Introduction
Multiple myeloma is a biologically diverse,
malignant disease involving the plasma cells. The condition is
characterized by the unrestrained proliferation of monoclonal
plasma cells within the bone marrow (1,2).
Plasma cells develop from B cells which arise from
the white blood cells as part of the immune system, and function in
the production and regulation of antibodies (2). Aberrations in the development of plasma
cells results in malignant diseases, which in turn results in
overproduction of non-functional immunoglobulin (Ig) chains or
intact Igs (3). The monoclonal IgM
protein that is found in the serum or urine of patients with
multiple myeloma is due to the uncontrolled proliferation of
myeloma cells (3). Specific heavy
and light chain Ig classes can be determined by electrophoresis,
and immune-electrophoresis or immunofixation techniques (3).
The criteria for diagnosis of myeloma include the
following: i) Urine or serum IgM-protein levels ≥30 g/l; ii)
myelogram showing a population of plasma cells ≥10%: iii)
Monoclonal plasma cells present in bone marrow biopsies; and iv)
evidence of end-stage organ damage, such as hypercalcaemia, renal
dysfunction, anaemia, osteolytic bony lesions or extramedullary
manifestations of myeloma (3-5).
The majority of patients (>90%) with multiple myeloma present
with IgM-proteins in the serum or urine at the initial presentation
(6,7).
At the time of diagnosis extramedullary
manifestations of myeloma are uncommon. There are different
variants of myeloma, namely solitary bone plasmacytoma which is
characterized by localized disease, and extramedullary plasmacytoma
which involves soft tissue outside the skeletal system (6,7).
It is important to differentiate myeloma from other
pathologies such as plasmablastic lymphoma, plasma cell leukaemia
and monoclonal gammopathies. Human immunodeficiency virus (HIV)
positive patients suffer from immune dysregulation, and as a
result, may have an increased risk of malignancies, such as myeloma
and lymphoma. A study published in Brazil found that there were
greater numbers of neoplasias (25.1 vs. 15.9%, respectively) and
lymphoid neoplasias (6.2 vs. 2.4%, respectively) in HIV positive
patients following anti-retroviral therapy (ART) than before the
patients were prescribed ARTs (8).
In the present case report, an HIV positive patient
with an initial manifestation of extramedullary myeloma involving
the parotid gland is described. The patient had been on ARTs for 2
years. Additionally, Medline, Embase, Scopus and Google Scholar
were searched using the key words: ‘Parotid extramedullary myeloma
AND HIV’ as part of the literature review.
Case report
The present study was performed in accordance with
the University of the Witwatersrand Human Research Ethics protocol.
Written consent was obtained for the publication of this case
report and any accompanying images.
A 46-year-old female presented Charlotte Maxeke
Johannesburg Academic Hospital with a 6-month history of a painful
mass on the left side of the face, involving the parotid area, with
rapid growth in the preceding month. There was associated fever,
body pains and difficulty opening the mouth. This resulted in
difficulty in masticating and she also reported pain on swallowing.
She did not notice any weakness of her face on the left side, or
complain of any visual problems, such as visual disturbance, eye
pain or an inability to close the eye on the affected side. The
patient was diagnosed with HIV infection 2 years prior and had been
on highly active antiretroviral therapy since then. The therapeutic
regimen for HIV consisted of a single fixed dose combination tablet
of Tenofovir (300 mg), Emtricitabine (200 mg) and Efavirenz (600
mg) daily. There was no history of previous head and neck
malignancy or tuberculosis infection.
