Introduction
Both Selaginella tamariscina (Beauv.) and
Selaginella pulvinata (Hook.et Grev.) are recorded in the
Chinese Pharmacopoeia as Juanbai (1), a traditional Chinese herb. Selaginella,
described as a ‘whorlleaf stonecrop plant’, has the ability to
recuperate even after a long period of drought (2). According to traditional Chinese
medicine, Selaginella is mild-natured, pungent, distributes to the
liver and heart meridians, and improves blood circulation.
Selaginella has been shown to exhibit antioxidant (3,4),
antidiabetic (5,6), anti-tumor (7) and angiogenic properties (8). The primary active components in
selaginella are flavonoids, which comprise ~2.85% of the plant
matter (9).
The physiological basis of cognitive impairment
(CI), also referred to as cognitive deficit or cognitive
disability, include aging, shrinkage or degeneration of brain
tissue. These processes may result from abnormalities in the levels
and actions of neurotransmitters and receptors, aggregation and
modification of proteins in brain tissue, chronic cerebral ischemic
injuries and diseases that result in insufficient blood supply to
the brain (10). In addition,
external factors such as infection, poisoning and metabolic
dysfunction can lead to CI. CI is most common in individuals >60
years old. If not well controlled, CI will develop into senile
dementia.
Models of memory impairment are useful for
evaluating the effects of drugs on learning and memory processes.
In the present study, memory impairment was induced using
scopolamine, sodium nitrite or ethanol. Scopolamine is a
competitive muscarinic cholinergic receptor antagonist that impairs
memory acquisition (11). Sodium
nitrite increases methemoglobin content in the blood, resulting in
a lowered carrying capacity of oxygen, thus leading to brain tissue
hypoxia, which impairs memory consolidation (12). Ethanol inhibits cortical activity,
conditioned reflexes, RNA synthesis and protein synthesis, thereby
impairing memory retrieval (13).
Materials and methods
Experimental animals
A total of 200 Kunming male and female mice,
weighing 18-22 g, [animal certificate no. SCXK-(Ji) 2010-0005] were
purchased from the Experimental Animal Center of Jilin University
School of Basic Medicine. The present study was approved by the
Animal Care and Welfare Committee of Changchun University of
Chinese Medicine (Changchun, China) (approval no. 2012021). The
mice were housed, 5 per cage, under standard laboratory conditions
(temperature, 25±2̊C; humidity, 60±5%; 12 h dark/light cycle), with
free access to standard rodent chow and water.
Total flavonoids of Selaginella
pulvinata (TFSP) acute toxicity test
Mice were observed for 3 days prior to testing, and
those displaying abnormal appearance or abnormal behavior were
excluded from further analysis. In a preliminary test, 6 mice were
administered the maximum volume (40 ml/kg) of the maximum
concentration (10.5 g/100 ml) of TFSP once, and observed for 14
days; and no deaths occurred. Therefore, this maximal dose was used
in an acute toxicity test, in which TFSP was administered by gavage
at day 0, and observed for 14 days. Throughout the test, the hair,
behavior, autonomic activity, respiration, oral and nasal
secretions, diet, urine and time of death of each animal were
observed. Animal weights were measured every other day. At the end
of the experiment, mice were sacrificed by cervical dislocation,
and the internal organs were harvested for examination.
Preparation of TFSP suspension
TFSP was prepared as described previously (14). Briefly, the high-dose suspension (40
mg/ml) was prepared by mixing 2.8 g TFSP powder with 70 ml
distilled water. Medium (20 mg/ml) and low (10 mg/ml) doses were
then diluted from the high dose suspension by adding distilled
water accordingly. The prepared suspensions were stored at 4̊C.
Experimental model
Test animals were habituated to the test environment
for 3 days before being randomly divided into groups. For each
memory impairment model type, there were 5 groups (n=10 per group):
control, CI, high-dose TFSP, medium-dose TFSP and low-dose TFSP.
The control groups were administered distilled water by gavage and
injected intraperitoneally with normal saline. The CI groups were
administered distilled water by gavage and injected
intraperitoneally with either scopolamine hydrobromide (5 mg/kg) or
sodium nitrite (90 mg/kg) or 45% ethanol (10 ml/kg). The TFSP
groups were administered with the indicated doses of TFSP
suspension via gavage and injected intraperitoneally with either
scopolamine hydrobromide (5 mg/kg) or sodium nitrite (90 mg/kg) or
45% ethanol (10 ml/kg). Drugs were administered once a day for 4
weeks.
Step-down test
Mice were placed in the control box of the step-down
instrument for 1 min. Then, at the onset of a 5-min training
period, a 32 V electric current was applied to the floor. Mice
respond by jumping onto the platform to escape the electric shock.
Subsequently, when a mouse descended back down such that both of
its feet touched the floor at the same time, it received a shock.
Each time a mouse descended down to the electrified floor, it was
considered an error. The following day, testing was conducted; each
mouse was placed on the platform, and the latency of the first jump
as well as number of jumps within a 5-min testing period were
recorded.
Tissue collection and preparation of
tissue sections
After the behavioral test on the last day,
retroorbital blood was collected and then the mice were
decapitated. Hippocampi were removed immediately and fixed in 10%
formaldehyde at room temperature for 1 week. The fixed tissues were
rinsed repeatedly with water and stored in 10% EDTA until
decalcification was complete [the decalcifying solution was
monitored and ammonium oxalate (5% g/v) was added until it did not
turn turbid]. Tissue blocks were dehydrated in an ascending series
of ethanol (80, 90, 95 and 100%) washed in xylene and embedded in
paraffin before being sectioned transversely (4-5 µm thickness).
The sections were then dewaxed in xylene, rehydrated in ethanol
(100, 95, 90 and 80%), stained with hematoxylin-eosin and toluidine
blue at room temperature for 5 min, and observed under a light
microscope at a magnification of x40.
Measurement of biochemical
indicators
The mice brains were separated and frozen at -20̊C.
Brains were unfrozen, 100 mg of each brain was weighed and
homogenized with pre-cooled normal saline, then centrifuged at
1,000 x g for 5 min at 4̊C. The levels of memory-related molecules
in the supernatant were assessed using specific kits according to
the manufacturer's protocol [total superoxide dismutase assay kit,
malondialdehyde (MDA) assay kit (cat. no. 20120916), nitric oxide
(NO) assay kit (cat. no. 20120803), total nitric oxide synthase
(NOS) assay kit (cat. no. 20121019), acetylcholine assay kit (cat.
no. 20121029), acetylcholinesterase assay kit (cat. no. 20120822),
choline acetyltransferase assay kit (cat. no. 20120902), reduced
glutathione (GSH) assay kit (cat. no. 20120910) and glutathione
peroxidase (GSHPx) assay kit (cat. no. 20121025)]. Kits were
purchased from Nanjing Jiancheng Institute of Biology) (Nanjing,
China).
