Introduction
Heat stress response is a complicated process that
protects an organism from potential injuries. The response involves
the activation of the neuroendocrine axis and the secretion of
stress hormones (1). It has been
reported that heat stress may elicit a range of coordinated
autonomic physical responses to maintain the balance of the
organism (2). The organism in turn,
possesses a ‘thermostat’ like function to respond to changes in the
environmental temperature, by increasing the body temperature,
heart rate and cardiac output and decreasing the organism's
activity (3). Stress factors trigger
a succession of cascade responses, including the
hypothalamic-pituitary-adrenal axis (HPA axis). The HPA axis is a
key component of the physiological response to heat stress, and is
composed of the paraventricular nucleus (PVN) of the hypothalamus,
the hypophysis and the adrenal cortex (4). Therefore, the HPA axis serves a vital
role in the stress response (5).
Corticotropin-releasing hormone (CRH), which is synthesized and
secreted by the neuroendocrine neurons of the hypothalamus,
stimulates the release of adrenocorticotropic hormone (ACTH).
Glucocorticoids, primarily cortisol (COR), are synthesized in the
adrenal cortex following stimulation by ACTH. In contrast,
glucocorticoids act on the hypothalamus and pituitary gland by
inhibiting the secretion of CRH and ACTH to normalize COR secretion
(6).
Neuropeptide Y (NPY) is one of the most abundant
polypeptides present in the central nervous system (CNS). NPY can
be detected in the hypothalamus, amygdaloid nucleus and
hippocampus. The arcuate nucleus of the hypothalamus contains the
highest concentration of NPY (7). As
a neurotransmitter, NPY serves an important role in regulating the
stress-related behavior and adaptation of the organism to
environmental challenges (8,9). In addition, NPY is involved in the
central mechanism of regulating psychological and physiological
stress, which serves as a protective factor, termed the ‘stress
factor’ (10). It has been shown
that CRH and NPY exert opposite effects (11). CRH was primarily discovered in 1955
and was initially detected in the PVN of the hypothalamus (12,13). CRH
acts as an important physiological regulator in initiating stress
responses and it is the most potent ACTH secretagogue. Several
depressive disorders have been attributed to CRH action (14,15).
Proopiomelanocortin (POMC) is a precursor peptide
that induces the production of several types of bioactive
neuropeptides, such as the opioid peptide, α-melanocyte-stimulating
hormone and ACTH (16,17). COR is the primary glucocorticoid
hormone and the major end product of the HPA axis (18). COR is secreted in accordance with the
host organism's natural circadian rhythm under non-stressful
conditions (18). However, in
response to stress, COR is released throughout the body (19). Due to a negative feedback mechanism,
COR concentration is not increased unconventionally under heat
stress conditions (20). ACTH is a
derivative of POMC, and it affects COR release, indicating a
possible association between ACTH and COR (21).
It has been confirmed that both the HPA axis, but
also the sympathetic nervous system (SNS) regulate the normal
functioning of the body (18,22). The
SNS can promote an abundant release of epinephrine (EPI) into the
bloodstream. EPI is a prototypical stress hormone released from the
adrenal medulla into the peripheral circulation to maintain the
balance of the organism and respond to stressful stimuli (23). In addition, EPI increases blood
pressure and glucose concentration in the blood (24). The SNS is more sensitive and responds
faster than the HPA axis in stress-responsive biological systems
(25). Emerging evidence has
suggested that the expression of protective proteins, namely heat
shock proteins (HSPs), is upregulated in organisms exposed to high
temperatures (26). HSPs belong to a
group of highly conserved proteins and act as molecular chaperones
(27). HSPs are classified according
to their molecular weights and homology in the 110, 90, 70 and 60
kDa classes (28). Several studies
have shown that HSP70 is the most abundant, important and inducible
protein among the members of the HSP family, and it promotes heat
resistance and protects cells from damage (29-31).
Along with continuing climate change, more attention
has been paid to the impact of the thermal environment on the
organism. However, to the best of our knowledge, there are no
studies assessing the effects of different time and temperature
exposures on NPY and POMC mRNA expression levels in the
hypothalamus and hypophysis. Additionally, whether the combination
of the genetic background with stress-related factors affects the
response of the organism to heat stress has not yet been
elucidated. The aim of the present study was to investigate the
changes in POMC and NPY mRNA expression levels. Therefore, the
association between the expression of POMC, NPY and stress-related
factors under different heat stress conditions was explored using
different models. Furthermore, the effects of different intensities
of heat stress on the spleen, thymus, hypophysis and hypothalamus
weight was also investigated.
