Introduction
Trauma is the fifth leading cause of death,
resulting in more fatalities than diabetes and infectious diseases
in China, and thus places a substantial burden on healthcare
systems across the world (1). A
recent retrospective study which included >500,000 Chinese
subjects reported that the population-weighted incidence rate of
traumatic fractures in the general population was ~3.2 per 1,000
individuals (1).
Despite the considerable developments in terms of
internal and external fixation systems, bone fractures may still
fail to heal under certain circumstances, including bone non-union
or pseudarthrosis, causing painful and delayed bone healing
(2). Clinical studies focusing on
facilitating bone healing and restoration of normal biomechanical
properties following bone fracture have shown that such methods may
allow patients to recover and return to normal life relatively
quicker than conventional methods (1-3).
Healing of fractures is initiated by the
inflammatory cascade, followed by the recruitment of various immune
and mesenchymal cells, as well as the formation of hematomas that
further develop into vascularized and innervated granulation tissue
(4). Following this initial stage of
repair, callus tissue, characterized by the formation of woven
bone, which may bridge the injury sites, is formed, followed by the
bone remodelling phase (5). Although
the inflammatory response is essential and beneficial to initiate
bone repair, dysregulated or chronic inflammation may severely
impair bone healing (6). Previous
studies have shown that macrophages and other interleukin
(IL)-17-producing γδ T cells promote bone healing (7,8), and
that cytotoxic T cells may impair bone repair (9). IL-10-producing B cells, which suppress
excessive and/or prolonged inflammation, may also contribute to
bone healing (4). However, the
underlying mechanisms of the effects of immune reaction on bone
homeostasis during fracture healing remains to be determined.
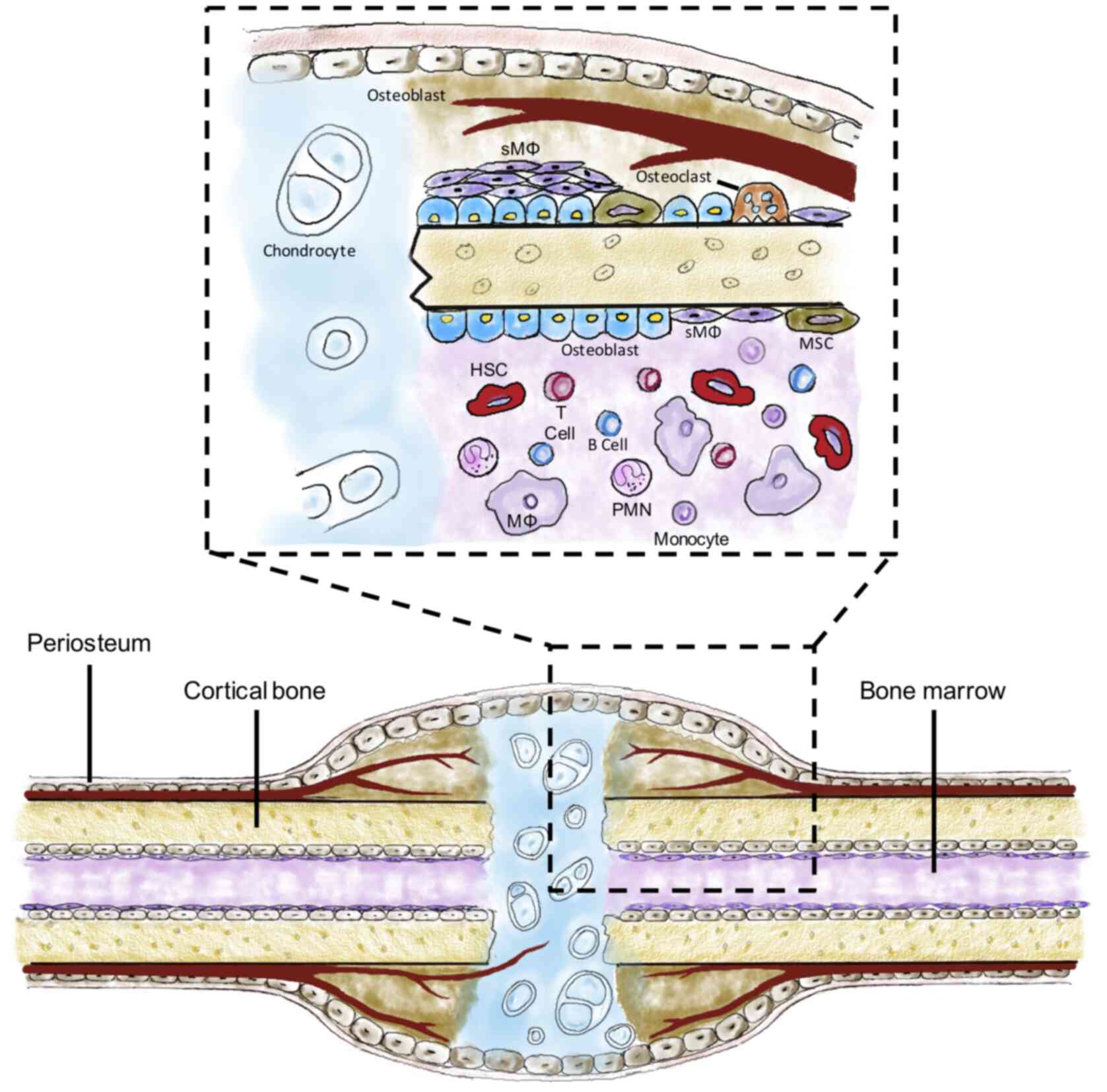
In recent years, tissue-resident macrophages have
been garnered increasing attention, not only because of their
important roles in innate immunity, but also in homeostasis and
regeneration (6,10-12).
Multiple subsets of tissue-resident macrophages have been
identified in different organs or tissues, including microglial
cells in the brain, Kupffer cells in the liver and Langerhans cells
in the skin (13). Bone-resident
macrophages are divided into erythroblastic island macrophages,
haematopoietic stem cell niche macrophages and skeletal macrophages
(sMΦ) (4,6,14,15).
sMΦ, also called osteal macrophages or osteomacs, have been
reported to significantly contribute to bone homeostasis and
regeneration (16,17).
The aim of the present review was to systematically
