Introduction
Psoriasis is an autoimmune skin disease that affects
0.91% of the population in the USA and 8.5% in Norway (1). Certain patients with psoriasis will
develop psoriatic arthritis (PsA) that causes pain and functional
impairment, bringing additional burden to these individuals
(2). It is commonly observed that
individuals with a family history of PsA (3), psoriasis in the nail (4), scalp (5)
or intergluteal region (5) are at an
increased risk of developing arthritis; however there are no
established serum markers to predict this risk.
PsA is one of the spondyloarthritides that are
seronegative, as there are no autoantibodies which assist with
diagnosis (6). However, despite the
fact that the CASPAR classification criteria for diagnosis of PsA
includes the presence of negative rheumatoid factor (RF) (6), certain patients do present positive for
this autoantibody (7) and its
significance is not completely understood.
Anti-cyclic citrullinated peptides (CCPs) are
autoantibodies that recognize citrulline-containing peptides
(8). Citrulline may be formed as the
result of posttranslational modifications
(citrullination/deamination) of arginine, which are catalyzed by
intracellular enzymes, such as peptidylarginine deaminases
(8). Anti-CCP antibodies are present
in the sera of 60-80% of patients with rheumatoid arthritis (RA)
with a specificity of 85-99% (8). In
some patients with PsA, anti-CCP antibodies have also been detected
(9). A study by Perez-Alamino et
al (9) showed that 13.5% of
their 81 patient cohort were positive for anti-CCP antibodies, and
that the presence of these antibodies was more common in patients
with erosive arthritis.
The aim of the present study was to evaluate the
frequency of presence of anti-CCP antibodies in patients with
psoriasis with and without arthritis in a cohort recruited from
Southern Brazil. Additionally, the clinical characteristics between
patients with PsA with and without anti-CCPs antibodies were
compared.
Materials and methods
Ethical approval
The present study was approved by the Local
Committee of Ethics in Research of the Sociedade Evangélica
Beneficente de Curitiba (approval no. CAAE 73205317.2.0000.0103)
and all participants provided signed informed consent. All
procedures involving participants were performed in accordance with
the ethical standards of the institutional and national research
committees, and the 1964 Helsinki declaration including its later
amendments or comparable ethical standards (10).
Sample and data collection
Patients with psoriasis with and without arthritis
were included in the present study. PsA patients were classified
according to the CASPAR criteria (11). This was a convenience sample that
included all patients that attended the hospital for a regular
appointment during a period of 10 months (between September 2018
and July 2019) that agreed to participate in the present study. As
controls, self-declared healthy individuals from the hospital
staff, paired for sex and age were used. Epidemiological and
clinical data, and data on the presence of RF and treatment
information were obtained retrospectively through analysis of
medical records. Serum sample and data collection were performed
between September 2018 and July 2019. These patients attended the
Rheumatology and Dermatology Clinics of the Mackenzie University
Hospital in Curitiba, Brazil periodically to monitor the
disease.
The inclusion criteria were: Patients who had a
diagnosis of psoriasis confirmed by a dermatological clinician.
Patients with arthritis had to fulfil the criteria outlined in the
CASPAR Classification system (6).
Pregnant patients, individuals <18 years of age and those
diagnosed under the age of 16 years were excluded.
Simultaneously with blood collection, Psoriasis Area
Severity Index (PASI) and body surface area (BSA) (12), nail involvement were determined in
the patients with psoriasis, and they were asked to answer a
quality of life questionnaire SF-12 (Short Form Health Survey-12)
(13).
PASI is an index used to express the severity of
psoriasis; it combines the severity (erythema, induration and
desquamation) and percentage of affected skin. PASI score ranges
from 0 (no disease) to 72 (maximal disease) (12). BSA classifies the severity of skin
psoriasis according to the amount of affected surface area. Values
<3% are considered as mild disease, between 3-10% is considered
moderate and >10% is considered severe (12). SF-12 is a survey used to evaluate the
quality of life, with 12 questions that are divided into physical
and mental status; it ranges from 0 (worst case scenario) to 100
(best case scenario) (13).
