Introduction
Glia make up the majority of the cells present in
the brain. They do not fire action potentials like neurons, but
instead surround and ensheath neuronal cell bodies, axons and
synapses to protect and maintain the microenvironment throughout
the nervous system. Of these glial cells, microglia exhibit unique
and specific characteristics in the central nervous system
(1). Microglia serve as the resident
innate immune cells in the brain and respond quickly to brain
injuries and immunological stimuli, changing from resting branched
microglia into activated, amoeboid microglia, undergoing dramatic
alterations in morphology, which is thought to favor phagocytosis
and mobility (2). In order to
understand the cell biology of microglia more directly, an
increasing number of studies are being performed in vitro
using both primary microglial cultures and immortalized cell
lines.
Mitogen-activated protein kinase (MAPK)-mediated
signaling serves a critical role in the activation of microglia,
including modulation of the p38, extracellular signal-regulated
kinase (ERK) and JNK cascade (3).
The p38 cascade has been implicated in cell death triggered by
cytokines, growth factor withdrawal and environmental stressors
(4). A previous in vivo study
showed that in chronic pain models, p38 phosphorylation in the
spinal cord was upregulated (5,6), which
has been reported to be exclusively expressed on microglia
(7). The ERK pathway, which is
activated by growth factors and hormones, is also involved in
mediating cellular proliferation, transformation and
differentiation (8). Numerous
studies have been performed to assess whether certain compounds are
able to inhibit microglial activation in vitro, and the
effectiveness of these compounds is assessed by measuring the
expression of phospho-(p-)p38 and p-ERK as the functional
activation markers of microglia (9-11).
For in vitro studies, certain studies have cultured
microglia in specific media, whereas others have used serum-free
media as the control group (12,13), in
which case the effect of serum can be disregarded.
In the present study, it was hypothesized that
microglial cells could be activated by serum deprivation. The
effect of serum deprivation on morphological changes, and on the
expression of p-p38 and p-ERK was assessed in primary microglia,
BV-2 cell and astrocytes.
Materials and methods
Cell culture
Cell culture preparation experiments were conducted
between 8:00 am and 4:00 pm. The experimental protocols were
performed in accordance with the Animals in Research: Reporting
in vivo Experiments (ARRIVE) guidelines (14), and approved by the Institutional
Animal Care and Use Committee of Peking University (approval no.
LA2012-34).
Primary cultures of cortical microglia were prepared
from newborn (within 24 h after birth) ICR mice (15). Mice were sacrificed by decapitation
and the brains were removed, followed by careful elimination of
meninges and blood vessels under a dissecting microscope in
ice-cold DMEM (Gibco; Thermo Fisher Scientific). After mechanical
dissociation, the cell suspension was passed through 70-µm nylon
filters (Spectra/Mesh; Spectrum Medical Industries). FBS [10%
(v/v); Hyclone; Cytvia] was added to the medium containing filtered
cell suspensions. The mixed cell suspensions were first seeded at
1x107 cells/ml in 75-mm2 flasks coated with
0.25% poly-D-lysine. Flasks were then incubated at 37˚C, with 95%
air/5% CO2 (v/v) and 95% humidity. Culture medium was
changed twice per week with DMEM containing 10% (v/v) FBS. After
12-14 days, when the cells were present as two separate layers, the
mixed glial culture was placed on a Forma Orbital Shaker (250 rpm;
37˚C; Thermo Fisher Scientific, Inc.) for 4 h. Following agitation,
the suspended cells were collected, pooled, centrifuged (room
temperature at 500 x g) and re-suspended. Cells were seeded at
3x105 cells/ml in 35-mm2 tissue culture
dishes (Corning, Inc.) for identification and 12-well plates for
the other experiments.
BV-2 cells were obtained from the Cell Bank of the
Chinese Academy of Medical Sciences and grown in DMEM supplemented
with 10% FBS. After 3-5 days of seeding the cells in 60-mm dishes,
cells were cultured until they were close to 100% confluent. Cells
were split 1:5 when they reached confluence, using trypsin solution
in PBS. The cells were then were seeded into 35-mm dishes 1 day
prior to subsequent experiments.
Primary cultures of cortical astrocytes were
prepared from newborn (within 24 h after birth) ICR mice as
previously described (16). Briefly,
mice were sacrificed by decapitation and the brain cortexes were
removed, followed by careful elimination of meninges and blood
vessels under a dissecting microscope in ice-cold modified DMEM.
After mechanical dissociation, the cell suspension was passed
through a 70-µm nylon and then 10-µm filters (Spectra/Mesh;
Spectrum Medical Industries). FBS (10%) was added to the medium
containing filtered cell suspensions. These mixed cell suspensions
were seeded onto 35-mm dishes at 1x106 cells/ml. Culture
medium was changed twice per week with DMEM containing 10% (v/v)
FBS. After 4 weeks, these astrocytes became mature and ready for
use for the subsequent experiments.
Treatment and cell morphology
analysis
The control group were the cells cultured under
normal conditions. Cells which were treated with LPS by directly
adding LPS (Sigma-Aldrich; Merck KGaA) to the media were considered
as the positive control. The remaining two groups were cell
cultures in which media was removed and replaced with serum-free
medium or serum-free medium containing 100 ng/ml LPS. A light
microscope (magnification, x40) was used for observing and
capturing images of the morphological changes in cells following
treatment. Cell morphology was observed at different time points
(within 24 h).