summarize the contribution of sMΦ in bone repair, and evaluate
their potential as a therapeutic target for promoting bone
regeneration and other bone diseases.
Materials and methods
Search strategy
A systematic search of the PubMed and
Embase® databases (from inception to December
23rd, 2019) for studies investigating the function of
sMΦ in bone injury repair was performed. This review was performed
in accordance with the Preferred Reporting Items for Systematic
Reviews and Meta-Analyses guidelines (18), with search key words including
‘osteal tissue macrophages’, ‘bone resident macrophages’, ‘skeletal
macrophages’, ‘bone resident macrophage’ and ‘bone fractures’. The
detailed search strategy is presented in Table I including a list of all search items
used, names of the database searched and the publication period
included.
 | Table ISearch strategy for studies published
from inception of the database to December 23rd 2019. |
Table I
Search strategy for studies published
from inception of the database to December 23rd 2019.
| A,
PubMeda | Number of
results |
|---|
| (‘Osteal tissue
macrophages’ OR ‘osteal tissue macrophage’ OR ‘osteal macrophage’
OR ‘osteal macrophages’ OR ‘bone resident macrophages’ OR ‘bone
resident macrophage’ OR ‘tissue resident macrophages’ OR ‘tissue
resident macrophage’ OR ‘skeletal macrophages’ OR ‘skeletal
macrophage’ OR ‘osteomacs’ OR ‘osteomac’ OR ‘resident tissue
macrophages’ OR ‘resident tissue macrophage) AND (Broken Bones’ OR
‘Bone, Broken’ OR ‘Bones, Broken’ OR ‘Broken Bone’ OR ‘Bone
Fractures’ OR ‘Bone Fracture’ OR ‘Fracture, Bone’ OR ‘Spiral
Fractures’ OR ‘Fracture, Spiral’ OR ‘Fractures, Spiral’ OR ‘Spiral
Fracture’ OR ‘Torsion Fractures’ OR ‘Fracture, Torsion’ OR
‘Fractures, Torsion’ OR ‘Torsion Fracture’ OR ‘Fracture’ OR
‘Fractures’ OR ‘Fractures, Bone’) | 76 |
| B,
Embase® |
| i) ‘osteal tissue
macrophages’ OR ‘osteal tissue macrophage’ OR ‘osteal macrophage’
OR ‘osteal macrophages’ OR ‘bone resident macrophages’ OR ‘bone
resident macrophage’ OR ‘tissue resident macrophages’ OR ‘tissue
resident macrophage’ OR ‘skeletal macrophages’ OR ‘skeletal
macrophage’ OR ‘osteomacs’ OR ‘osteomac’ OR ‘resident tissue
macrophages’ OR ‘resident tissue macrophage’.mp. | 933 |
| ii) exp
fracture/ | 275,697 |
| iii) ‘bone
fracture’ OR ‘bone fractures’ OR ‘fracture’ OR ‘fractures’.mp. | 366,616 |
| iv) ii and iii | 373,991 |
| v) i and iv | 17 |
Inclusion and exclusion criteria
Studies were included if they met the following
criteria: i) Relevant to evaluating the effect of sMΦ in bone
repair or regeneration; ii) full-length research articles were
available; and iii) studies were published in English. Reviews,
correspondences, case reports, expert opinions and editorials were
all excluded.
