depends on the mode of delivery (14,15). To
date, there have been no studies investigating any possible
association between protease activity in the fetal intestine during
intrauterine development of the fetus and the mode of delivery, to
the best of our knowledge.
The aim of the present study was to assess the
changes in the activities of proteases and aminopeptidases, which
are a specific pool of proteolytic enzymes present in the meconium
of healthy infants, born either vaginally or by a cesarean section,
and to investigate whether there are any statistically significant
associations between their activity and the mode of delivery.
Materials and methods
Ethics
The present study was approved by the Medical Ethics
Committee at the Central Clinical Hospital of the Ministry of the
Interior (Warsaw, Poland) (approval no. 71/2011), and was performed
in accordance with the Declaration of Helsinki (16). Written informed consent was obtained
from the parents or guardians prior to inclusion of infants in the
study.
Profile of neonates
A total of 30 infants born in the Clinical
Department of Obstetrics, Female Diseases and Gynecological
Oncology, Central Clinical Hospital of the Ministry of the
Interior, Warsaw, were included in the present study. Multiple
birth neonates, infants born before 35 weeks of gestation or those
with a birth weight of <2 kg were excluded from the present
study. The health conditions of the pregnant women and infants were
assessed by the attending physician.
Of the 30 infants, 13 were female and 17 were male;
14 were delivered by vaginal birth and 16 were delivered by
cesarean section; the mean gestational age was 38.9±1.4 weeks
(standard deviation), median gestational age was 39 weeks (range,
36-41 weeks); the mean birth weight was 3,409±512.5 g, median,
3,445 g (range, 2,260-4,710 g); Apgar scores were (min 1/3/5/10):
10/10/10/10 (n=21); 9/10/10/10 (n=1); 9/9/10/10 (n=1); 9/9/9/10
(n=2); 8/9/10/10 (n=5). The Apgar score is a test performed on
newborns that is repeated every few mins following birth. The test
is scored between 0-10, and measures baby's heart rate, breathing,
muscle tone, reflex response and appearance.
Sample collection
The meconium was collected in the hospital ward and
consisted of all consecutive portions. A total of 110 meconium
samples from 30 infants were collected from the nappy with a
disposable spatula and transferred into 50 ml graduated plastic
containers. The empty containers were weighed prior to adding
meconium and reweighed after filling. The date, time and weight of
each meconium collection were recorded. Collection was considered
complete, when, on inspection, the dark-greenish-black color of the
material had changed to the yellowish-brown color characteristic of
stool. Passing of meconium stopped between 24-52 h postnatally.
Between 2-5 meconium portions were collected from each infant.
Analytical-grade distilled water was added to each meconium sample
up to a homogenate volume of 45 ml in three stages (10 ml, 10 ml
and then up to 45 ml). Meconium homogenates were stored at -80̊C.
Prior to assessment of protease and aminopeptidase activity, each
homogenate sample was thoroughly mixed and individually diluted
with analytical-grade distilled water to a final concentration of
0.5 mg meconium per ml after thawing.
Assay of protease activity
The protease activity in meconium was determined
using an EnzChek Protease assay kit (Molecular Probes Inc.). This
commercial kit did not involve any separation steps and can be used
to measure the kinetics of a variety of exo- and endopeptidases
continuously over a wide range of pH values. The full
substrate-dependent kinetic analysis of the meconium protease
activity was performed. A set of increasing concentrations of
BODIPY FL casein solutions as a substrate was prepared (2.5, 3.75,
5.0, 7.5, 10, 20, 30 and 40 µg/ml). Separate stock solutions for
each BODIPY FL casein concentration were prepared (a total of 8
different concentrations). A sample with the reaction mixture
prepared for the measurement contained 100 µl BODIPY FL casein at
increasing concentrations and 100 µl of individually prepared
meconium homogenate solution at a final concentration 0.5 mg/ml.
Thus the increasing concentrations of substrates were added into a
series of 8 wells and each well contained 100 µl meconium
homogenate. Samples were assessed in triplicate in a 96-well plate.
At time 0 h and after 1 h of incubation at room temperature.
Fluorescence was measured at 485 nm (excitation) and 530 nm
(emission) using a Synergy H1 Hybrid Reader (BioTek Instruments,
Inc.). The activity of proteases in meconium was calculated as the
increase in relative fluorescence units (RFU)/h. For each sample, a
mean RFU/h was calculated. Autoclaved samples were used as the
negative controls.