Western blot analysis for p-p38 and
p-ERK in cell culture
After washing with ice-cold PBS three times, cell
cultures were lysed in ice-cold lysis buffer [150 mM NaCl, 0.1%
(w/v) NP-40, 50 mM Tris (pH 8.0), 0.5% (w/v) sodium deoxycholate,
1% (w/v) SDS, 1 mM DTT, 0.1 mM PMSF and protease inhibitor cocktail
(Roche Diagnostics)]. Following treatment, the culture plates were
briefly centrifuged at room temperature; supernatants were
collected and stored for western blot analysis. A Lowry protein
assay was used to measure the total protein concentration. The
proteins were boiled and analyzed using standard western blotting
procedures. Briefly, proteins (20 µg per lane) were loaded on a 12%
SDS-gel, resolved using SDS-PAGE and transferred onto PVDF
membranes (EMD Millipore). The PVDF membranes were blocked with 5%
(w/v) non-fat milk in TBST buffer [0.1 M Tris-HCl (pH 8.0), 0.9%
(w/v) NaCl and 0.1% (v/v) Tween-20], and the target proteins were
probed with diluted primary antibodies against p-p38 (1:1,000; cat.
no. 9211; Cell Signaling Technology, Inc.), p-ERK (1:1,000; cat.
no. 9101; Cell Signaling Technology, Inc.) in blocking solution
overnight at 4˚C. The membranes were then incubated with a mouse
anti-rabbit secondary antibody conjugated to horseradish peroxidase
(1:2,000; cat. no. sc-2357; Santa Cruz Biotechnology, Inc.) for 1 h
at room temperature. Finally, ECL western blot detection kit
(Thermo Fisher Scientific, Inc.) was used to visualize the
expression of the target proteins. Densitometry analysis was
performed using Quantity One analysis software v4.6.6 (Bio-Rad
Laboratories, Inc.), and the density of bands was normalized to the
density of internal control and expressed as the fold of change
compared with the control group.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism version 5 (GraphPad Software, Inc.). All data are presented
as the mean ± the standard error of the mean. Differences between
groups (quantification of western-blot results) were compared using
a one-way ANOVA and corrected using a Bonferroni's correction.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Morphological changes of microglia,
BV-2 cells and astrocytes following serum deprivation and LPS
treatment
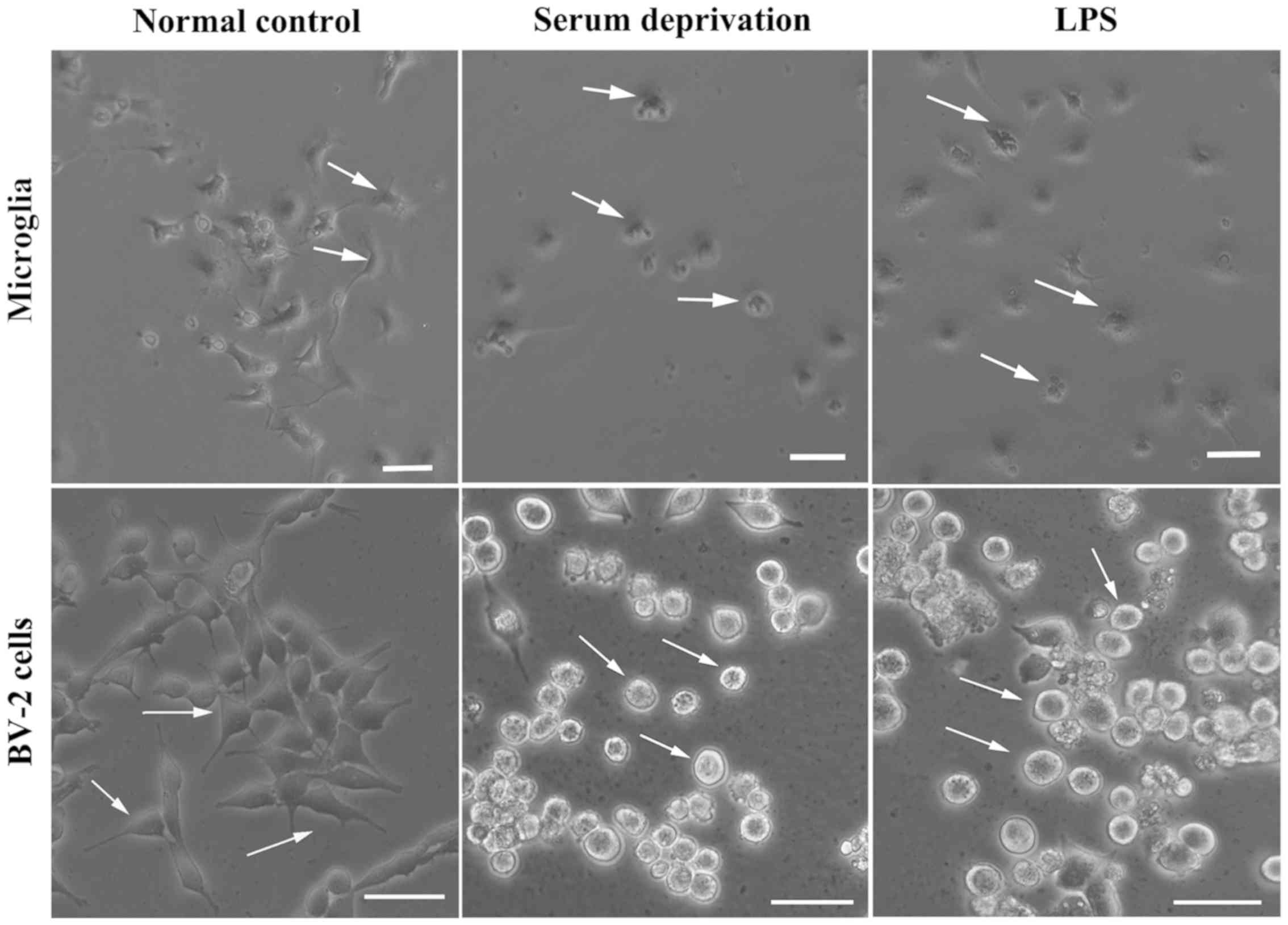
The morphology of microglia change when
transitioning from their resting state to an activated state
(5,6). In the present study, microglia became
rounded and there were several light vesicles present in the body
of microglia following LPS treatment (Data S1). Microglia reacted
rapidly following LPS treatment; within several minutes. The
processes became shorter and the body became notably rounder
compared with the resting state. Subsequently, whether serum had
any effect on the morphological changes of microglia was assessed
(Fig. 1). After 24 h of serum
deprivation with or without LPS treatment, the processes of the
microglia became shorter and the microglia transformed into an
ameboid-like morphology, suggesting that they had become activated.