Quality assessment and statistical
analysis
The Systematic Review Centre for Laboratory animal
Experimentation tool (19) was used
to assess the risk of bias of included studies, with the types of
bias including: Selection bias, performance bias, attrition bias,
detection bias, reporting bias and other biases. The response was
defined as ‘Low risk of bias’ or ‘High risk of bias’ for each item
in the checklist. For ideal methodological quality, the percentage
of ‘Low risk of bias’ was required to be ≥80% (20). If it was not possible to make a
judgment based on the present information, a rating of ‘Unclear
risk of bias’ was assigned. Finally, a sum of the percentage of
bias for each study was calculated.
Data extraction
Data extraction was performed by two reviewers
independently. Disagreements were resolved by consensus or
discussion amongst co-investigators. The extracted data were
characteristics of the study samples, general and detailed
methodology characteristics, and study results.
Statistical analysis
All statistical analysis was performed using SPSS
version 25 (IBM Corp.). A one-way ANOVA and Brown-Forsythe test
were used to compare groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
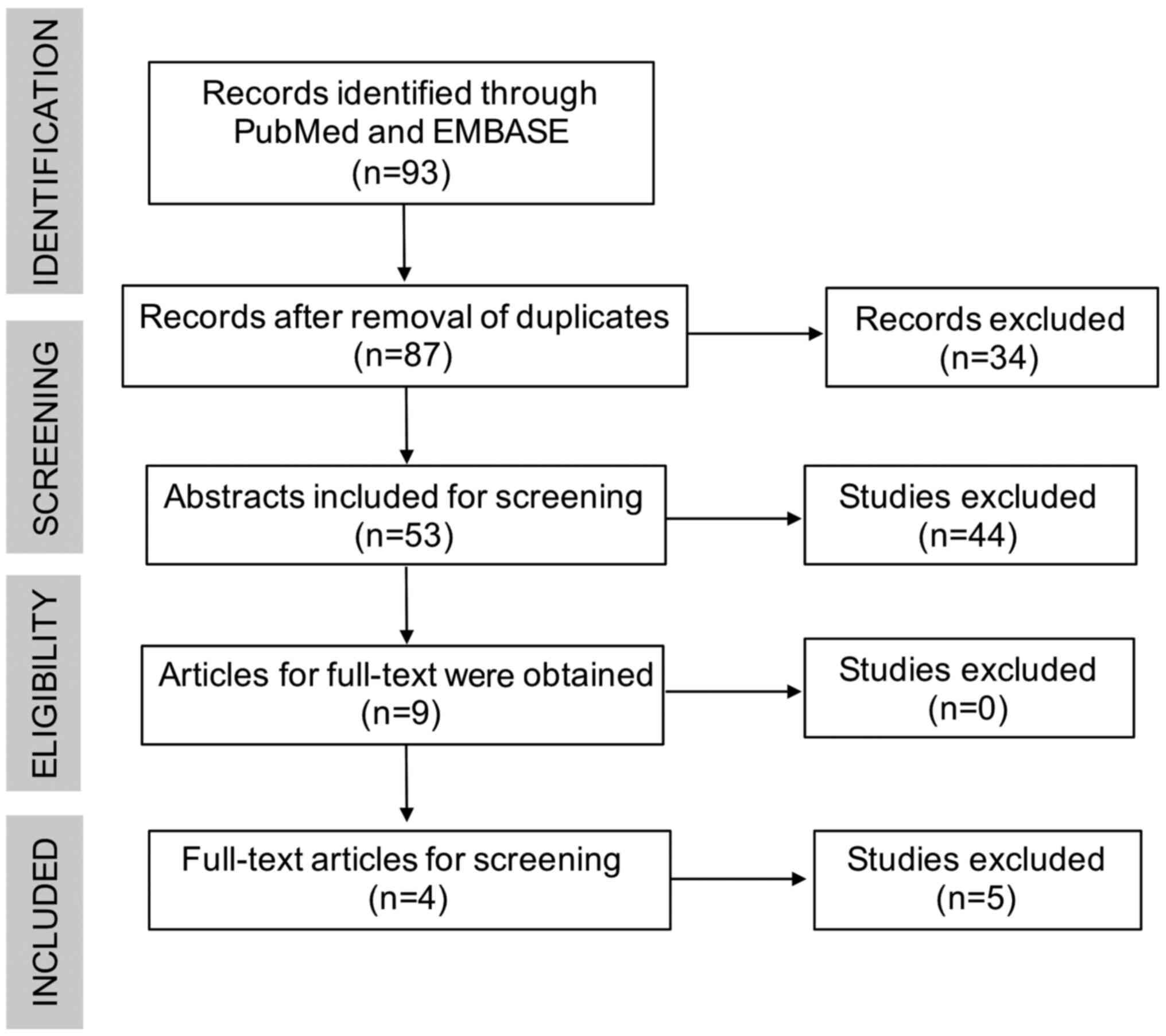
Details of the study selection process are presented
in Fig. 1. The systematic search
resulted in retrieval of 93 articles. After removing duplicates, 87
articles remained for first-stage screening. By reviewing the
titles and abstracts, 3 articles were deemed irrelevant. No
additional articles were included by checking the references. A