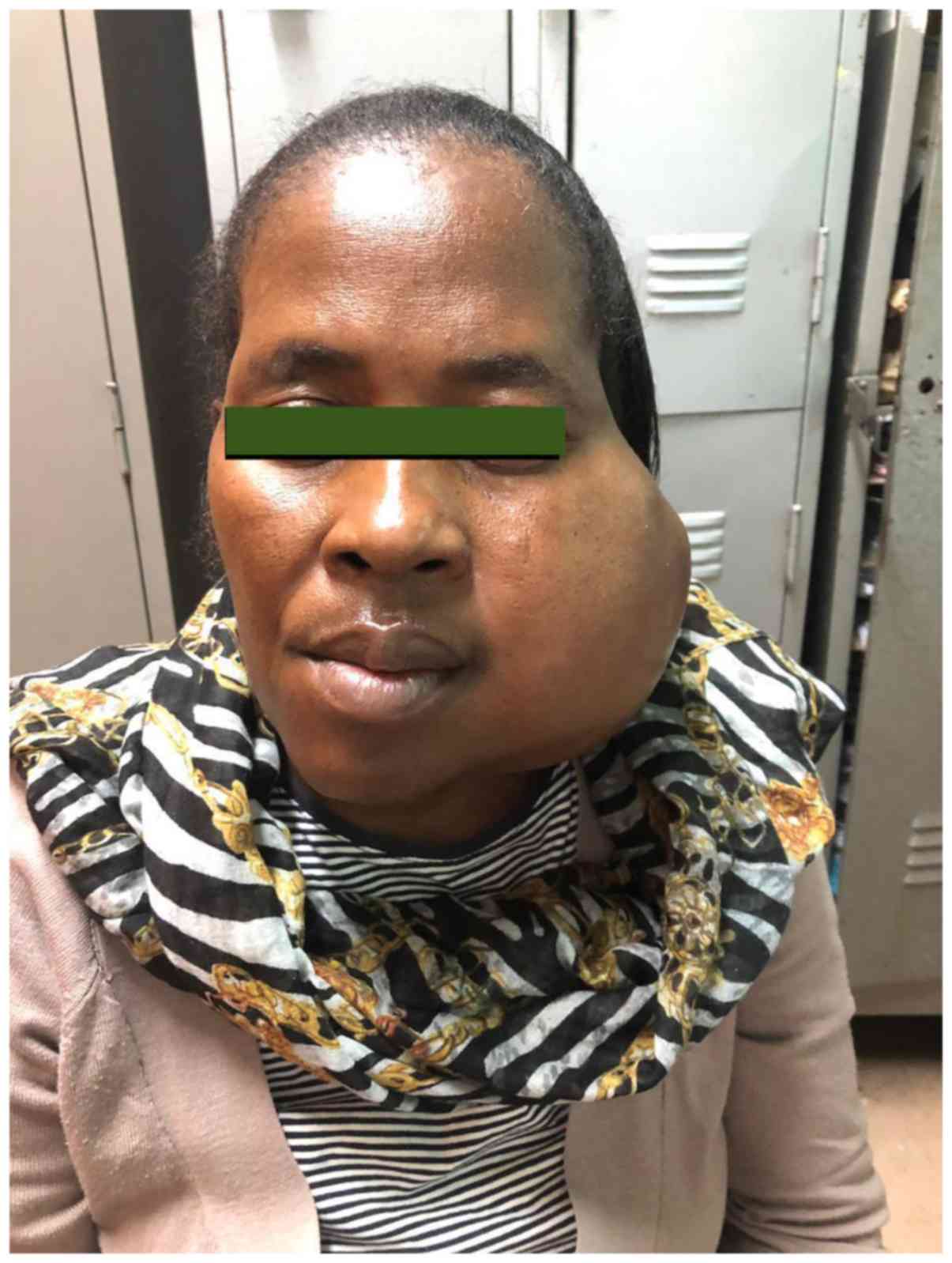
Clinical examination revealed a large, ~8x10 cm left
parotid mass, extending to the left side of the neck. The mass was
firm, fixed to underlying structures and tender on palpation. The
overlying skin was intact. The sizeable mass resulted in loss of
the nasolabial fold on the affected side; examination of the nose
showed no presence of a tumour within the nasal cavity and central
position of the nasal septum. There was associated trismus with
visible intraoral extension of the tumour, as the mucosal-covered
soft tissue mass appeared to be growing from the maxillary area on
the left side. There was medialisation of the left tonsil. However,
there were no signs of acute airway compromise. The facial nerve
was intact and there were no features of orbital extension of the
mass such as proptosis or loss of visual acuity. The rest of the
cranial nerve examination was essentially normal. There were no
neck nodes palpable (Fig. 1).