We also detected if this phenomenon appeared in the microglial cell
line, BV-2. After 24 h treatment of serum deprivation, the
morphology of BV-2 cells looked the same as the LPS-treated group,
whose processes disappeared and bodies became fattened and
contained vesicles. However, LPS did not result in an immediate
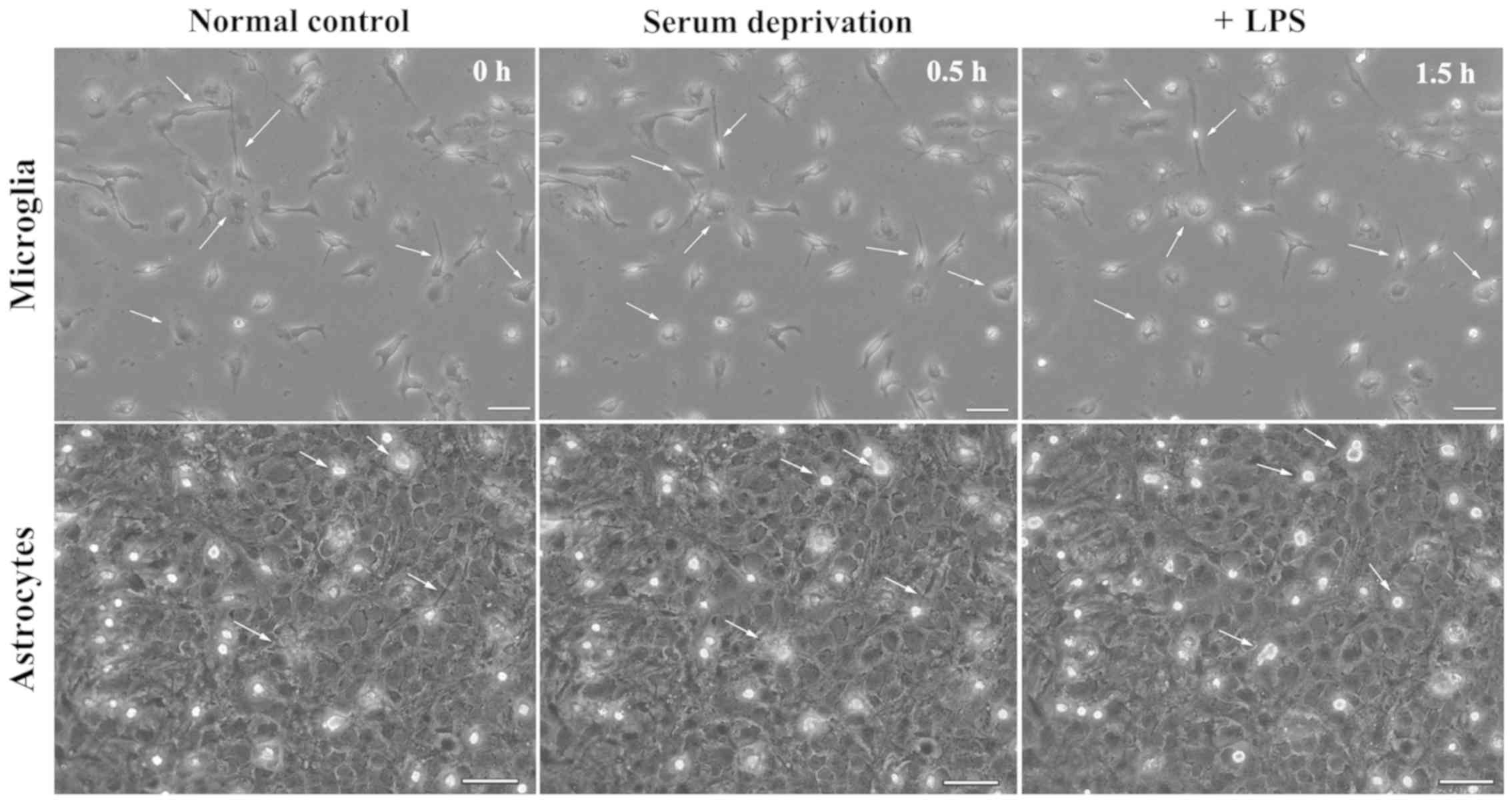
effect on the morphology of astrocytes, nor did serum deprivation
treatment (Fig. 2). In astrocyte
cultures, several microglia were present on the upper layer.
Following serum deprivation, the morphology of microglia remained
dynamic, but the morphology of astrocytes was stable. Addition of
LPS to the cell culture medium showed that the morphology of
microglia exhibited a morphology indicative of activation, while
the morphology of astrocytes did not change (Fig. 2).