Materials and methods
Animals and heat exposure groups
Male Sprague-Dawley (SD) rats weighing 200±20 g,
were obtained from the Laboratory Animal Center of Ningxia Medical
University (Yinchuan, China). Animals were housed 3-4 per plastic
cage with ad libitum access to water and food with a 12/12-h
light/dark cycle. The cages were cleaned once every 2 days.
Following a 1 week habituation period, 60 healthy male SD rats were
randomly divided into four groups: Control (CN); moderate strength
6 h (MS6); moderate strength 24 h (MS24); and high strength 6 h
(HS6) groups. The rats in the CN group were exposed to a
temperature of 24±2˚C for 24 h, and all experimental groups were
compared with the same CN group. Rats in the heat exposure groups
were placed in an intelligent artificial climate chamber (ZRS-JSW;
Hangzhou Pnshar Technology Co., Ltd.). Rats in the MS6 and MS24
groups were exposed to a temperature of 32±1˚C for 6 and 24 h,
respectively. Rats in the HS6 group were maintained at 38±1˚C for 6
h. Heat stress was repeated for 14 consecutive days. Heat exposure
for 6 and 24 h was considered moderate and long-time exposure,
respectively. Finally, heat exposure at 32˚C and 38˚C was
considered moderate and high temperature exposure, respectively.
All animal experimental procedures were approved by Ningxia Medical
University Institutional Review Board (approval no.
NXMU-2017-030).
Blood sample and organ collection
Following heat exposure, all rats were subjected to
intraperitoneal anesthesia with 20% urethane (20 g powdered
urethane dissolved in 100 ml deionized water), and blood samples
were obtained from the posterior vena cava. Plasma was separated
from blood by centrifugation at 4˚C at 4,500 x g for 15 min, and
the supernatant was stored at -80˚C for subsequent analyses. The
spleen, thymus, hypophysis and hypothalamus were successively
collected following intravenous blood collection. Hypophysis and
hypothalamus tissues were stored in a refrigerator at -80˚C after
liquid nitrogen freezing and were used for gene expression analysis
assays.
Relative organ weights
measurement
Spleen, thymus, hypophysis and hypothalamus were
removed and precisely weighed using an electronic analytical
balance (JA2003N; Shanghai Yoke Instrument Co., Ltd.). Relative
weight was calculated as follows: Relative weight=weight of the
organ/weight of the rat.
Measurement of COR, EPI, CRH and HSP70
in the plasma
The plasma concentrations of CRH (CSB-E08038r), COR
(CSB-E05112r), EPI (CSB-E08678r) and HSP70 (CSB-E08308r) were
determined using ELISA (Cusabio Technology LLC). The optical
density (OD value) was determined at 450 nm using an universal
microplate reader (Bio-Rad Laboratories, Inc.). A standard curve
was constructed for each component to determine their
concentrations. The concentration values of CRH, COR and HSP70 are
expressed in ng/ml and that of EPI in pg/ml.
RNA isolation and reverse
transcription-quantitative (q)PCR
Total RNA was isolated from the hypophysis (for
measurement of POMC) and hypothalamus (for measurement of NPY)
using an RNA extraction kit (Axygen; Corning, Inc.) according to
the manufacturer's protocol. RNA integrity was assessed using 2.0%
agarose gel electrophoresis. Subsequently, cDNA was synthesized
using a cDNA Synthesis kit (Transgen Biotech Co., Ltd.) according
to the manufacturer's protocol, and stored at -80˚C for subsequent
analysis. The qPCR reactions were performed in a total volume of 25
µl containing 1 µl cDNA, 0.5 µl forward primer (10 µM), 0.5 µl
reverse primer (10 µM), 12.5 µl 2x qPCR SuperMix and 10.5 µl
ddH2O, and amplified on a real-time PCR system
(FTC-3000; Funglyn Biotech, Inc.). The qPCR thermocycling
conditions were as follows: 94˚C for 5 min (initial denaturation);
followed by 36 (for NPY) or 42 (for POMC) cycles of amplification
at 94˚C for 30 sec (denaturation), 62˚C for 30 sec (annealing) and
72˚C for 30 sec (extension). The qPCR primer sequences for POMC,
NPY and β-actin are listed in Table
I. β-actin was used as the loading control. The relative gene
expression levels were quantified using the 2-ΔΔCq
method (32), where ΔΔCq=ΔCq
(sample)-ΔCq (control) and ΔCq=Cq (target gene)-Cq (reference
gene).