Erythrocyte sedimentation rate (ESR), C reactive
protein (CRP), Ankylosing Spondylitis Disease Activity Score
(ASDAS)-ESR (14) and ASDAS-CRP
(14) were measured to evaluate
articular inflammatory activity. ASDAS is a composite instrument
that takes into account duration of morning stiffness, degree of
back and peripheral pain (or swelling), patient's global assessment
and C reactive protein (for ASDAS-CRP) or ESR (for ASDAS ESR).
ASDAS is scored as follows: <1.3, inactive disease; 1.3-<2.1,
low disease activity; 2.1-<3.5, high disease activity; and
>3.5, very high disease activity (15).
A total of 91 patients with psoriasis (53.6% female;
mean age 54.4±12.1 years; age range 21-76 years) and 100 healthy
controls (51% female; mean age 52.8±10.8 years; age range 18-77
years) were recruited for the present study.
Determination of anti-CCP
antibodies
A total of 5 ml venous blood from each patient and
control was collected. Serum aliquots were stored at -80˚C until
the assays were performed. Serum levels of anti-CCP were measured
using a commercial ELISA kit (cat. no. ORG301 anti-CCP high
sensitive; Orgentech Diagnostika) according to the manufacturer's
protocol. The cut-off point was set as 20 units/ml.
Statistical analysis
Anti-CCP positivity between patients with psoriasis
(with and without arthritis) and controls was compared. In
addition, epidemiologic and clinical data of PsA patients positive
or negative for anti-CCP antibodies was compared. For comparisons,