Temporal profile of p-p38 expression
following LPS treatment
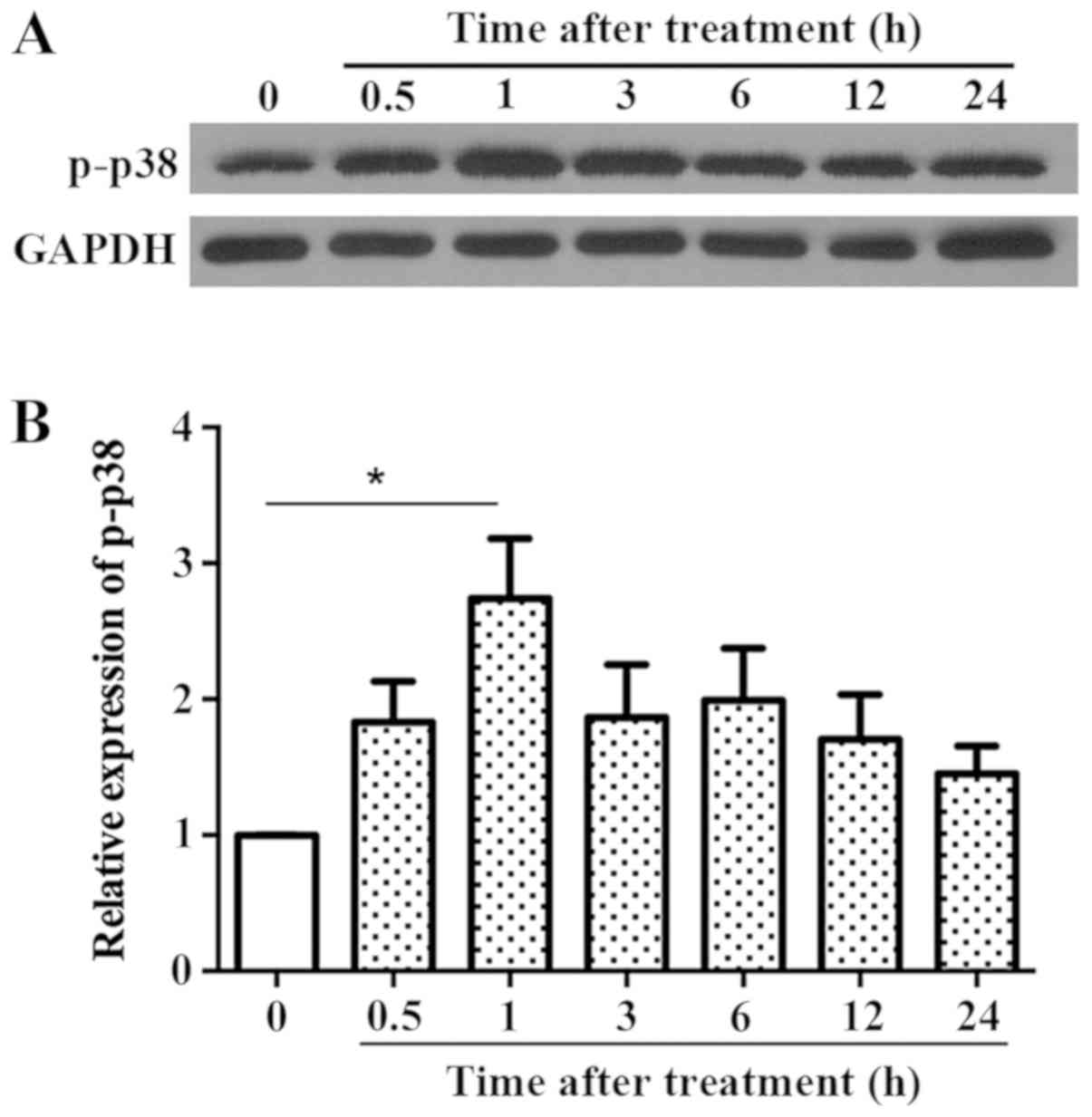
LPS treatment resulted in rapid upregulation of
p-p38. The time of peak p-p38 expression was thus determined.
Fig. 3 shows that the expression of
p-p38 increased within 30 min and peaked at 1 h, which was
significantly different from the control group; after which it
decreased gradually, but remained higher than the control group.
Thus, a time point of 1 h was used for subsequent experiments.
Effect of serum deprivation on p-p38
and p-ERK expressions in microglia, BV-2 and astrocytes
