Introduction
Cogon grass (Imperata cylindrica/I.
cylindrica) is a member of the Gramineae family and is
ubiquitously found in nature, but is often considered useless and
treated as a weed (1). However, this
plant is widely utilized within traditional medicine, particularly
in Asia (2). Various studies have
shown that I. cylindrica exhibits numerous beneficial
biological properties, as it contains glycoside, triterpenoids,
flavonoids, phenolic compounds, tannins and proteins, which display
antioxidant properties in vitro (3-5).
Cogon grass reeds are also suitable for use as an alternative
medication to treat hypercholesterolemia (6). Patients with hypercholesterolaemia are
at increased risk of developing cardiovascular diseases or
suffering from a stroke, which can result in death at a young age,
adding to the global disease burden (7).
Various studies have shown that hypercholesterolemia
and cardiovascular disease are associated with the overproduction
of reactive oxygen species (8-10).
These free radicals facilitate lipid peroxidation within cell
membranes, which results in the production of radical lipid
peroxides and various other free radicals (11). Free radicals possess unpaired
electrons, which are unstable and exhibit a tendency to absorb
electrons from other molecules within close proximity to them in
order to achieve stability (12,13).
This can cause damage to various components of the cell (14).
Phenolic compounds located within I.
cylindrica roots, such as flavonoids, oligostilbenoid and
phenolic acids, are compounds that display strong antioxidant
activity (15). Antioxidants serve
an important role in the process of scavenging free radicals and
breaking down oxidation chain reactions both in vitro and
in vivo (14). The
antioxidant activity of phenolic compounds is induced primarily via
the presence of hydroxyl groups (-OH) within their aromatic ring
structures, mediating redox reactions in a specific way to
neutralize free radicals (16).
In the present study, flavonoid compounds from I.
cylindrica roots were isolated and purified through several
stages, and in vivo tests were used to assess the effects of
I. cylindrica on lipid profile levels in a rat model of
hypercholesterolaemia.
Materials and methods
I. cylindrica samples
The plant material used for isolation and
identification in the present study was I. cylindrica roots
obtained from the Punjul village, Karangrejo District, Tulungagung
Regency, and farmland in Malang, East Java, Indonesia. The plant
material utilized for in vivo evaluation of lipid-lowering
properties was identified and confirmed by the Department of
Biology, Faculty of Science and Technology, Universitas Airlangga,
Indonesia.
Isolation and identification of I.
cylindrica root compounds
A total of 850 g I. cylindrica roots powder
was extracted by maceration using 96% methanol as a solvent three
times, 24 h each, at room temperature. Subsequently, the filtrate
and the residue were separated by filtering through a Whatman 42
filter paper (Whatman, plc; GE Healthcare) with a pore size of 2.5
µm. The methanol extract was then evaporated with a rotary vacuum
evaporator (BÜCHI® R210; BÜCHI Labortechnik, AG) to
yield a thick extract.
The thick methanol extract was then added to water
and partitioned with n-hexane (1:1), followed by
partitioning with ethyl acetate (1:1). The separation was performed
using thin-layer chromatography (TLC). The ethyl acetate fraction
was screened for flavonoids using Willstatter reagents, and the
target flavonoid compound to be isolated was found in this
fraction.
A total of 14 g ethyl acetate viscous extract
obtained was absorbed in 28 g silica gel G 60 of size 0.2-0.5 mm.
The compounds were then separated using gravity column
chromatography. The eluent used was n-hexane ethyl acetate,
starting with a ratio of 9:1 with an increasing polarity gradient,
followed by 100% ethyl acetate and 100% methanol. From this
separation process, 24 fractions were obtained, which were then
grouped into 6 main fractions based on similar retention factor
(Rf) values in a TLC test using n-hexane and ethyl acetate
(2:8) eluate, termed fractions A, B, C, D, E and F.
The combined fractions of B and C were then targeted
for this study and were separated and purified further. The
combined fractions were absorbed in silica gel as above. Then, the
compounds were separated by gravity column chromatography using a
hexane-chloroform mixture eluent, starting with a ratio of 7:3,
with an increasing polarity gradient, followed by 100% chloroform,
100% ethyl acetate and 100% methanol. Thus, 121 fractions were
obtained, which were then grouped into five main fractions based on
similar Rf values in a TLC test using 100% chloroform eluent,
termed fractions G, H, I, J and K.
The combined fractions of I and J were then absorbed
in silica gel. The compounds were separated by gravity column
chromatography using the chloroform-n-hexane eluent,
starting with a ratio of 19:1 with an increasing polarity gradient,
followed by 100% chloroform and chloroform-ethanol eluent starting
at 19:1, with an increasing polarity gradient to a ratio of 19:1,
followed by 100% ethyl acetate and 100% methanol. From the elution
process, 64 fractions were obtained, which were subsequently
grouped based on similar Rf values in a TLC test using
chloroform-ethanol (97:3) eluent into four main fractions, termed
fractions L, M, N and O, in which the target compound was in the M
and N fractions.
The M and N fractions were combined and further
purified by gravity column chromatography using n-hexane
ethyl acetate (9:1) as the eluent to obtain a pure yellow compound
with a yield of 5 mg. The pure compounds obtained after showing a
single spot on TLC were then identified using proton core nuclear
magnetic resonance spectroscopy (1H-NMR) and carbon core
nuclear magnetic resonance spectroscopy (13C-NMR). NMR
spectroscopic analysis was performed using NMR JEOL ALPHA 500, 1H
500 MHz and 13C 125 MHz, at the Faculty of Pharmacy, Meijo
University, Nagoya, Japan.
Determination of total phenolic
content
The total phenolic content was determined using the
Folin-Ciocalteu method (17,18). The ethanol extract/ethyl acetate
fraction of I. cylindrica was oxidized using Folin-Ciocalteu
reagent (Merck KGaA) and the reaction was neutralized using 20%
Na2CO3. Absorbance was measured by exciting
with blue light at a wavelength of 760 nm after 60 min, employing
standard gallic acid. Total phenolic content is presented in units
of g gallic acid equivalent (GAE) per kg of I. cylindrica
extract/fraction. Measurements were taken three times, and the mean
was taken (19).