Assay of aminopeptidase activity
Maximal potential aminopeptidase activity (Vmax) was
measured using a fluorogenic substrate (17), according to a modified version of a
previously described protocol (18,19).
Briefly, 10 µl of the substrate L-leucine-7-amido-4-methylcoumarin
hydrochloride (Leu-MCA; Sigma-Aldrich; Merck KGaA) solution in
ethanol was added to 190 µl meconium homogenate (at a concentration
of 0.5 mg meconium/ml), resulting in solutions with final Leu-MCA
concentrations of 1.0, 2.5, 5.0, 10, 15, 20, 25 or 30 µM. Separate
stock solutions for each Leu-MCA concentration were prepared (a
total of 8 concentrations). For preparation of the calibration
standard solution, 7-amino-4-methylcoumarin (MCA) was used to
measure the fluorescence. Final MCA concentrations in ethanol were
4.688, 9.375, 18.75, 37.5, 75, 150, 300 or 600 nM. A calibration
curve was prepared individually for each meconium homogenate.
Samples were loaded in triplicate into a 96-well plate. At time 0 h
and after 1 h of incubation at room temperature, the fluorescence
of the MCA product was determined spectrofluorometrically at 380 nm
(excitation) and 460 nm (emission) using a Synergy H1 Hybrid Reader
(BioTek Instruments, Inc.). The aminopeptidase activity calculated
from the standard curve is given in nM/l/h.
Data analysis
Both methods of enzyme activity measurement used the
enzymatic hydrolysis of substrates and detection of the
fluorescence of the products. The tested enzyme-substrate system
followed first-order Michaelis–Menten kinetics (20). Plotting reaction velocity (v) against
substrate concentration [S] resulted in a rectangular
hyperbola-shaped relationship, described by the equation:
v=Vmax x[S]/(Km +[S]), where Km is
the Michaelis constant and Vmax is the maximal potential
activity. Km represents the concentration of substrate
which permits the enzyme to achieve half Vmax.
Plots of reaction velocity against substrate
concentration for proteases and aminopeptidases were constructed
and Vmax and Km were calculated. Non-linear
regression analysis was used to calculate the kinetic parameters of
enzymatic reactions using Origin 6.1 (OriginLab Corporation).
Aminopeptidases identified in the meconium samples
(21) are listed in Table I according to their classification
system in the following online databases; uniprot.org,
brenda-enzymes.org and merops.sanger.ac.uk.
 | Table IAminopeptidases identified in meconium
samples. |
Table I
Aminopeptidases identified in meconium
samples.
| Classification
system | | |
|---|
| UniProtKB | BRENDA | MEROPS | Enzyme name | Abbreviation |
|---|
| P28838 | EC 3.4.11.1 | M17.001 | Leucyl
aminopeptidase, cytosol aminopeptidase | LAP, cAP |
| P15144 | EC 3.4.11.2 | M01.001 | Aminopeptidase N or
M, alanine aminopeptidase, alpha-aminoacyl-peptide hydrolase,
alanine or leucine arylamidase, leucine-beta-naphthylamidase | AP-N, AAP, CD13 |
| Q9UIQ6 | EC 3.4.11.3 | M01.011 | Placental leucine
aminopeptidase, oxytocinase, cystine aminopeptidase | P-LAP, CAP |
| Q9H4A4 | EC 3.4.11.6 | M01.014 | Aminopeptidase B | AP-B |
| Q07075 | EC 3.4.11.7 | M01.003 | Aminopeptidase A,
glutamyl aminopeptidase, cystyl aminopeptidase | AP-A |
| O43895 | EC 3.4.11.9 | M24.005 | Aminopeptidase P,
X-Pro aminopeptidase | AP-P |
Statistical analysis
Statistical analysis was performed using Statistica
Version 12 (StatSoft Inc.). The results are presented as the mean ±
standard deviation, or median and range. A Shapiro-Wilk's test was
performed to assess the normality of distribution. A Mann-Whitney
U-test and a Kruskal-Wallis with a Fisher's LSD post hoc test or an
ANOVA with a post-hoc Tukey's test, were used to compare the
activity of peptidases and aminopeptidases. A Spearman's rank-order
correlation test was performed to determine the relationship
between the activity of proteases and aminopeptidases in the first
and last meconium portions. P<0.05 was considered to indicate a
statistically significant difference.