 | Table IPrimer sequences, GenBank accession
codes and expected product sizes. |
Table I
Primer sequences, GenBank accession
codes and expected product sizes.
| Genes | Sequence, 5'-3' | GenBank accession
no. | Base pairs |
|---|
|
Proopiomelanocortin | | NM_139326 | 203 |
|
Forward |
CCTGCTTCAGACCTCCATAGAC | | |
|
Reverse |
AGCGGAAGTGACCCATGAC | | |
| Neuropeptide Y | | NM_012614 | 107 |
|
Forward |
GCTCTGCGACACTACATCAATC | | |
|
Reverse |
GCATTTTCTGTGCTTTCTCTCA | | |
| β-actin | | NM_031144 | 207 |
|
Forward |
CACCCGCGAGTACAACCTTC | | |
|
Reverse |
CCCATACCCACCATCACACC | | |
Statistical analysis
Experimental data were analyzed using SPSS version
16.0 (SPSS, Inc.). All results are expressed as the mean ± standard
deviation and where compared using a one-way ANOVA followed by a
post-hoc SNK-test to compare the differences between two samples.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Relative organ weights
The relative organ weights are listed in Table II. The measurements showed that the
relative weight of the spleen in the MS24 group was significantly
lower compared with that in the CN and MS6 groups (CN, P=0.002;
MS6, P=0.031; P<0.05). Similarly, the weight of the hypothalamus
in the MS24 group was significantly lower than that in the CN group
(P=0.002; P<0.05). Compared with the CN group, hypophysis
(P=0.049; P<0.05) and hypothalamus (P=0.028; P<0.05) weights
were significantly decreased and increased, respectively. In the