Assessment of in vivo lipid-lowering
properties
The present study was approved by the Animal Care
and Use Committee, Faculty of Medicine Universitas Airlangga
(Surabaya, Indonesia). All procedures were performed in accordance
with the Guide for the Care and Use of Laboratory Animals published
by the National Institutes of Health (20). A total of 28 male Wistar rats aged
2-3 months, weighing 180-200 g in a healthy condition were used in
the present study. The animals were acclimatized to the laboratory
conditions for 7 days, provided ad libitum access to food
and water, and maintained under a 12 h light/dark cycle at room
temperature. After acclimatization, the animals were divided
randomly into four groups. The present study utilized a randomized
post-test only control group experimental design. Group 1 (K0): A
negative control group that received a control diet (CD); group 2
(K1): A positive control group that received a high cholesterol
diet (HD); group 3 (K2): A treatment group that was administered a
HD and the ethanol extract of I. cylindrica at a dose of 15
mg/200 g body weight (BW); and group 4 (K3): A treatment group that
was administered a HD and the ethyl acetate I. cylindrica
fraction at a dose of 15 mg/200 g BW [based on Suratman et
al (21) with
modifications].
Rats were used in the present study as they have
similar physiological and behavioural characteristics as humans
(22). The anatomical structure of
rats is slightly different from those of other mammals, for
examples, the oesophagus directly empties into the stomach such
that the rat cannot spit out its food and they possess no
gallbladder ties. At the age of 3 months, these rats are mature and
their anatomy and physiology are optimal; the male rat does not
possess an oestrus cycle, so it does not affect blood cholesterol
levels (23,24).
For the in vivo test, re-extracts were
created by replacing the methanol with ethanol in the
aforementioned extraction procedures to obtain the ethyl acetate
fraction. Ethanol was used due to its very low toxicity. The
present study employed a single dose of 15 mg/200 g BW, according
to the method described by Suratman et al (21) with slight modifications. Ethanol
extracts were composed of various compounds, while the ethyl
acetate fraction was a fraction comprised of semipolar compounds.
The mean weight ± standard deviation of the rats was 197.28±10.78
g, thus, each rat received 15 mg of the extract, which was
dissolved in 2 ml carboxymethyl cellulose sodium (1 ml/100 g
BW).
Hypercholesterolemia was determined on the 15th day
following induction with a HD, which was considered sufficient
based on the increase in total blood cholesterol levels >54
mg/dl, with a normal value of 10-54 mg/dl (23,24).
Preliminary tests demonstrated that the
administration of HD for 14 days resulted in increased blood
cholesterol levels, and hypercholesterolemia persisted via
continuation of a HD until the 30th day (data not shown). Thus, on
days 15-30, the treatment group was administered 15 mg/200 g BW
(K2) ethanol extract and 15 mg/200 g BW (K3) ethyl acetate
fraction. The administration of HD within the control group (K1)
and the treatment group (K2 and K3) was continued until the 30th
day.
On the day of the sacrifice (end of 5th week/day