Plain X-ray revealed multiple osteolytic lesions
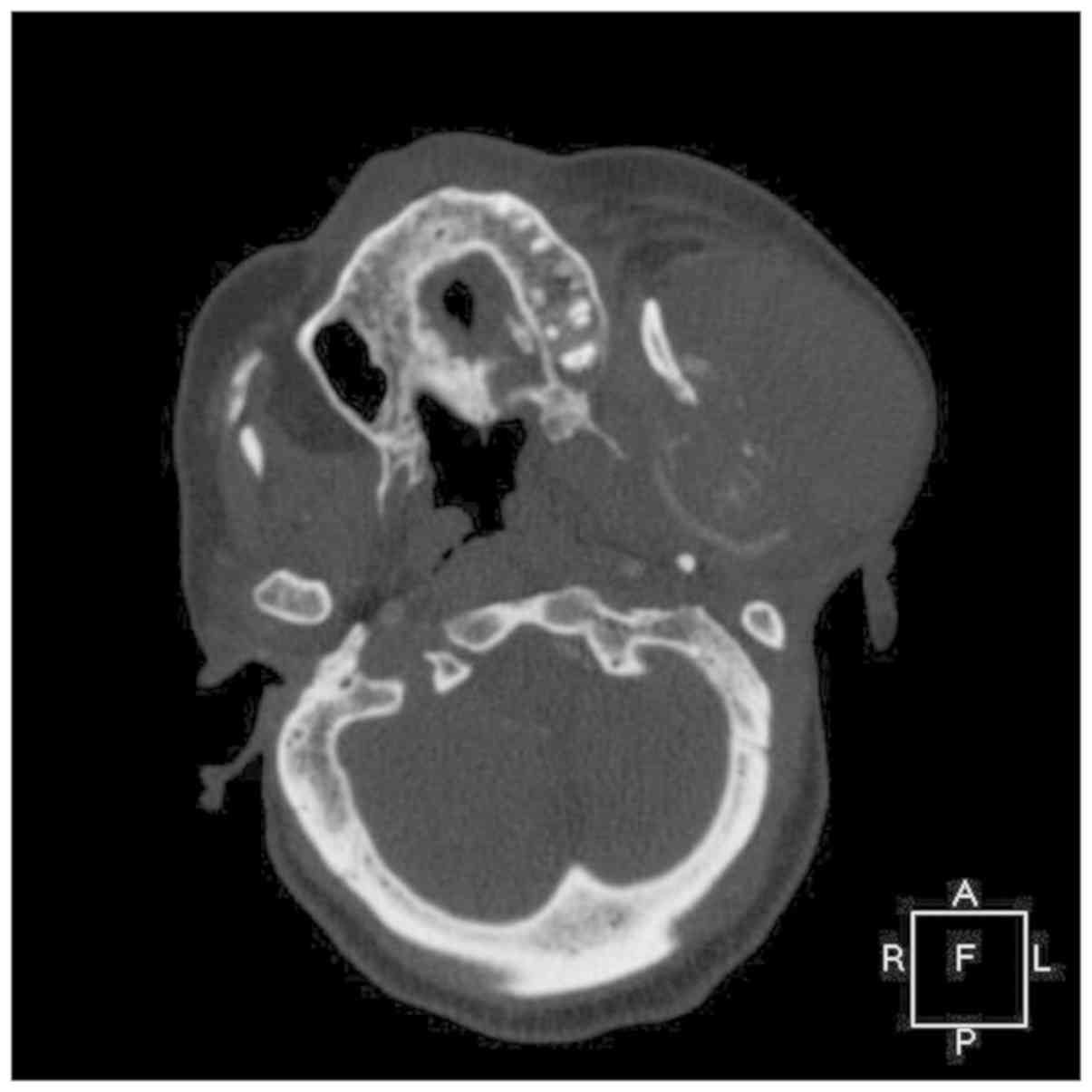
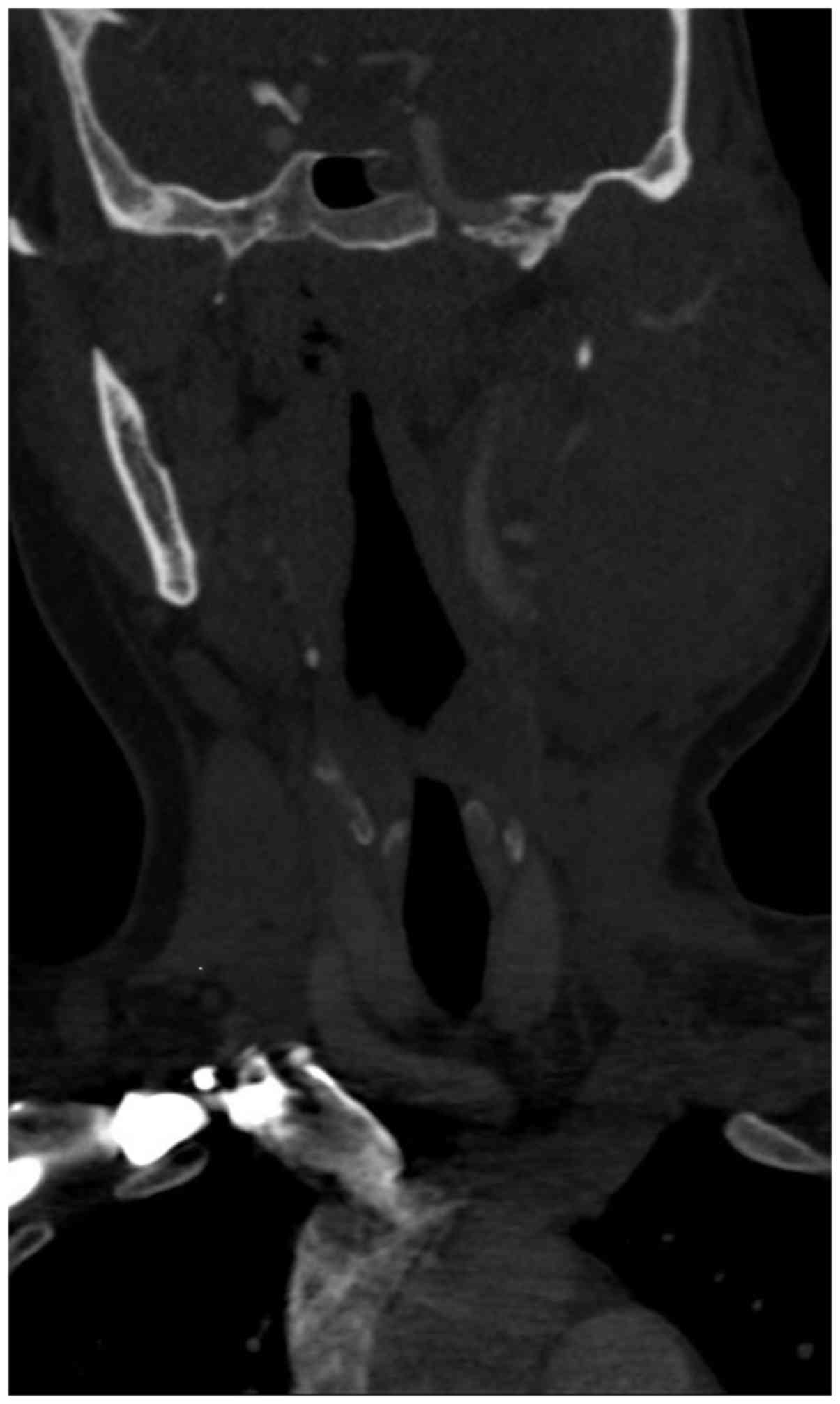
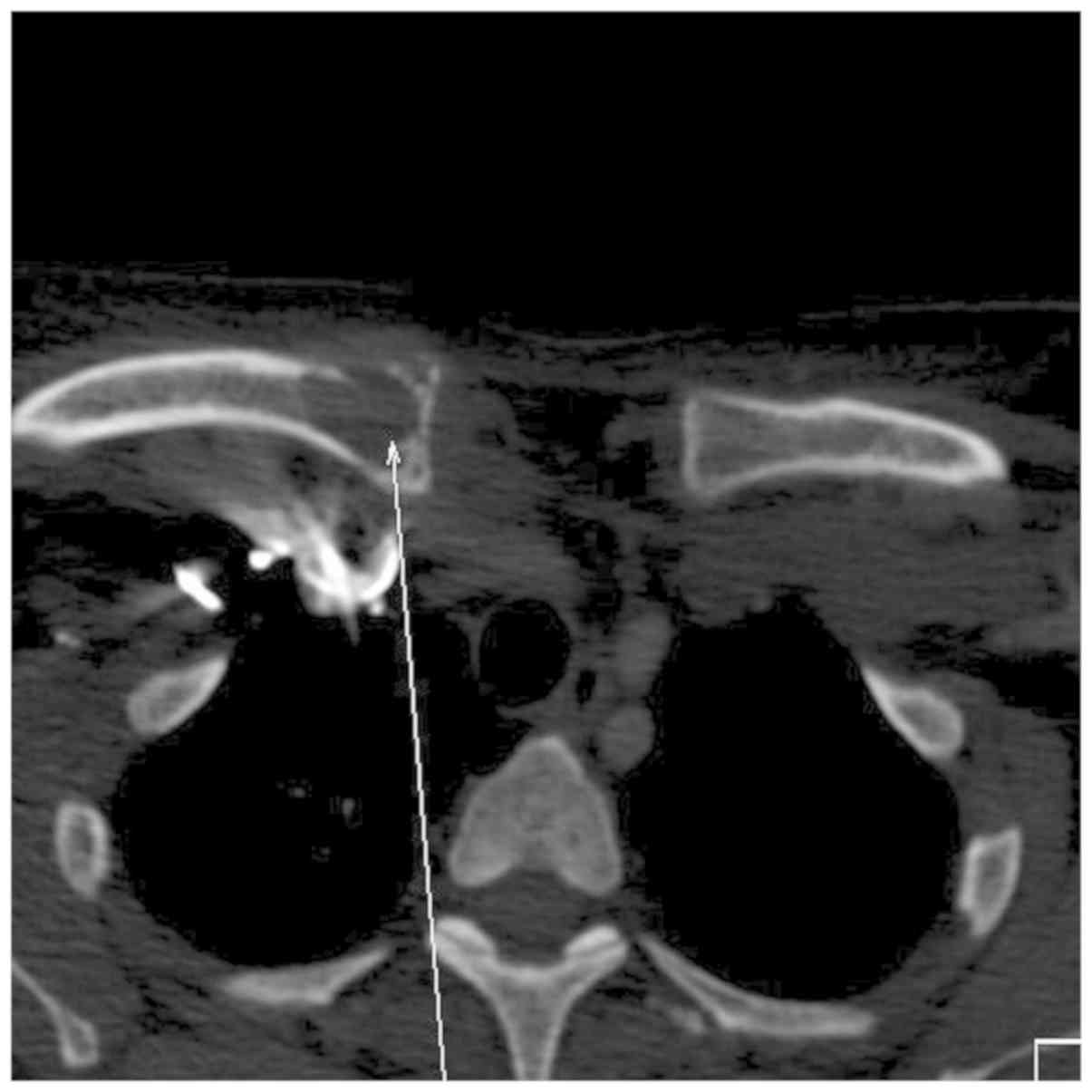
involving the skull. A contrast computed tomography scan showed a
large, solid, fairly well circumscribed left neck mass, which
extended to the skull base superiorly and inferiorly to the hyoid
bone (Figs. 2, 3 and 4).
The mass was ~8.0x8.2x10.0 cm in size. The left
masseter muscle and left parotid gland was indistinguishable from
the mass and inseparable from the sternocleidomastoid muscle
posteriorly. There was no encasement by the mass of any major neck
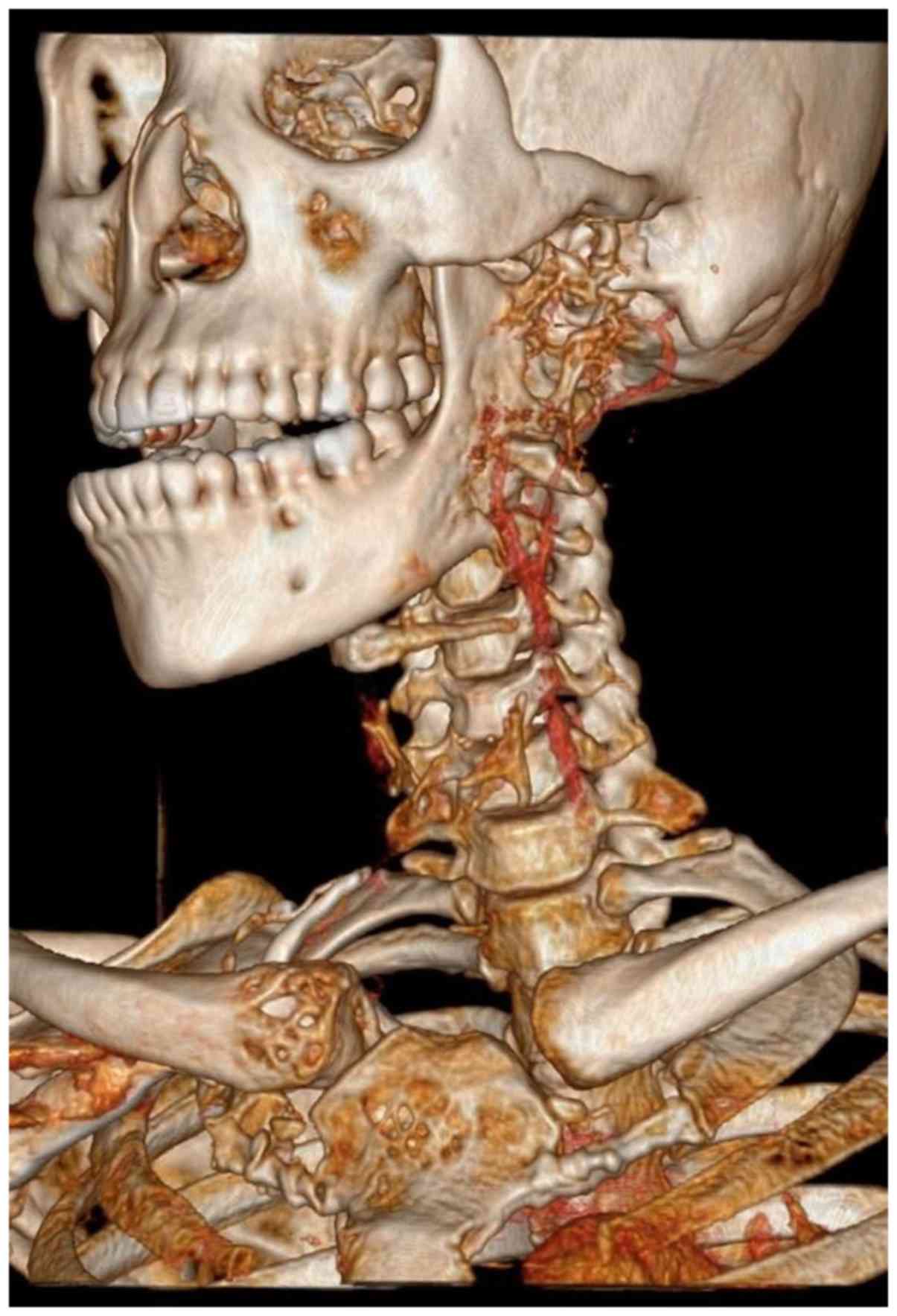
vessels. There was significant erosion and destruction of the
underlying left ramus of the mandible, and lytic lesions involving
the ribs, sternum, clavicle, and vertebrae (Fig. 5). There was associated significant
ipsilateral neck nodes. Thus, a malignant neoplasm involving the
parotid gland was suspected.
A fine needle aspirate of the parotid mass showed
the presence of numerous plasma cells, suggestive of plasma cell
dyscrasia. A haematologist was consulted, and a suspicion of