Results
Table II shows the
activity of aminopeptidases (103 nM/l/h) in successive
percentage ranges of meconium weight. It was assumed that the
weight of meconium excreted after birth varied among neonates, but
was always equal to 100% of the weight of meconium formed and
accumulated in the intestine during gestation. Total meconium
weight per neonate ranged from 0.579-32.086 g (mean, 10.92±8.1;
median, 9.19). Each subsequent portion of meconium collected from
an individual neonate represented the specific activity of an
aminopeptidase and a specific range (%) of the total meconium
weight. Subsequently, for each of the calculated percentage ranges,
the value corresponding to 50% of this range was calculated. This
value was assigned to one of the four percent ranges: 0-25, 26-50,
51-75 and 76-100%. The number of aminopeptidases activity
measurements in percent ranges varied as the number of meconium
portions excreted, and the total weight of meconium accumulated in
the fetal intestine were neonate-specific and differed among
infants. Thus, aminopeptidase activities in the 0-25 and 76-100%
ranges did not invariably correspond to the measurements in the
first and last meconium.
 | Table IIActivity of aminopeptidases stratified
by ranges of percentages in meconium samples collected from 30
neonates. |
Table II
Activity of aminopeptidases stratified
by ranges of percentages in meconium samples collected from 30
neonates.
| | | Aminopeptidase
activity (103 nM/l/h) | |
|---|
| Percentage | n | Mean ± standard
deviation | Median | Range | Kruskal-Wallis-ANOVA
P-value |
|---|
| 0-25% | 29 | 1.27±0.99 | 0.98 | 0.10-4.31 | |
| 26-50% | 23 | 1.08±0.68 | 1.13 | 0.01-3.01 | P=0.702 |
| 51-75% | 21 | 1.87±1.79 | 1.10 | 0.05-6.64 | |
| 76-100% | 37 | 1.31±1.15 | 1.02 | 0.11-5.29 | |
The median values were closer than the mean values
in sequential percent ranges and there were no statistically
significant differences in the aminopeptidase activity values
demonstrated within these ranges (P=0.702).
Table III shows the
activity of proteases and aminopeptidases in the first and last