HS6 group, there was no statistically significant difference in the
weight of the thymus amongst the different groups. Although the
weights of the spleen, hypophysis and hypothalamus in the MS6
group, hypophysis in the MS24 group and spleen in the HS6 group
were lower compared with those in the CN group, no statistically
significant differences were observed.
 | Table IIRelative organ weights. |
Table II
Relative organ weights.
| Organs | Control | MS6 | MS24 | HS6 |
|---|
| Spleen,
x10-3 g | 2.458±0.265 | 2.350±0.303 |
2.112±0.194a,b | 2.293±0.201 |
| Thymus,
x10-3 g | 2.259±0.478 | 2.225±0.360 | 2.334±0.251 | 2.039±0.288 |
| Hypophysis,
x10-5 g | 4.182±0.908 | 3.966±0.671 | 4.233±0.633 |
3.510±0.778a |
| Hypothalamus,
x10-5 g | 6.465±1.518 | 7.461±1.213 |
5.702±0.934b |
7.708±1.228a |
Plasma concentration of CRH, COR, EPI
and HSP70
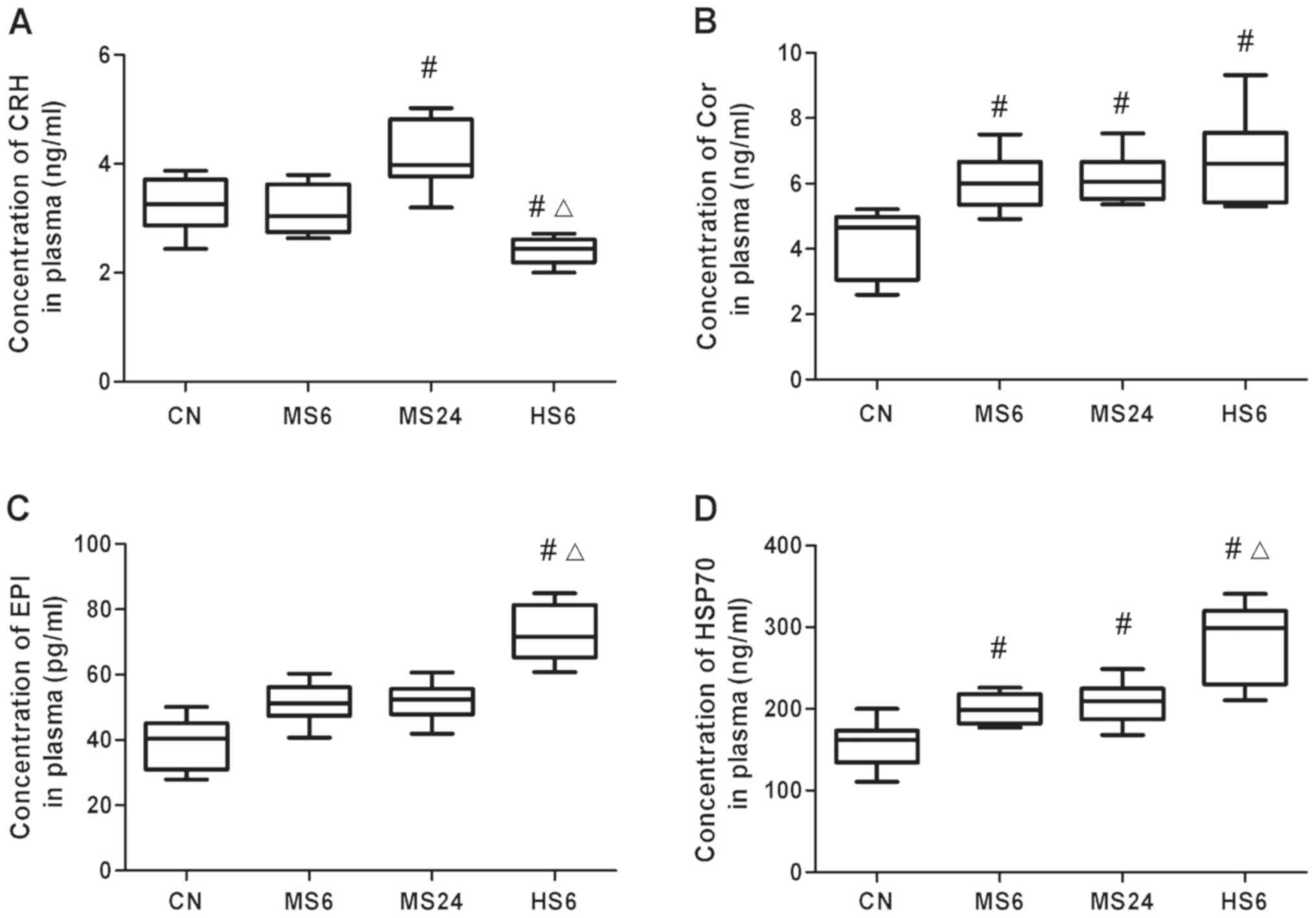
Following exposure of rats at an ambient temperature
of 38˚C, CRH levels were decreased in the HS6 group compared with
those in the CN and MS6 groups (CN, P=0.004; MS6, P<0.001).
Additionally, CRH levels were increased in the MS groups, and most
notably in the MS24 group (P=0.025; Fig.
1A).
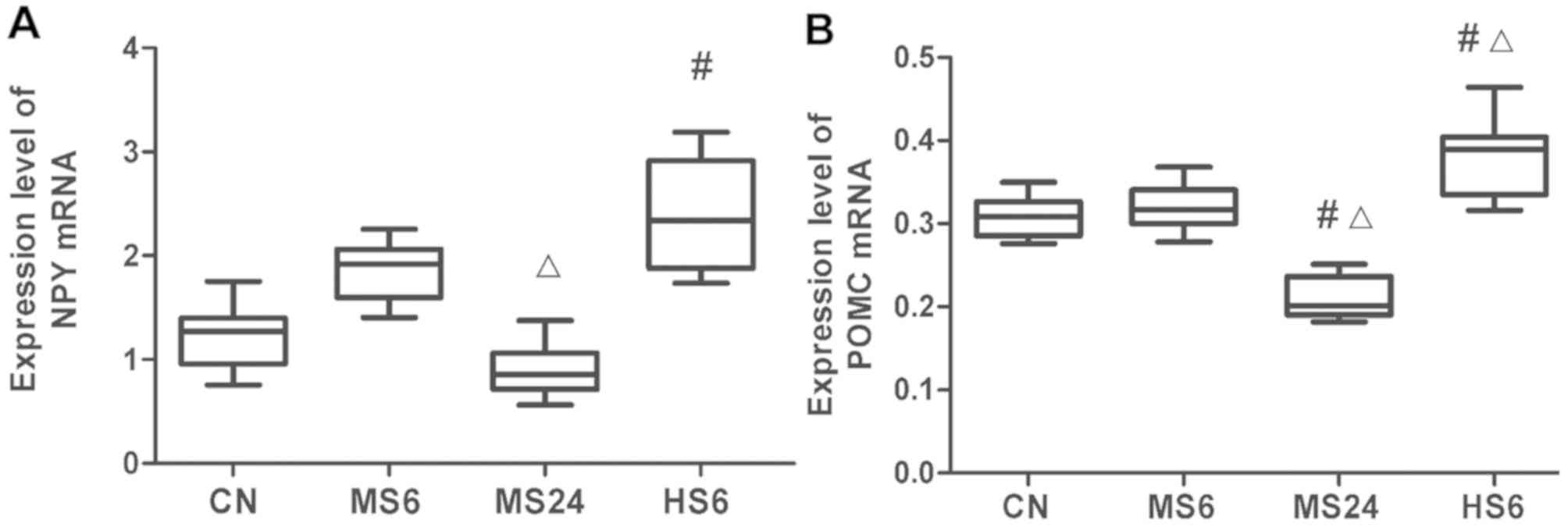 | Figure 1mRNA expression levels of NPY in the
hypothalamus and POMC in the hypophysis. (A) NPY and (B) POMC mRNA
expression levels in the hypothalamus and hypophysis, respectively.