multiple myeloma was raised based on the multiple bony lytic
lesions. Blood results showed a low haemoglobin level of 9.8 g/dl,
a white blood cell count of 5.6x109/l, a platelet count
of 278x109/l, serum calcium levels of 2.23 mmol/l, serum
phosphorous levels of 1.22 mmol/l, urea levels of 2.2 mmol/l and
creatinine levels of 60 µmol/l. The CD4 count was 519 cells/µl. The
total serum protein concentration was high (98 g\l) and albumin
concentration was low (31 g/l).
There was an abnormal serum free light chain assay
result with a free κ-light chain concentration of 40.9 mg/l and a
free λ-light chain concentration of 245 mg/l, with a κ:λ ratio of
0.17; thus, increased paraprotein levels were detected. A
monoclonal band of IgG λ was detected on immunofixation. Protein
electrophoresis demonstrated paraprotein levels of 1 in 32 g/l,
with a monoclonal band present in the γ region.
Urine analysis was therefore performed which
revealed monoclonal (Bence-Jones) paraprotein levels >30 g/l.
Urine immunofixation electrophoresis demonstrated increased free λ
chain levels.
Bone marrow aspirate showed plasmacytosis comprised
12% of the myelogram. There was no increase in the number of blast
cells. The plasma cells were mature, and some cells were
binucleated. There were left shifted neutrophils, indicative of a
deficiency in vitamin B12 or Folate, and erythropoiesis was
decreased. Trephine biopsy was deemed inadequate for histological
evaluation.
Cytogenetic evaluation showed a normal female
karyotype of 46XX. Core biopsy of the parotid mass demonstrated
cores comprising a malignant neoplasm seen infiltrating into the
soft tissue. There was focal representation of parotid salivary
gland parenchyma. The neoplasm was arranged in sheets of markedly
pleomorphic and mature plasmacytoid cells. The neoplastic cells
were enlarged and contained bizarre cellular and nuclear
morphological features. The nuclei were eccentrically placed with
dense chromatin and prominent nucleoli. There was abundant
amphophilic cytoplasm. Examination in 10 high-power fields revealed
6 mitotic characteristics and immunohistochemistry analysis of the
specimen was used to show these characteristics: -LCA, which
highlights reactive lymphocytes; -CD3, which highlights reactive
T-cells; -CD38, where positive staining indicates tumour cells;
-CD138, where positive staining indicates tumour cells; -Cyclin D1,
where positive nuclear staining of tumour cells; -Ki67, where
positive staining indicates a proliferation index >60%.
Fluorescent in situ hybridization showed λ-light chain
restriction.
These findings were therefore consistent with a
diagnosis of multiple myeloma, Durie-Salmon stage IIIA (5). The patient was referred to the Medical
Oncology unit for chemotherapy. She was prescribed 7 cycles of
Melphalan 9 mg/m2 per Os day 1-4 and Prednisone 100 mg
per Os day 1-4. This was repeated every 4 weeks. A total of 2
months after initiation of chemotherapy, she reported to have been
tolerating the treatment well. There was marked clinical reduction
in the size of the parotid mass, which now measured 4x3 cm and
chemotherapy was continued. Trismus and difficulty in mastication
was reduced and there was no longer visible intraoral extension of
the mass on examination. Unfortunately, the patient was lost to
long-term follow up and it is unclear whether the patient had a
complete response to treatment or relapsed as a result of
defaulting the treatment.