total of 9 relevant articles were identified, the full text of
which were assessed for eligibility. Finally, 4 articles that met
all of the inclusion criteria were identified and included in the
present systematic review (10,16,17,21).
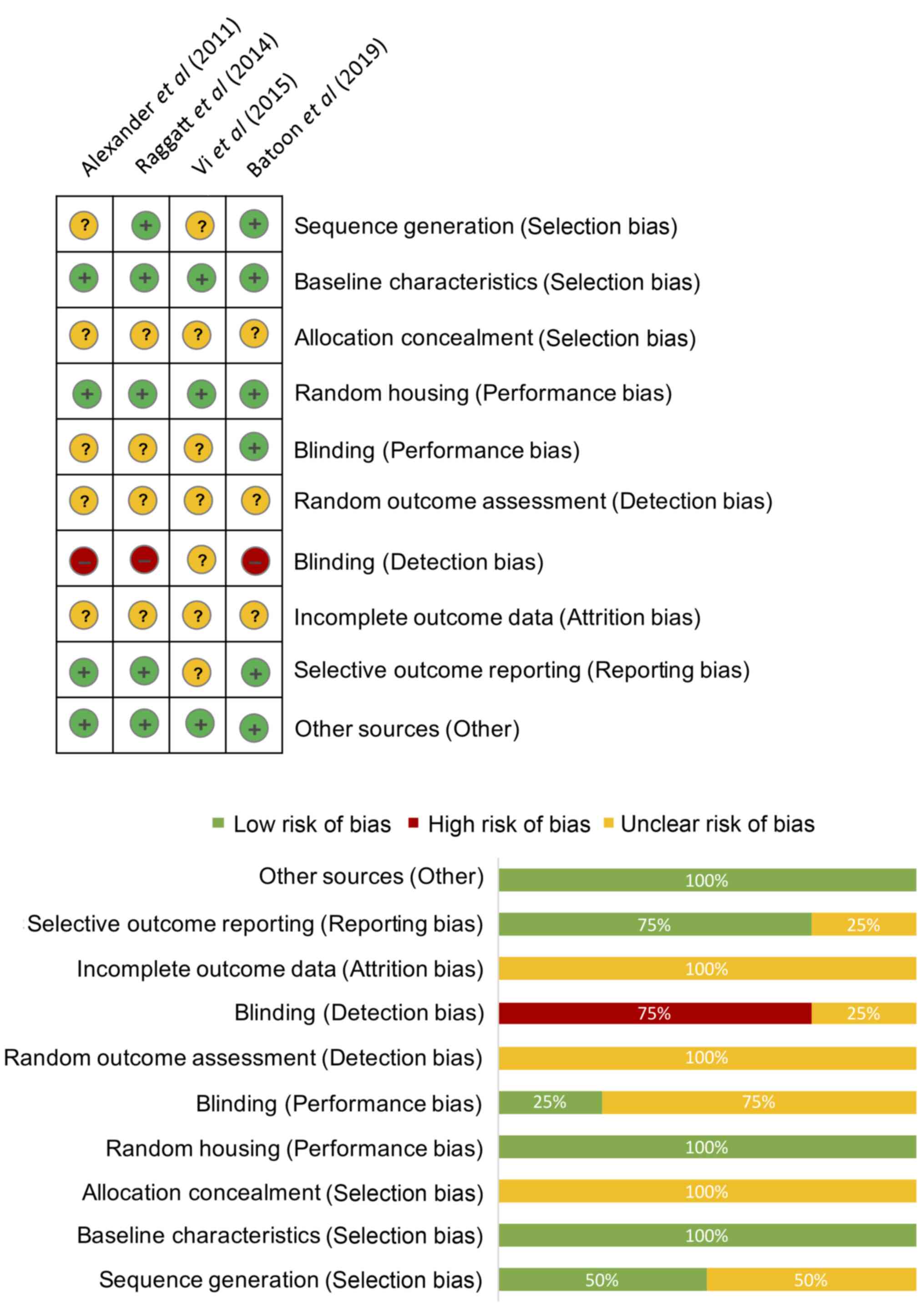
The results of the risk of bias assessment are
presented in Fig. 2. The mean
percentage of low risk bias was 45% [95% confidence interval (CI),
12.6-77.4%], the mean percentage of high risk bias was 7.5%, (95%
CI, 0.0-24.5%) and the mean percentage of unclear bias was 45.0%,
(95% CI, 12.6-77.4%). P<0.05 in the Brown-Forsythe test
indicated there was a significant difference between these 3 bias
groups. There was a relatively high risk of bias associated with
the blinding of the investigators and animals, since none of the
assessors in these studies were blinded, and reports on allocation,
random outcome assessment and incomplete outcome data were not well
documented. There was a low risk of bias for baseline
characteristics, random housing, selective information and other
potential biases in the studies evaluated.
Table II presents
the major characteristics of the studies included in the present
systematic review. In all of the studies, mice were used as the
experimental animals, with an age of 11-13 weeks. Of the four
studies, three utilized the tibial fracture model and the remaining
study used a femoral fracture model. Furthermore, three studies
used immunohistochemistry combined with flow cytometry for
identification and characterization of sMΦ. Specific surface
markers used to define sMΦ were F4/80+,
Mac-2-/low, TRAP-,