#P<0.05 vs. CN; ΔP<0.05 vs. MS6 group.
CN, Control; MS6, moderate strength 6 h; MS24, moderate strength 24
h; HS6, high strength 6 h; CRH, corticotrophin; COR, cortisol; EPI,
epinephrine; HSP70, heat shock protein 70; POMC,
proopiomelanocortin; NPY, neuropeptide Y. |
COR concentration was elevated following heat stress
in the MS6, MS24 and HS6 groups compared with the CN group (MS6,
P=0.042; MS24, P=0.020; HS6, P=0.012). In addition, the COR levels
in the HS6 group were higher than those in the MS6 group; however,
the differences were not significant. Furthermore, no significant
differences were found between the high and moderate temperature
exposure groups when exposed for the same duration of time. The
increase in COR concentration was similar between the MS6 and MS24
groups (Fig. 1B).
Regarding EPI plasma levels, its concentration was
significantly elevated in the HS6 group (P=0.003) compared with the
CN and MS6 groups (P=0.02). In both the MS6 and MS24 groups, EPI
was increased compared with the control; however, no significant
differences were observed (Fig.
2C).
Finally, HSP70 plasma levels were raised following
both moderate and high temperature exposure compared with the
control group (MS6, P=0.019; MS24, P=0.005; HS6, P<0.001);
however, the difference between the MS6 and MS24 groups was not
significant. High temperature exposure induced HSP70 expression in
the plasma compared with the moderate temperature exposure under
the same exposure time (P=0.008; Fig.
2D).
mRNA expression levels of NPY and POMC. NPY
mRNA expression levels in the hypothalamus are presented in
Fig. 2A. In the MS6 and MS24 groups,
NPY mRNA expression was significantly upregulated and
downregulated, respectively, compared with that in the MS6 group
(P=0.014). In addition, high temperature exposure for 6 h
significantly increased NPY expression compared with the CN group
(P=0.005).
As shown in Fig. 2B,
qPCR analysis showed that POMC mRNA expression levels were
significantly increased in the HS6 group compared with the CN
(P=0.022) and MS6 (P=0.029) groups; however, there was no
statistically significant differences between the CN and MS6
groups. In contrast, POMC expression was significantly reduced in
the MS24 group compared with the CN (P=0.005) and MS6 (P=0.002)
groups.
Discussion
The present study investigated the association
between stress-related factors and different intensities of heat
stress on the expression of POMC and NPY in high-temperature
environments. The spleen and thymus are involved in the immune
response (33,34). It has been reported that heat stress
may cause atrophy of the spleen and thymus to different degrees,
which is attributed to the apoptosis of the internal organs
(35). In the present study, the
effects of different intensities of heat stress on spleen, thymus,
hypophysis and hypothalamus weight were first determined. The
results showed that the relative spleen weight was significantly
decreased in the long-term heat exposure group. In addition, in the
long-term heat exposure group, increasing heat stress levels
increased the relative hypothalamus weight and decreased the
relative weight of the hypophysis. These results indicated that
there was an opposite effect of heat stress on the hypothalamus and
hypophysis.