Statistical analysis
All results are presented as mean ± standard
deviation. Data were compared across the three groups using a
one-way ANOVA with a post-hoc Tukey-Kramer test. P<0.05 was
considered to indicate a statistically significant difference.
Results
TFSP acute toxicity test
Of the 20 test mice (10 each male and female)
administered the maximum volume (40 ml/kg) of TFSP suspension (10.5
g/100 ml) by gavage daily for 14 days, none died or exhibited
abnormal reactions, and no abnormalities were observed in their
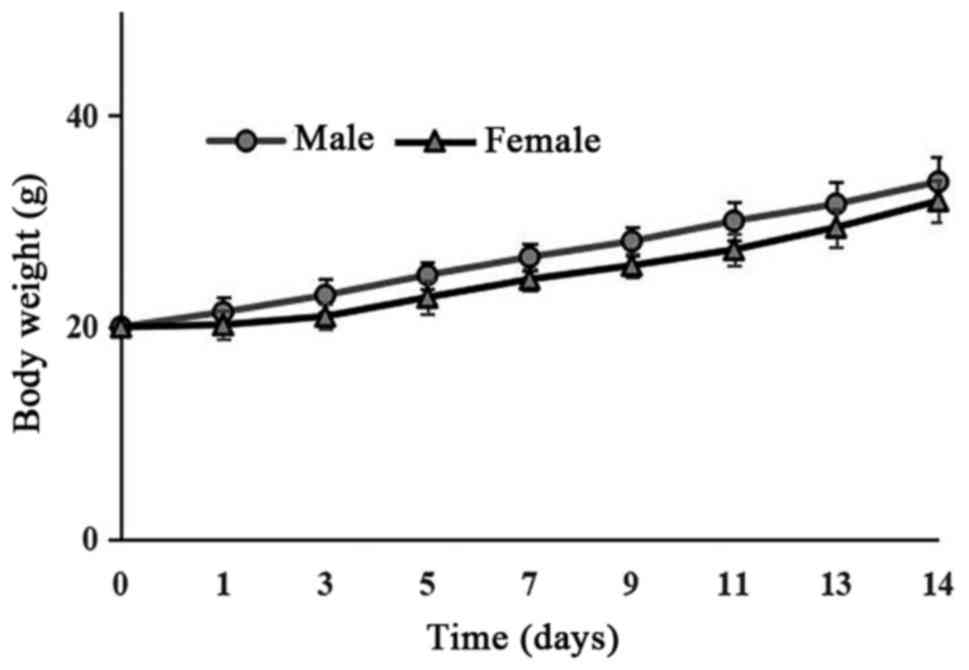
dissected organs (data not shown). The body weights of the mice
increased gradually (Fig. 1). Thus,
the experimental doses of TFSP used were 1/5 (800 mg/kg), 1/10 (400
mg/kg) and 1/20 (200 mg/kg) of the maximum dose, all of which were
considered to be safe.
Behavioral effects of TFSP on
scopolamine model mice
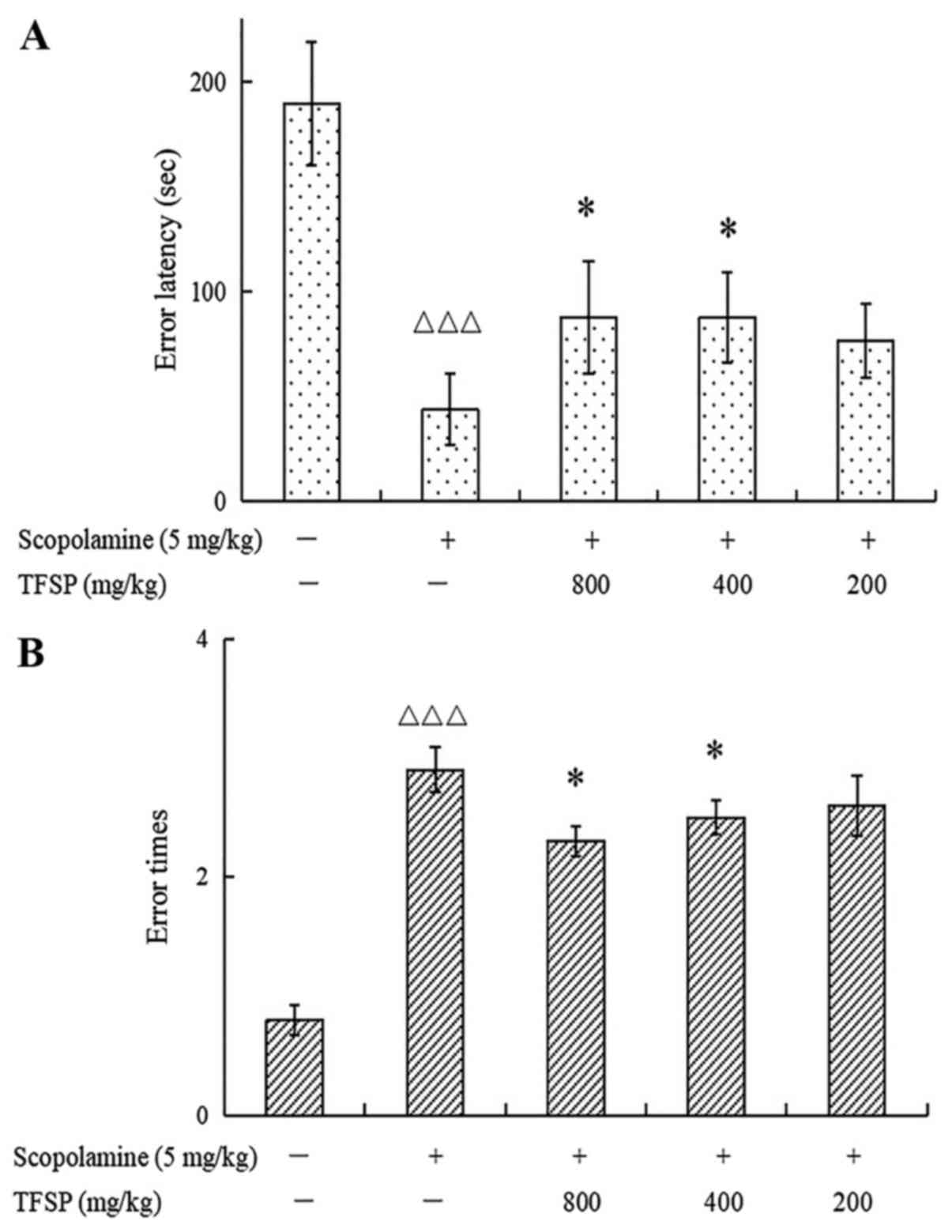
The scopolamine group displayed shorter mean latency
to error and a greater mean number of errors compared with the
control group (both P<0.001). Mice treated with high and medium
doses of TFSP had significantly longer error latencies and fewer
errors compared with those treated with scopolamine alone
(P<0.05; Fig. 2).