CD169+, Ly6G- and CD115low. In
addition, all of the studies concluded that sMΦ have an essential
role in fracture healing, and the mechanisms are summarized in
Fig. 3.
 | Table IISummary of studies included in the
systematic review. |
Table II
Summary of studies included in the
systematic review.
| First author, year
(Refs.) | Species
investigated | Sample
characteristics | Bone injury
model | Identification
methods of macrophages | Markers of sMΦ
used | Role of sMΦ in bone
repair |
|---|
| Alexander et
al, 2011(17) | Mouse | 11-12 weeks
old | Tibial
fracture | FC, IHC | F4/80+, Mac-2-/low,
TRAP- | i) Participated in
intramenbranous ossification. ii) Required for CT1+ matrix
deposition and bone mineralization. |
| Raggatt et
al, 2014(21) | Mouse | 11-12 weeks
old | Femoral
fracture | FC, IHC | F4/80+, Mac-2- | Promoted anabolism
during endochondral callus formation. |
| Vi et al,
2015(10) | Mouse | 12 weeks old | Tibial
fracture | IHC | F4/80+, TRAP- | Maintained bone
homeostasis and promoted fracture repair by enhancing the
differentiation of mesenchymal progenitors. |
| Batoon et
al, 2019(16) | Mouse | 11-13 weeks
old | Tibial
fracture | FC, IHC | CD169+, F4/80+,
Ly6G-, CD115 low | Supported
osteoblasts during both bone homeostasis and repair. |
Discussion
The aim of the present study was to summarize the
results of previous studies assessing the role of sMΦ in bone
healing. Previous studies supported the involvement of sMΦ in
fracture healing, and identified the underlying cellular and
molecular mechanisms and their utility in novel immunoregulatory
therapy in bone regeneration.
