Introduction
Obesity has become a serious global health concern
(1). Prolonged obesity results in
several diseases, such as diabetes, hypertension, atherosclerosis,
coronary heart disease and hyperlipidemia (2). Thus, safe and effective methods for
weight loss are urgently required. Several studies have shown that
weight loss is significantly increased under high-altitude or
hypoxic conditions compared with normoxic conditions (3,4). Hypoxic
training has been suggested to be beneficial in several clinical
conditions, such as coronary artery disease and chronic obstructive
pulmonary disease (5).
At present, there have been some developments in
increasing fat metabolism under hypoxic conditions. Studies have
shown that hypoxia can induce lipolysis and inhibit fat synthesis
(6) and influence Wnt/β-catenin
signaling (7). Wnt/β-catenin
signaling is a molecular switch that governs adipogenesis (8). Adipogenesis is suppressed by activating
the Wnt/β-catenin signaling pathway, which represses the expression
of PPARγ and C/EBPα (9). Several
different microRNAs (miRNAs/miRs) regulate Wnt/β-catenin signaling
in numerous diseases (10,11).
miRNAs are a class of small noncoding RNAs, 18-22 nt
in length, are ubiquitously present in eukaryotes and can regulate
protein expression at the mRNA level (12-14).
miR-122 and miR-33 regulate cholesterol and fatty acid metabolism,
and miR-370 affects lipid metabolism by regulating miR-122
expression (15,16). In addition, miR-378/378* (miR-378* is
derived from the same hairpin RNA precursor as miR-378, and both
are located in the first intron of peroxisome PPARγ coactivator-1β)
(17), miR-27, miR-103/107 and
miR-613 participate in the regulation of lipid metabolism (18-21).
miR-92a regulates spheroid formation and malignant progression in
ovarian cancer via the Wnt signaling pathway (22). However, the effect of miR-92a on the
Wnt signaling pathway in hypoxic rats is unclear.
The aim of the present study was to investigate the
regulatory role of miR-92a in the Wnt/β-catenin signaling pathway.
Microarray analysis, reverse transcription-quantitative (RT-q)PCR,
western blotting and dual luciferase reporter assays were used to
detect miRNA-92a, frizzled (Fzd)10 and c-Myc gene expression in the
epididymal fat of hypoxic rats. The results of the present study
provide evidence that the novel lipolysis suppressor miR-92a
regulated Fzd10/Wnt/β-catenin signaling under hypoxic
conditions.
Materials and methods
Animal model and tissues
A total of 60 3-week old male Sprague-Dawley rats
were purchased from Vital River Laboratory Animal Technology Co.,
Ltd. All rats were housed at 22.0±0.5˚C in standard cages with a 12
h light-dark cycle. The rats weighed 92±6 g and were separated into
two groups: Control animals (n=15), which received standard rat
chow (fat content: 10% energy); and the high-fat diet group (HF
group; n=45), which received a high-fat diet (fat content: 40%
energy).
The epididymal fat was obtained from the hypoxic
rats as described in our previous study (23). The obese rats exercised on a
treadmill for 2 weeks to adapt to the training program. The
training speed was elevated from 16 meters/min to 25 meters/min,
and the exercise time was gradually prolonged from 20 to 60 min/day
over 2 weeks by 5 min every 2 days. A total of 20 rats were
selected based on their body weight (57±5 g) and these were
randomly assigned to a normoxic sedentary group (n=10) or hypoxic
training group (n=10). All 20 animals were continued on a high-fat
diet. The rats in the normoxic group were housed under normoxic
conditions (21% oxygen) without exercise, whereas the rats in the
hypoxic group were housed with 13.6% oxygen (equivalent to an
altitude of 3,500 meters) (24), and
trained at a speed of 20 meters/min for 1 h a day, 6 days per week
for 4 weeks. For the rats in the hypoxic group, sample collection
was scheduled 24-36 h after the last session of exercise training
to eliminate the effect of acute exercise. After an overnight fast,
the rats in the two groups were weighed and then anesthetized with
an intraperitoneal injection of 10% chloral hydrate (350 mg/kg body
weight). Subsequently, the epididymal fat was excised and washed in
precooled saline for follow-up experiments. No abnormal changes
(such as peritonitis) were observed in the viscera during rat
dissection. All animal procedures were approved by the Ethics
Committee of Shandong Sport University. All animal experiments were
performed in accordance with the Guide for the Care and Use of
Laboratory Animals (Chinese Edition) published by the Ministry of
Health of the People's Republic of China (25).
Cell culture and reagents
Mouse 3T3-L1 preadipocytes were cultured in DMEM
supplemented with 10% FBS and penicillin/streptomycin (100
units/ml; all purchased from Gibco; Thermo Fisher Scientific, Inc.)
and incubated at 37˚C in 5% CO2.
The following reagents and instruments were used:
miRcute miRNA Isolation kit (Tiangen Biotech Co., Ltd.), miRcute
miRNA cDNA First Strand Synthesis kit (Tiangen Biotech Co., Ltd.),
miRcute MiRNA Quantitative Fluorescence Detection kit (cat. no.