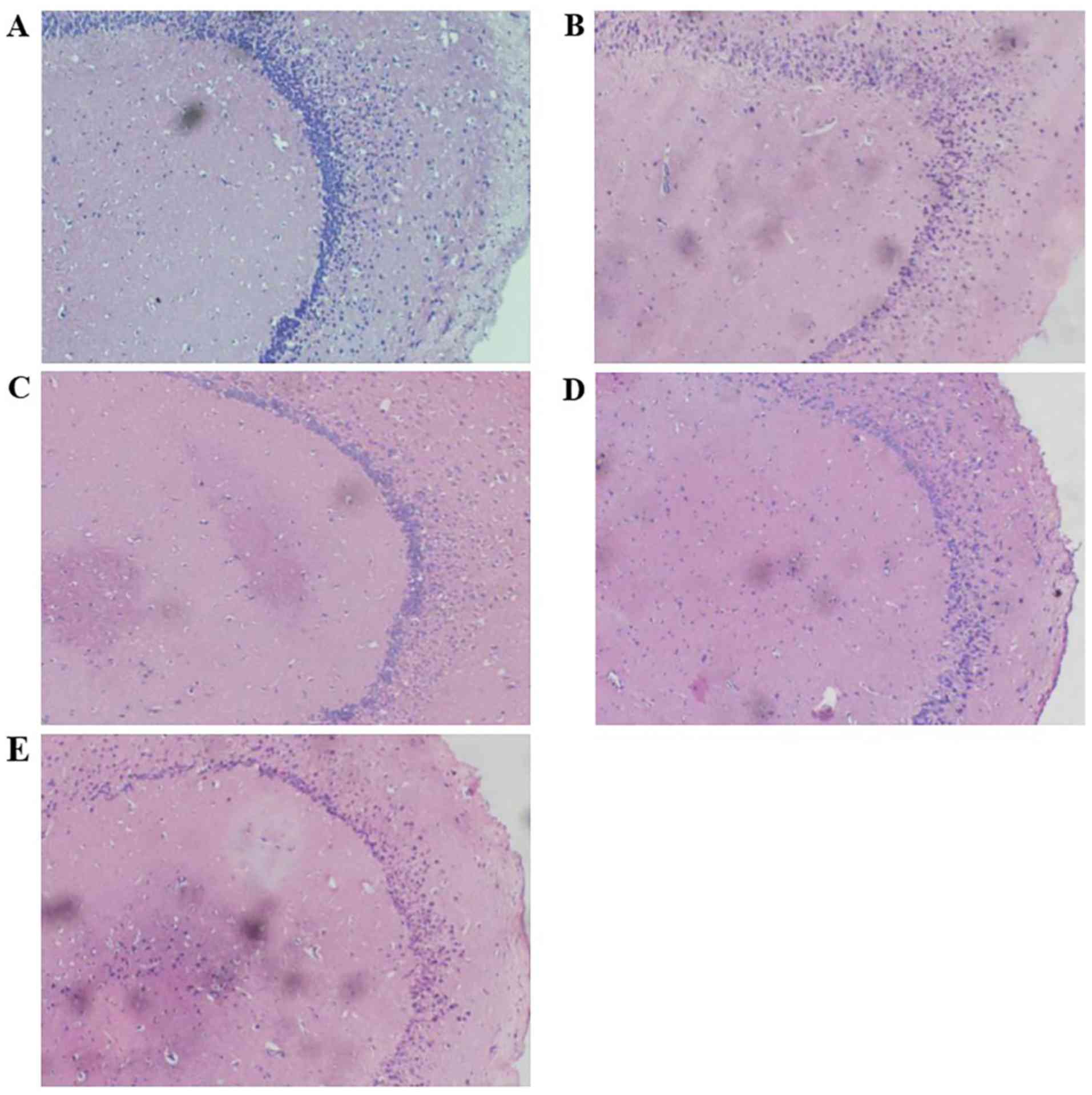
Pathological analysis showed that scopolamine reduced hippocampal
neuron counts significantly, and the cells observed were sparsely
arranged and disordered (Fig. 3B).
These effects were partially reversed in mice brains treated with
TFSP (400 and 800 mg/kg; Fig.
3C-E).
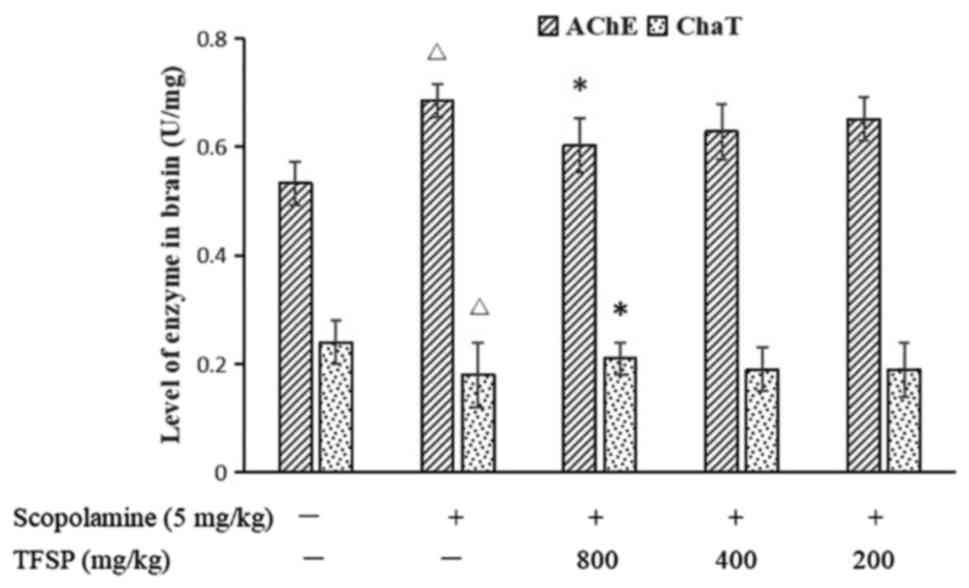
Effects of TFSP on cholinergic
molecules in brain tissue
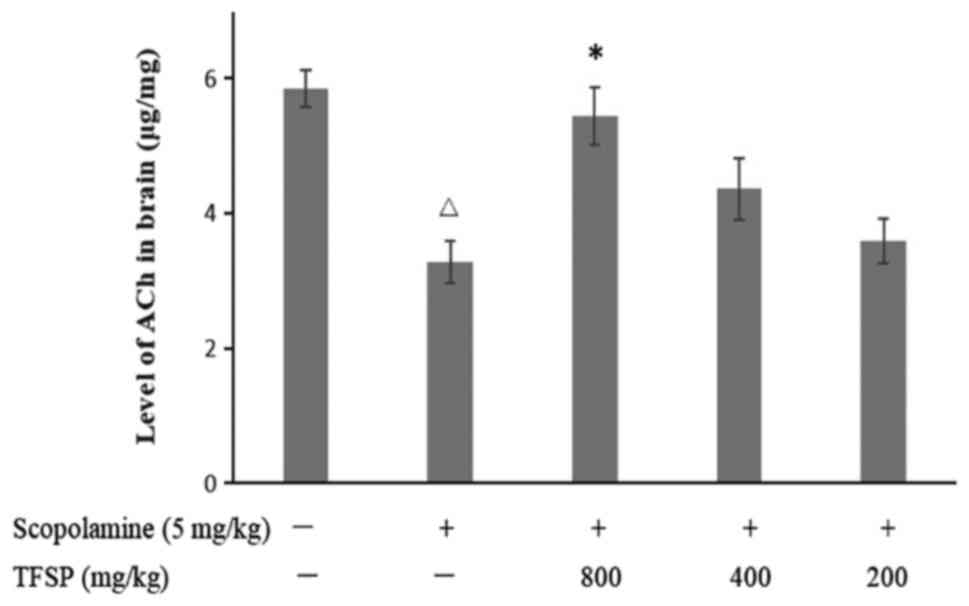
Mice treated with scopolamine had lower levels of
acetylcholine in the brain compared with the untreated controls. A
high-dose of TFSP increased the levels of acetylcholine in the
brain (P<0.05; Fig. 4), reduced
the activity of the acetylcholinesterase and increased the activity
of the acetylcholine-biosynthesizing enzyme choline
acetyltransferase (both P<0.05, Fig.