meconium portions, and the ratio of enzyme activity of first to
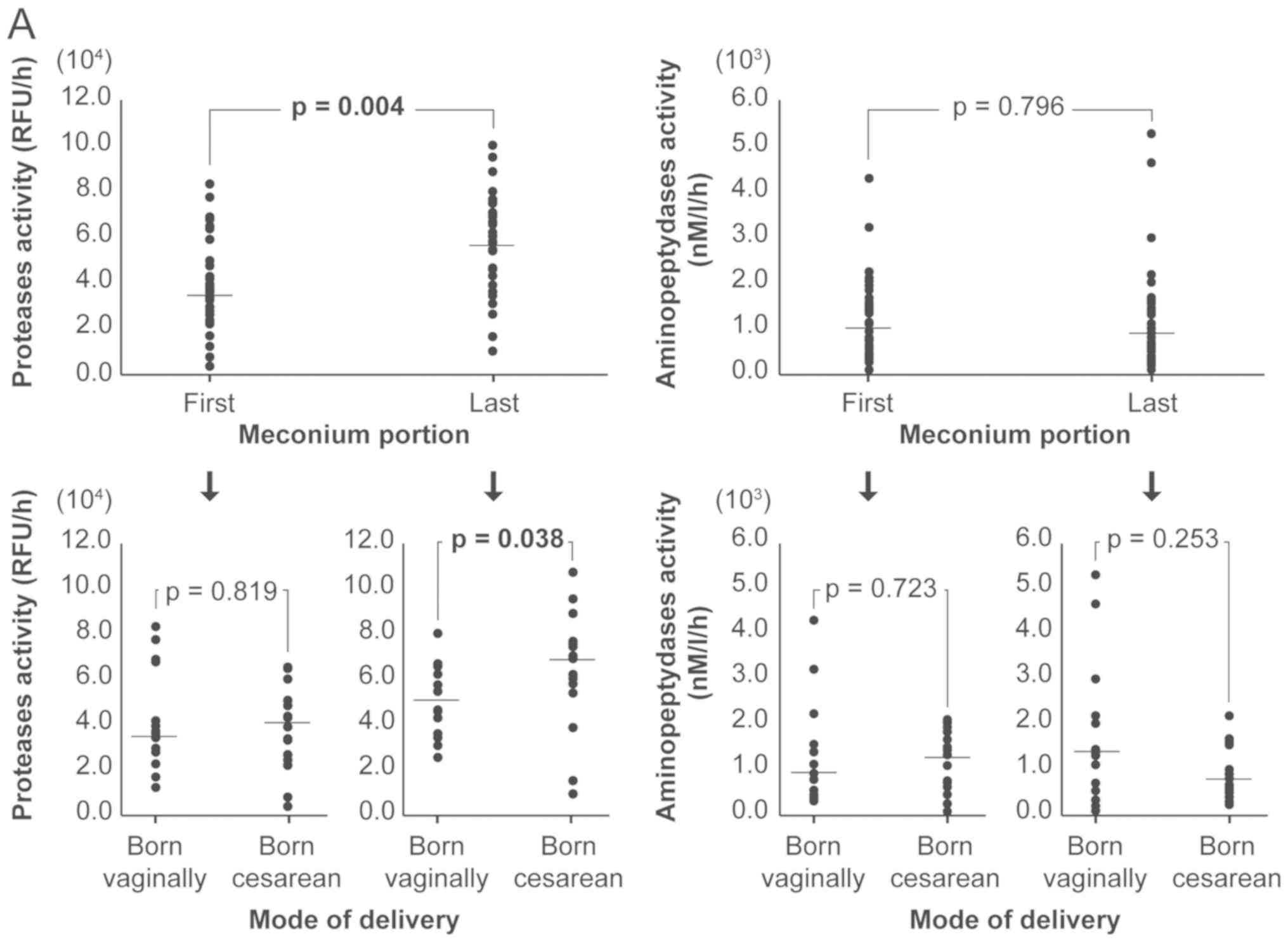
last meconium portion. Fig. 1 shows
the distribution of the measurements in individual infants,
including the mode of delivery.
 | Table IIIRatio of protease and aminopeptidase
activities in the first and last meconium. |
Table III
Ratio of protease and aminopeptidase
activities in the first and last meconium.
| | Protease activity
(104 RFU/h) | Aminopeptidase
activity (103 nM/l/h) |
|---|
| Meconium | Mean ± SD | Median | Range | Mean ± SD | Median | Range |
|---|
| First | 3.99±2.03 | 3.79 | 0.35-8.34 | 1.24±0.94 | 1.04 | 0.09-4.31 |
| Last | 5.76±2.24 | 5.92 | 0.98-10.66 | 1.29±1.22 | 0.91 | 0.11-5.29 |
| | Ratio for
protease | Ratio for
aminopeptidase |
| Ratio | Mean ± SD | Median | Range | Mean ± SD | Median | Range |
| First/last | 0.76±0.48 | 0.77 | 0.08-2.68 | 1.35±1.04 | 0.99 | 0.15-4.11 |
A statistically significant increase in the activity
of proteases was demonstrated between the first and last meconium
sample (P=0.004), and between the meconium the last portions from
the neonates born vaginally and those delivered by a cesarean
section (P=0.038). The activities of both proteases (meconium from
24 neonates) and aminopeptidases (meconium from 15 neonates) were
lower in the first meconium portion compared with the last meconium
portion. In 5 neonates, the activity of proteases in the first
meconium portion was slightly higher compared with the last
meconium portion and the ratios did not exceed 1.25, except in only
one neonate (born by a cesarean section) where the ratio was 2.68.
Unlike protease activity, the activity of aminopeptidases was
significantly higher in the first meconium portion compared with
the last meconium portion in 8 neonates, and the ratio exceeded 2,
the maximum ratio observed was 4.11 in a neonate born vaginally.
Statistically significant differences were observed in the first to
last meconium portion ratio for proteases and aminopeptidases in
the entire study group (P=0.014) and in neonates born by cesarean
birth (P=0.008).