36), the rats were anesthetized by a 0.1 ml/100 g BW
intraperitoneal injection of a cocktail of drugs (ketamine 50
mg/kg, xylazine 2 mg/kg and acepromazine 0.5 mg/kg) (25). All rats were sacrificed by collecting
the blood from the cardiac vein, and following drawing of blood,
the arteries were cut to ensure termination of the animals.
HD was composed of 12.5% casein, 20% pork oil, 1%
cholesterol, 0.25% cholic acid, 20,52% sucrose, 41,23% flour, 3,5%
salt mixture and 1% multivitamins [based on and modified from
Mohamed et al (26)]. The
administration of HD lasted for one month. The CD was composed of
12,5% casein, 10% corn oil, 23,3% sucrose, 46,7% flour, 3,5% salt
mixture, 1% multivitamins and 3% fibre (26). The feed was formed into pellets and
was provided ad libitum every day for 30 days. Determination
of total cholesterol, low-density lipoprotein (LDL) cholesterol,
and high-density lipoprotein (HDL) cholesterol levels was
outsourced to Surabaya Regional Health Laboratory were enzymatic
methods and spectrophotometry were used.
Statistical analysis
Data were analysed using SPSS version 23 (IBM,
Corp.). Comparisons between total cholesterol, HDL and LDL levels
were assessed using ANOVA, followed by a least significant
difference test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Identification of isolated
compounds
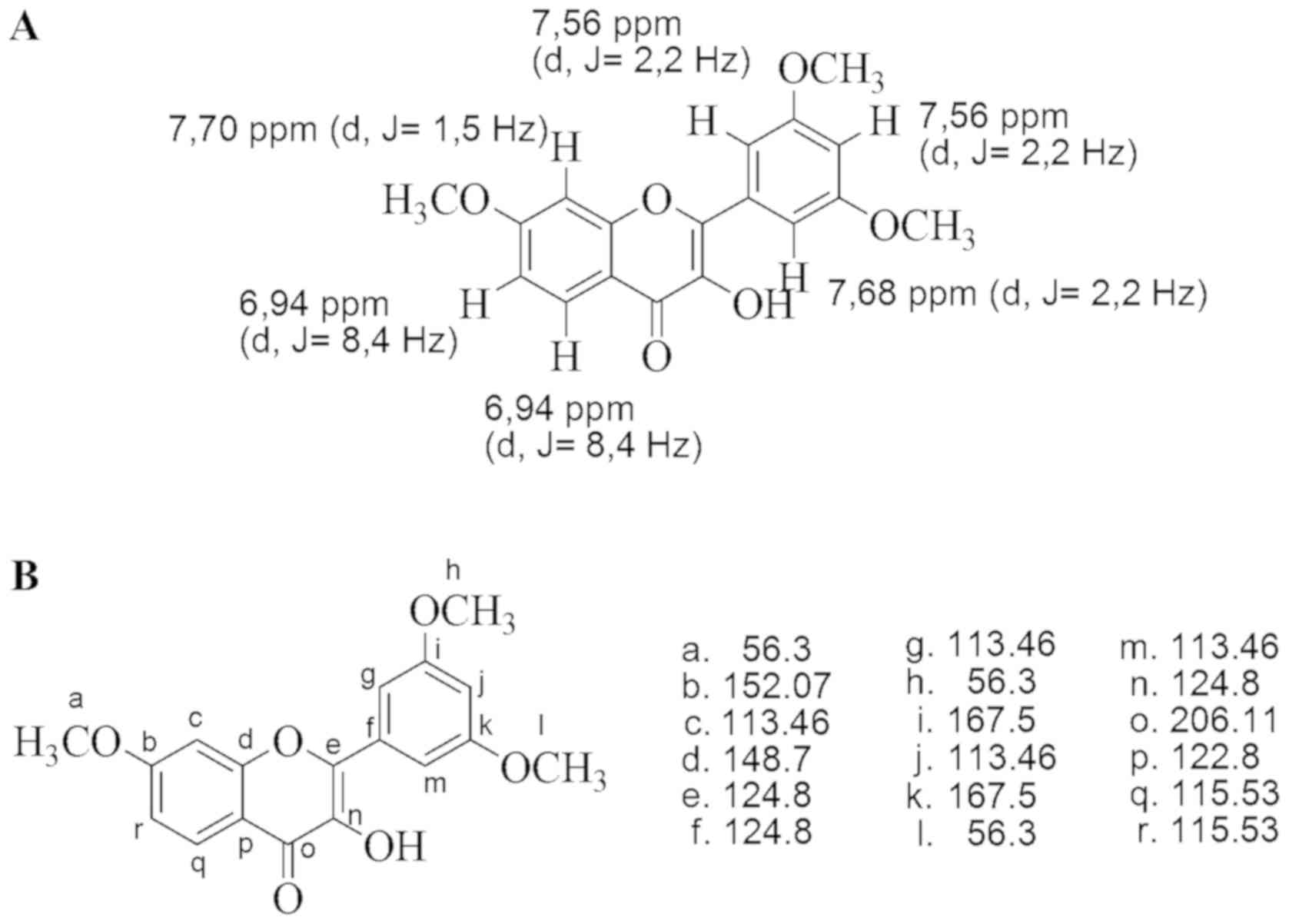
The signals in 13C-NMR [insolvent
(CD3)2 CO] presented at chemical shift (δ) in parts per
million (ppm) values of 206.11, 167.5, 152.07, 148.07, 124.85,
122.89, 115.53, 113.46 and 56.3. The signal at δ 206.11 ppm was the
C-carbonyl signal, whereas the signals at δ 167.5, 152.07, 148.07,
124.85, 122.89, 115.53 and 113.46 ppm represented 14 carbon atoms
in a compound comprised 12 carbon atoms from the two aromatic rings
on rings A and B (which were subdivided into five C-aryloxy atoms
and 7 aromatic carbon atoms) and two ethylenic carbon signals on
ring C. A shift of δ 56.3 ppm indicated the presence of a methoxy
carbon signal (OCH3).
1H-NMR proton spectroscopy analysis in
the CDCl3 solvent revealed that the isolated flavonoid
has 6 aromatic protons at δ ppm 7.70 [1H, doublet (d), J=1.5 Hz],
7.68 (1H, d, J=2.2 Hz), 7.56 (2H, d, J=2.2 Hz) and 6.94 (2H, d,
J=8.4 Hz). The 6-proton-signal aromatic was a doublet distributed
over positions, such that for the meta position, the signal was δ
7.68 ppm (1H, d, J=2.2 Hz) and δ 7.56 ppm (2H, d, J=2.2 Hz),
whereas the proton doublet signal δ 7.70 ppm (1H, d, J=1.5 Hz)
represented a mutual position at an ortho position to the proton
signal δ 6.94 ppm (2H, d, J=8.4 Hz). Signals at δ ppm 7.56 and 6.94
each represented two protons (visible from the signal intensity,
which was two times higher than signals at δ ppm 7.70 and 7.68). A
singlet signal at d ppm 3.87 (9H, s) indicated the presence of
three OCH3 substituents.
The results of 1H-NMR analysis were as
follows: d ppm 7.68 (1H, d, J=2.2 Hz), d ppm 7.56 (2H, d, J=2.2
Hz), d ppm 7.70 (1H, d, J=1.5 Hz) and d ppm 6.94 (2H, d, J=8.4 Hz)
(Fig. 1).
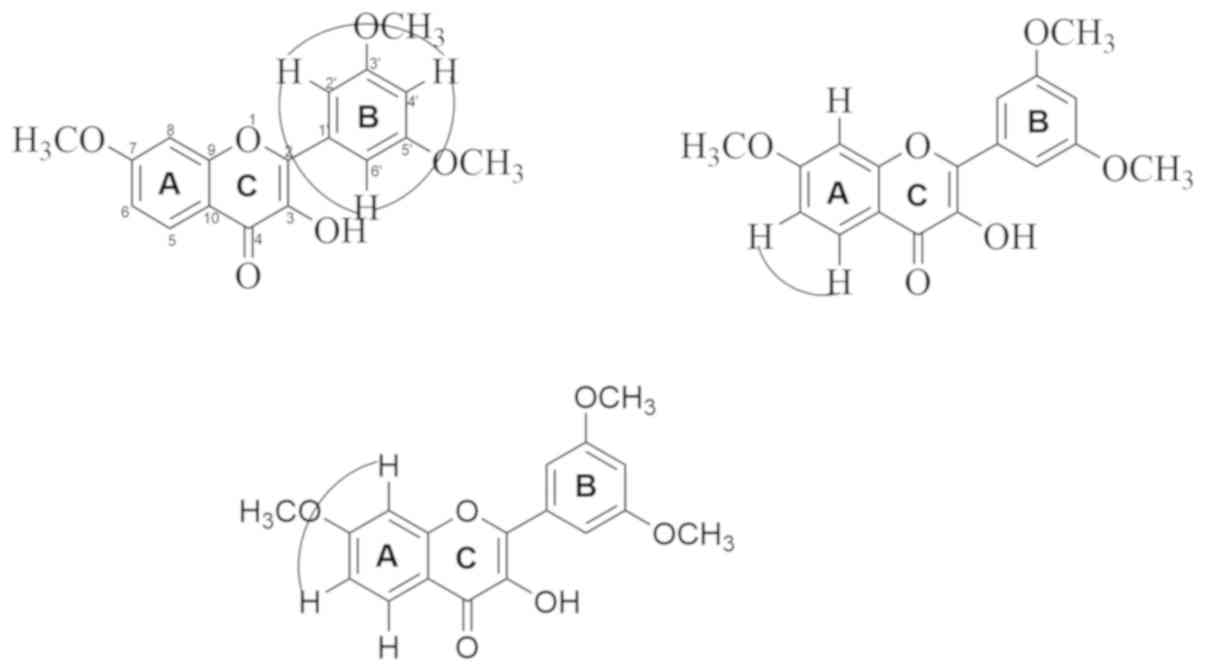
Tables I and II display the NMR spectral analysis of
isolated compounds in comparison to previous studies covering a
structure similar to the isolated compounds (27,28).