5). Medium and low doses of TFSP did not exert any significant
effects.
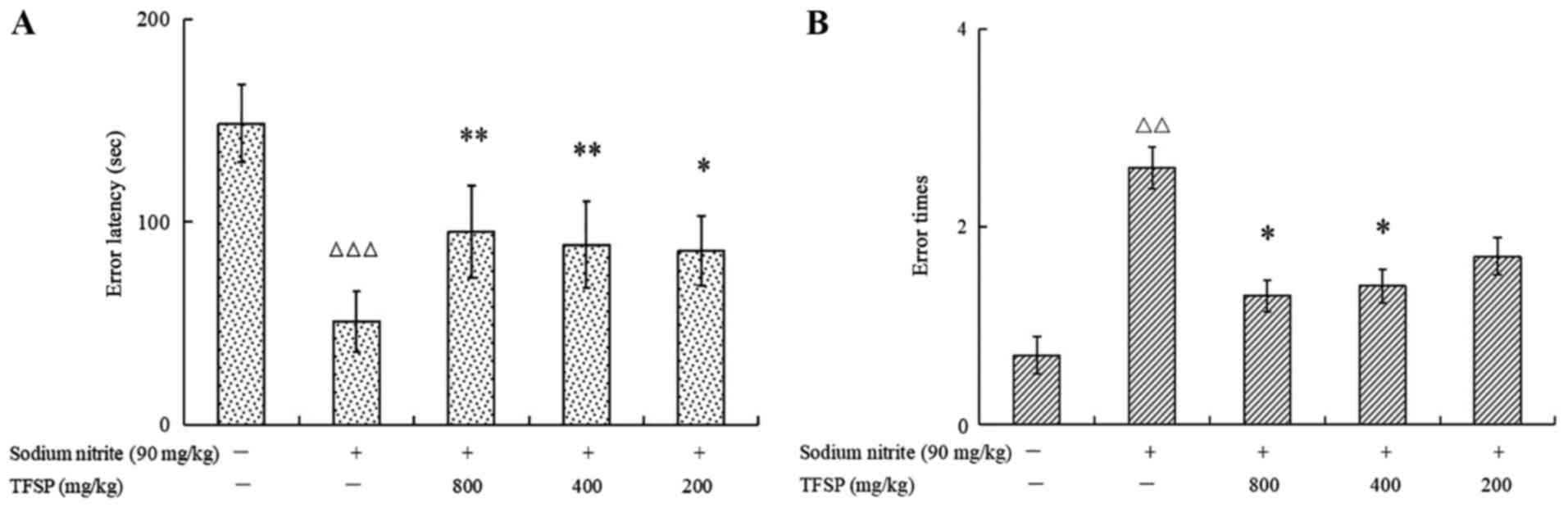
Behavioral effects of TFSP on mice
treated with sodium nitrite
Mice treated with sodium nitrite displayed a
significantly shorter mean error latency and a greater mean number
of errors compared with the untreated controls (P<0.01; Fig. 6). All TFSP doses prolonged error
latency significantly, and the high and medium doses of TFSP
reduced the number of errors significantly compared with the model
group (P<0.05).
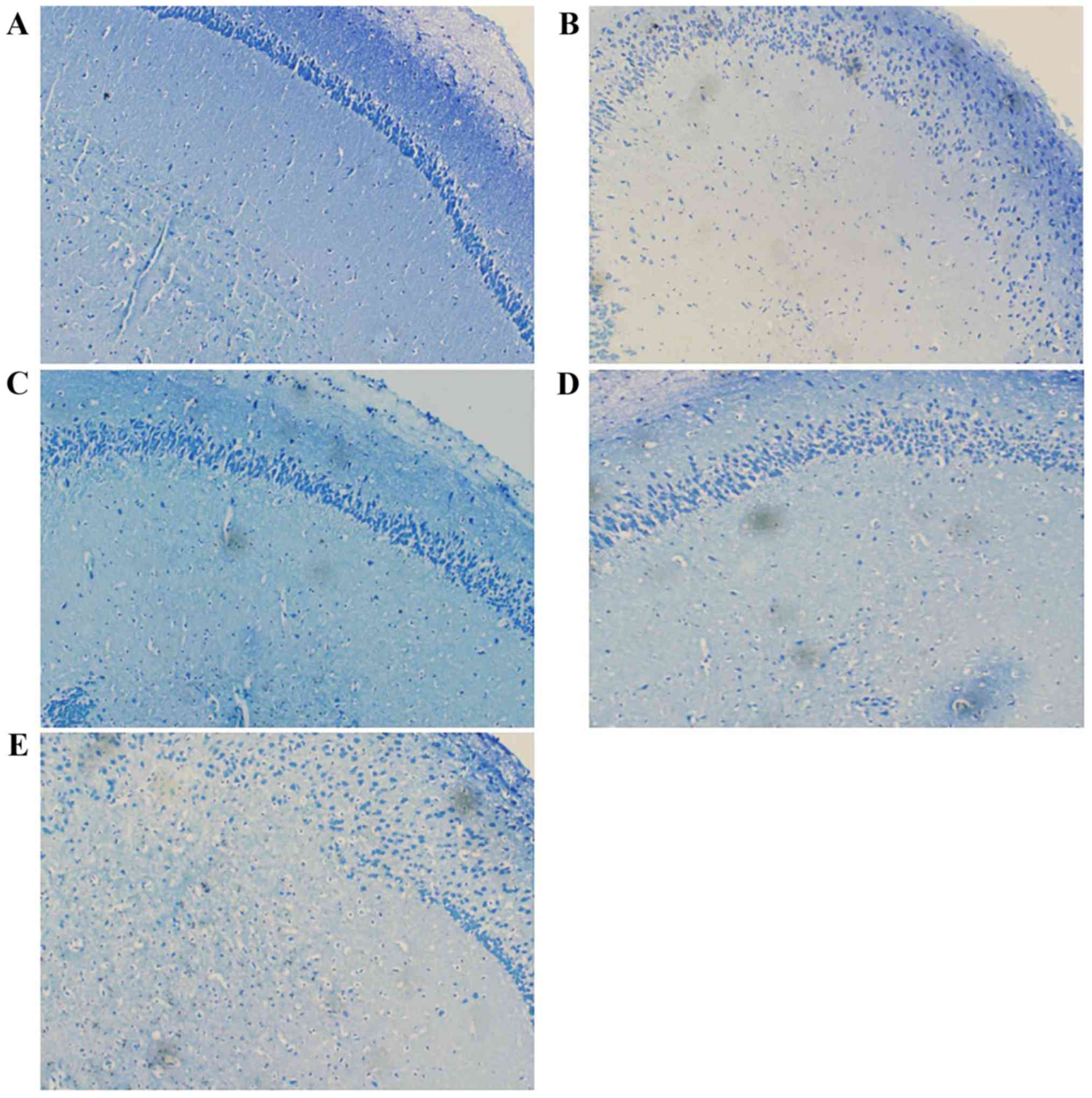
Pathological images of toluidine blue staining
showed that mice injected with sodium nitrite had fewer hippocampal
neurons, and the cells were sparsely arranged and disordered.