the data was collected in a frequency and contingency table. To
compare nominal data, a Fisher's exact test was used. To compare
numerical data, an unpaired t-test or Mann-Whitney U test was used.
Data distribution was evaluated using a Shapiro Wilk's test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
A total of 91 patients with psoriasis were included
in the present study, of which 43 (47.2%) had arthritis and 48
(52.7%) did not. For the control group, 100 healthy controls were
recruited matched for age and sex. In the PsA subgroup, 44.1% of
the patients had axial involvement and 88.3% had peripheral
involvement (41.8% had the polyarticular form; 46.5% had the
oligoarticular form). Dactylitis was present in 37.1%, distal
interphalangeal involvement in 6.9% and enthesitis in 69.7%, with
several patients exhibiting more than one manifestation.
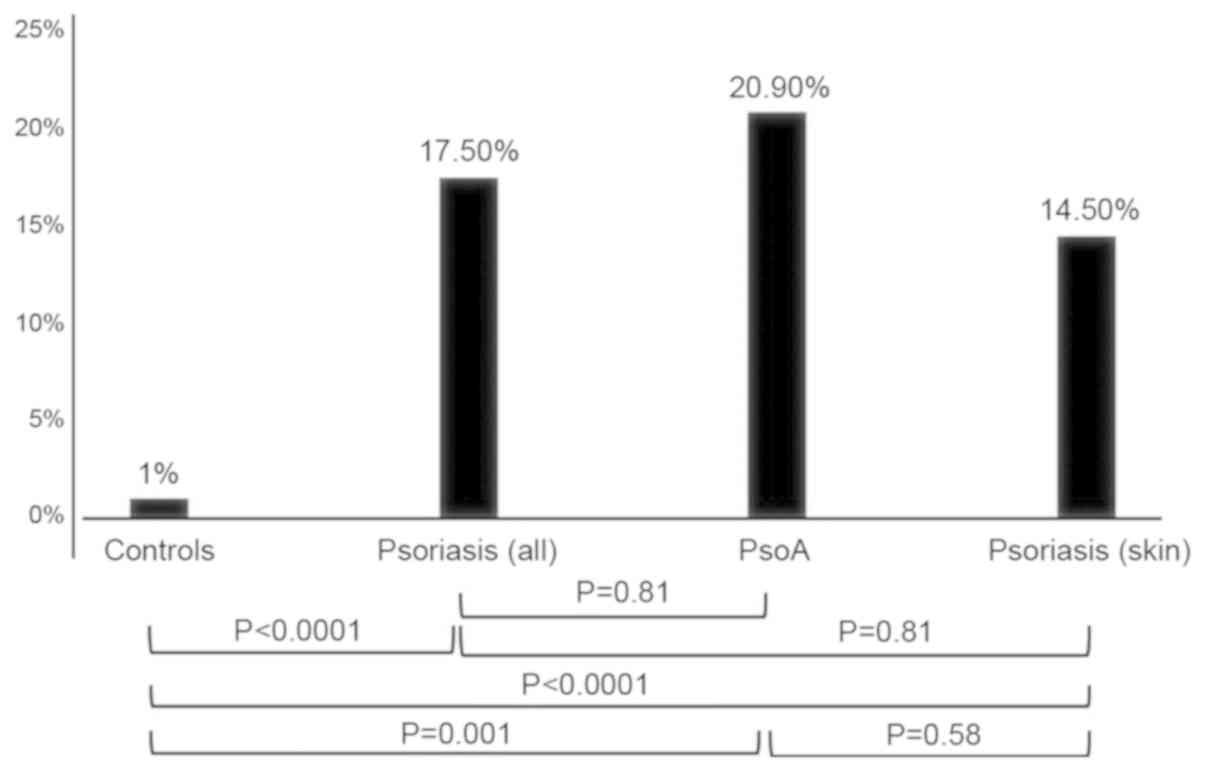
The presence of anti-CCP antibodies in the studied
samples is shown in Fig. 1. Anti-CCP
antibodies were found in 17.5% of the psoriasis group and in 1% of
the control group (P<0.001). In patients with PsA, 20.9% were
positive for anti-CCP antibodies and 14.5% of patients without
joint disease were positive (P=0.58).
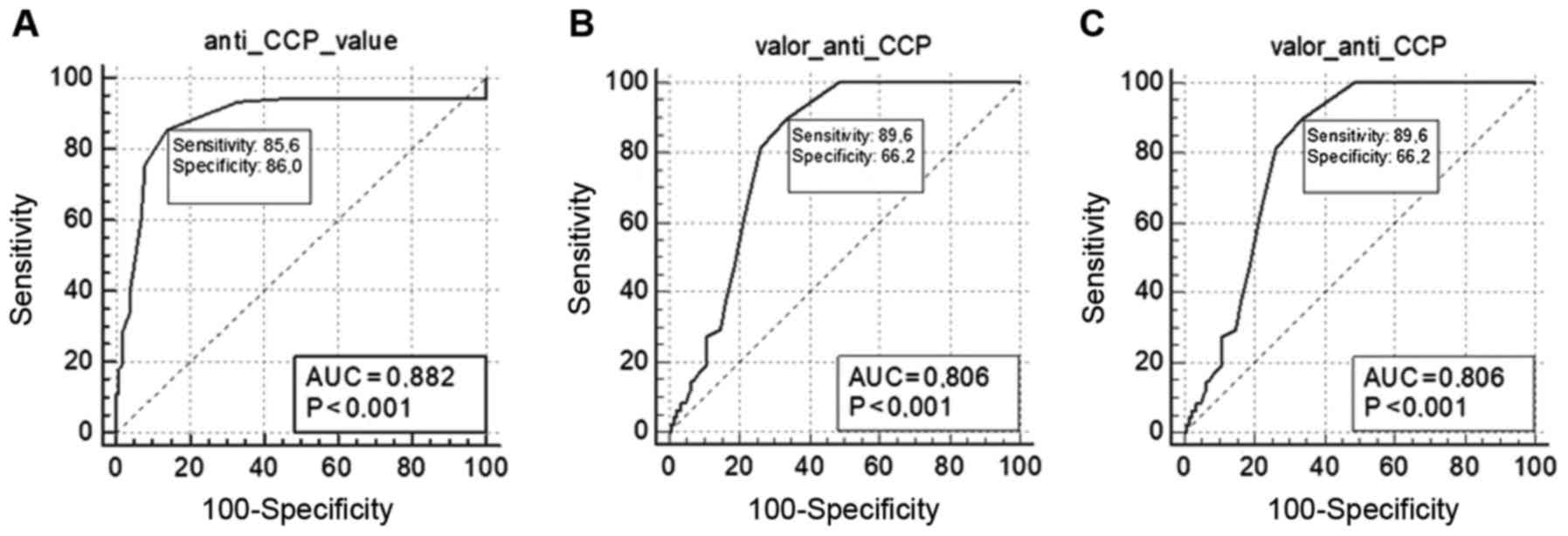
The sensitivity and specificity of anti-CCP
antibodies in each studied group is shown in Fig. 2A for the entire psoriasis group (with
and without arthritis), Fig. 2B for
patients with PsA and Fig. 2C for
patients with psoriasis without arthritis.