Alexander et al (17) assessed the effects of sMΦ and
inflammatory macrophages in bone healing and showed that
F4/80+Mac-/low sMΦ
formed a distinctive canopy-like structure over cuboidal
osteoblasts located on the surface of new bone. The number of
F4/80+Machigh inflammatory macrophages was
considerably lower than that of sMΦ during the early and late
anabolic phases of tibial fracture repair, which heals primarily
via intramembranous ossification (16). F4/80+ macrophages were
present in all phases of fracture healing and were required for
matrix deposition and bone mineralization. Systematic depletion of
F4/80+ macrophages notably suppressed bone deposition
and mineralization (21).
Furthermore, due to the relationship in the lineage of macrophages
and osteoclasts, osteoclasts were specifically ablated using
osteoporotegerin treatment to study the effect of an absence of
osteoclasts on bone healing (21).
It was shown that osteoporotegerin treatment resulted in
significantly impaired bone resorption, but did not compromise
CT1+ woven bone deposition, which further confirmed the
importance of F4/80+ macrophages that were prominently
sMΦ in bone healing (22). The
systematic depletion approach of macrophages using lysozyme
M-driven Cre recombinase, Csf1r promoter, clodronate liposome or
antibody is also able to reduce inflammatory macrophages and
osteoclasts (23). Therefore,
specific ablation of sMΦ by targeting a specific surface marker in
fracture models is necessary (23).
In addition, macrophages were shown to promote
endochondral callus formation following bone fracture (21). In a mouse femoral fracture model,
which primarily heals via endochondral ossification, Batoon et
al (15) found that
F4/80+ Mac-2+ inflammatory macrophages were
abundant in the granulation tissue, which was fully established 7
days after fracture surgery. However, the presence of sMΦ, defined
as F4/80+Mac-2 cells-, were relatively rare at this
reparative stage. Furthermore, during soft-to-hard callus
transition, both sMΦ and inflammatory macrophages were abundantly
present in the maturing callus. F4/80+ macrophage depletion at the
start of the early anabolic phase significantly impeded soft callus
formation and the progression of anabolism in endochondral
ossification (15). Furthermore,
Alexander et al (14)
suggested that macrophages have a significant influence on both
cartilage and bone deposition during endochondral ossification. The
presence of F4/80+ macrophages throughout the entire
process of fracture repair and macrophage deficiency may result in
smaller fracture calluses, but increased fibrotic calluses, which
results in delayed bone repair (10).
The crosstalk between sMΦ and
osteoblasts/osteoclasts is currently being investigated. Batoon
et al (16) demonstrated that
CD169, a cell surface antigen expressed by mature tissue-resident
macrophages, may be used to discriminate osteoclasts and sMΦ.
CD169+ sMΦ depletion may significantly compromise
osteoblastogenesis and bone repair in bone injury, primarily via
promoting both endochondral ossification or intramembranous
ossification (16). Furthermore,
increasing the proliferation of sMΦ in callus tissue by
administering colony-stimulating factor-1, which may target sMΦ and
promote its proliferation, was reported to promote bone repair
(17,21). Although the mechanisms by which sMΦ
promotes fracture healing remain elusive, the NF-κB signalling
pathway, bone morphogenetic proteins and oncostatin M are thought
to be essential in sMΦ-mediated osteogenesis (24,25).
Ablation of sMΦ was indicated to significantly impair osteocalcin
expression and osteoblast mineralization in vivo and in
vitro (11). Furthermore, the
interaction between sMΦ and osteoclasts may also be a point of
interest. Macrophage-deficient mice exhibited functionally active
osteoclast activities, but were characterized by decreased sMΦ at
the bone surface and impaired bone formation (10,26).
These results emphasize the importance of sMΦ in bone healing, and
highlight the potential role of sMΦ as a therapeutic target for
bone regeneration. Thus, a more in-depth understanding from a
global perspective of molecular profiles and phenotypes adopted by
sMΦ in the bone environment is required.
An increasing number of studies have shown that
tissue-resident macrophages are able to adopt tissue-specific
phenotypes and functions and may acquire self-renewal capacity
(10,16,17,21).