Treatment with TFSP appeared to ameliorate these effects (Fig. 7).
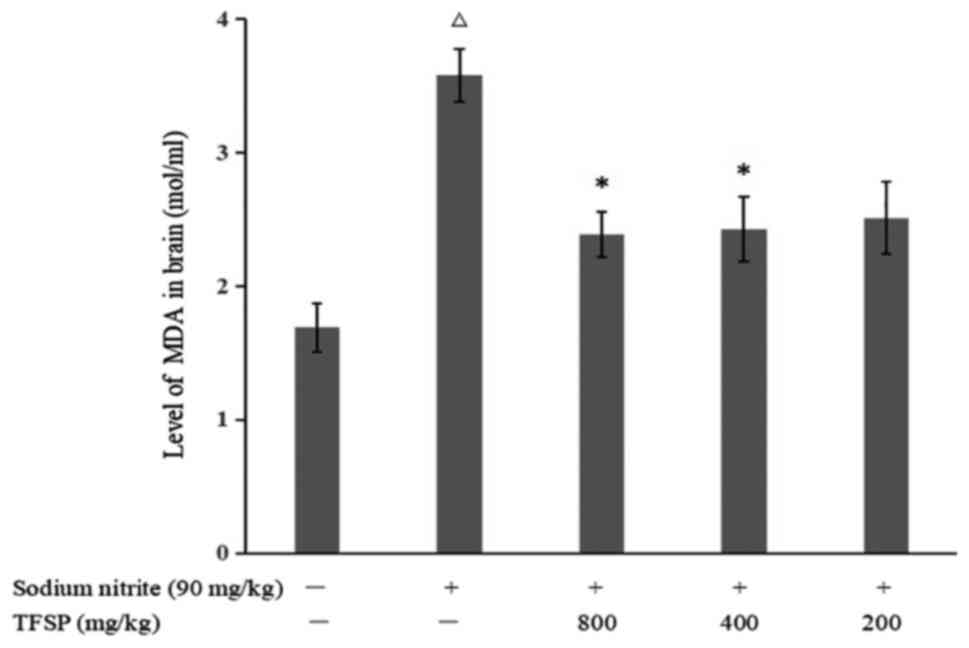
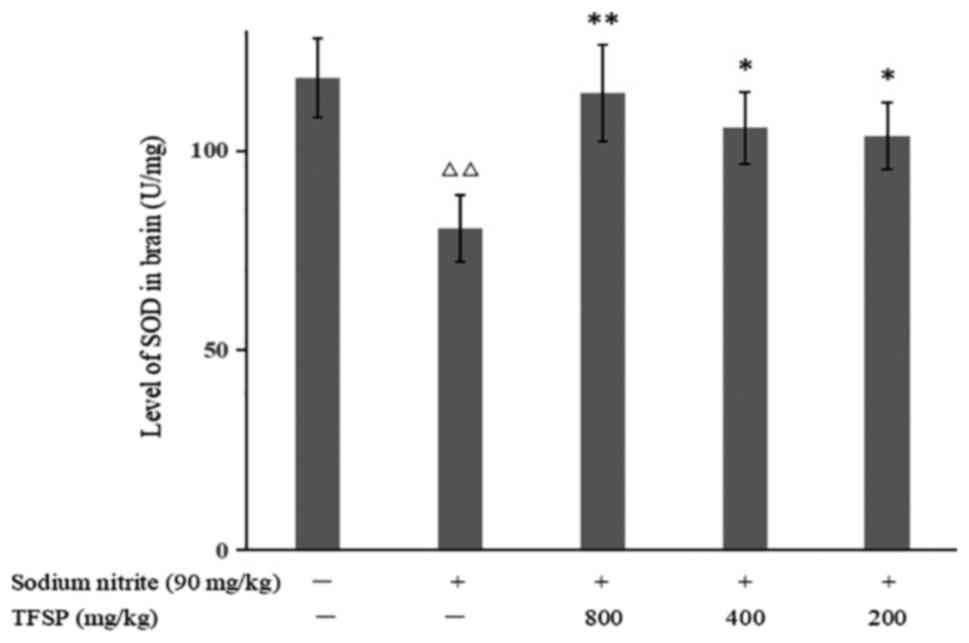
Effects of TFSP on MDA content and SOD
activity in brain tissue of mice treated with sodium nitrite
Compared with the control group, the MDA content in
the brains of CI mice model was increased (P<0.05), and SOD
activity was decreased (P<0.01), suggesting that sodium nitrite
induced oxidative stress. TFSP significantly reduced MDA content
(P<0.05, Fig. 8) and increased
SOD activity (800 mg/kg, P<0.01; 400 and 200 mg/kg, P<0.05
Fig. 9).