Anti-CCP antibodies were more commonly present in
patients with a polyarticular form of PsA compared with those with
skin disease only (44.4 vs. 14.5%, respectively; P=0.009), and
there was a trend of increased frequency in patients with
polyarticular arthritis compared with olygoarticular peripheral
arthritis (5 vs. 33.3%; P=0.06).
The results of analysis of samples from patients
with PsA with and without anti-CCP antibodies are shown in Table I. The presence of anti-CCP was
significantly higher in patients with polyarthritis (P=0.002;
OR=19.20, 95% CI=2.1-174.4). In addition, psoriatic patients with
oligoarthritis showed lower positivity for anti-CCP (P=0.02;
OR=0.09; 95% CI=0.01-0.87).
 | Table IComparison of anti-CCP positive and
negative psoriatic arthritis patients. |
Table I
Comparison of anti-CCP positive and
negative psoriatic arthritis patients.
| Characteristics | Anti-CCP positive,
n=9 | Anti-CCP negative,
n=34 | P-value |
|---|
| Sex, n | | | 0.47e |
|
Male | 5 | 14 | |
|
Female | 4 | 20 | |
| Age,
yearsc | 54.2±12.4 | 54.4±11.2 | 0.95g |
| Ethnic
background | | | |
|
Caucasains | 88.90% | 80% | |
|
African
descendent | 11.10% | 20% | 1.00e |
| Smoker | 57.10% | 48.10% | 1.00e |
| Skin disease
duration, monthsd | 348 (78-448) | 132.0 (80.0-240) | 0.18h |
| Articular disease
duration, monthsd | 60 (36.0-132.0) | 76.0
(36.0-108.0) | 0.99h |
| Rheumatoid factor
positive | 11.10% | 0 | 0.20e |
| Form of
arthritis | | | |
|
Axial +
peripheral (oligo or polyarthritis) | 66.60% | 29.40% | 0.15e |
|
Only axial
involvement | 0 | 14.70% | 0.56e |
|
Only
peripheral involvement | 100% | 85.20% | 0.56f |
|
Polyarthritis | 88.80% | 29.40% | 0.002b,e,i |
|
Oligoarthritis | 11.10% | 55.80% | 0.02a,e,j |
|
Distal
interphalangeal joint involvement | 11.10% | 5.80% | 0.51e |
|
Dactylitis | 28.50% | 39.20% | 0.68e |
|
Entesitis | 88.80% | 64.70% | 0.23e |
|
Nail
involvement | 48.80% | 58.30% | 1.00e |
| CRP,
mg/dld | 7.8 (2.0-22.1) | 2.4 (1.1-5.5) | 0.08h |
| ESR,
mm/hd | 27.6
(13.7-67.5) | 30.0
(21.0-40.5) | 0.94h |
| Conventional DMARD
use | 66.60% | 63.30% | 1.00e |
| Biological drugs
use | 44.40% | 54.80% | 0.71e |
| ASDAS
ESRd | 3.60
(1.37-14.9) | 2.65
(1.34-3.30) | 0.28h |
| ASDAS
CRPd | 2.13
(1.17-4.78) | 1.69
(1.09-2.64) | 0.52h |
| Quality of
life | | | |
|
SF12-physical
domainc | 31.7±11.6 | 36.5±8.8 | 0.25g |
|
SF-12-mental
domainc | 47.7±14.1 | 41.1±11.05 | 0.21g |
| PASId | 0.80 (0-3.0) | 1.20 (0-4.15) | 0.92h |
| BSAd | 1.0 (0-4.0) | 2.0 (0-5.0) | 0.97h |
Discussion
The results of the present study show that the
prevalence of anti-CCP antibodies in patients with psoriasis was
significantly higher than patients without psoriasis, and this
increase in prevalence was not altered by the complication of
arthritis. However, patients who were anti-CCP positive and had
arthritis exhibited a higher prevalence of polyarticular forms of
arthritis.