FP401; Tiangen Biotech Co., Ltd.), SuperReal PreMix SYBR-Green
(cat. no. FP204; Tiangen Biotech Co., Ltd.), TIANScript II cDNA
First Strand Synthesis kit (cat. no. KR107; Tiangen Biotech Co.,
Ltd.) and RT-qPCR amplifier (BIOER FQD-96A; Hangzhou Bioer Co.,
Ltd.). Fzd10 cat. no. ab199428), glycogen synthase kinase 3β
(GSK3β; cat. no. ab75745; Abcam), adenomatous polyposis coli (APC)
(cat. no. ab40778), c-Myc (cat. no. ab39688; Abcam), β-actin (cat.
no. ab8227; Abcam) and β-catenin (cat. no. ab32572; Abcam) primary
antibodies, and the secondary goat anti-rabbit antibody (cat. no.
ab6721; Abcam) were all purchased from Abcam. TRIzol reagent (cat.
no. 10606ES60) was purchased from Yisheng Biology and a
Bicinchoninic Acid (BCA) Protein assay reagent kit (cat. no.
RTP7102) was purchased from Zhongke Ruitai. All plasmids/agomiRs
were designed and synthesized by Shanghai Sangon BioTech Co., Ltd.
Xfect transfection reagent was purchased from Takara Bio, Inc.
miRNA microarray
Microarray assays were performed to analyze the
changes in the expression of miRNAs between the hypoxic and
normoxic rats, as described previously (23). Significance analysis of microarrays
was performed to identify the significantly altered miRNA. The
miRNAs with an absolute fold change (hypoxic/normoxic group)
>1.5 and P<0.05 was considered significantly differently
expressed.
RT-qPCR
Epididymal fat was collected, and total RNA was
extracted using TRIzol using phenol-chloroform extraction. RNA
integrity was examined using 8% gel electrophoresis, and RNA purity
was assessed by determining the 260/280 ratio by spectrophotometry.
RNA was reverse transcribed into cDNA using a TIANScript II cDNA
First Strand Synthesis kit according to the manufacturer's
protocol. The primer sequences of Fzd10, c-Myc, GAPDH and miRNA-92a
for RT-qPCR are shown in Table I.
GAPDH was used as the internal control for Fzd10 and c-Myc, and U6
was used as the internal control for miRNA-92a. The PCR protocol
for Fzd10 and c-Myc was as follows: Pre-denaturation for 10 min at
95˚C; followed by 40 cycles of denaturation for 30 sec at 95˚C,
annealing for 20 sec at 55˚C, and extension for 30 sec at 72˚C. The
reaction conditions for miRNA-92a were: Pre-denaturation at 95˚C
for 5 min; followed by 40 cycles of denaturation at 95˚C for 20 sec
and annealing at 60˚C for 30 sec. The relative levels of Fzd10,
c-Myc and miRNA-92a were calculated using the
2-ΔΔCq method (26).
 | Table ISequences of the primers used in the
present study. |
Table I
Sequences of the primers used in the
present study.
| Genes | Sequences |
|---|
| Frizzled 10 |
|
Forward |
5'-CTCTCCATGTGCTACTGCGT-3' |
|
Reverse |
5'-ACCCACCATAAAGAGCTGGC-3' |
| c-Myc |
|
Forward |
5'-GAAACGGCGAGAACAGTTGA-3' |
|
Reverse |
5'-CCAAGGTTGTGAGGTTGAGCAGC-3' |
| GAPDH |
|
Forward |
5'-GCAAGTTCAACGGCACAGT-3' |
|
Reverse |
5'-GCCAGTAGACTCCACGACAT-3' |
| microRNA-92a |
|
Forward |
5'-CTGTCCTGTTATTGAGCACTGGTCT ATGG-3' |
|
Reverse |
5'-AAGACATTAGTAACCCACCCCCAT TCC-3' |
| U6 |
|
Forward |
5'-AACGCTTCACGAATTTGCGT-3' |
|
Reverse |
5'-CTCGCTTCGGCAGCACA-3' |
Western blotting
Total protein was extracted using RIPA protein
lysate (Beyotime Institute of Biotechnology), and protein
concentration was measured using a BCA protein assay kit.
Subsequently, proteins were loaded on a 10% SDS-gel, resolved using
SDS-PAGE and transferred to a polyvinylidene difluoride membrane.
The membrane was blocked using 5% skimmed milk for 2 h. After
blocking, primary antibodies against Fzd10 (1:1,000), c-Myc
(1:1,000), GSK3β (1:1,000), APC (1:1,000), β-catenin (1:5,000) or
β-actin (1:2,500) were added and incubated overnight at 4˚C.
Subsequently, the secondary antibody (1:10,000) was added and
incubated at room temperature for 1 h. Signals were visualized
using enhanced chemiluminescence reagent (Thermo Fisher Scientific,
Inc.). The developed film was scanned using a gel imaging system
(Ketagalan GL) and analyzed using ImageJ version 1.8.0 (National
Institutes of Health). The expression levels of Fzd10 and c-Myc
were calculated relative to the expression of β-actin.
Bioinformatics prediction of the
regulatory upstream miRNA of Fzd10
Bioinformatics prediction was used to identify the
upstream miRNA for Fzd10. miRwalk2(27) and TargetScan prediction software was
used for bioinformatics prediction (28).
Dual luciferase reporter assay
The wild and mutant types of miRNA-92a binding
sequence in the 3'-untranslated region (UTR) region of the Fzd10
gene was constructed by in vitro chemical synthesis. The
cleavage sites of Spe-1 and HindIII were added on both ends.
The two DNA fragments were cloned into pMIR-REPORT luciferase
plasmids. The plasmids with wild-type 3'-UTR and mutant 3'-UTR
sequences were transfected into 3T3-L1 cells, respectively.
Subsequently, agomiR-92a (100 nM) was transfected and incubated for
24 h. The fluorescence values were measured using a GloMax 20/20
luminometer (Promega Corporation). Renilla fluorescent activity was
used as an internal control, and all procedures were performed
according to the manufacturer's protocol.