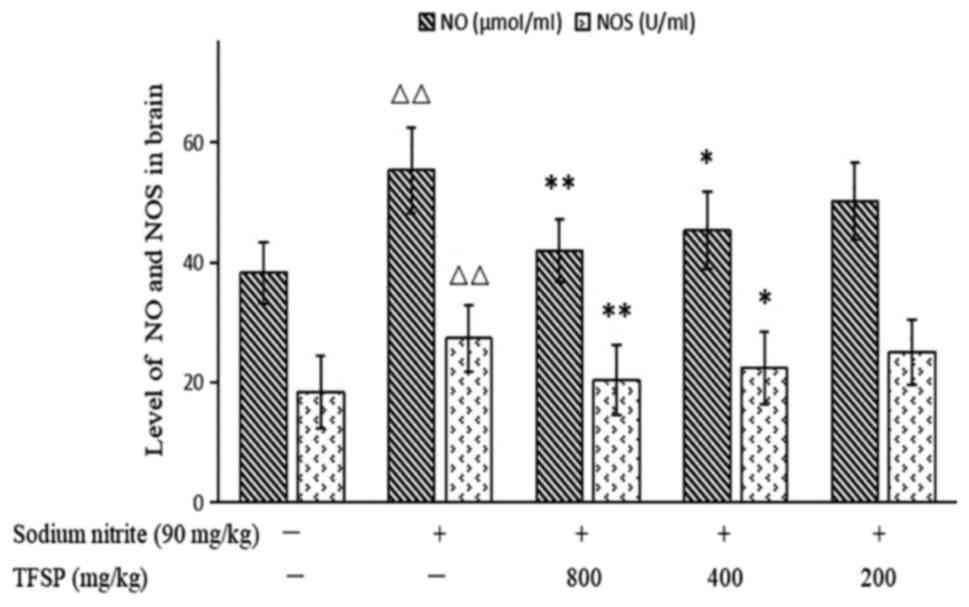
Effects of TFSP on NO content and NOS
activity in brain tissues of mice treated with sodium nitrite
Levels of NO and activity of NOS were higher in the
brains of the model mice compared with the untreated controls
(P<0.01). High and medium doses of TFSP significantly reduced NO
content (P<0.05) and NOS activity (P<0.01; Fig. 10).
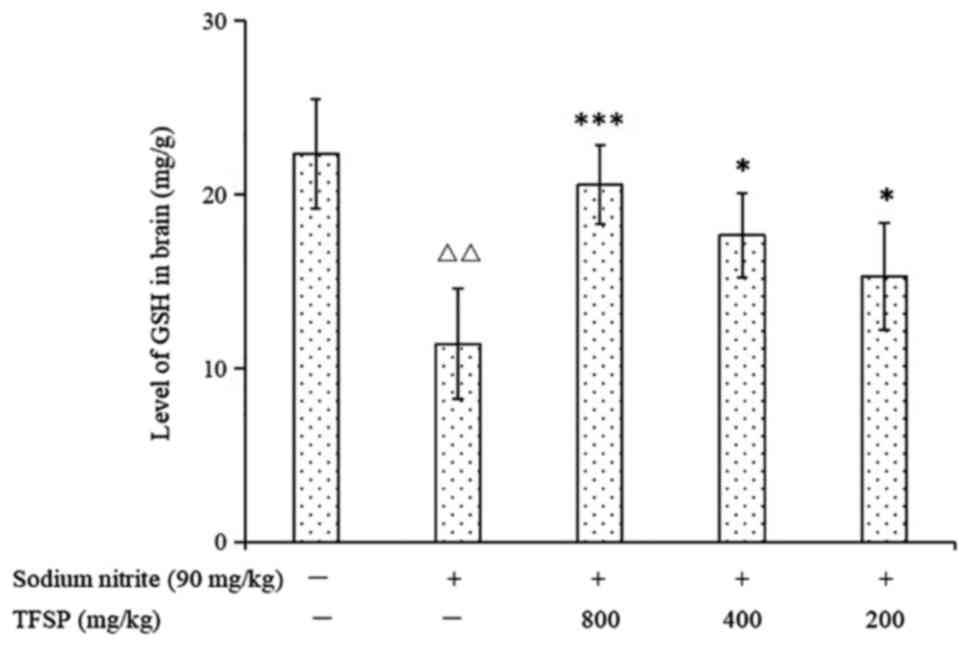
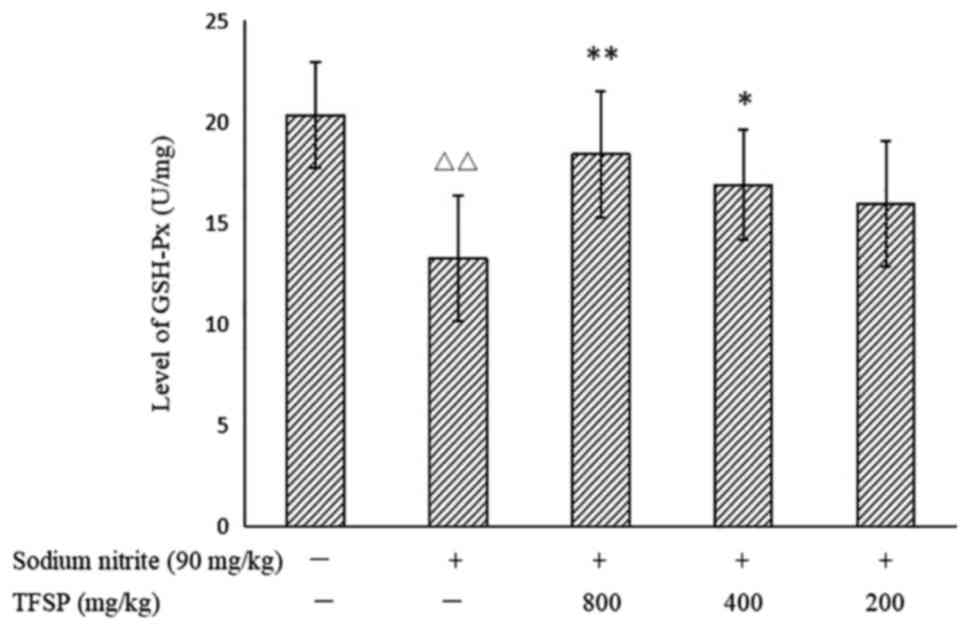
Effects of TFSP on GSH content and
GSH-Px activity in brain tissues of mice treated with sodium
nitrite
Levels of GSH and GSH-Px activity were significantly
lower in the brains of the model mice compared with the untreated
controls (P<0.01), suggesting that the brain tissues of the
model group were in a state of oxidative damage. Treatment with
medium and high doses of TFSP increased GSH content (Fig. 11) and GSH-Px activity significantly
(Fig. 12).