From the analysis, the recommended structure for the compound as a
result of isolation is shown in Fig.
2, noting the chemical shift data for each H and C atom.
 | Table IComparison of δ 1H-NMR for
isolated flavonol compounds based on previous studies. |
Table I
Comparison of δ 1H-NMR for
isolated flavonol compounds based on previous studies.
| H position | d Isolated
compound | d Quercetin
(27) | d Kaempferol
(27) |
|---|
| H1 | - | - | |
| H2 | - | - | - |
| H3 | - | - | - |
| H4 | - | - | - |
| H5 | 6.94 ppm, d,
J=8.4 Hz | - | - |
| H6 | 6.94 ppm, d,
J=8.4 Hz | 6.37 ppm, d,
J=2.5 Hz | 6.2 ppm, d,
J=8 Hz |
| H7 | - | - | - |
| H8 | 7.70 ppm, d,
J=1.5 Hz | 6.14 ppm, d,
J=2.5 Hz | 6.4 ppm, d,
J=8 Hz |
| H9 | - | - | - |
| H10 | - | - | - |
| H1' | - | - | - |
| H2' | 7.56 ppm, d,
J=2.2 Hz | 7.64 ppm, d,
J=8.5 Hz | 8.0 ppm, d,
J=8 Hz |
| H3' | - | - | 6.9 ppm, d,
J=8 Hz |
| H4' | 7.56 ppm, d,
J=2.2 Hz | - | - |
| H5' | - | 6.85 ppm, d,
J=8.5 Hz | 6.9 ppm, d,
J=8 Hz |
| H6' | 7.68 ppm, d,
J=0.2 Hz | 7.49 ppm, q,
J=8.5 Hz | (8.0 ppm, d,
J=8 Hz |
| H-OMe | 3.87 ppm, s | - | - |
| OH | - | - | - |
 | Table IIComparison of δ 13C-NMR
for isolated flavonol compounds based on previous studies. |
Table II
Comparison of δ 13C-NMR
for isolated flavonol compounds based on previous studies.
| C position | Isolated compound
δ, ppm | Quercetin δ, ppm
(27) | Quercetin δ, ppm
(28) |
|---|
| C2 | 124.8 | 156.6 | 146.8 |
| C3 | 124.8 | 136.2 | 135.5 |
| C4 | 206.11 | 176.3 | 175.8 |
| C5 | 115.53 | 161.2 | 160.7 |
| C6 | 115.53 | 98.8 | 98.2 |
| C7 | 152.07 | 164.5 | 163.9 |
| C8 | 113.46 | 93.7 | 93.3 |
| C9 | 148.7 | 148.2 | 156.2 |
| C10 | 122.8 | 103.4 | 103.1 |
| C1' | 124.8 | 120.4 | 122.1 |
| C2' | 113.46 | 116.1 | 115.3 |
| C3' | 167.5 | 145.5 | 145.0 |
| C4' | 113.46 | 147.2 | 147.6 |
| C5' | 167.5 | 115.5 | 115.6 |
| C6' | 113.46 | 122.4 | 120.0 |
Total phenolic content
Total phenolic content is expressed as g GAE/kg
extract by comparing the standard gallic acid curve with the
absorbance curve of the sample. Both ethanol extracts and ethyl
acetate fractions were screened using Willstater reagent, and they
produced an orange colour, indicating a positive result for the
presence of a flavonoid compound. The determination of total
phenolic content was performed using the Folin-Ciocalteau method.
In the ethanol extract I. cylindrica, the total phenolic
content was 545.67 g GAE/kg extract. In the ethyl acetate fraction
of I. cylindrica, total phenolic content was 682.33 g GAE/kg
extract.
In vivo lipid-lowering properties
The effect of extracts were assessed in vivo
for 5 weeks. At the end of the 5th week/day 36, the animals were
sacrificed and ~5 ml blood was collected from the cardiac vein.
At the end of the treatment (end of 5th week/day
36), animals from each group (K0, K1, K2 and K3) were weighed.
Total cholesterol, HDL and LDL levels were assessed in the blood
samples. The results showed that the hypercholesterolemia diet in
the experimental group increased cholesterol levels significantly
(Table III and Fig. 3).