Multiple studies have confirmed the essential roles of macrophages
in skeletal homeostasis and bone repair (10,16,17,21);
however, direct evidence of the function of sMΦ in bone biology
remains insufficient, due to the heterogeneity of macrophage
clusters and the lack of sMΦ-specific biomarkers (27). With the development of cutting-edge
techniques, including optimized next-generation sequencing
technologies (28), for use in life
science investigations, a single-cell sequencing approach may be a
suitable means of profile the involved macrophages, thus assisting
in the identification of the heterogeneity of sMΦ during fracture
repair.
The present systematic review provided an overview
of the roles of sMΦ in bone healing. Several biomarkers defining
sMΦ were identified based on the available literature. The present
study is limited by the high risk of bias with regard to blinding
and sequence generation in the reviewed studies. Another limitation
is that due to the shortage of sufficient studies on this topic,
the importance of sMΦ in fracture healing may be under- or
overestimated.
In conclusion, a growing body of evidence strongly
supports the notion that
F4/80+Mac-2-CD169+ sMΦ may serve
as a promising therapeutic target for immunoregulatory therapy in
bone repair, due to their essential role in bone formation and
homeostasis. Further investigation aiming to modulate sMΦ, with the
aim of promoting bone regeneration, are required.
Acknowledgements
Not applicable.
Funding
This study was supported by funding from the MWLC
Associate Member Programme, Ming Wai Lau Center of Regenerative
Medicine of Karolinska Institute (grant no. TK1914020), CUHK
Research Committee Funding (grant no. 2018.020) and Hong Kong
Government Research Grant Council, General Research Fund (Reference
no. 14104620) to CW Lee.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZW and OKSL conceived and designed the study. ZW
acquired the data. ZW, LYS, YFW, ZH, YD, CWL and SMK analyzed and
interpreted the data. ZW wrote the manuscript. ZW, SMK, and OKSL
revised the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen W, Lv H, Liu S, Liu B, Zhu Y, Chen X,
Yang G, Liu L, Zhang T, Wang H, et al: National incidence of
traumatic fractures in China: A retrospective survey of 512 187
individuals. Lancet Glob Heal. 5:e807–e817. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Schneider E, Goldhahn J and Burckhardt P:
The challenge: Fracture treatment in osteoporotic bone. Osteoporos
Int. 16:1–2. 2005.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Holmes D: Non-union bone fracture: A
quicker fix. Nature. 550(S193)2017.PubMed/NCBI View
Article : Google Scholar
|
|
4
|
Loi F, Córdova LA, Pajarinen J, Lin TH,
Yao Z and Goodman SB: Inflammation, fracture and bone repair. Bone.
86:119–130. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Marsell R and Einhorn TA: The biology of
fracture healing. Injury. 42:551–555. 2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ono T and Takayanagi H: Osteoimmunology in
bone fracture healing. Curr Osteoporos Rep. 15:367–375.
2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tsukasaki M and Takayanagi H:
Osteoimmunology: Evolving concepts in bone-immune interactions in
health and disease. Nat Rev Immunol. 19:626–642. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ono T, Okamoto K, Nakashima T, Nitta T,
Hori S, Iwakura Y and Takayanagi H: IL-17-producing γδ T cells
enhance bone regeneration. Nat Commun. 7(10928)2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lorenzo J: Interactions between immune and
bone cells: New insights with many remaining questions. J Clin
Invest. 106:749–752. 2000.PubMed/NCBI View
Article : Google Scholar
|
|
10
|
Vi L, Baht GS, Whetstone H, Ng A, Wei Q,
Poon R, Mylvaganam S, Grynpas M and Alman BA: Macrophages promote
osteoblastic differentiation in-vivo: Implications in fracture
repair and bone homeostasis. J Bone Min Res. 30:1090–1102.
2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Chang MK, Raggatt LJ, Alexander KA,
Kuliwaba JS, Fazzalari NL, Schroder K, Maylin ER, Ripoll VM, Hume
DA and Pettit AR: Osteal tissue macrophages are intercalated
throughout human and mouse bone lining tissues and regulate
osteoblast function in vitro and in vivo. J Immunol. 181:1232–1244.
2008.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Michalski MN and McCauley LK: Macrophages
and skeletal health. Pharmacol Ther. 174:43–54. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Epelman S, Lavine KJ and Randolph GJ:
Origin and functions of tissue macrophages. Immunity. 41:21–35.
2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Alexander KA, Raggatt LJ, Millard S,
Batoon L, Chiu-Ku Wu A, Chang MK, Hume DA and Pettit AR: Resting
and injury-induced inflamed periosteum contain multiple macrophage
subsets that are located at sites of bone growth and regeneration.