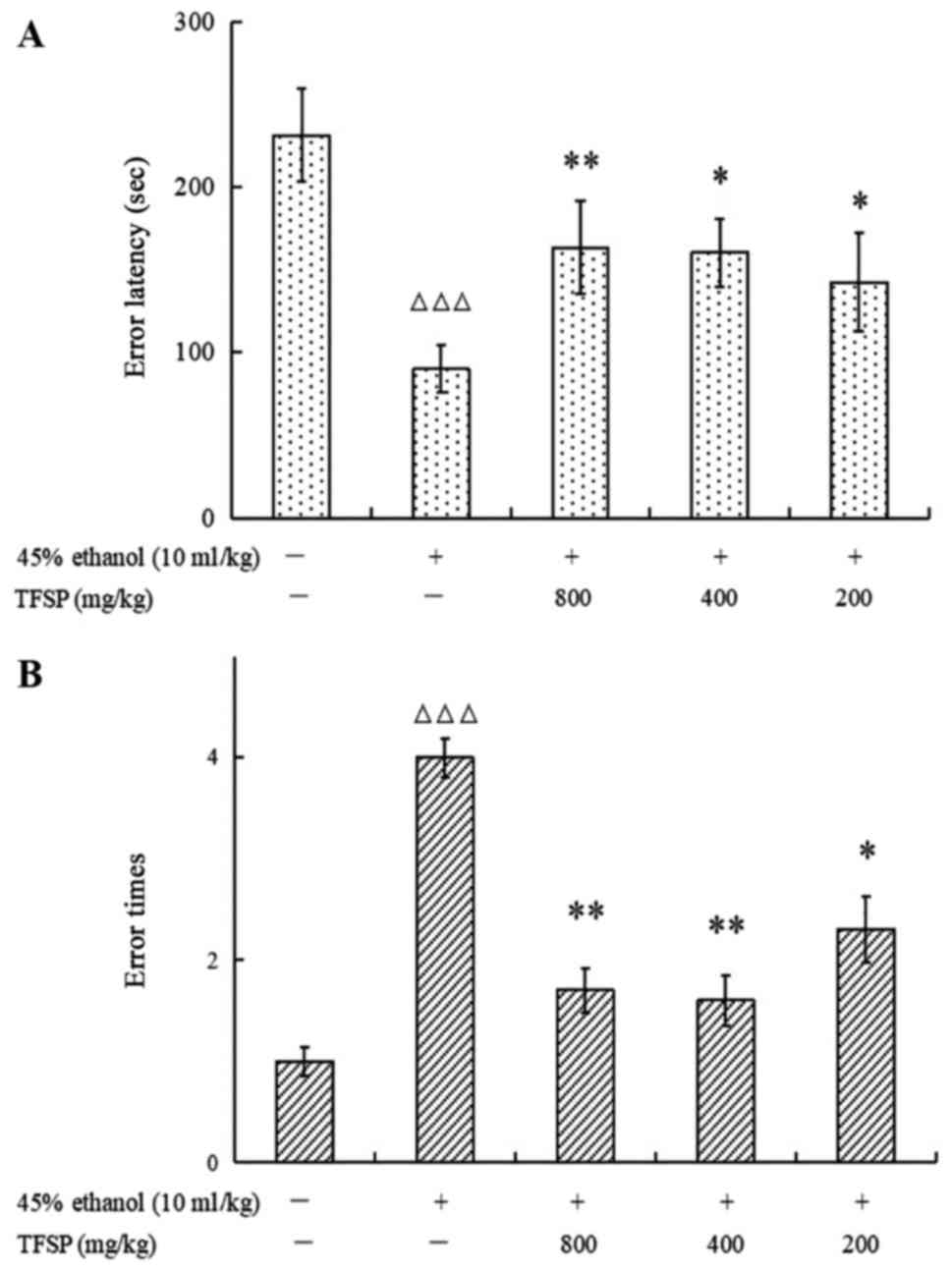
TFSP improves impairments in memory
retrieval induced by ethanol
Both the latency to error and the number of errors
were significantly worse in ethanol-treated mice compared with the
controls (P<0.001). Compared with the model group, all three
TFSP doses significantly prolonged error latency (P<0.01) and
reduced the number of errors (P<0.05; Fig. 13).
Discussion
TFSP contributes to the improvement of behavior in
mice with memory impairments induced by scopolamine, sodium nitrite
or 45% ethanol. TFSP treatment increased error latencies and
reduced the number of errors in the step-down behavioral test.
Furthermore, TFSP appeared to protect against hippocampal damage
induced by these treatments as it associated with a reduced loss
and disordering of hippocampal cells. These results suggest that
TFSP may ameliorate CI at a cellular level.
The content of acetylcholine in brain tissue is
closely associated with cognitive function, and is dependent on the
relative activities of acetylcholinesterase and choline
acetyltransferase. Activated acetylcholinesterase breaks down
acetylcholine, whereas activated choline acetyltransferase promotes
synthesis of acetylcholine (15).
Scopolamine mimics the memory dysfunction caused by insufficient
acetylcholine by blocking muscarinic receptors (16,17). The
results of the present study showed that TFSP may exhibit
therapeutic effects on scopolamine-induced memory dysfunction via
regulation of these key cholinergic enzymes.
Sodium nitrite leads to CI by inducing hypoxia
(18), and also contributes to brain
damage by disrupting redox reactions and producing large quantities
of peroxides and free radicals (such as MDA and NO) (19). The resulting hypoxic environment
decreases the activity of antioxidant enzymes, such as SOD and
GSH-Px, resulting in an accumulation of peroxidation products and
free radicals, thereby further aggravating tissue damage. The
results of the present study demonstrated that TFSP promotes
favorable regulation of peroxides, free radicals and antioxidant
enzymes, supporting the protection and repair of brain tissue, and
thus cognition.
NO acts as reactive oxygen, and produces opposing
effects on acetylcholine release in neuronal Aplysia synapses,
dependent on the excitatory or the inhibitory nature of the synapse
(20). In the present study, TFSP
reduced NO and acetylcholinesterase levels in the serum and
alleviated cognitive dysfunction in a similar manner to benazepril
hydrochloride does (21).
Estrogen serves an important role in cognition
(22). Flavonoids in Selaginella
pulvinata have estrogenic (23,24) and
neuroprotective effects (25), that
may have contributed to the relief of CI observed in the present
study. It may also improve the cholinergic system through its
estrogen-like effects, although this requires further study to
confirm.
In conclusion, TFSP showed no significant acute
toxicity in mice, and exerted a protective effect in several mouse
models of CI. TFSP was associated with improvements in both
cytological and behavioral measures. These results suggest that
TFSP may be a promising treatment for CI. Further investigations on
TFSP should focus on the active component(s), and additional mouse
models of cognition, such as a maze test should be used to evaluate
efficacy, before assessing its suitability in humans.
Acknowledgements
We would like to thank Mr Quancheng Zhao (Jilin
Academy of traditional Chinese Medicine), Mrs Xiyan Ou, Mr Xiaobing
Li and Mr Yunlu Ding (Chunchun University of Chinese Medicine), Mr
Xuefeng Lv (Songyuan Food and Drug Administration) and Mrs Lianping
Wang (Xiuzheng Pharmaceutical Group Co., Ltd.) for their
experimental help.