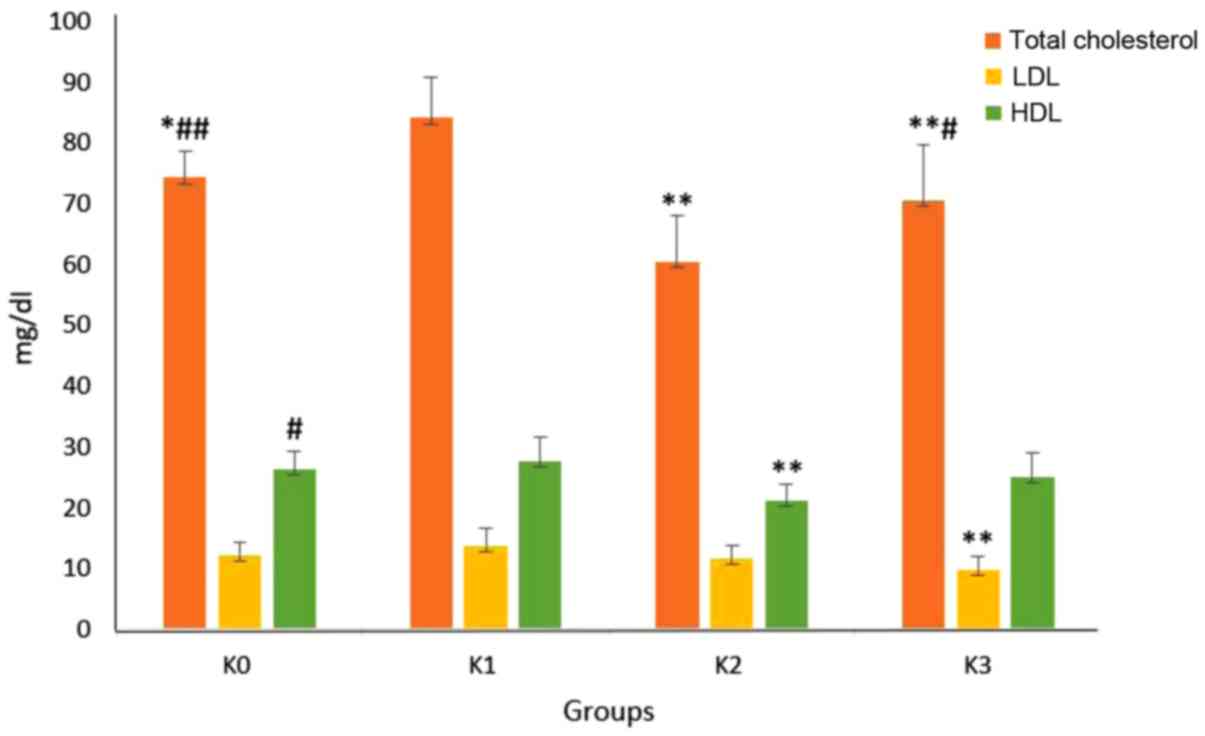 | Figure 3Total cholesterol, LDL and HDL levels
in the experimental animal model. Data are presented as the mean ±
standard deviation. N=6 per group. *P<0.05,
**P<0.01 vs. K1; #P<0.05,
##P<0.01 vs. K2. LDL, low density protein; HDL,
high-density protein; CD, control diet; HD, hypercholesterol diet;
BW, body weight; HD, high cholesterol diet; I. cylindrica,
Imperata cylindrica; K0, negative control group fed a
control diet; K1, positive control fed HD; K2, treatment group fed
a HD and the ethanol extract of I. cylindrica at a dose of
15 mg/200 g BW; K3, treatment group administered a HD and the ethyl
acetate I. cylindrica fraction at a dose of 15 mg/200 g
BW. |
 | Table IIITotal cholesterol, LDL and HDL levels
in vivo. |
Table III
Total cholesterol, LDL and HDL levels
in vivo.
| Variables | K0a | K1a | K2a | K3a |
|---|
| Total
cholesterol |
74.29±4.3b,e | 84.14±6.6 |
60.43±7.6c |
70.57±9.2c,d |
| LDL | 12.14±2.1 | 13.71±2.9 | 11.57±2.3 |
9.86±2.2c |
| HDL |
26.29±3.1d | 27.57±4.1 |
21.14±2.8c | 25±4.1 |
Effect of ethanol extract and ethyl
acetate fraction on total cholesterol levels
Treatment with the ethanol extract (15 mg/200 g BW;
K2) lowered the total cholesterol levels significantly (P=0.001;
mean ± standard deviation, 60.43±7.6 mg/dl) compared with the
positive control group (K1). Treatment with the ethyl acetate
fraction (15 mg/200 g BW; K3) also lowered the total cholesterol
levels significantly (P=0.002; mean ± standard deviation, 70.57±9.2
mg/dl) compared with the positive control group (K1) (Table III and Fig. 3).
Effect of ethanol extract and ethyl
acetate fraction on LDL levels
Treatment with the ethanol extract (K2) reduce LDL
levels but the difference was not significant (P=0.109; mean ±
standard deviation, 11.57±2.3 mg/dl) compared with the positive
control group (K1). The ethyl acetate fraction (K3) significantly
reduced LDL levels (P=0.006; mean ± standard deviation, 9.86±2.2
mg/dl) compared with the positive control group (K1) (Table III and Fig. 3).
Effect of ethanol extract and ethyl
acetate fraction on HDL levels
The ethanol extract (K2) significantly reduced HDL
levels (P=0.003; mean ± standard deviation, 21.14±2.8 mg/dl
compared with the positive control group (K1). The ethyl acetate
fraction (K3) did not significantly reduce HDL levels (P=0.190;
mean ± standard deviation, 25±4.1 mg/dl compared with positive the
control group (K1) (Table III and
Fig. 3).
Discussion
Based on the results of the analysis of
1H-NMR and 13C-NMR, the isolated compound was
considered to be a flavonoid of the flavonol group, and consisted
of 18 carbon atoms and 16 hydrogen atoms, termed
7,3',5'-trimethoxyflavonol. The ethyl acetate fraction of I.
cylindrica extraction was rich in flavonoid compounds.
A comparison of total serum cholesterol levels
between the CD (K0) and HD-fed (K1) groups showed that the HD
induced hypercholesterolemia in vivo. The present study
demonstrated that a diet of pork fat and pure cholesterol for 4
weeks was successful in raising rat blood cholesterol levels in the
K1 group.