Immunohistochemistry
Immunohistochemistry was performed as previously
described (29). Briefly, 5 µm
paraffin-embedded tissue sections from rats were deparaffinized in
xylene, rehydrated in a series of decreasing ethanol solution, and
then washed in PBS. Tissue sections were further quenched
sequentially using 3% hydrogen peroxide for 15 min and incubated in
10% normal goat serum (cat. no. G9023; Sigma-Aldrich; Merck KGaA)
for 45 min at room temperature. The slides were then incubated at
4˚C overnight with rabbit antiFzd10 antibody (1:500). The slides
were then rinsed with PBS and incubated with horseradish
peroxidase-conjugated goat anti-rabbit immunoglobulin G antibody
(1:1,000; cat. no. ab6721; Abcam) for 30 min at 37˚C.
3,3'-Diaminobenzidine (chromogenic reagent; OriGene Technologies,
Inc.) was used as the chromogen, and hematoxylin (Sigma-Aldrich;
Merck KGaA) was used for nuclear counterstaining. For the negative
controls, the primary antibodies were omitted. Experiments were
repeated at least three times.
Statistical analysis
All data were analyzed using SPSS version 18.0 (SPSS
Inc.). All data are presented as the mean ± standard deviation.
Comparison between groups was performed using a one-way ANOVA
followed by a post-hoc Tukey's test. P<0.05 was considered to
indicate a statistically significant difference.
Results
miRNA-92a expression in epididymal
fat
After 12 weeks of feeding, the HF group showed a 10%
higher average weight compared with the C group. Lee's index, fat
mass, and body fat/body weight ratio were all significantly
elevated (P<0.05) in the HF group, demonstrating that an obese
fat model was successfully established as described previously
(23). The differential expression
levels of miRNAs were determined using a criterion of fold change
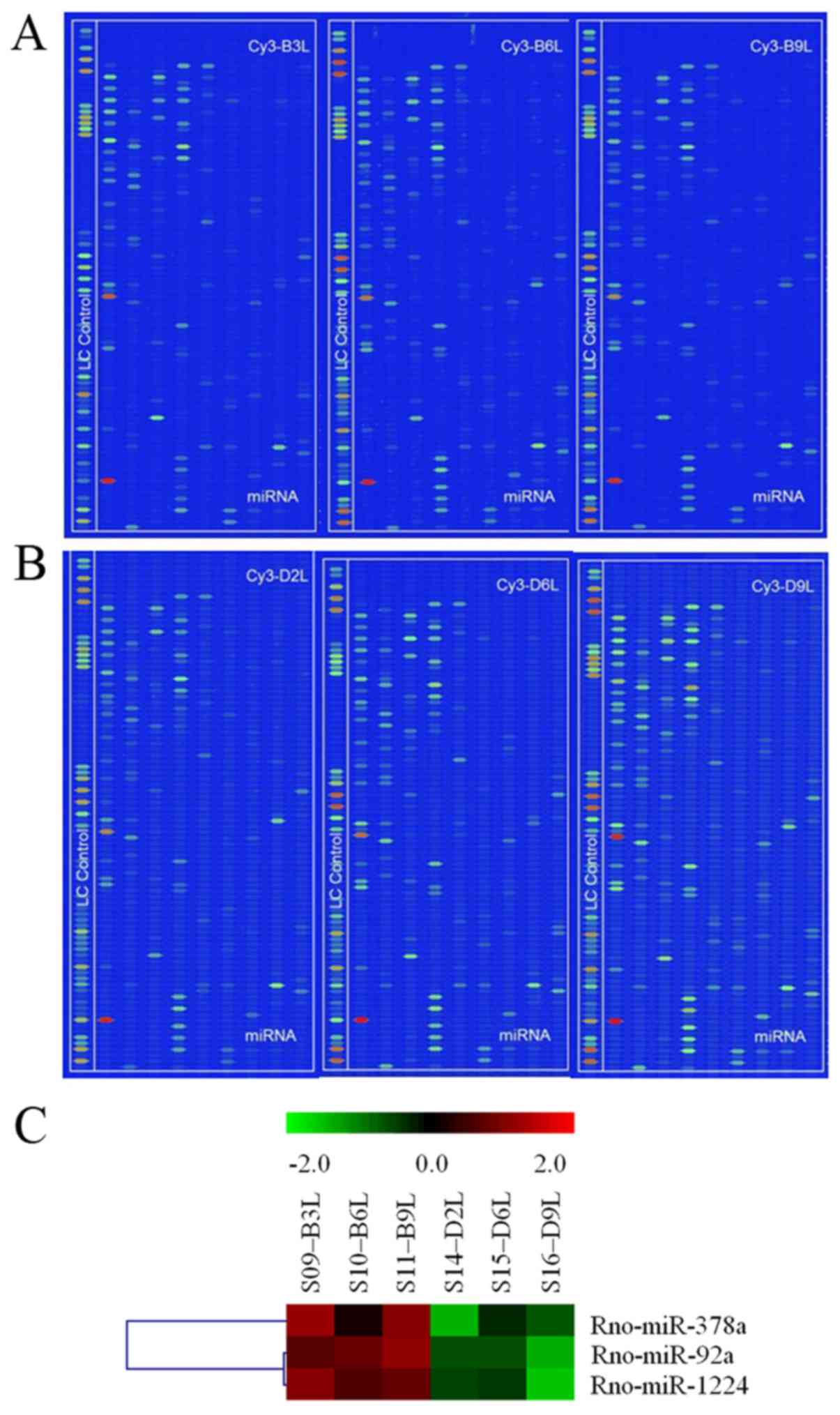
>1.5 and P<0.05. miRNA microarray results showed that the
fold changes of the expression levels of 22 miRNAs in 722 mature
miRNAs were increased >1.5 fold between the hypoxic and normoxic
rats. The hypoxic rats showed lower miRNA-92a expression levels
compared with the normoxic rats (Fig.