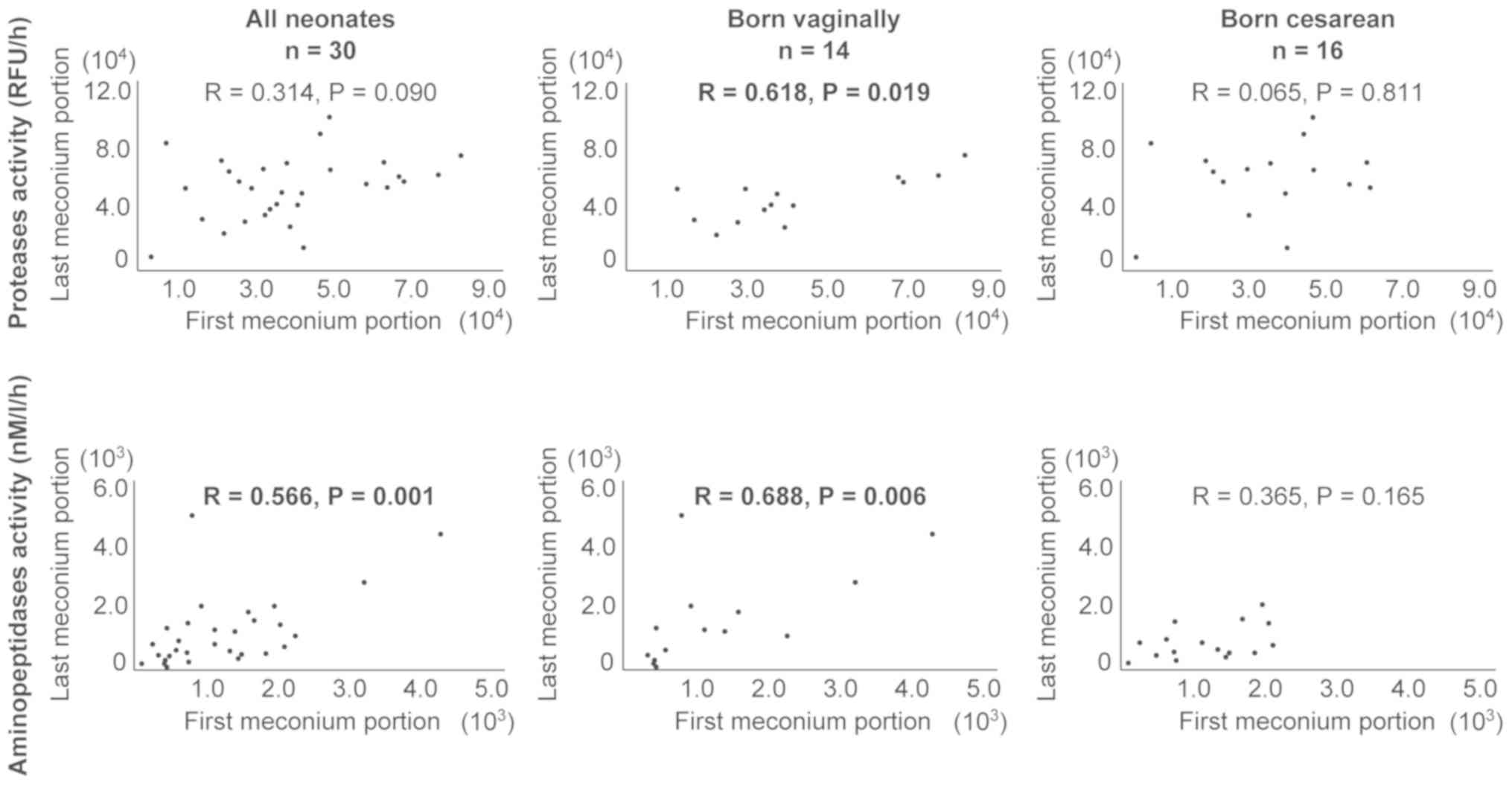
Fig. 2 shows the
association between the activity of proteases and of
aminopeptidases in the first and last meconium portion, including
the mode of delivery. Vaginal birth was associated with higher
Spearman's correlation coefficients values relative to the entire
study group for both proteases (R=0.618 and R=0.314, respectively)
and aminopeptidases (R=0.688, R=0.566, respectively).
Discussion
The assessment of protease and aminopeptidase
activity in the meconium of healthy infants, and the
interrelationship of these enzymes was demonstrated for the first
time in the present study, to the best of our knowledge. The
results showed that during fetal intrauterine development, activity
of these enzymes may be associated with the mode of delivery.