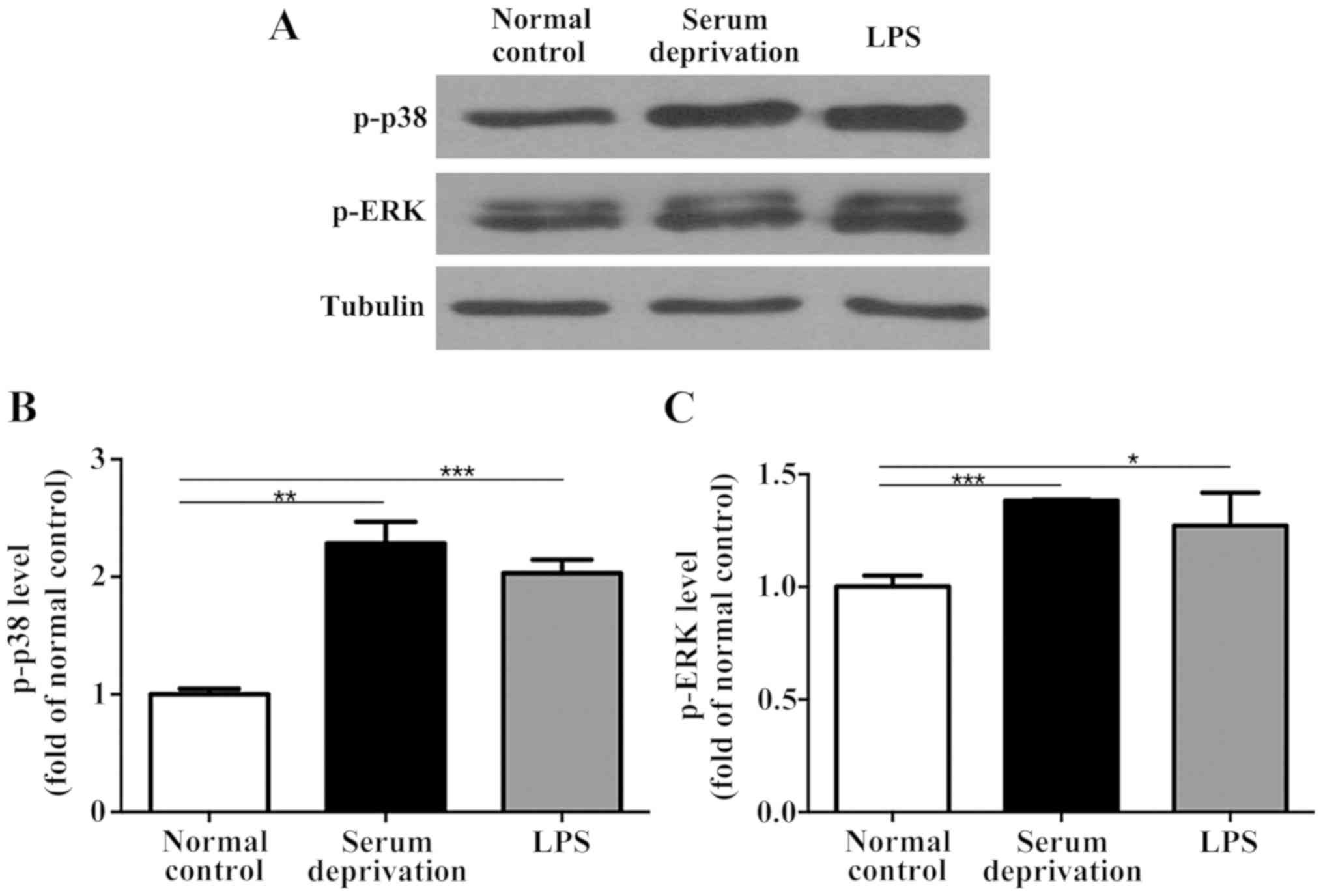
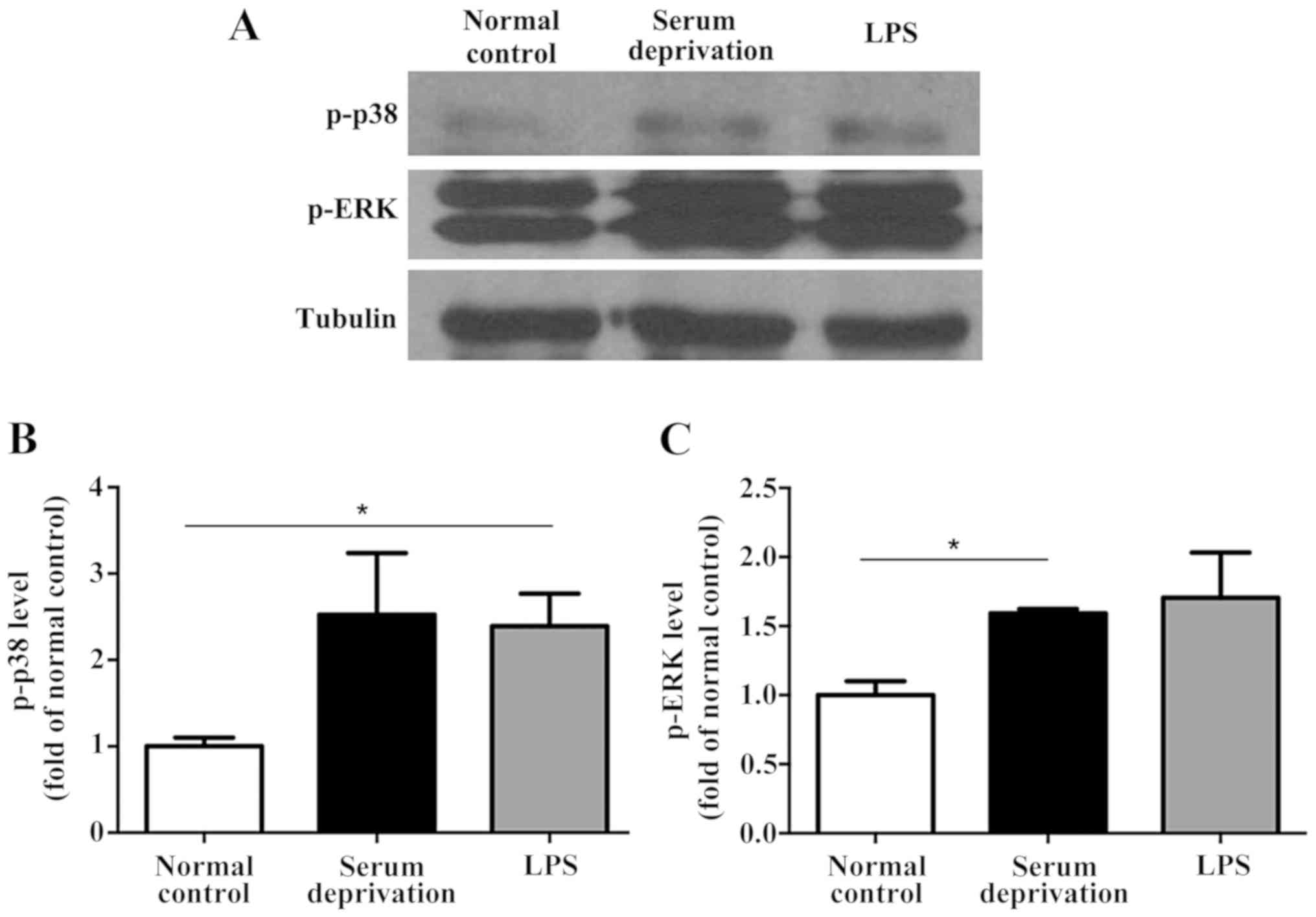
In the primary microglia culture, serum deprivation
and LPS treatment both increased the expression levels of p-p38 and
p-ERK compared with the normal control (Fig. 4). No significant differences were
observed between the serum deprived cultures and those treated with
LPS. Additionally, in the BV-2 cells, although the effect of serum
deprivation was not as prominent as that in the primary microglia,
serum deprivation still resulted in upregulated expression of both
p-p38 and p-ERK significantly (Fig.