The results demonstrated that the administration of
the ethanol extract (K2) or the ethyl acetate fraction (K3) of
I. cylindrica was capable of significantly reducing total
cholesterol levels compared with the HD group (K1). The
administration of the ethyl acetate fraction of I.
cylindrica also significantly reduced LDL levels, and in groups
treated with the ethanol extract, I. cylindrica extract did
not significantly reduce cholesterol levels compared with the HD
group. The administration of the ethyl acetate fraction of I.
cylindrica did not exert a significant effect on HDL levels
(P>0.05), and in the group administered with the ethanol extract
of I. cylindrica, HDL levels were significantly reduced
compared with the group fed an HD.
Based on previous studies, screening of the ethanol
extracts of I. cylindrica showed the presence of tannins,
saponins, flavonoids, alkaloids, cardiac glycosides, coumarin and
terpenoids (29,30). It has also been demonstrated that
I. cylindrica is a good source of antioxidants, including a
high content of flavonoid and total phenolic compounds (4). Zhou et al (31) also showed that Rhizomes
Imperata extract is rich in polyphenols, which also exhibit
antioxidant activity.
The decrease in total cholesterol levels in the
group administered ethanol extract was larger compared with the
group administered the ethyl acetate fraction. Compounds contained
within the ethanol extracts include flavonoids and saponins
(32). Saponin binds with
cholesterol and bile to form strong complex compounds and cannot be
reabsorbed (33,34). This results in cholesterol
elimination and increased bile excretion. The body attempts to
increase the conversion of cholesterol into bile acids, thus
reducing blood cholesterol levels (35).
The LDL cholesterol levels in the group administered
the ethyl acetate fraction exhibited a larger decrease compared
with the group administered the ethanol extract. In the ethyl
acetate fraction, there were only semipolar compounds such as
flavonoids, which reduce blood cholesterol levels by inhibiting
cholesterol synthesis and increasing LDL receptor expression
(36,37). Several studies have shown that the
consumption of isoflavones induces a decrease in plasma cholesterol
in C57BL/6 mice, increasing the activity of LDL receptors on HepG2
cells (37,38). This is likely due to the effect of
flavonoids on sterol regulatory element-binding protein 2(37). The ethyl acetate fraction consists of
only semipolar compounds, and this is hypothesized to have a direct
effect on LDL reduction, while the ethanol extract contains several
complex compounds which exhibit antagonistic interactions between
each other. Therefore, the effects of active constituents may be
masked by other compounds in this complex mixture, thus making the
LDL reduction effect of ethyl acetate fraction more potent than
that of the ethanol extract. This phenomenon also occurs in
mixtures of natural product (39).
Semipolar compounds are considered to be more effective in
regulating LDL uptake. The effect of reducing LDL cholesterol using
the ethyl acetate fraction was better compared with the ethanol
extract. It is hypothesized that the semipolar compounds increase
LDL uptake, which can affect the regulation of LDL.
Flavonoids are reported in the literature to
regulate apolipoprotein-B (apoB) secretion and cellular cholesterol
homeostasis in human hepatoma cell lines, reducing ester
cholesterol mass, inhibiting acyl-CoA: Cholesterol acyltransferase
expression, inhibiting microsomal triglyceride transfer protein
activity, and furthermore, inhibiting hepatic lipid synthesis by
inhibiting apoB and increasing apoA levels in HepG2 cells (40).
Across all groups, the increase or decrease in HDL
cholesterol levels could not be exclusively attributed to the
administration of HD or the extract as it was suspected that the
conditions of experimental animals were still in a compensated
state; the positive control group (K1) showed high levels of HDL
compared with other groups (K0, K2 and K3). This finding was
supported by Dauqan et al (41), where, the normal stressed group of
mice exhibited an increase in HDL levels, and Hayek et al
(42) who suggested that a diet of
saturated fat and a cholesterol diet facilitated an increase in HDL
and Apo-A1 cholesterol levels.
Hayek et al (42) also suggested that a high-fat diet
increased HDL levels, facilitated by an adaptation mechanism that
is reflected in the increase in the flux of HDL, cholesterol ester
(CE) and transport rate necessary when a high metabolic burden is
created following a high-fat or high-cholesterol diet. A high-fat
diet may reverse the transport of cholesterol via the HDL pathway.
The administration of a high-fat diet can increase
apolipoprotein-A1 (Apo-A1) levels via a posttranscriptional
mechanism by increasing the translational ability of Apo-A1 mRNA
and decreasing intracellular Apo-A1 degradation. High-fat diets can
reduce the rate of catabolism of the HDL-CE and Apo-A1 fractions,
which may be caused by an increase in HDL size. The duration of the
diet is also considered a factor that influences the increase in
HDL levels within the group fed a high-fat diet (42,43).
This is further supported by Hayek et al (42), where administration of high-fat diets
provided for 4 weeks resulted in an increase in HDL levels.
In general, the ethyl acetate fraction resulted in
the most favourable effects, as the lipid-lowering profile included
reduced total cholesterol and LDL levels, compared with the ethanol
extract. This result is supported by the total phenolic contents of
the two extracts, as the total phenolic content was higher in the
ethyl acetate fraction compared with the ethanol extract.
The results of the present study are supported by
previous studies that have shown the presence of an association
between the intake of flavonoids such as flavones and flavonols and
a decreased risk of coronary artery disease; even data on the
intake of anthocyanins and flavanones showed reduced mortality
rates from coronary cardiovascular diseases (CVDs) (44-46).
A meta-analysis demonstrated that the consumption of three cups of
tea per day reduced the risk of CVD by 11%, while the consumption
of red wine reduced the risk of CVD by 32% (47). A high intake of flavonoids from herbs
was associated with a reduced risk of developing cardiovascular
disease. The mechanism underlying their beneficial effects however,
remain unclear, but current evidence suggests that flavonoids can
affect cardiovascular risk factors (35).
A limitation of the present study was the use of a
single dose for assessing the effects of the extracts and the
fraction. Thus, the dose-dependency and optimal dose were not
determined. Furthermore, quantitative tests of various compounds in
the extracts and fractions were not performed; thus, synergy
calculations could not be performed.