Increases in the protease activity between the first and last
meconium portions, observed in 80% of the infants included in the
study, confirmed the results of our previous (22). Peptidases are a highly diverse class
of enzymes and the MEROPS database is an essential source of
information regarding peptidases, their substrates and inhibitors
(23). In total, 22 human
aminopeptidases are described in the MEROPS database and 6 of these
were present in meconium (Table I)
(21). In addition, proteomic
analysis has shown a total of 265 enzymatic proteins in meconium,
25% of which are proteases, and of these, ~9% are aminopeptidases
(data not shown). The activities of aminopeptidases assayed in the
present study represent a pool of six enzymes with important roles
in fetal development, such as the regulation of pressure, neuronal
cell function in the brain, learning and memory, or final digestion
of peptides (8,11). Meconium aminopeptidases are identical
to placental aminopeptidases (8),
but an additional leucyl aminopeptidase (EC 3.4.11.1) is present in
meconium which may be derived from activated lymphocytes (24). As the activities of aminopeptidases
showed no apparent changes between successively collected portions
of meconium, this may suggest a similar degree of their involvement
throughout gestation. However, as a pool of aminopeptidases was
assayed, the actual involvement of individual enzymes may vary at
different stages of gestation. To date, the activity of these
aminopeptidases has not been investigated in meconium. Placental
leucine aminopeptidase (P-LAP) appears to be the most frequently
studied aminopeptidase (6-8,11,25-27).
In the serum of normal pregnant women, P-LAP shows a characteristic
pattern of activity. P-LAP activities in the serum of normal
pregnant women are constant during the early stages of pregnancy,
then rise between the 18th and 37th weeks, peaking at 38 weeks of
gestation. The activities are flat or slightly decreased just
before labor and then further decrease within 4 weeks after
delivery (25). The possible sources
of serum P-LAP include the placenta, umbilical vessels, decidua
and/or the fetus (25). However,
whether meconium may be an additional source of serum P-LAP, or if
serum P-LAP is present in the meconium has not been determined, to
the best of our knowledge. High expression levels of P-LAP have
been reported in the lung, kidney, heart and pancreas (26), and it is involved in hormonal control
of uterine contraction, osmoregulation and blood glucose level
regulation (27). Aminopeptidase N
serves a role in signal transduction, inflammation, immunological
responses, matrix degradation, chemokines and cytokines processing
(28). Aminopeptidases are an
important subgroup of peptidases which serve a key role in a
variety of processes, such as regulation of hormone levels, protein
maturation, inactivation of proteins and protein digestion
(25-29).
Further research is required to determine the activity of each of
the aminopeptidases identified in meconium in the present study,
and to measure their activities in the maternal serum and/or
amniotic fluid. Individual assessment of specific aminopeptidases
and other proteases is essentially hampered by the need for
specific substrates or selective inhibitors. Research is currently
being performed in this area, particularly for aminopeptidase N
(28), and another study used
artificial substrates for aminopeptidase A, aminopeptidase B and
aminopeptidase N (29).
The source of proteases and aminopeptidases in
meconium has not been definitively established, to the best of our
knowledge. They are hypothesized to be derived from the mother,
placenta, amniotic fluid and/or fetus, but other studies have also
demonstrated aminopeptidase activity is associated with bacteria
(4,30). The paradigm regarding the sterile
fetal environment is now being challenged based on evidence showing
the presence microbial communities in meconium (31). A previous study demonstrated that the
diversity of the meconium microbiome was higher in vaginally
delivered infants compared with infants born by cesarean section,
with the Propionibacterium species being the most abundant
in the former and Bacillus licheniformis in the latter
(14). Another study found
significant associations between specific intestinal bacterial
groups and fecal protease activity with a significant difference
between the microbiotas of high vs. low fecal protease activity
samples, suggesting that the difference in microbial communities
between these groups is based on both dominant and low abundance
bacterial taxa present in fecal samples (4). However, the meconium microbiome may
also be dependent on race or geographical differences as well as
the interval of meconium collection (14).
The health condition of all neonates included in the
present study was assessed and considered good by the attending
physician. There was a stronger association between the activities
of proteases and aminopeptidases in the first vs. the last meconium
in vaginally born infants compared with the entire study group,
which may suggest in utero homeostasis of processes
catalyzed by these enzymes, while in infants born by a cesarean
section, this homeostasis may have been disturbed. In the present
study, 10 of the 16 infants born by a cesarean section were born by
an emergency cesarean section (fetal asphyxia or failure to
progress), while 6 infants were born by an elective cesarean
section. Considering the rising rates of cesarean sections, both
elective and emergency (32),
additional studies are required to characterize and differentiate
protease and individual aminopeptidase activities associated with
the mode of delivery, particularly for infants born by cesarean
section. It may also be important to consider the pharmacological
aspects when analyzing individual aminopeptidases.