5). LPS treatment also increased the expression levels of p-p38
and p-ERK compared with the control group. However, no significant
differences in the expression of p-p38 and p-ERK were detected in
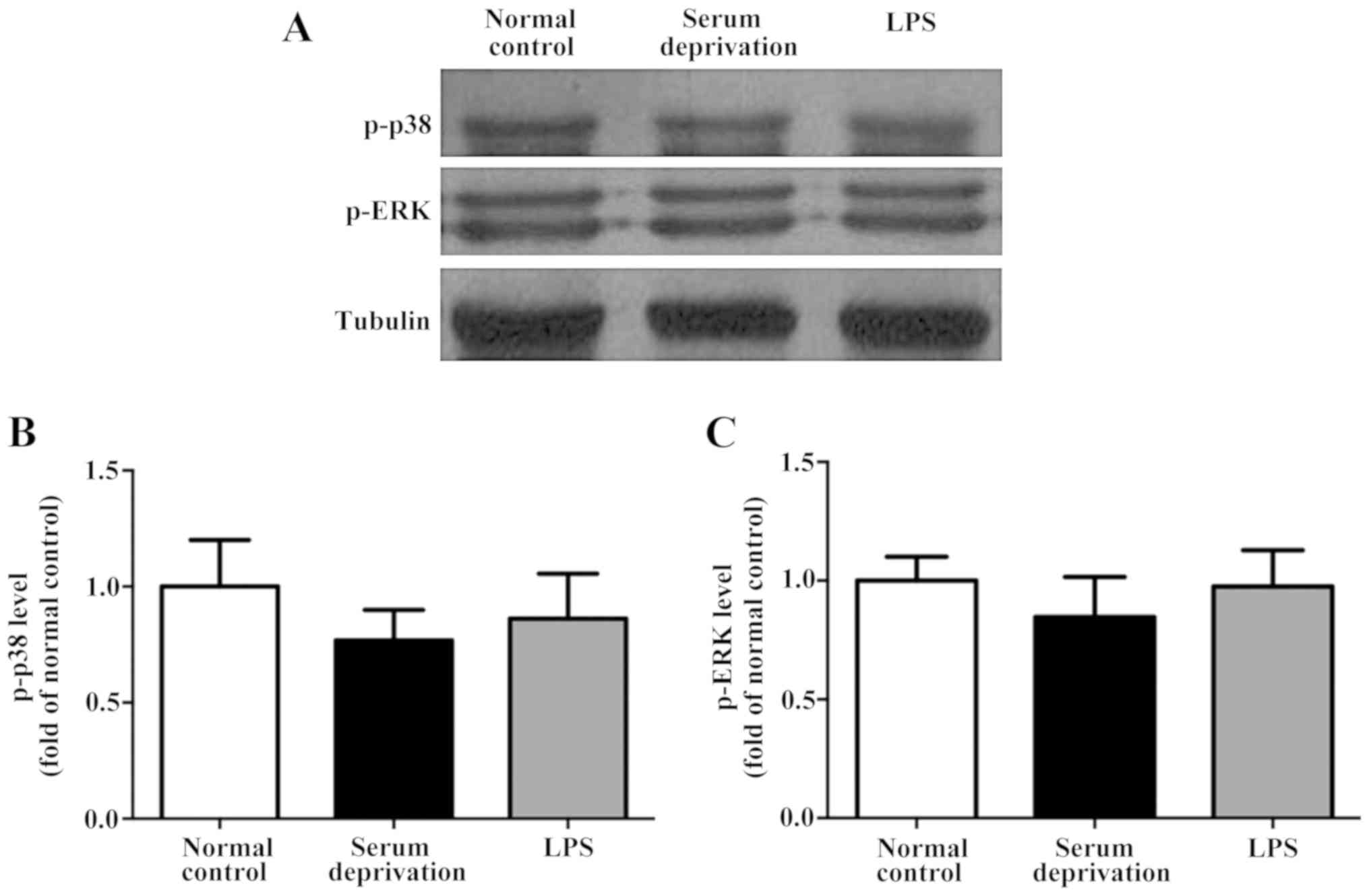
astrocytes following serum deprivation for 1 h. Furthermore, LPS
did not increase the expression levels of p-p38 and p-ERK in the
astrocytes (Fig. 6). Together, the
results suggest that the effect of serum deprivation on the primary
microglia and BV-2 cells were specific and not applicable to
astrocytes.
Discussion
In the present study, it was found that: i) Once
serum was depleted, the processes of primary microglia and BV-2
cells became shorter and the body became more rounded in shape,
indicative of activation; ii) serum deprivation significantly
increased the expression of p-p38 and p-ERK in the primary
microglia and BV-2 cell culture, and iii) both morphological
changes and upregulation of p-p38 and p-ERK caused by serum
deprivation were not observed in the primary astrocytes within the
limited time period assessed.
Microglia serve as immune cells monitoring the
brain's microenvironment for any damage (17). It has been reported that the rapid
and constitutive motion of microglial processes contributes to
their function (18). Toll-like
receptors (TLRs) are expressed by several cells in the innate
immune system, and form part of a larger family of so-called
pattern recognition receptors, which sense danger signals in the
form of pathogen-associated molecular patterns (19). LPS is a common inflammogen that is
used to activate the glia via TLR4 in several animal models of
inflammation-mediated neuro-degeneration, including both in
vivo and in vitro models (20,21). In
the present study, LPS-treated microglia were used as a positive
activation control.
It was observed that the processes of microglia
became shorter and there was an increase in the presence of
phagocytic vesicles in the cell body following LPS treatment.
Interestingly, similar morphological changes were detected in both
the microglia and BV-2 cells following serum deprivation alone
(Fig. 1). These morphological
changes observed were consistent with the majority of previous
reports on microglia activation (5,6,22). The effects on morphological changes
in the cultured microglia in the presence of serum in vitro
have been reported previously. Microglia cultured in the presence
of serum are considered to be activated microglia, as the shape of
the microglia was altered when serum deprived (23,24).
Additionally, it was shown that it was difficult to interpret the
morphological changes in cultured cells in vitro, due to the
possibility of transient changes in cell morphology. Activated
macrophages may exhibit increased phagocytic activity compared with
‘resting’ cells (25). Of interest,
microglia have been shown to switch to a phenotype contributing to
neuronal damage without undergoing notable morphological changes
(26). Thus, it is not sufficient to
judge the influence of serum deprivation on microglia based only on
morphological alterations. The state of microglia and the influence
of serum on microglia also depend on the isolation and culture
method of microglia (27).
MAPKs are critical regulators of pro-inflammatory
cytokines which are involved in the inflammatory process. Of the
several MAPKs, p38 has been crucial for anti-inflammatory drug
discovery, due to its importance in the production of the
pro-inflammatory cytokines and other mediators (28). p-p38 and p-ERK are commonly used in
in vitro studies as activation markers of primary microglia,
microglial cell lines and astrocytes (29,30).
Serum-deprivation has been shown to selectively upregulate the
expression of certain genes (31-33).