Eker et al (16) studied patients with PsA and found
that the prevalence of patients with anti-CCP antibodies was 20.6%
in the 44 patient cohort recruited from Turkey. Inanc et al
(17) studied patients with PsA, and
found that the prevalence of patients with anti-CCP antibodies was
12.5%. Conversely, Korendowych et al (18) did not find any differences in
anti-CCP antibody levels in patients with PsA compared with the
control group. Differences in the results may be due to the
influence of the genetic background of the studied populations, or
due to the commercial kits used for anti-CCP antibody detection. In
addition, it is necessary to take into account that PsA is a very
polymorphic disease with several forms of clinical presentations
(5). Different proportions of these
various forms in the different samples may have influenced the
results.
Very few studies have determined the presence of
anti-CCP antibodies in patients with skin disease without joint
involvement, as was performed in the present study. Of the previous
studies where skin involvement without joint involvement was
assessed, the prevalence of anti-CCPs antibodies in patients with
PsA was higher in patients with arthritis compared with patients
without arthritis (19,20). In the present study, the proportion
of patients with anti-CCP antibodies was similar between patients
with skin-only involvement when compared with patients with PsA.
However, when considering only patients with the polyarticular form
of PsA, the prevalence of anti-CCP antibodies was higher in
patients with arthritis. Again, the different proportions of
arthritis subtypes may have served a role in the observed results.
The presence of anti-CCP antibodies in patients with RA is known to
precede manifestation of clinical disease, occasionally by several
years (8,21). The same phenomena may be observed in
patients with PsA. Similarly, it is possible that some of the
positive patients in the present study with just skin disease will
develop arthritis in the future.
In patients with RA, anti-CCP positivity is
implicated in the pathogenesis of the disease, and is associated
with a worse disease prognosis (8).
The presence of anti-CCP antibodies is associated with HLA-DRB1
alleles containing a shared epitope. In PsA, it is generally
accepted that when this autoantibody is present, the disease is
more aggressive (9). Associations
between HLA DRB1 alleles (18,22) with
the polyarticular forms (17,23-25)
have also been found. However, the association between the presence
of anti-CCP with higher degrees of erosion is contested (17,18,23-24), as is the
necessity for initially treating patients with immunobiologics
(18,23). Behrens et al (24) suggested that anti-CCP antibodies
mediate bone destruction in PsA through an osteocatabolic effect.
In the present study, a higher prevalence of the anti-CCP
antibodies in polyarticular forms was observed, but there was no
increased requirement for therapeutic intervention in the present
study, and the quality of life was not altered, suggesting no
effect on disease severity. However, a tendency towards higher
levels of CRP was observed, showing that the presence of anti-CCP
antibodies may be associated with increased inflammatory activity.
The degree of bone erosion was not analyzed in the present study,
making this a limitation. Other limitations were the
cross-sectional design of the study, and the small sample size,
which may have resulted in a statistical bias.