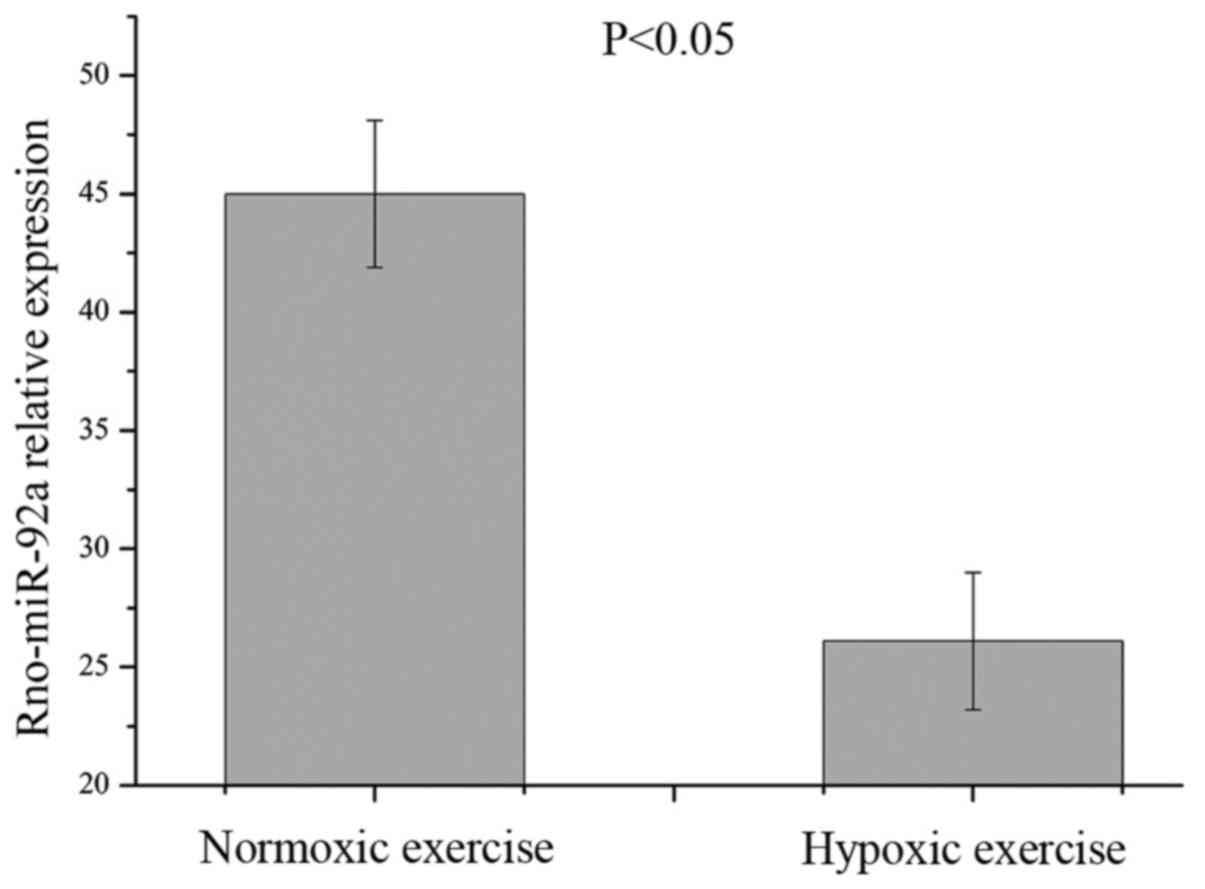
1). RT-qPCR results also showed that the expression levels of
miRNA-92a significantly decreased in the epididymal fat of the
hypoxic rats compared with that of the normoxic rats (Fig. 2).