The present study demonstrated that serum deprivation significantly
increased the expressions levels of p-p38 and p-ERK in primary
microglia and BV-2 cell cultures, and this was not observed in
primary astrocytes in the limited time period of assessment.
Culture medium supplemented with serum provides a migratory
stimulus for BV2 microglia (34).
Serum deprivation is not only a simple stressful condition compared
with others forms of mimicked stresses. For example, serum
depletion but not hypoxia and glucose depletion increased mRNA,
protein and enzyme activity of SPHK2, and this was dependent on
activation of the JNK/CREB signaling pathway, although the cells
grew slowly compared with cells grown in supplemented culture
(35). Serum deprivation caused cell
death, nuclei condensation and upregulation of Bax expression in
cultured microglia. SB203580, a specific inhibitor of p38MAPK, was
able to reverse cell death (36). It
is also hypothesized that overstimulation or overactivation of
microglia results in microglial apoptosis (37,38).
Serum exposure influences microglial survival, specification and
function (24). MTT analysis (data
not shown) was used to assess the cell viability caused by serum
deprivation, which did not cause significant levels of cell death.
Thus, 24 h was selected as the longest time point for observation
of morphological changes. Cell death may have increased if serum
deprivation was prolonged. It is speculated that serum deprivation
causes the activation of microglia over a short period of time,
while microglia may eventually die due to longer periods without an
adequate nutrient supply.
In summary, the present study showed that microglia
responded quickly to external stimuli both in vivo and in
vitro. The biological responses of microglia were different to
that of astrocytes to a certain degree. In serum-deprived
conditions, the morphology of microglia changed such that the
processes became shorter and vesicles were present in the cell
body. The expressions levels of p-p38 and p-ERK were also
upregulated in both primary microglia culture and BV-2 cell lines.
However, all the aforementioned changes were not observed in
astrocytes. In addition to the changes in the levels of
phosphorylated MAPKs and morphology, other changes may also occur
in the cell lines following serum deprivation and/or LPS treatment,
and these require further study.
Supplementary Material
Dynamic morphological changes of
primary microglia in 8 h following lipopolysaccharide treatment.
Scale bar, 20 μm.
Supplementary Data
Acknowledgements
We would like to thank Professor Albert Cheung Hoi
Yu and his lab (Peking University and Department of Neurobiology,
Peking University Health Science Center) for their aid with the
cell culture.
Funding
The present study was supported by a grant from the
Discipline Construction Fund of Beijing Stomatological Hospital
(grant no. 17-09-12).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YY and KYF conceived and designed the experiments.
YY performed the experiments, analyzed the data and wrote the
manuscript. Both authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The experimental protocol was performed in
accordance with the Animals in Research: Reporting in vivo
Experiments (ARRIVE) guidelines, and approved by the Institutional
Animal Care and Use Committee of Peking University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lund H, Pieber M, Parsa R, Han J,
Grommisch D, Ewing E, Kular L, Needhamsen M, Espinosa A, Nilsson E,
et al: Competitive repopulation of an empty microglial niche yields
functionally distinct subsets of microglia-like cells. Nat Commun.
9(4845)2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Chen SX, Wang SK, Yao PW, Liao GJ, Na XD,
Li YY, Zeng WA, Liu XG and Zang Y: Early CALP2 expression and
microglial activation are potential inducers of spinal IL-6
up-regulation and bilateral pain following motor nerve injury. J
Neurochem. 145:154–169. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Johnson GL and Lapadat R:
Mitogen-activated protein kinase pathways mediated by ERK, JNK, and
p38 protein kinases. Science. 298:1911–1912. 2002.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kyriakis JM and Avruch J: Mammalian
mitogen-activated protein kinase signal transduction pathways
activated by stress and inflammation. Physiological Rev.
81:807–869. 2001.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Fu KY, Light AR, Matsushima GK and Maixner
W: Microglial reactions after subcutaneous formalin injection into
the rat hind paw. Brain Res. 825:59–67. 1999.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Tan YH, Li K, Chen XY, Cao Y, Light AR and
Fu KY: Activation of Src family kinases in spinal microglia
contributes to formalin-induced persistent pain state through p38
pathway. J Pain. 13:1008–1015. 2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tsuda M, Mizokoshi A, Shigemoto-Mogami Y,
Koizumi S and Inoue K: Activation of p38 mitogen-activated protein
kinase in spinal hyperactive microglia contributes to pain
hypersensitivity following peripheral nerve injury. Glia. 45:89–95.
2004.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Grewal SS, York RD and Stork PJ:
Extracellular-signal-regulated kinase signalling in neurons. Curr
Opin Neurobiol. 9:544–553. 1999.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Chen HL, Jia WJ, Li HE, Han H, Li F, Zhang
XL, Li JJ, Yuan Y and Wu CY: Scutellarin exerts anti-inflammatory
effects in activated microglia/brain macrophage in cerebral
ischemia and in activated BV-2 microglia through regulation of
MAPKs signaling pathway. Neuromolecular Med. 22:264–277.
2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chen S, Lyu C, Zhou J, Huang S, Zhang Y,
Liu G, Liu K, Chen D, Hu Y, Zhou L and Gu Y: TLR4 signaling pathway
mediates the LPS/ischemia-induced expression of monocytechemotactic
protein-induced protein 1 in microglia. Neurosci Lett. 686:33–40.
2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Xu ZZ, Berta T and Ji RR: Resolvin E1
inhibits neuropathic pain and spinal cord microglial activation
following peripheral nerve injury. J Neuroimmune Pharmacol.
8:37–41. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kloss CU, Bohatschek M, Kreutzberg GW and
Raivich G: Effect of lipopolysaccharide on the morphology and
integrin immunoreactivity of ramified microglia in the mouse brain
and in cell culture. Exp Neurol. 168:32–46. 2001.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Pahan K, Sheikh FG, Namboodiri AM and
Singh I: Lovastatin and phenylacetate inhibit the induction of
nitric oxide synthase and cytokines in rat primary astrocytes,
microglia, and macrophages. J Clin Invest. 100:2671–2679.