Studies have shown that patients with PsA who are
positive for anti-CCP antibodies may indeed be patients with RA and
psoriasis, as separate conditions, rather than RA being a
complication of psoriasis (23,25).
Differentiation between patients with PsA from patients with RA and
psoriasis as separate conditions may be quite difficult,
particularly in those without distal interphalangeal involvement
with the presence of RF. In the present study, only one patient was
positive for RF. This patient was male with association of a
polyarticular form of arthritis and axial involvement-with severe
bilateral sacroiliitis and extensive syndesmophytosis, making a RA
diagnosis unlikely. However, in the other patients this possibility
still remains, and was not addressed in the present study.
In conclusion, the prevalence of anti-CCP antibodies
was more common in patients with psoriasis and with PsA compared
with healthy controls. Additionally, these autoantibodies were more
common in patients with the polyarticular form of arthritis.
Additional studies are required, ideally prospectively designed
with larger sample sizes, to determine the value of anti-CCP
autoantibodies more accurately in the diagnosis and prognosis of
patients with psoriasis and PsA.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
CG, TS, APBC, JS, VM and RN conceived and designed
the study, and acquired the data. CG, TS and RN analyzed and
interpreted the data. CG, TS and RN drafted the manuscript and
revised it critically for important intellectual content. All the
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Local
Committee of Ethics in Research of the Sociedade Evangélica
Beneficente de Curitiba (approval no. CAAE 73205317.2.0000.0103)
and all participants provided signed informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Parisi R, Symmons DP, Griffiths CE and
Ashcroft DM: Identification and Management of Psoriasis and
Associated ComorbidiTy (IMPACT) project team. Global epidemiology
of psoriasis: A systematic review of incidence and prevalence. J
Invest Dermatol. 133:377–385. 2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Henes JC, Ziupa E, Eisfelder M, Adamczyk
A, Knaudt B, Jacobs F, Lux J, Schanz S, Fierlbeck G, Spira D, et
al: High prevalence of psoriatic arthritis in dermatological
patients with psoriasis: A cross-sectional study. Rheumatol Int.
34:227–234. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Tey HL, Ee HL, Tan AS, Theng TS, Wong SN
and Khoo SW: Risk factors associated with having psoriatic
arthritis in patients with cutaneous psoriasis. J Dermatol.
37:426–430. 2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Raposo I and Torres T: Nail psoriasis as a
predictor of the development of psoriatic arthritis. Actas
Dermosifiliogr. 106:452–457. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Rouzaud M, Sevrain M, Villani AP,
Barnetche T, Paul C, Richard MA, Jullien D, Misery L, Le Maître M,
Aractingi S, et al: Is there a psoriasis skin phenotype associated
with psoriatic arthritis? Systematic literature review. J Eur Acad
Dermatol Venereol. 28 (Suppl 5):S17–S26. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Taylor W, Gladman D, Helliwell P,
Marchesoni A, Mease P and Mielants H: CASPAR Study Group:
Classification criteria for psoriatic arthritis: Development of new
criteria from a large international study. Arthritis Rheum.
54:2665–2673. 2006.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Khondker L and Khan SI: Association of
rheumatoid factor and uric acid with psoriatic arthritis: A review.
Mymensingh Med J. 23:609–613. 2014.PubMed/NCBI
|
|
8
|
Kurowska W, Kuca-Warnawin EH, Radzikowska
A and Maśliński W: The role of anti-citrullinated protein
antibodies (ACPA) in the pathogenesis of rheumatoid arthritis. Cent
Eur J Immunol. 42:390–398. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Perez-Alamino R, Garcia-Valladares I,
Cuchacovich R, Iglesias-Gamarra A and Espinoza LR: Are anti-CCP
antibodies in psoriatic arthritis patients a biomarker of erosive
disease? Rheumatol Int. 34:1211–1216. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
WMA declaration of Helsinki-ethical
principles for medical research involving human subjects.
https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/.