In summary, a pure compound was extracted from I.
cylindrica roots in the form of a yellow powder. Based on the
results of the analysis by 1H-NMR and
13C-NMR, the isolated compound was shown to be a
flavonoid, which consisted of 18 carbon atoms and 16 hydrogen atoms
and was termed 7,3',5'-trimethoxyflavonol. The in vivo tests
showed that the ethyl acetate fraction of I. cylindrica
reduced total cholesterol and LDL level more effectively than the
ethanol extract but did not affect HDL levels in a rat model of
hypercholesterolemia.
Acknowledgements
We would like to thank Associate Professor Yoshiaki
Takaya (Faculty of Pharmacy, Meijo University, Nagoya, Japan) for
his support.
Funding
The present study was supported by a Grant-in-Aid
from Dato' Sri Prof. Dr. Tahir through the Tahir Professorship
Program.
Availability of data and materials
The datasets used and/or analysed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
SKh conceived and designed the study. NSA, ANK, SKh,
SKu and SSu performed the sample collection. SKh, CDKW and SSo
performed the laboratory experiments. SKh and CDKW analysed the
data and wrote the manuscript. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
the Faculty of Medicine, University of Airlangga (Surabaya,
Indonesia) (approval no. 027/EC/KEPK/FKUA/2012).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hidayat S and Rachmadiyanto AN:
Utilization of Alang-Alang (Imperata cylindrica (L.)
Raeusch.) as Traditional Medicine in Indonesian Archipelago. Proc
1st Satreps Conf. 1:82–89. 2017.
|
|
2
|
Subositi D and Widodo H: Genetic diversity
of Cogon grass (Imperata cylindrica (L.) Beauv) based on the
intersimpel marking of the sequence repeat (ISSR). Jurnal Ilmu-Ilmu
Hayati. 17:115–122. 2018.
|
|
3
|
Khaerunnisa S: Utilization of bioactive
compounds from alang-alang root (Imperata cylindrica) as an
antioxidant, 2009.
|
|
4
|
Lalthanpuii PB, Zarzokimi and
Lalchhandama K: Some phytochemical analyses of different extracts
of the cogon grass Imperata cylindrica from Mizoram, India.
Sci Vis. 18:120–124. 2018.
|
|
5
|
Padma R, Parvathy NG, Renjith V and Rahate
KP: Quantitative estimation of tannins, phenols and antioxidant
activity of methanolic extract of Imperata cylindrica. Int J
Res Pharm Sci. 4:73–77. 2013.
|
|
6
|
Anggraeni N, Syamsunarno MRA, Mukarromah
GR, Zada A, Triatin RD, Pamela Y and Dhianawaty D: Low Serum
cholesterol in Mice Pre-treated with Imperata cylindrica L.
after Acute Olive Oil Gavage. KnE Life Sci. 3(460)2017.
|
|
7
|
National Heart Foundation of Australia:
The economic burden of Hypercholesterolaemia. Australia, 2018.
|
|
8
|
Alinde OBL, Esterhuyse AJ and Oguntibeju
OO: Role of reactive oxygen species in the pathogenesis of
cardiovascular disease. Sci Res Essays. 7:4151–4159. 2012.
|
|
9
|
Malekmohammad K, Sewell RDE and
Rafieian-Kopaei M: Antioxidants and atherosclerosis: Mechanistic
aspects. Biomolecules. 9(301)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Panth N, Paudel KR and Parajuli K:
Reactive oxygen species: A key hallmark of cardiovascular disease.
Adv Med. 2016(9152732)2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Singh UN, Kumar S and Dhakal S: Study of
oxidative stress in hypercholesterolemia. Int J Contemp Med Res.
4:2454–7379. 2017.
|
|
12
|
Phaniendra A and Latha P: Free radicals:
Properties, sources, targets, and their implication in various free
radicals: Properties, sources, targets, and their implication in
various diseases. Ind J Clin Biochem. 30:11–26. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Das Sarma A, Rahaman Mallick A and Ghosh
A: Free radicals and their role in different clinical conditions:
An overview. Int J Pharma Scie Res. 1:185–192. 2010.
|
|
14
|
Abdul Qadir M, Shahzadi SK, Bashir A,
Munir A and Shahzad S: Evaluation of phenolic compounds and
antioxidant and antimicrobial activities of some common herbs. Int
J Anal Chem. 2017(6)2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Tungmunnithum D, Thongboonyou A, Pholboon
A and Yangsabai A: Flavonoids and other phenolic compounds from
medicinal plants for pharmaceutical and medical aspects: An
overview. Medicines. 5(93)2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Rajput B, Golave A, Yadav S and Jadhav JP:
Total phenolic concentrations and antioxidant activities in Drimia
sp. J Herbs Spices Med Plants. 24:28–36. 2018.
|
|
17
|
Sánchez-Rangel Carlos J, Jorge BJ, Heredia
JB, Zevallos LC and Jacobo-Velázquez DA: The Folin-Ciocalteu assay
revisited: Improvement of its speci fi city for total phenolic
content determination. Anal Methods. 5:5990–5999. 2013.
|
|
18
|
Blainski A, Lopes G and de Mello J:
Application and analysis of the folin ciocalteu method for the
determination of the total phenolic content from Limonium
Brasiliense L. Molecules. 18:6852–6864. 2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Mohamed OS, Said MM, Ali ZY, Atia HA and
Mostafab HS: Improving effect of dietary oat bran supplementation
on oxidative stress induced by hyperlipidemic diet. Researcher.
3:1–10. 2011.
|
|
20
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals. Guide for the Care and Use of Laboratory Animals. 8th
edition. Washington (DC), National Academies Press (US); 2011.
Available from: https://www.ncbi.nlm.nih.gov/books/NBK54050/
doi: 10.17226/12910.
|
|
21
|
Suratman S, Listyawati S and Sutarno S:
Physical characteristics and NaCl content of urine white male rat
(Rattus norvegicus L.) after orally intakes of cogon grass rhizome
(Imperata cylindrica L.) extract. Biofarmasi J Nat Prod
Biochem. 1:7–12. 2003.
|
|
22
|
Gilbert G and Lickliter R: The various
roles of animal models in understanding human development. Soc Dev.