1997.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kilkenny C, Browne WJ, Cuthill IC, Emerson
M and Altman DG: Improving bioscience research reporting: The
ARRIVE guidelines for reporting animal research. PLoS Biol.
8(e1000412)2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Giulian D and Baker TJ: Characterization
of ameboid microglia isolated from developing mammalian brain. J
Neurosci. 6:2163–2178. 1986.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Gao K, Wang CR, Jiang F, Wong AY, Su N,
Jiang JH, Chai RC, Vatcher G, Teng J, Chen J, et al: Traumatic
scratch injury in astrocytes triggers calcium influx to activate
the JNK/c-Jun/AP-1 pathway and switch on GFAP expression. Glia.
61:2063–2077. 2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Nimmerjahn A, Kirchhoff F and Helmchen F:
Resting microglial cells are highly dynamic surveillants of brain
parenchyma in vivo. Science. 308:1314–1318. 2005.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Parkhurst CN and Gan WB: Microglia
dynamics and function in the CNS. Curr Opin Neurobiol. 20:595–600.
2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kawai T and Akira S: The role of
pattern-recognition receptors in innate immunity: Update on
Toll-like receptors. Nat Immunol. 11:373–384. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
20
|
Carvey PM, Chang Q, Lipton JW and Ling Z:
Prenatal exposure to the bacteriotoxin lipopolysaccharide leads to
long-term losses of dopamine neurons in offspring: A potential, new
model of Parkinson's disease. Front Biosci. 8 (Suppl):S826–S837.
2003.PubMed/NCBI View
Article : Google Scholar
|
|
21
|
Gao JJ, Diesl V, Wittmann T, Morrison DC,
Ryan JL, Vogel SN and Follettie MT: Regulation of gene expression
in mouse macrophages stimulated with bacterial CpG-DNA and
lipopolysaccharide. J Leukoc Biol. 72:1234–1245. 2002.PubMed/NCBI
|
|
22
|
Stence N, Waite M and Dailey ME: Dynamics
of microglial activation: A confocal time-lapse analysis in
hippocampal slices. Glia. 33:256–266. 2001.PubMed/NCBI
|
|
23
|
Nakamura Y, Si QS and Kataoka K:
Lipopolysaccharide-induced microglial activation in culture:
Temporal profiles of morphological change and release of cytokines
and nitric oxide. Neurosci Res. 35:95–100. 1999.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Bohlen CJ, Bennett FC, Tucker AF, Collins
HY, Mulinyawe SB and Barres BA: Diverse requirements for microglial
survival, specification, and function revealed by defined-medium
cultures. Neuron. 94:759–773.e8. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Mosser DM: The many faces of macrophage
activation. J Leuko Biol. 73:209–212. 2003.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Perry VH: Stress primes microglia to the
presence of systemic inflammation: Implications for environmental
influences on the brain. Brain Behav Immu. 21:45–46.
2007.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Collins HY and Bohlen CJ: Isolation and
culture of rodent microglia to promote a dynamic ramified
morphology in Serum-free medium. J Vis Exp: 57122, 2018 doi:
10.3791/57122.
|
|
28
|
Olajide OA, Bhatia HS, de Oliveira AC,
Wright CW and Fiebich BL: Inhibition of neuroinflammation in
LPS-activated microglia by cryptolepine. Evid Based Complement
Alternat Med. 2013(459723)2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Hu LF, Wong PT, Moore PK and Bian JS:
Hydrogen sulfide attenuates lipopolysaccharide-induced inflammation
by inhibition of p38 mitogen-activated protein kinase in microglia
J. Neurochem. 100:1121–1128. 2007.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wang MJ, Jeng KC, Kuo JS, Chen HL, Huang
HY, Chen WF and Lin SZ: c-Jun N-terminal kinase and, to a lesser
extent, p38 mitogen-activated protein kinase regulate inducible
nitric oxide synthase expression in hyaluronan fragments-stimulated
BV-2 microglia. J Neuroimmunol. 146:50–62. 2004.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Huang Z, Huang G, Li Q and Jin J: p38
mitogen-activated protein kinase/activator protein-1 involved in
serum deprivation-induced human alkaline ceramidase 2 upregulation.
Biom Rep. 3:225–229. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Celestino AT, Levy D, Maria Ruiz JL and
Bydlowski SP: ABCB1, ABCC1, and LRP gene expressions are altered by
LDL, HDL, and serum deprivation in a human doxorubicin-resistant
uterine sarcoma cell line. Biochem Biophys Res Commun. 457:664–668.
2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Jiang P, Ren YL, Lan Y, Li JL, Luo J, Li J
and Cai JP: Phagocytosis of platelets enhances endothelial cell
survival under serum deprivation. Exp Biol Med (Maywood).
240:876–883. 2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Omar Zaki SS, Kanesan L, Leong MYD and
Vidyadaran S: The influence of serum-supplemented culture media in
a transwell migration assay. Cell Biol Int. 43:1201–1204.
2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Mizutani N, Omori Y, Tanaka K, Ito H,
Takagi A, Kojima T, Nakatochi M, Ogiso H, Kawamoto Y, Nakamura M,
et al: Increased SPHK2 transcription of human colon cancer cells in
serum-depleted culture: The involvement of CREB transcription
factor. J Cell Biochem. 116:2227–2238. 2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Koyama Y, Kimura Y, Yoshioka Y, Wakamatsu
D, Kozaki R, Hashimoto H, Matsuda T and Baba A: Serum-deprivation
induces cell death of rat cultured microglia accompanied with
expression of Bax protein. Jpn J Pharmacol. 83:351–354.
2000.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Kingham PJ, Cuzner ML and Pocock JM:
Apoptotic pathways mobilized in microglia and neurones as a
consequence of chromogranin A-induced microglial activation. J
Neurochemistry. 73:538–547. 1999.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Liu B, Wang K, Gao HM, Mandavilli B, Wang
JY and Hong JS: Molecular consequences of activated microglia in
the brain: Overactivation induces apoptosis. J Neurochemistry.
77:182–189. 2001.PubMed/NCBI View Article : Google Scholar
|