Most HSPs are generally stress-inducible as they
play a particularly important cytoprotective role in cells exposed
to stressful conditions (28). HSP70
is considered the most abundant and widely studied protein and its
concentration is significantly altered in response to stressful
stimuli (36). The results of the
present study demonstrated that the HSP70 plasma levels were
elevated in response to heat stress, particularly under exposure to
high temperatures. This finding suggested that heat stress-induced
damage promoted upregulation of HSP70 in order to protect the
organs. Emerging evidence has shown that EPI mediates stress
responses by initiating sympathetic nervous system to allow the
host organism to resist stress (23). The results of the present study
demonstrated that EPI levels were increased in the high temperature
exposure group. By contrast, exposure to a moderate temperature did
not increase EPI levels, irrespective of the exposure time,
suggesting that only high heat stress elevated EPI plasma
concentrations.
NPY and CRH neuropeptides are two independent stress
factors of the hypothalamus that act by binding to their respective
receptors. Currently known NPY receptors have seven kinds,
including NPY Y1-Y7. The most abundant Y1 and Y2 receptors are the
main regulators of NPY's anti-stress response (37). Under stress, CRH is mainly mediated
through its receptors, the main receptors are R1 and R2, and R1 is
mainly involved in the beginning of the HPA axis reaction (38). Long term exposure to stress stimuli
makes an organism vulnerable to chronic stress, which in turn
inhibits the NPY system and downregulates NPY expression, thus
attenuating its protective effect. CRH promotes stress-related
behaviors, whereas NPY exhibits anti-stress related effects. It has
been suggested that under heat stress conditions, NPY responds to
the harmful effects of stress by releasing CRH (39). Therefore, the expression levels of
NPY and CRH under different heat stress conditions were determined.
The results indicated that NPY mRNA expression levels in the CNS
could be altered in response to heat stress. As a result,
moderate-time heat stress exposure upregulated NPY expression to
protect the body. At the same time, there was an inverse
association between CRH concentration and NPY mRNA expression
levels. This finding confirmed the opposing behaviors of CRH and
NPY in response to heat stress. Therefore, NPY may moderate the
expression and release of CRH, while CRH inhibits NPY
expression.
COR is an important stress hormone that is regulated
by ACTH and protects the body from stress damage (40). Additionally, ACTH promotes the
release of COR, which in turn suppresses the release of ACTH
through a negative feedback mechanism to maintain COR balance. ACTH
is a derivative of POMC (41). The
results of the present study showed that the mRNA expression levels
of POMC were also altered in the CNS under heat stress conditions.
Furthermore, exposure to high temperatures resulted in upregulation
of POMC expression compared with exposure to a moderate temperature
for the same duration. COR and POMC were both increased in the
moderate-time exposure group, however, POMC expression was
decreased when COR concentration increased after the long-time
exposure. This finding may be attributed to the association between
COR and ACTH; COR levels may not have been high enough in the
moderate-time exposure group to inhibit ACTH via the negative
feedback mechanism. By contrast, in the case of the long-time
exposure group, the levels of COR were high enough to inhibit ACTH
in the remaining exposure time in order to maintain the balance of
COR concentration. The aforementioned inhibitory effect was
accompanied by POMC downregulation.
Overall, the present study investigated the changes
in expression of POMC, NPY and heat stress-related factors at
different heat stress intensities. Following long-term heat stress
exposure, the mRNA expression levels of both NPY and POMC were
decreased. Furthermore, the relative weights of the pituitary and
hypothalamus were inversely proportional to CRH plasma
concentration and NPY gene expression, respectively. In addition,
COR concentration was directly proportional to POMC expression in
all heat stress groups except the MS24 group. Therefore, the
present study suggested that heat damage caused by long-term heat
exposure may be involved in NPY and POMC downregulation.
In conclusion, the results of the present study
demonstrated that different heat stress intensities modulated NPY
and POMC mRNA expression. Therefore, NPY and POMC downregulation
may be partially associated with long-time heat exposure-induced
injuries.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural
Science Foundation of China (grant no. 81760055) and the Natural
Science Foundation of Ningxia (grant nos. 2020AAC03149 and
2020AAC03141).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
GL conceived and designed the experiments. YG
provided theoretical guidance and revised the manuscript. NZ
performed the experiments and prepared the manuscript. LM performed
the experiments and analyzed the data. XC and LZ provided
experimental technical assistance. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
All animal experimental procedures were approved by
Ningxia Medical University Institutional Review Board (Yinchuan,
China) (approval no. NXMU-2017-030).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bagath M, Krishnan G, Devaraj C, Rashamol
VP, Pragna P, Lees AM and Sejian V: The impact of heat stress on
the immune system in dairy cattle: A review. Res Vet Sci.