In summary, in the present study, it was
demonstrated that there was an absence of significant dynamic
changes in the aminopeptidase activity during meconium accumulation
in the fetal intestine. In infants born vaginally, compared with
those born by a cesarean section, there was a stronger association
between the activities of proteases and aminopeptidases in the
first vs. the last meconium portion, which may suggest a more
prominent homeostatic effect of processes catalyzed by these
enzymes in the fetal intestine or even in the intrauterine
environment. These results, however, should be confirmed in a
larger number of infants, taking into account the type of cesarean
section (elective vs. emergency) and whether this affects the
activity of enzymes. Additionally, the activity of individual
aminopeptidases identified in meconium should be determined.
Acknowledgements
The authors would like to thank Dr Donat Jaguś at
The Clinical Department of Obstetrics, Female Diseases and
Gynecological Oncology, Central Clinical Hospital of the Ministry
of the Interior in Warsaw for systematic documentation and valuable
medical tips.
Funding
This work was supported by a Research Project Funded
by The National Center for Science (approval no.
DEC-2011/01/B/NZ7/00648).
Availability of data and materials
The datasets used and/or analyzed during this study
are available from the corresponding author upon reasonable
request.
Authors' contributions
ES and BL-M conceived and designed the study and
drafted the initial manuscript. ES analyzed the data. PW collected
materials, and measured and analyzed the data. BK measured and
analyzed the data. JŻ-D and AJ provided medical supervision of
meconium collection and medical documentation. All authors have
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
This study was approved by the Medical Ethics
Committee (approval no. 71/2011) at the Central Clinical Hospital
of the Ministry of the Interior (Warsaw, Poland), in accordance
with the Declaration of Helsinki. Written informed consent was
obtained from the parents prior to inclusion of infants in the
study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pérez-Silva JG, Español Y, Velasco G and
Quesada V: The Degradome database: Expanding roles of mammalian
proteases in life and disease. Nucleic Acids Res. 44:D351–D355.
2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
López-Otín C and Bond JS: Proteases:
Multifunctional enzymes in life and disease. J Biol Chem.
283:30433–30437. 2008.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Antalis TM, Shea-Donohue T, Vogel SN,
Sears C and Fasano A: Mechanisms of disease: Protease functions in
intestinal mucosal pathobiology. Nat Clin Pract Gastroenterol
Hepatol. 4:393–402. 2007.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Carroll IM, Ringel-Kulka T, Ferrier L, Wu
MC, Siddle JP, Bueno L and Ringel Y: Fecal protease activity is
associated with compositional alterations in the intestinal
microbiota. PLoS One. 8(e78017)2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Seshagiri PB, Lalitha HS, Mishra A and
Sireesha GV: Embryo-endometrial proteases during early mammalian
development. Indian J Exp Biol. 41:756–763. 2003.PubMed/NCBI
|
|
6
|
Mizutani S and Tomoda Y: Effects of
placental proteases on maternal and fetal blood pressure in normal
pregnancy and preeclampsia. Am J Hypertens. 9:591–597.
1996.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Mizutani S, Wright J and Kobayashi H: A
new approach regarding the treatment of preeclampsia and preterm
labor. Life Sci. 88:17–23. 2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Mizutani S: Physiological roles of
placental proteases in feto-placental homeostasis. Nagoya J Med
Sci. 61:85–95. 1998.PubMed/NCBI
|
|
9
|
Townsend R, O'Brien P and Khalil A:
Current best practice in the management of hypertensive disorders
in pregnancy. Integr Blood Press Control. 9:79–94. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kattah AG and Garovic VD: The management
of hypertension in pregnancy. Adv Chronic Kidney Dis. 20:229–239.
2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sanderink GJ, Artur Y and Siest G: Human
aminopeptidases: A review of the literature. J Clin Chem Clin
Biochem. 26:795–807. 1988.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Byzia A, Szeffler A, Kalinowski L and Drag
M: Activity profiling of aminopeptidases in cell lysates using a
fluorogenic substrate library. Biochimie. 122:31–37.
2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Park BY and Lee BK: Use of meconium in
perinatal epidemiology: Potential benefits and pitfalls. Ann
Epidemiol. 24:878–881. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Shi YC, Guo H, Chen J, Sun G, Ren RR, Guo
MZ, Peng LH and Yang YS: Initial meconium microbiome in Chinese
neonates delivered naturally or cesarean section. Sci Rep.