Bioinformatics and dual luciferase
reporter assay
Bioinformatics prediction was performed to identify
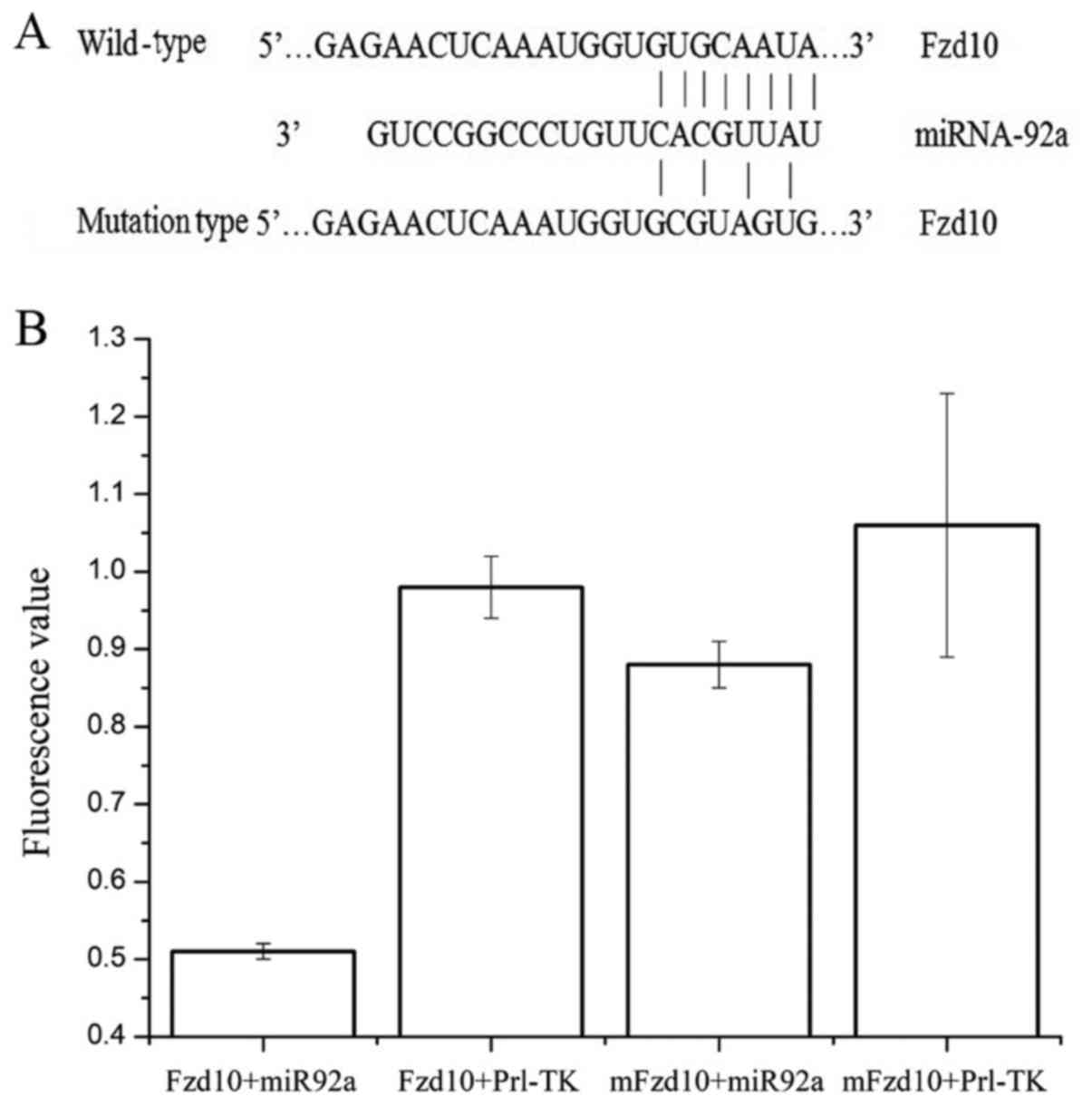
the target gene of miRNA-92a. The Fzd10 gene was determined as a
potential target gene of miRNA-92a. The predicted wild and mutated
binding sequence of Fzd10 is shown in Fig. 3A. Dual luciferase reporter assays
were performed to determine whether or not miRNA-92a could directly
target Fzd10. Results showed that the fluorescence values
significantly decreased after co-transfection of agomiRNA-92a and
pMIR-REPORT-Fzd10 (Fzd10+miR92a group; P<0.05; Fig. 3B). No significant change in
fluorescence value was observed after co-transfection of
agomiRNA-92a and pMIR-REPORT-mFzd10 (mFzd10+miR92a group, where
mFzd10 represents the mutant FZD10; P>0.05). There was no
significant differences in the fluorescence values observed between
the internal control Fzd10+pRL-TK group and the mFzd10+pRL-TK group
(P>0.05). These results suggest that miRNA-92a can directly bind
to the 3'-UTR region of Fzd10 to regulate its expression.
Wnt/β-catenin signaling pathway
expression
Fzd10 is the receptor of Wnt1, Wnt3a, Wnt4 and Wnt5a
(30). Wnt3a, Wnt4 and Wnt5a are
involved in fat cell differentiation and fat formation (31,32). The
c-Myc gene is the downstream gene of the Wnt/β-catenin signaling
pathway (33). As miRNA-92a targets
Fzd10, the effect of hypoxic training on the Wnt/β-catenin
signaling pathway was assessed.
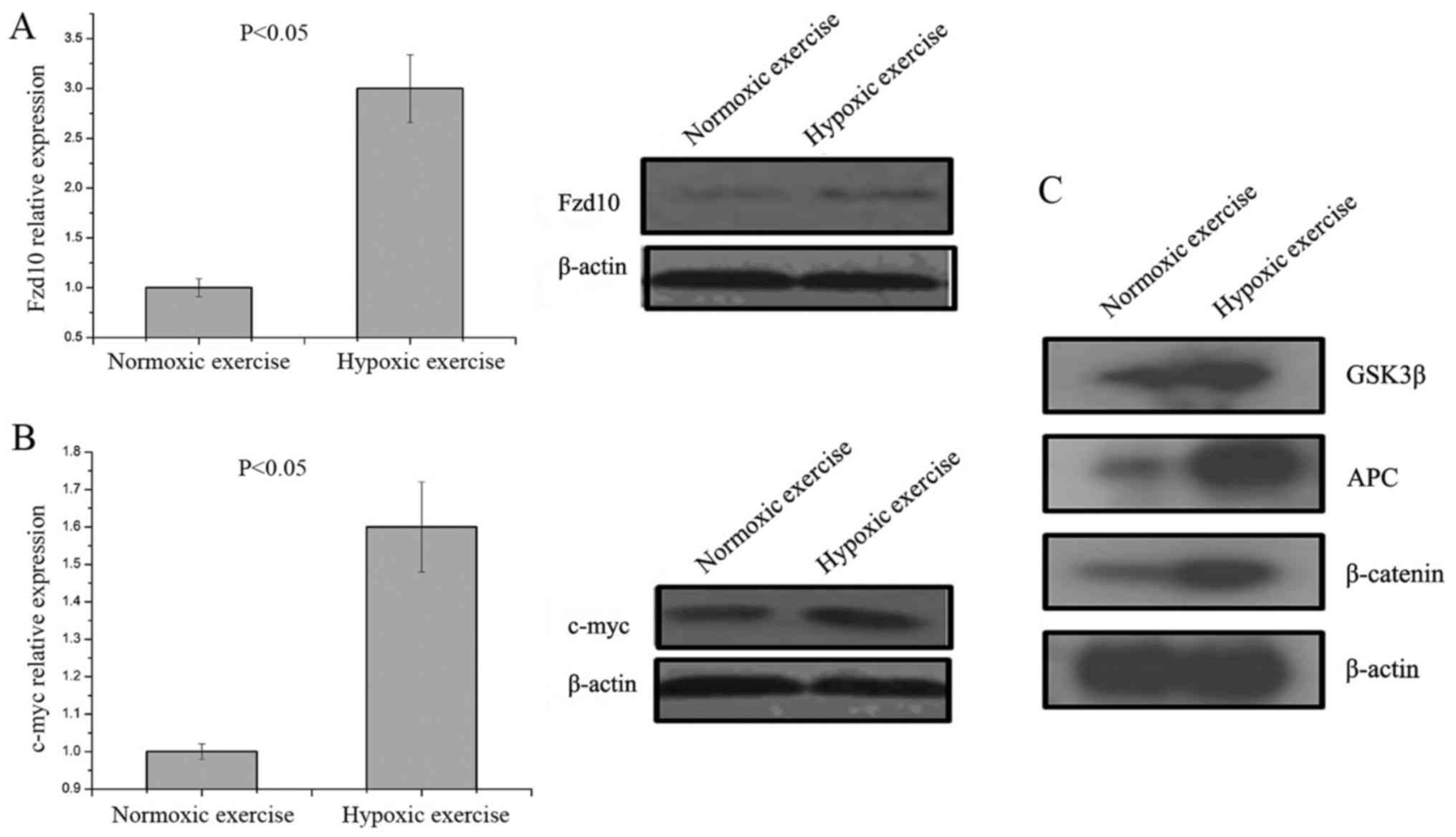
The changes in Fzd10 expression were comparatively
analyzed and shown in Figs. 4A and
5. The mRNA and protein expression levels of Fzd10 in the
epididymal fat of the hypoxic rats was significantly increased
compared with the epididymal fat of the normoxic rats (P<0.05).
Experimental results also suggested that c-Myc expression levels
increased in the epididymal fat of the hypoxic rats (Fig. 4B). Furthermore western blotting
experiments were performed, and the results showed that the
expression levels of GSK3β, APC and β-catenin in the epididymal fat
of the hypoxic rats were significantly increased compared with
epididymal fat of the normoxic rats (P<0.05; Fig. 4C).
Discussion
To date, several studies have shown that hypoxia can
induce lipolysis and inhibit fat synthesis (6). Hypoxia treatment (1% O2)
reduces the expression of fatty acid transport proteins (such as
FABP and CD36) and transcription factors (such as PPARγ and
C/EBPα), and reduces free fatty acid (FFA) intake by adipocytes
(34). Energy consumption in muscles
or elsewhere in the body increases during exercise, which further
reduces energy from being stored as fat. Thus, the above studies
may explain how the rats lost significant amounts of weight during
the hypoxic training. van Tienen et al (35) found that Wnt5b is a potent enhancer