Literature review
Multiple myeloma accounts for 1% of all types of
cancer and 10% of all haematological cancers (6,7). It is
slightly more common in males, and was found to be more common in
African Americans compared with Caucasians (8). At the time of diagnosis, 1-2% of
patients present with the condition occurring outside the bone
marrow, and 8% of the patients develop extramedullary
manifestations during the course of the disease with a median age
of presentation of 72 years (9,10).
HIV results in immunodeficiency that predisposes
patients to a variety of disorders, including plasma cell disorders
(11). Studies have shown a 4.5-fold
increased risk of multiple myeloma in patients infected with HIV,
with the presentation being more aggressive and disseminated
compared with patients who were not co-infected with HIV (11,12).
There is an increased incidence of multiple myeloma
in patients with HIV/acquired immune deficiency syndrome according
to Grulich et al (13). The
pathophysiology underlying the development of plasma cell disorders
is unclear, but may be associated with chronic antigenic
stimulation from HIV and other viral co-infections, elevated serum
IL-6 levels and Epstein-Barr virus-driven proliferation of infected
B cells (14-16).
HIV infection is a risk factor for both aggressive
clinical behaviour and unusual clinical presentation of
extramedullary myeloma cases. de Camargo Moraes et al
(17) described a case of an HIV
positive patient that presented with swelling of the palate, and
left gingival fornix in the maxilla confirmed the diagnosis with
well-differentiated plasma cells and restriction of the
λ-light-chain. A retrospective cohort study, performed in a single
centre, reported that HIV-positive patients presented with multiple
myeloma at a significantly younger age, and presented with less
osteolytic lesions, renal impairment, and lower neutrophil counts
(18). All HIV-positive patients
presented with paraproteins of the IgG subtype, suggesting a
possible relationship between multiple myeloma and an IgG response
to HIV antigens (18). The patients
had significantly increased CD4 counts, with a low prevalence of
abnormal free κ/λ ratios (18). The
study further showed that HIV co-infection did not significantly
affect the stage of the disease multiple myeloma presented at, nor
did it affect the incidence of pathological fractures, bone marrow
plasmacytosis or changes in the lymphocytic counts (18).
Cauda et al (19) suggested that HAART may lead to a
reduction in IgM-proteins in certain HIV-infected patients with
monoclonal gammopathy. Additional studies are required to determine
whether HAART treatment may delay the progression from monoclonal
gammopathy to plasma cell malignancy.
There have been reports that HAART alone can lead to
the complete remission of smouldering multiple myeloma, however
there are no accepted guidelines for managing HIV positive patients
with multiple myeloma (20). This is
due to the paucity of clinical data and HIV positive patients with
multiple myeloma being excluded from clinical trials.
In conclusion, there is no consensus regarding a
treatment protocol for HIV positive patients receiving HAART who
present with multiple myeloma. However, based on the present
clinical case report and a review of the relevant literature the
treatment should include high dose chemotherapy agents. Although
multiple myeloma is considered incurable, all patients should be
started on treatment with the goal of preventing further
complications.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analysed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
SMa, conceived and designed the report, and wrote
the manuscript. SMu collected and analysed the data, and
contributed to writing the manuscript. Both authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was performed in accordance with
the University of the Witwatersrand Human Research Ethics
protocol.
Patient consent for publication
Written consent was obtained for the publication of
this case report and any accompanying images. A copy of the written
consent is available for review on reasonable request.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dezube BJ, Aboulafia DM and Pantanowitz L:
Plasma cell disorders in HIV-infected patients: From benign
gammopathy to multiple myeloma. AIDS Read. 14:372–374, 377-379.
2004.PubMed/NCBI
|
|
2
|
Roodman GD: Mechanisms of bone metastasis.