13:311–325. 2004.
|
|
23
|
Suryaningtyas W, Prasetyo R and Dewi B:
Research and laboratory techniques in experimental animals and
human. Airlangga University Press, Surabaya, 2015.
|
|
24
|
Chow PKH, Ng RTH and Ogden BE: Using
Animal Models in Biomedical Research. World Scientific Publishing,
Singapore, pp 1-308, 2008.
|
|
25
|
Institutional Animal care and use
committee: Anesthesia (Guideline). Available from: https://animal.research.uiowa.edu/iacuc-guidelines-anesthesia.
|
|
26
|
Mohamed DA, Hamed TE and Al-Okbi SY:
Reduction in hypercholesterolemia and risk of cardiovascular
diseases by mixtures of plant food extracts: A study on plasma
lipid profile, oxidative stress and testosterone in rats. Grasas Y
Aceites: 61: 2010 doi: 10.3989/gya.021210.
|
|
27
|
Awaad S, Mohamed N, Maitland D and Soliman
G: Anti-ulcerogenic activity of extract and some isolated
flavonoids from Desmostachia bipinnata (L.) Stapf. Rec Nat Prod.
2(76)2008.
|
|
28
|
Markham K: How to identify flavonoids.
Institut Teknologi Bandung, Bandung, 1988.
|
|
29
|
Krishnaiah D, Devi T, Bono A and Sarbatly
R: Studies on phytochemical constituents of six Malaysian medicinal
plants. J Med Plants Res. 3:067–072. 2009.
|
|
30
|
Babu RH and Savithramma N: Phytochemical
screening of underutilized species of Poaceae. An Int J. 1:947–951.
2013.
|
|
31
|
Zhou X, Wang J, Jiang B, Shang J and Zhao
C: A study of extraction process and in vitro antioxidant activity
of total phenols from Rhizoma imperatae. Afr J Traditional
Complementary Alternative Med. 10:175–178. 2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Akpakpan A, Ukpong EJ and Willie I:
Phytochemical screening of the ethanol and aqueous extracts of
dicliptera verticillata leaves phytochemical screening of the
ethanol and aqueous extracts of dicliptera verticillata Leaves,
2017.
|
|
33
|
Afrose S, Hossain S, Salma U, Miah AG and
Tsujii H: Dietary karaya saponin and Rhodobacter capsulatus
exert hypocholesterolemic effects by suppression of hepatic
cholesterol synthesis and promotion of bile acid synthesis in
laying hens. Cholesterol. 2010(7)2010.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Shi Y, Guo R, Wang X, Yuan D, Zhang S,
Wang J, Yan X and Wang C: The regulation of alfalfa saponin extract
on key genes involved in hepatic cholesterol metabolism in
hyperlipidemic rats. PLoS One. 9(e88282)2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Vinarova L, Vinarov Z, Atanasov V,
Pantcheva I, Tcholakova S, Denkov N and Stoyanov S: Lowering of
cholesterol bioaccessibility and serum concentrations by saponins:
In vitro and in vivo studies. Food Funct. 6:501–512.
2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kobayashi S: The effect of polyphenols on
hypercholesterolemia through inhibiting the transport and
expression of Niemann-Pick C1-Like 1. Int J Mol Sci.
20(4939)2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zeka K, Ruparelia K, Arroo R, Budriesi R
and Micucci M: Flavonoids and their metabolites: Prevention in
cardiovascular diseases and diabetes. Diseases. 5(19)2017.
View Article : Google Scholar
|
|
38
|
Millar CL, Duclos Q and Blesso CN: Effects
of dietary flavonoids on reverse cholesterol transport, HDL
metabolism, and HDL function. Adv Nutr. 8:226–239. 2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Caesar LK and Cech NB: Synergy and
antagonism in natural product extracts: When 1 + 1 does not equal
2. Nat Prod Rep. 36:869–888. 2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Roza JM, Xian-Liu Z and Guthrie N: Effect
of citrus flavonoids and tocotrienols on serum cholesterol levels
in hypercholesterolemic subjects. Altern Ther Health Med. 13:44–48.
2007.PubMed/NCBI
|
|
41
|
Dauqan EMA, Abdullah A, Sani HA and
Selangor B: Lipid profile and antioxidant enzymes in normal and
stressed rat fed with palm olein school of biosciences and
biotechnology, school of chemical sciences and food technology,
faculty of science and technology. Am J Appl Sci. 9:1071–1078.
2012.
|
|
42
|
Hayek T, Ito Y, Azrolan N, Verdery RB,
Aalto-Setmla K, Walsh A and Breslow JL: Dietary fat increases high
density lipoprotein (HDL) levels both by increasing the transport
rates and decreasing the fractional catabolic rates of HDL
cholesterol ester and apolipoprotein (Apo) A-I. Presentation of a
new animal model and mechanistic studies in human Apo A-I
transgenic and control mice. J Clin Invest. 91:1665–1671.
1993.PubMed/NCBI View Article : Google Scholar
|
|
43
|
San Mauro Martín I, Collado Yurrita L,
Cuadrado Cenzual MÁ, Ciudad Cabañas MJ and Mendive Dubourdieu P:
Role of ApoA1 on High-density lipoprotein: An intervention with
plant sterols in patients with hypercholesterolemia. Nutr Hosp.
31:494–499. 2014.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Wallace TC: Anthocyanins in cardiovascular
disease prevention anthocyanins in cardiovascular disease 1. Adv
Nutr. 2:1–7. 2011.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Grassi D, Desideri G and Ferri C:
Flavonoids: Antioxidants against atherosclerosis. Nutrients.
2:889–902. 2010.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Cassidy A, Bertoia M, Chiuve S, Flint A,
Forman J and Rimm EB: Habitual intake of anthocyanins and
flavanones and risk of cardiovascular disease in men. Am J Clin
Nutr. 104:587–594. 2016.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Vauzour D, Rodriguez-Mateos A, Corona G,
Oruna-Concha MJ and Spencer JPE: Polyphenols and human health:
Prevention of disease and mechanisms of action. Nutrients.
2:1106–1131. 2010.PubMed/NCBI View Article : Google Scholar
|