126:94–102. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
He X, Lu Z, Ma B, Zhang L, Li J, Jiang Y,
Zhou G and Gao F: Chronic heat stress alters hypothalamus
integrity, the serum indexes and attenuates expressions of
hypothalamic appetite genes in broilers. J Therm Biol. 81:110–117.
2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Jay O and Brotherhood JR: Occupational
heat stress in Australian workplaces. Temperature (Austin).
3:394–411. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Baker JD, Ozsan I, Rodriguez Ospina S,
Gulick D and Blair LJ: Hsp90 Heterocomplexes regulate steroid
hormone receptors: From stress response to psychiatric disease. Int
J Mol Sci. 20(79)2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Rimoldi S, Lasagna E, Sarti FM, Marelli
SP, Cozzi MC, Bernardini G and Terova G: Expression profile of six
stress-related genes and productive performances of fast and slow
growing broiler strains reared under heat stress conditions. Meta
Gene. 6:17–25. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Spencer RL and Deak T: A users guide to
HPA axis research. Physiol Behav. 178:43–65. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Morales-Medina JC, Dumont Y and Quirion R:
A possible role of neuropeptide Y in depression and stress. Brain
Res. 1314:194–205. 2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Li Q, Bartley AF and Dobrunz LE:
Endogenously released neuropeptide Y suppresses hippocampal
short-term facilitation and is impaired by stress-induced anxiety.
J Neurosci. 37:23–37. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Reichmann F and Holzer P: Neuropeptide Y:
A stressful review. Neuropeptides. 55:99–109. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sayed S, Van Dam NT, Horn SR, Kautz MM,
Parides M, Costi S, Collins KA, Iacoviello B, Iosifescu DV, Mathé
AA, et al: A randomized dose-ranging study of neuropeptide Y in
patients with posttraumatic stress disorder. Int J
Neuropsychopharmacol. 21:3–11. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sah R and Geracioti TD: Neuropeptide Y and
posttraumatic stress disorder. Mol Psychiatry. 18:646–655.
2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Backstrom T and Winberg S: Central
corticotropin releasing factor and social stress. Front Neurosci.
7(117)2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhou JJ, Gao Y, Zhang X, Kosten TA and Li
DP: Enhanced hypothalamic NMDA receptor activity contributes to
hyperactivity of HPA axis in chronic stress in male rats.
Endocrinology. 159:1537–1546. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhou JN and Fang H: Transcriptional
regulation of corticotropin-releasing hormone gene in stress
response. IBRO Rep. 5:137–146. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhang S, Lv F, Yuan Y, Fan C, Li J, Sun W
and Hu J: Whole-brain mapping of monosynaptic afferent inputs to
cortical CRH neurons. Front Neurosci. 13(565)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Carney BC, Dougherty RD, Moffatt LT,
Simbulan-Rosenthal CM, Shupp JW and Rosenthal DS: Promoter
methylation status in pro-opiomelanocortin (POMC) does not
contribute to dyspigmentation in hypertrophic scar. J Burn Care
Res. 41:339–346. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Duque-Díaz E, Alvarez-Ojeda O and Coveñas
R: Enkephalins and ACTH in the mammalian nervous system. Vitam
Horm. 111:147–193. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Koss KJ and Gunnar MR: Annual research
review: Early adversity, the hypothalamic-pituitary-adrenocortical
axis, and child psychopathology. J Child Psychol Psychiatry.
59:327–346. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Szczepek AJ, Dietz GPH, Reich U, Hegend O,
Olze H and Mazurek B: Differences in Stress-induced modulation of
the auditory system between Wistar and Lewis rats. Front Neurosci.
12(828)2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Dmitrieva NO, Almeida DM, Dmitrieva J,
Loken E and Pieper CF: A day-centered approach to modeling
cortisol: Diurnal cortisol profiles and their associations among
U.S. adults. Psychoneuroendocrinology. 38:2354–2365.
2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Skobowiat C, Nejati R, Lu L, Williams RW
and Slominski AT: Genetic variation of the cutaneous HPA axis: An
analysis of UVB-induced differential responses. Gene. 530:1–7.
2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Chen X, Gianferante D, Hanlin L, Fiksdal
A, Breines JG, Thoma MV and Rohleder N: HPA-axis and inflammatory
reactivity to acute stress is related with basal HPA-axis activity.