N Engl J Med. 350:1655–1664. 2004.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Longo DL and Anderson KC: Plasma cell
disorders. In: Harrison's principles of internal medicine. Kasper
DL, Braunwald E, Fauci AS, Hauser SL, Longo DL and Jameson JL
(eds). 16th edition. McGraw-Hill, New York, NY, pp657-658,
2005.
|
|
4
|
Van Riet I: Homing mechanisms of myeloma
cells. Pathol Biol (Paris). 47:98–108. 1999.PubMed/NCBI
|
|
5
|
The International Myeloma Working Group:
Criteria for the classification monoclonal gammopathies, multiple
myeloma and related disorders: A report of the international
myeloma working group. Br J Haematol 121: 749-757, 2003.
|
|
6
|
Knobel D, Zouhair A, Tsang RW, Poortmans
P, Belkacémi Y, Bolla M, Oner FD, Landmann C, Castelain B and
Ozsahin M: Rare Cancer Network Prognostic factors in solitary
plasmacytomas of the bone: A multicenter rare cancer network study.
BMC Cancer. 6(118)2006.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ozsahin M, Tang RW, Poortmans P, Belkacémi
Y, Bolla M, Dinçbas FO, Landmann C, Castelain B, Buijsen J,
Curschmann J, et al: Outcomes and patterns of failure in solitary
plasmacytoma: A multicenter rare cancer network study of 258
patients. Int J Radiat Oncol Biol Phys. 64:210–217. 2006.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Micheletti AR, Macedo AC, Silva GB, Silva
AC, Silva-Vergara ML, Murta EF and Adad SJ: Benign and malignant
neoplasias in 261 necropsies for HIV-positive patients in the
period of 1989 to 2008. Rev Inst Med Trop Sao Paulo. 53:309–314.
2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Diamond T, Levy S, Day P, Barbagallo S,
Manoharan A and Kwan YK: Biochemical, histomorphometric and
densitometric changes in patients with multiple myeloma: Effects of
glucocorticoid therapy and disease activity. Br J Haematol.
97:641–648. 1997.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Nilsson-Ehle H, Holmdahl C, Suurküla M and
Westin J: Bone scintigraphy in the diagnosis of skeletal
involvement and metastatic calcification in multiple myeloma. Acta
Med Scand. 211:427–432. 1982.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Terpos E, Berenson J, Cook RJ, Lipton A
and Coleman RE: Prognostic variables for survival and skeletal
complications in patients with multiple myeloma osteolytic bone
disease. Leukemia. 24:1043–1049. 2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Vincent Rajkumar S: Multiple myeloma: 2014
Update on diagnosis, risk-stratification, and management. Am J
Hematol. 89:999–1009. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Grulich AE, Li Y, McDonald A, Correll PK,
Law MG and Kaldor JM: Rates of non-AIDS-defining cancers in people
with HIV infection before and after AIDS diagnosis. AIDS.
16:1155–1161. 2002.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ikezoe T, Saito T, Bandobashi K, Yang Y,
Koeffler HP and Taguchi H: HIV-1 protease inhibitor induces growth
arrest and apoptosis of human multiple myeloma cells via
inactivation of signal transducer and activator of transcription 3
and extracellular signal-regulated kinase 1/2. Mol Cancer Ther.
3:473–479. 2004.PubMed/NCBI
|
|
15
|
Carraway H and Ambinder RF: Plasma cell
dyscrasia, Hodgkin lymphoma, HIV, and Kaposi sarcoma-associated
herpesvirus. Curr Opin Oncol. 14:543–545. 2002.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Voelkerding KV, Sandhaus LM, Kim HC,
Wilson J, Chittenden T, Levine AJ and Raska K Jr: Plasma cell
malignancy in the acquired immune deficiency syndrome. Association
with Epstein-Barr virus. Am J Clin Pathol. 92:222–228.
1989.PubMed/NCBI View Article : Google Scholar
|
|
17
|
de Camargo Moraes P, Thomaz LA, Montalli
VA, Junqueira JL, Ribeiro CM and Oliveira LB: Extramedullary
plasmacytoma diagnosed in an HIV-positive patient by an unusual
clinical presentation. Case Rep Dent. 2016(6305173)2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
De Groot JJB, Webb MJ, Raubenheimer JE,
Struwig MC and Louw VJ: Concomitant HIV infection in newly
diagnosed multiple myeloma patients is hard to recognise and should
be tested for routinely in areas of high endemicity. S Afr Med J.
107:781–787. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Cauda R, Lucia MB, Marasca G, Rutella S,
Petrucci MT, La Verde G and Gastaldi R: Beneficial effect of highly
active antiretroviral therapy (HAART) in reducing both HIV viral
load and monoclonal gammopathy. Eur J Haematol. 63:134–135.
1999.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Gimeno LSE, Abella E, Perez-Vila E,
Cervera M, Montero M, Gimenez MT, Knobel H and Besses C: Complete
remission of smoldering myeloma in a HIV patient after highly
antiretroviral therapy. Haematologica. 92 (Suppl 2)(S485)2007.
|