Immunol Cell Biol. 95:7–16. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Batoon L, Millard SM, Raggatt LJ and
Pettit AR: Osteomacs and bone regeneration. Curr Osteoporos Rep.
15:385–395. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Batoon L, Millard SM, Wullschleger ME,
Preda C, Wu AC, Kaur S, Tseng HW, Hume DA, Levesque JP, Raggatt LJ
and Pettit AR: CD169+ macrophages are critical for
osteoblast maintenance and promote intramembranous and endochondral
ossification during bone repair. Biomaterials. 196:51–66.
2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Alexander KA, Chang MK, Maylin ER, Kohler
T, Müller R, Wu AC, Van Rooijen N, Sweet MJ, Hume DA, Raggatt LJ
and Pettit AR: Osteal macrophages promote in vivo intramembranous
bone healing in a mouse tibial injury model. J Bone Min Res.
26:1517–1532. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
18
|
Liberati A, Altman DG, Tetzlaff J, Mulrow
C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J
and Moher D: The PRISMA statement for reporting systematic reviews
and meta-analyses of studies that evaluate health care
interventions: Explanation and elaboration. J Clin Epidemiol.
62:e1–e34. 2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hooijmans CR, Rovers MM, De Vries RBM,
Leenaars M, Ritskes-Hoitinga M and Langendam MW: SYRCLE's risk of
bias tool for animal studies. BMC Med Res Methodol.
14(43)2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Stiuso P, Scognamiglio I, Murolo M,
Ferranti P, De Simone C, Rizzo MR, Tuccillo C, Caraglia M,
Loguercio C and Federico A: Serum oxidative stress markers and
lipidomic profile to detect NASH patients responsive to an
antioxidant treatment: A pilot study. Oxid Med Cell Longev.
2014(169216)2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Raggatt LJ, Wullschleger ME, Alexander KA,
Wu AC, Millard SM, Kaur S, Maugham ML, Gregory LS, Steck R and
Pettit AR: Fracture healing via periosteal callus formation
requires macrophages for both initiation and progression of early
endochondral ossification. Am J Pathol. 184:3192–3204.
2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Van Rooijen N, Sanders A and Van Den Berg
TK: Apoptosis of macrophages induced by liposome-mediated
intracellular delivery of clodronate and propamidine. J Immunol
Methods. 193:93–99. 1996.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Arai F, Miyamoto T, Ohneda O, Inada T,
Sudo T, Brasel K, Miyata T, Anderson DM and Suda T: Commitment and
differentiation of osteoclast precursor cells by the sequential
expression of c-Fms and receptor activator of nuclear factor κB
(RANK) receptors. J Exp Med. 190:1741–1754. 1999.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Mise-Omata S, Alles N, Fukazawa T, Aoki K,
Ohya K, Jimi E, Obata Y and Doi T: NF-κB RELA-deficient bone marrow
macrophages fail to support bone formation and to maintain the
hematopoietic niche after lethal irradiation and stem cell
transplantation. Int Immunol. 26:607–618. 2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhang Y, Xu J, Ruan YC, Yu MK, O'Laughlin
M, Wise H, Chen D, Tian L, Shi D, Wang J, et al: Implant-derived
magnesium induces local neuronal production of CGRP to improve
bone-fracture healing in rats. Nat Med. 22:1160–1169.
2016.PubMed/NCBI View
Article : Google Scholar
|
|
26
|
Wintges K, Beil FT, Albers J, Jeschke A,
Schweizer M, Claass B, Tiegs G, Amling M and Schinke T: Impaired
bone formation and increased osteoclastogenesis in mice lacking
chemokine (C-C motif) ligand 5 (Ccl5). J Bone Miner Res.
28:2070–2080. 2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Schlundt C, El Khassawna T, Serra A,
Dienelt A, Wendler S, Schell H, van Rooijen N, Radbruch A, Lucius
R, Hartmann S, et al: Macrophages in bone fracture healing: Their
essential role in endochondral ossification. Bone. 106:78–89.
2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Debnath S, Yallowitz AR, McCormick J,
Lalani S, Zhang T, Xu R, Li N, Liu Y, Yang YS, Eiseman M, et al:
Discovery of a periosteal stem cell mediating intramembranous bone
formation. Nature. 562:133–139. 2018.PubMed/NCBI View Article : Google Scholar
|