8(3255)2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Nagpal R, Tsuji H, Takahashi T, Kawashima
K, Nagata S, Nomoto K and Yamashiro Y: Sensitive quantitative
analysis of the meconium bacterial microbiota in healthy term
infants born vaginally or by cesarean section. Front Microbiol.
7(1997)2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
World Medical Association: World medical
association declaration of Helsinki: Ethical principles for medical
research involving human subjects. JAMA 310: 2191-2194, 2013.
|
|
17
|
Hoppe HG: Significance of exoenzymatic
activities in the ecology of brackish water: Measurements by means
of methylumbelliferyl-substrates. Mar Ecol Prog Ser. 11:299–308.
1983.
|
|
18
|
Kiersztyn B, Siuda W and Chróst RJ:
Persistence of bacterial proteolytic enzymes in lake ecosystems.
FEMS Microbiol Ecol. 80:124–134. 2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chróst RJ: Microbial ectoenzymes in
aquatic environments. In: Overbeck J & Chróst RJ, eds. Aquatic
microbial ecology: Biochemical and molecular approaches.
Springer-Verlag: New York, 47-78, 1990.
|
|
20
|
Johnson KA and Goody RS: The original
Michaelis constant: Translation of the 1913 Michaelis-Menten paper.
Biochemistry. 50:8264–8269. 2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lisowska-Myjak B, Skarżyńska E, Wojdan K
and Nasierowska-Guttmejer A: Protein and peptide profiles in
neonatal meconium. J Obstet Gynaecol Res. 45:556–564.
2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Skarżyńska E, Kiersztyn B, Wilczyńska P,
Jakimiuk A and Lisowska-Myjak B: Total proteolytic activity and
concentration of alpha-1 antitrypsin in meconium for assessment of
the protease/antiprotease balance. Eur J Obstet Gynecol Reprod
Biol. 223:133–138. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Rawlings ND, Barrett AJ, Thomas PD, Huang
X, Bateman A and Finn RD: The MEROPS database of proteolytic
enzymes, their substrates and inhibitors in 2017 and a comparison
with peptidases in the PANTHER database. Nucleic Acids Res.
46:D624–D632. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Sugaya N, Takeuchi Y and Kanno T:
Increased cytosol aminopeptidase in serum in measles infection.
Clin Chem. 34:212–213. 1988.PubMed/NCBI
|
|
25
|
Yamahara N, Nomura S, Suzuki T, Itakura A,
Ito M, Okamoto M, Tsujimoto M, Nakazato M and Mizutani S: Placental
leucine aminopeptidase/oxytocinase in maternal serum and placenta
during normal pregnancy. Life Sci. 66:1401–1410. 2000.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kobayashi H, Nomura S, Mitsui T, Ito T,
Kuno N, Ohno Y, Kadomatsu K, Muramatsu K, Nagasaka T and Mizutani
S: Tissue distribution of placental leucine
aminopeptidase/oxytocinase during mouse pregnancy. J Histochem
Cytochem. 52:113–121. 2004.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Matsumoto H and Mori T: Changes in cystine
aminopeptidase (oxytocinase) activity in mouse serum, placenta,
uterus and liver during pregnancy or after steroid hormone
treatments. Zoolog Sci. 15:111–115. 1998.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Grzywa R, Oleksyszyn J, Salvesen GS and
Drag M: Identification of very potent inhibitor of human
aminopeptidase N (CD13). Bioorg Med Chem Lett. 20:2497–2499.
2010.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Gard PR, Fidalgo S, Lotter I, Richardson
C, Farina N, Rusted J and Tabet N: Changes of renin-angiotensin
system-related aminopeptidases in early stage Alzheimer's disease.
Exp Gerontol. 89:1–7. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Gonzales T and Robert-Baudouy J: Bacterial
aminopeptidases: Properties and functions. FEMS Microbiol Rev.
18:319–344. 1996.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Neu J and Rushing J: Cesarean versus
vaginal delivery: Long-term infant outcomes and the hygiene
hypothesis. Clin Perinatol. 38:321–331. 2011.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Arboleya S, Suárez M, Fernández N,
Mantecón L, Solís G, Gueimonde M, de Los Reyes and Gavilán CG:
C-section and the neonatal gut microbiome acquisition: Consequences
for future health. Ann Nutr Metab. 73 (Suppl 3):17–23.
2018.PubMed/NCBI View Article : Google Scholar
|