of adipogenic capacity and functions by stimulating PPARγ and aP2,
and by inhibiting the Wnt/β-catenin signaling pathway. Hypoxia
influences the Wnt/β-catenin signaling pathway and miRNA expression
(36).
miRNAs are important post-transcriptional regulators
of lipid metabolism (37). In the
present study, the miRNA expression levels in the epididymal fat
obtained from the hypoxic rats were analyzed using microarray
analysis (23). The results showed
that the miR-92a expression levels decreased significantly in the
hypoxic rats compared with the normoxic rats. Furthermore, RT-qPCR
results confirmed that the miR-92a expression levels in the fat
tissues of the hypoxic rats was lower compared with the normoxic
rats.
miR-92a is a key regulator and a diagnostic
biomarker that participates in several diseases. In several types
of cancer, miR-92a can regulate tumorigenesis and metastasis
(38). Numerous studies have
confirmed that miR-92a also participates in lipid metabolism
(39). The bioinformatics results of
the present study showed that miR-92a targets Fzd10, a key acceptor
involved in the Wnt/β-catenin signaling pathway, and this was
confirmed by the dual luciferase reporter assay. Wnt signaling is
mediated by Fzd receptors at the cell surface and can be modulated
by secreted frizzled-related proteins and other molecular
antagonists (30). Wnt/β-catenin
signaling is a molecular switch that governs adipogenesis (8). Hypoxia can also induce animal lipolysis
(6) and inhibited miR-92a expression
in the present study. The results of the present study showed that
miR-92a downregulation resulted in upregulated Fzd10 expression in
the hypoxic rats, and that Fzd10 overexpression activated
Wnt/β-catenin signaling and further induced lipid mobilization.
Subsequent experimental results confirmed that Fzd10
and c-Myc expression levels were upregulated in the fat tissues of
the hypoxic rats, suggesting that the Wnt/β-catenin signaling
pathway was activated. However, inhibition of Wnt3a, Wnt4, or Wnt5a
expression prevents the accumulation of triacylglycerol and
decreased the expression of adipogenesis-related genes (31). Based on the results of the present
study, it cannot be concluded whether miR-92a regulates fat
metabolism via Wnt. c-Myc, a transcription factor located
downstream of the Wnt/β-catenin signaling pathway, was upregulated
in the fat tissues of the hypoxic rats. Inhibition of PPARγ
expression reduces FFA intake of adipocyte (34), and the activation of the
Wnt/β-catenin signaling pathway can significantly attenuate the
upregulation of PPARγ and increase the levels of phospho-β-catenin
(40). Thus, miR-92a may have
enhanced fat loss through influencing PPARγ expression and the
Wnt/β-catenin signaling pathway under hypoxic conditions.