Accessed July 17, 2020.
|
|
11
|
Zlatkovic-Svenda M, Kerimovic-Morina D and
Stojanovic RM: Psoriatic arthritis classification criteria: Moll
and wright, ESSG and CASPAR-a comparative study. Acta Reumatol
Port. 38:172–178. 2013.PubMed/NCBI
|
|
12
|
Finlay AY: Current severe psoriasis and
the rule of tens. Br J Dermatol. 152:861–867. 2005.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Andrade TL, Camelier AA, Rosa FW, Santos
MP, Jezler S and Pereira e Silva JL: Applicability of the 12-item
short-form health survey in patients with progressive systemic
sclerosis. J Bras Pneumol. 33:414–422. 2007.(In English,
Portuguese). PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lukas C, Landewé R, Sieper J, Dougados M,
Davis J, Braun J, van der Linden S and van der Heijde D: Assessment
of SpondyloArthritis international Society. Development of an
ASAS-endorsed disease activity score (ASDAS) in patients with
ankylosing spondylitis. Ann Rheum Dis. 68:18–24. 2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Assesment of Spondyloarthritis
International Society: ASDAS calculator. https://www.asas-group.org/clinical-instruments/asdas-calculator/.
Accessed November 10, 2018.
|
|
16
|
Eker YÖ, Pamuk ÖN, Pamuk GE, Dönmez S and
Çakır N: The frequency of anti-CCP antibodies in patients with
rheumatoid arthritis and psoriatic arthritis and their relationship
with clinical features and parameters of angiogenesis: A
comparative study. Eur J Rheumatol. 1:67–71. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Inanc N, Dalkilic E, Kamali S,
Kasapoglu-Günal E, Elbir Y, Direskeneli H and Inanc M: Anti-CCP
antibodies in rheumatoid arthritis and psoriatic arthritis. Clin
Rheumatol. 26:17–23. 2007.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Korendowych E, Owen P, Ravindran J,
Carmichael C and McHugh N: The clinical and genetic associations of
anti-cyclic citrullinated peptide antibodies in psoriatic
arthritis. Rheumatology (Oxford). 44:1056–1060. 2005.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Alenius GM, Berglin E and Rantapää
Dahlqvist S: Antibodies against cyclic citrullinated peptide (CCP)
in psoriatic patients with or without joint inflammation. Ann Rheum
Dis. 65:398–400. 2006.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Candia L, Marquez J, Gonzalez C, Santos
AM, Londoño J, Valle R, Zabaleta J, Yaqub Z and Espinoza LR: Low
frequency of anticyclic citrullinated peptide antibodies in
psoriatic arthritis but not in cutaneous psoriasis. J Clin
Rheumatol. 12:226–229. 2006.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lee AN, Beck CE and Hall M: Rheumatoid
factor and anti-CCP antibodies in rheumatoid arthritis: A review.
Clin Lab Sci. 21:15–18. 2008.PubMed/NCBI
|
|
22
|
Popescu C, Zofotă S, Bojincă V and Ionescu
R: Anti-cyclic citrullinated peptide antibodies in psoriatic
arthritis-cross-sectional study and literature review. J Med Life.
6:376–382. 2013.PubMed/NCBI
|
|
23
|
Maejima H, Aki R, Watarai A, Shirai K,
Hamada Y and Katsuoka K: Antibodies against cyclic citrullinated
peptide in Japanese psoriatic arthritis patients. J Dermatol.
37:339–345. 2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Behrens F, Koehm M, Thaçi D, Gnann H,
Greger G, Maria Wittig B and Burkhardt H: Anti-citrullinated
protein antibodies are linked to erosive disease in an
observational study of patients with psoriatic arthritis.
Rheumatology (Oxford). 55:1791–1795. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Grover C, Kashyap B, Daulatabad D, Dhawan
A and Kaur IR: Significance of anti-cyclic citrullinated peptide
autoantibodies in immune-mediated inflammatory skin disorders with
and without arthritis. Indian J Dermatol. 61:510–514.
2016.PubMed/NCBI View Article : Google Scholar
|