Funding
This study was funded by Sanya Institute and Local
Science and Technology Project (grant no. 2019YD11) and Hainan
Tropical Ocean University Scientific Research Starting Foundation
for PhD (grant no. RHDXB201708).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
LZ conceived the experiments, analyzed the results,
and wrote and edited the manuscript. YZ and YH wrote the
manuscript, performed the experiments and analyzed the results. HL,
CL, JX, NZ, and QL performed the experiments and analyzed the
results. YS conceived the experiments and wrote the manuscript. ZZ
designed the study, analyzed data and edited the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Animal Care
and Welfare Committee of Changchun University of Chinese Medicine
(Changchun, China) (approval no. 2012021).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chinese Pharmacopoeia Commission. Chinese
Pharmacopoeia, 2015.
http://db.ouryao.com/yd2015/view.php?v=txt&id=353.
|
|
2
|
Nanjing University of Chinese Medicine.
Great Dictionary of Chinese Medicine, 2006.
|
|
3
|
Zheng XK, Wang WW, Zhang L, Su CF, Wu YY,
Ke YY, Hou QW, Liu ZY, Gao AS and Feng WS: Antihyperlipidaemic and
antioxidant effect of the total flavonoids in Selaginella
tamariscina (Beauv.) Spring in diabetic mice. J Pharm
Pharmacol. 65:757–766. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zhang Z, Sun T, Niu JG, He ZQ, Liu Y and
Wang F: Amentoflavone protects hippocampal neurons:
Anti-inflammatory, antioxidative, and antiapoptotic effects. Neural
Regen Res. 10:1125–1133. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Su C, Yang C, Gong M, Ke Y, Yuan P, Wang
X, Li M, Zheng X and Feng W: Antidiabetic activity and potential
mechanism of amentoflavone in diabetic Mice. Molecules.
24(E2184)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zheng X, Ke Y, Feng A, Yuan P, Zhou Y, Yu
Y, Wang X and Feng W: The Mechanism by which amentoflavone improves
insulin resistance in HepG2 cells. Molecules. 21(pii:
E624)2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yang JS, Lin CW, Hsieh YS, Cheng HL, Lue
KH, Yang SF and Lu KH: Selaginella tamariscina (Beauv.)
possesses antimetastatic effects on human osteosarcoma cells by
decreasing MMP-2 and MMP-9 secretions via p38 and Akt signaling
pathways. Food Chem Toxicol. 59:801–807. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zheng XK, Ning TL, Wang XL, Liu CX, Liu YY
and Feng WS: Effects of total flavonoids and amntoflavone isolated
from Selaginella tamariscina on human umbilical vein
endothelial cells proliferation and VEGF expression. J Chin Pharm
Sci. 46:998–1002. 2011.
|
|
9
|
Yan Y, Zhu HY and Ma L: Content
determination of flavones in five genus of Selaginella from
Guizhou province, China. J Yunnan Univ (Natural Science Edition).
32:227–231. 2010.
|
|
10
|
Experts group of prophylaxis and treatment
cognitive impairment in China. Expert consensus on prevention and
treatment of cognitive impairment in China. Chinese Journal of
Internal Medicine 45: 171-173, 2006.
|
|
11
|
Klinkenberg I and Blokland A: The validity
of scopolamine as a pharmacological model for cognitive impairment:
A review of animal behavioral studies. Neurosci Biobehav Rev.
8:1307–1350. 2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Vohra BP and Hui X: Improvement of
impaired memory in mice by taurine. Neural Plast. 4:245–259.
2000.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Haab Lutte A, Huppes Majolo J, Reali
Nazario L and DaSilva RS: Early exposure to ethanol is able to
affect the memory of adult zebrafish: Possible role of adenosine.
Neurotoxicology. 69:17–22. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Li QJ, Wang LS and Wang LP: Study on
Extraction method of total flavonoids from Selaginella
tamariscina (Beauv.) Spring. J Changchun Univ Trad Chin Med.
28:355–356. 2012.
|
|
15
|
Park D, Yang YH, Bae DK, Lee SH, Yang G,
Kyung J, Kim D, Choi EK, Lee SW, Kim GH, et al: Improvement of
cognitive function and physical activity of aging mice by human
neural stem cells over-expressing choline acetyltransferase.
Neurobiol Aging. 34:2639–2646. 2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Li B, Guo DY and Li L: Behavioral
Comparison among three animal models mimicking Alzheimer's disease.
Chi J Exp Anim. 7:40–45. 1999.
|
|
17
|
Xu JM and Yu HY: Impact of different
dosage scopolamine on rat learning and mnemonic ability. J Suzhou
Univ (Health Sciences). 26:53–54. 2006.
|
|
18
|
Zhai ZL, Yan Y and Shang YZ: Improving
effects of brevisvapine on chemicals-induced memory impairment in
mice. Chin J Biochem Pharm. 33:61–63. 2012.
|
|
19
|
Liu XX, Zhao YY, Chen Chun S and Bai J:
Effects of extract of Alpinia officinarum Hance on learning
memory consolidation and free radical in mice. Chin Traditional Pat
Med. 32:1105–1108. 2010.
|
|
20
|
Mothet JP, Fossier P, Tauc L and Baux G:
Opposite actions of nitric oxide on cholinergic synapses: Which
pathways? Proc Natl Acad Sci USA. 93:8721–8726. 1996.https://pubmed.ncbi.nlm.nih.gov/?term=Opposite+actions+of+nitric+oxide+on+cholinergic+synapses%3A+Which+pathways%3F.
PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhao Z, Zhao X and Xu J: The effects of
benazepril hydrochloride on nitirc oxide, acetylcholine in serum
and cerebrospinal fluid of vascular dementia. Shanxi Med J.
4:457–460. 2019.https://xueshu.baidu.com/usercenter/paper/show?paperid=1p6k0jj0v46x0vx0ad6b06d06q344386&site=xueshu_se
or http://d.wanfangdata.com.cn/periodical/sxyyzz201904021.
|
|
22
|
Krolick KN, Zhu Q and Shi H: Effects of
estrogens on central nervous system neurotransmission: Implications
for sex differences in mental disorders. Prog Mol Biol Transl Sci.
160:105–171. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zheng XK, Zhang X, Wang XL, Zhang N, Yuan
PP and Feng WS: Effect of estrogen-like effective part of
Selaginella tarmariscina on bone metabolism in
ovariectomized rats. Zhong Yao Cai. 10:1826–1829. 2014.(In
Chinese). PubMed/NCBI
|
|
24
|
Zheng XK, Jiang Y, Pei SJ, Wang XL, Zhang
N, Ke YY, Zhai YY and Feng WS: Experimental study on estrogenic
activity of ten kinds of Selaginella tamariscina from ten
different place of origin. Xian Dai Zhong Yi Yao. 1:238–242.
2015.
|
|
25
|
Choi RC, Zhu JT, Yung AW, Lee PS, Xu SL,
Guo AJ, Zhu KY, Dong TT and Tsim KW: Synergistic action of
flavonoids, baicalein, and daidzein in estrogenic and
neuroprotective effects: A development of potential health products
and therapeutic drugs against Alzheimer's disease. Evid Based
Complement Alternat Med. 2013(635694)2013.PubMed/NCBI View Article : Google Scholar
|