In conclusion, the mRNA expression levels of miR-92a
in the hypoxic rats were decreased in the present study, and the
decrease may be associated with upregulation of Fzd10 expression.
This in-turn may result in lipolysis through the regulation of the
Wnt/β-catenin signaling pathway, and thus weight loss in the
rats.
Acknowledgements
Not applicable.
Funding
This study was supported by grants from the National
Natural Science Foundation of China (grant no. 31701042), The
Taishan Scholars Program of Shandong Province (grant no.
tsqn201909148), China Institute of Sport Science (grant no. 16-24),
Major Research Project of Shandong Province (grant no.
2017GSF18115) and Shandong Social Science Planning Fund Program
(grant no. 16CTYJ22).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JC and QS conceived the study and wrote the
manuscript. YY, ZS and XiT collected and analyzed the data and the
literature. LF designed the experiments and supervised the project.
XuT participated in writing the manuscript as well as designed and
supervised the project. All authors have read and approved the
final manuscript.
Ethics approval and consent to
participate
All animal procedures were approved by the Εthics
Committee of Shandong Sport University and the Shandong Province
Animal Investigational Committee. All animal experiments were
performed in accordance with the Guide for the Care and Use of
Laboratory Animals published by the Ministry of Health of the
People's Republic of China.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Poirier P and Despres JP: Exercise in
weight management of obesity. Cardiol Clin. 19:459–470.
2001.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Reinehr T and Roth CL: Is there a causal
relationship between obesity and puberty? Lancet Child Adolesc
Health. 3:44–54. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wee J and Climstein M: Hypoxic training:
Clinical benefits on cardiometabolic risk factors. J Sci Med Sport.
18:56–61. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Netzer NC, Chytra R and Küpper T: Low
intense physical exercise in normobaric hypoxia leads to more
weight loss in obese people than low intense physical exercise in
normobaric sham hypoxia. Sleep Breath. 12:129–134. 2008.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Burtscher M, Gatterer H, Szubski C,
Pierantozzi E and Faulhaber M: Effects of interval hypoxia on
exercise tolerance: Special focus on patients with CAD or COPD.
Sleep Breath. 14:209–220. 2010.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Dijk W, Mattijssen F, de la Rosa Rodriguez
M, Loza Valdes A, Loft A, Mandrup S, Kalkhoven E, Qi L, Borst JW
and Kersten S: Hypoxia-inducible lipid droplet-associated (HILPDA)
is not a direct physiological regulator of lipolysis in adipose
tissue. Endocrinology. 158:1231–1251. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ozturk E, Hobiger S, Despot-Slade E,
Pichler M and Zenobi-Wong M: Hypoxia regulates RhoA and
Wnt/β-catenin signaling in a context-dependent way to control
re-differentiation of chondrocytes. Sci Rep. 7(9032)2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ross SE, Hemati N, Longo KA, Bennett CN,
Lucas PC, Erickson RL and MacDougald OA: Inhibition of adipogenesis
by Wnt signaling. Science. 289:950–953. 2000.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kawai M, Mushiake S, Bessho K, Murakami M,
Namba N, Kokubu C, Michigami T and Ozono K: Wnt/Lrp/beta-catenin
signaling suppresses adipogenesis by inhibiting mutual activation
of PPARgamma and C/EBPalpha. Biochem Biophys Res Commun.
363:276–282. 2007.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhang HD, Jiang LH, Sun DW, Li J and Ji
ZL: The role of miR-130a in cancer. Breast Cancer. 24:521–527.
2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Rana MA, Ijaz B, Daud M, Tariq S, Nadeem T
and Husnain T: Interplay of Wnt β-catenin pathway and miRNAs in HBV
pathogenesis leading to HCC. Clin Res Hepatol Gastroenterol.
43:373–386. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Jiang XI, Luo Y, Zhao S, Chen Q, Jiang C,
Dai Y, Chen Y and Cao Z: Clinical significance and expression of
microRNA in diabetic patients with erectile dysfunction. Exp Ther
Med. 10:213–218. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Jia W, Wu Y, Zhang Q, Gao GE, Zhang C and
Xiang Y: Expression profile of circulating microRNAs as a promising
fingerprint for cervical cancer diagnosis and monitoring. Mol Clin
Oncol. 3:851–858. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Graziano A, Lo Monte G, Piva I, Caserta D,
Karner M, Engl B and Marci R: Diagnostic findings in adenomyosis: A
pictorial review on the major concerns. Eur Rev Med Pharmacol Sci.
19:1146–1154. 2015.PubMed/NCBI
|
|
15
|
Moore KJ, Rayner KJ, Suárez Y and
Fernández-Hernando C: microRNAs and cholesterol metabolism. Trends
Endocrinol Metab. 21:699–706. 2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Iliopoulos D, Drosatos K, Hiyama Y,
Goldberg IJ and Zannis VI: MicroRNA-370 controls the expression of
microRNA-122 and Cpt1α and affects lipid metabolism[S].