Psychoneuroendocrinology. 78:168–176. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Chauhan NR, Kapoor M, Prabha Singh L,
Gupta RK, Chand MR, Tulsawani R, Nanda S and Bala SS: Heat
stress-induced neuroinflammation and aberration in monoamine levels
in hypothalamus are associated with temperature dysregulation.
Neuroscience. 358:79–92. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Phadke D, Beller JP and Tribble C: The
disparate effects of epinephrine and norepinephrine on
hyperglycemia in cardiovascular surgery. Heart Surg Forum.
21:E522–E526. 2018.PubMed/NCBI View
Article : Google Scholar
|
|
25
|
Quas JA, Yim IS, Oberlander TF, Nordstokke
D, Essex MJ, Armstrong JM, Bush N, Obradović J and Boyce WT: The
symphonic structure of childhood stress reactivity: Patterns of
sympathetic, parasympathetic, and adrenocortical responses to
psychological challenge. Dev Psychopathol. 26:963–982.
2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Qiu Dingjie. Study on the variation of
liver hsp70 expression and mRNA abundance by heat stress in rats
Fujian Agriculture and Forestry University, 2010.
|
|
27
|
Zininga T, Ramatsui L and Shonhai A: Heat
shock proteins as immunomodulants. Molecules.
23(2846)2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Pockley AG and Henderson B: Extracellular
cell stress (heat shock) proteins-immune responses and disease: An
overview. Philos Trans R Soc Lond B Biol Sci.
373(20160522)2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Chen H, Wu Y, Zhang Y, Jin L, Luo L, Xue
B, Lu C, Zhang X and Yin Z: Hsp70 inhibits
lipopolysaccharide-induced NF-kappaB activation by interacting with
TRAF6 and inhibiting its ubiquitination. J FEBS Lett.
580:3145–3152. 2006.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Morimoto R, Sarge KD and Abravaya K:
Transcriptional regulation of heat shock genes. A paradigm for
inducible genomic responses. J Biol Chem. 267:21987–21990.
1992.PubMed/NCBI
|
|
31
|
Richter-Landsberg C and Goldbaum O: Stress
proteins in neural cells: Functional roles in health and disease.
Cell Mol Life Sci. 60:337–349. 2003.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zhang W, Yang H, Zhu L, Luo Y, Nie L and
Li G: Role of EGFR/ErbB2 and PI3K/AKT/e-NOS in Lycium barbarum
polysaccharides ameliorating endothelial dysfunction induced by
oxidative stress. Am J Chin Med. 47:1523–1539. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
He S, Yu Q, He Y, Hu R, Xia S and He J:
Dietary resveratrol supplementation inhibits heat stress-induced
high-activated innate immunity and inflammatory response in spleen
of yellow-feather broilers. Poult Sci. 98:6378–6387.
2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Liu J, Zhao H, Wang Y, Shao Y, Zhang L and
Xing M: Impacts of simultaneous exposure to arsenic (III) and
copper (II) on inflammatory response, immune homeostasis, and heat
shock response in chicken thymus. Int Immunopharmacol. 64:60–68.
2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Xinlong C: Apoptosis of rat thymus cells
induced by heat injury j. Foreign Med. 19:233–234. 1998.
|
|
36
|
Xu J, Tang S, Yin B, Sun J, Song E and Bao
E: Correction to: Co-enzyme Q10 and acetyl salicylic acid enhance
Hsp70 expression in primary chicken myocardial cells to protect the
cells during heat stress. Mol Cell Biochem. 461:213–214.
2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Xingguo W: Role of hypothalamic npy in
chronic stress-induced depression Nanchang University, 2013.
|
|
38
|
Ping W: Patterns of crh, crhr1 and crhr2
gene expression in brain and pituitary of rats induced by hypoxia
Zhejiang University, 2011.
|
|
39
|
Farzi A, Reichmann F and Holzer P: The
homeostatic role of neuropeptide Y in immune function and its
impact on mood and behaviour. Acta Physiol (Oxf). 213:603–627.
2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
In lewicz DP, Hill SR, Jav JL, West CH,
Zavosh AS and Sipols AJ: Effect of recurrent yohimbine on immediate
and post-hoc behaviors, stress hormones, and energy homeostatic
parameters. Physiol Behay. 129:186–193. 2014.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Zhou Y, Kruyer A and Ho A: Cocaine place
conditioning increases pro-opiomelanocortin gene expression in rat
hypothalamus. Neurosci Lett. 530:59–63. 2012.PubMed/NCBI View Article : Google Scholar
|