J Lipid Res. 51:1513–1523. 2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Eichner LJ, Perry MC, Dufour CR, Bertos N,
Park M, St-Pierre J and Giguère V: miR-378(*) mediates metabolic
shift in breast cancer cells via the PGC-1β/ERRγ transcriptional
pathway. Cell Metab. 12:352–361. 2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Trajkovski M, Hausser J, Soutschek J, Bhat
B, Akin A, Zavolan M, Heim MH and Stoffel M: MicroRNAs 103 and 107
regulate insulin sensitivity. Nature. 474:649–653. 2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ou Z, Wada T, Gramignoli R, Li S, Strom
SC, Huang M and Xie W: MicroRNA hsa-miR-613 targets the human
LXRalpha gene and mediates a feedback loop of LXRα autoregulation.
Mol Endocrinol. 25:584–596. 2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Gerin I, Bommer GT, McCoin CS, Sousa KM,
Krishnan V and MacDougald OA: Roles for miRNA-378/378* in adipocyte
gene expression and lipogenesis. Am J Physiol Endocrinol Metab.
299:E198–E206. 2010.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lin Q, Gao Z, Alarcon RM, Ye J and Yun Z:
A role of miR-27 in the regulation of adipogenesis. FEBS J.
276:2348–2358. 2009.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Chen MW, Yang ST, Chien MH, Hua KT, Wu CJ,
Hsiao SM, Lin H, Hsiao M, Su JL and Wei LH: The STAT3-miRNA-92-Wnt
signaling pathway regulates spheroid formation and malignant
progression in ovarian cancer. Cancer Res. 77:1955–1967.
2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lu YL, Jing W, Feng LS, Zhang L, Xu JF,
You TJ and Zhao J: Effects of hypoxic exercise training on microRNA
expression and lipid metabolism in obese rat livers. J Zhejiang
Univ Sci B. 15:820–829. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Xu J: The effects of hypoxic endurance
training on body weight and glucose metabolism in obesity rats
(unpublished PhD thesis). Shanghai Sport University, 2011.
|
|
25
|
National Research Council: Guide for the
care and use of laboratory Animals-Chinese Version. The National
Academies Press, Washington, DC, 1996.
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Dweep H and Gretz N: miRWalk2.0: A
Comprehensive Atlas of microRNA-target Interactions. Nat Methods.
12:697. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Nam JW, Rissland OS, Koppstein D,
Abreu-Goodger C, Jan CH, Agarwal V, Yildirim MA, Rodriguez A and
Bartel DP: Global analyses of the effect of different cellular
contexts on microRNA targeting. Mol Cell. 53:1031–1043.
2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kolkova Z, Noskova V, Ehinger A, Hansson S
and Casslén B: G protein-coupled estrogen receptor 1 (GPER, GPR 30)
in normal human endometrium and early pregnancy decidua. Mol Hum
Reprod. 16:743–751. 2010.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ring L, Neth P, Weber C, Steffens S and
Faussner A: β-Catenin-dependent pathway activation by both
promiscuous ‘canonical’ WNT3a-, and specific ‘noncanonical’ WNT4-
and WNT5a-FZD receptor combinations with strong differences in LRP5
and LRP6 dependency. Cell Signal. 26:260–267. 2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Nishizuka M, Koyanagi A, Osada S and
Imagawa M: Wnt4 and Wnt5a promote adipocyte differentiation. FEBS
Lett. 582:3201–3205. 2008.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kanazawa A, Tsukada S, Kamiyama M,
Yanagimoto T, Nakajima M and Maeda S: Wnt5b partially inhibits
canonical Wnt/beta-catenin signaling pathway and promotes
adipogenesis in 3T3-L1 preadipocytes. Biochem Biophys Res Commun.
330:505–510. 2005.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Chen C, Peng Y, Peng Y, Peng J and Jiang
S: miR-135a-5p inhibits 3T3-L1 adipogenesis through activation of
canonical Wnt/β-catenin signaling. J Mol Endocrinol. 52:311–320.
2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Faiss R, Girard O and Millet GP: Advancing
hypoxic training in team sports: From intermittent hypoxic training
to repeated sprint training in hypoxia. Br J Sports Med. 47 (Suppl
1):i45–i50. 2013.PubMed/NCBI View Article : Google Scholar
|
|
35
|
van Tienen FH, Laeremans H, van der Kallen
CJ and Smeets HJ: Wnt5b stimulates adipogenesis by activating
PPARgamma, and inhibiting the beta-catenin dependent Wnt signaling
pathway together with Wnt5a. Biochem Biophys Res Commun.
387:207–211. 2009.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Strillacci A, Valerii MC, Sansone P,
Caggiano C, Sgromo A, Vittori L, Fiorentino M, Poggioli G, Rizzello
F, Campieri M and Spisni E: Loss of miR-101 expression promotes
Wnt/β-catenin signalling pathway activation and malignancy in colon
cancer cells. J Pathol. 229:379–389. 2013.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Rayner KJ, Fernandez-Hernando C and Moore
KJ: MicroRNAs regulating lipid metabolism in atherogenesis. Thromb
Haemost. 107:642–647. 2012.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Li M, Guan X, Sun Y, Mi J, Shu X, Liu F
and Li C: miR-92a family and their target genes in tumorigenesis
and metastasis. Exp Cell Res. 323:1–6. 2014.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Giral H, Kratzer A and Landmesser U:
MicroRNAs in lipid metabolism and atherosclerosis. Best Pract Res
Clin Endocrinol Metab. 30:665–676. 2016.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Chen X, Luo Y, Jia G, Liu G, Zhao H and
Huang Z: The effect of arginine on the Wnt/β-catenin signaling
pathway during porcine intramuscular preadipocyte differentiation.
Food Funct. 8:381–386. 2017.
|