Introduction
Coronavirus disease 2019 (COVID-19), caused by
infection from severe acute respiratory syndrome-coronavirus 2
(SARS-CoV-2), has become a global pandemic and a serious global
public health emergency (1). The
prevention and control of COVID-19 is a considerable challenge
being faced by numerous governments worldwide, and this is
complicated by the high transmission efficiency, high mortality
rate, general susceptibility of the population, and the absence of
effective drugs and vaccines (2,3). As the
pathology of COVID-19 is similar to that of Middle East respiratory
syndrome coronavirus (MERS-CoV) and SARS-CoV infection (4,5), drugs
identified to be effective for these previous diseases, as well as
antivirals screened from in vitro experiments, are being
assessed or used clinically without sufficient clinical evidence.
These drugs include IFN-α and ribavirin, as well as
lopinavir/ritonavir (LPV/r) and Umifenovir as antiviral drugs.
Several studies have described the effects of antiviral on the
duration of viral shedding (6-8) and
other studies have assessed the differences in the duration of
viral shedding between patients with different degrees of severity
of infection (9). Recently, obesity
was identified as a risk factor for increased COVID-19 prevalence,
severity and lethality (10), and
modulation of zinc status may be beneficial in the management
COVID-19(11). However, these
studies did not consider the effects of both clinical and
clinicopathological factors on the duration of viral shedding. In
the present study, detailed data on inpatients with definite
clinical outcomes between January 20, 2020 and March 20, 2020 was
collected and reviewed the duration of viral shedding in COVID-19
infected patients in order to evaluate the impact of current
clinical and clinicopathological factors on viral shedding.
Clinicopathological factors refers to the patient's general
characteristics and medical history that are not related to this
hospitalization, such as sex, age, comorbid chronic diseases and
onset-hospitalization interval.
Materials and methods
Study design
Data on SARS-CoV-2 nucleic acid-positive
hospitalized patients were collected between January 20, 2020 and
March 20, 2020. Reverse transcription polymerase chain reaction
(RT-PCR) was used to test for SARS-CoV-2 nucleic acids from
respiratory tract secretions and throat swab specimens. The
patients underwent a RT-PCR test every 3 days during the first week
of hospitalization, and a nucleic acid test every day after a week
of hospitalization. A retrospective analysis of the SARS-CoV-2
shedding duration and the effect of currently used drugs on the
duration of viral shedding was performed. This study was exempt
from the need to obtain patient consent due to the retrospective
nature of the study, and was approved by the Medical Ethics
Committee of Zhengzhou University (approval no. 2020-KY-162).
Data collection
The age, sex, clinical symptoms, onset-drug
interval, onset-hospitalization interval, therapeutic drugs and
prognosis of each patient were collected. The median age of the 186
patients was 46.5 years (age range, 5-94 years). A total of 105
(56.5%) cases were males and 81 (43.5%) were females. Onset was
defined as the earliest time when clinical symptoms appeared. The
duration of viral shedding was defined as the time from onset to
the last positive test for SARS-CoV-2. The criteria for severe
cases (including critical) was defined as follows: Breathing
significantly faster, >30 times per minute in a resting state;
blood oxygen saturation <90%, or blood oxygen saturation notably
decreased in the analysis of arterial blood gas; and lung image
showing >50% apparent progression of lesions within 24-48 h.
Non-severe cases were diagnosed with mild respiratory infection
symptoms (defined as fever <39˚C, no breathing difficulties,
blood oxygen saturation ≥90%), without visible radiological changes
of the chest. The application of drugs was defined as patients
treated with a drug for ≥3 days.
Statistical analysis
All statistical analysis was performed using SPSS
version 21.0 (SPSS, Inc.). Categorical variables were expressed as
percentages. Normally distributed continuous variables are shown as
the mean ± standard deviation, and were compared using a Student's
t-test. Non-normally distributed continuous variables are presented
as the median, and a rank sum test was used for comparison.
Significant risk factors identified by univariate analyses were
further analysed by multivariate logistic regressions to identify
independent risk factors associated with a prolonged duration of
SARS-CoV-2 shedding [odds ratio (OR), 95% confidence interval
(CI)]. Rank correlation analysis was used to analyse the
correlation between onset-drug interval of LPV/r and the duration
of viral shedding. A two-sided P<0.05 was considered to indicate
a statistically significant difference.
Results
Characteristics and treatments
A total of 133 non-severe cases (71.5%) and 53
(28.5%) severe cases were enrolled in this study, including 10
deaths (5.4%). A total of 54 patients (29.0%) had a comorbid
chronic disease, and 31 patients exhibited ≥1 comorbid disease,
including 29 with hypertension, 18 with diabetes, 15 with coronary
heart disease and 7 with chronic lung disease. The median
onset-hospitalization interval for the 186 patients was 5 days
[interquartile range (IQR) 2-8 days]. LPV/r (adults, 400 mg/100 mg
bid po; children, 200 mg/50 mg bid po) was administered to 158
patients (84.9%) during hospitalization. A total of 140 patients
had a clear onset-drug interval, with a median period of 7 days
(IQR, 4-10 days). Umifenovir (200 mg tid po) was administered to 44
(23.7%) patients. A total of 18 patients (9.7%) were treated with
ribavirin (500 mg bid ivggt) during hospitalization. A total of 30
patients (16.1%) were treated with a corticosteroid during
hospitalization, at doses of ranging from 40-120 mg; however,
although the highest dose was 120 mg per day, the majority of
patients received <80 mg/day.
Duration of viral shedding
The duration of SARS-COV-2 shedding was 3-40 days,
and the median period was 13 days (IQR, 10-19 days). The median
duration of viral shedding in non-severe case was 12 days (IQR,
8-17 days), and the longest duration was 39 days. The median
duration of viral shedding in severe case was 17 days (IQR, 12-23
days), and the longest duration was 40 days; there was a
significant difference (P<0.001) between the two groups. In 5
deaths, the nucleic acid test remained positive. The different
characteristics of the COVID-19 patients with the duration of viral
shedding are outlined in Table
I.
 | Table ICharacteristics of COVID-19 patients
and the duration of viral shedding. |
Table I
Characteristics of COVID-19 patients
and the duration of viral shedding.
| | Viral shedding
duration after illness onset | |
|---|
| Factors | ≤14 days, n=115 | >14 days,
n=71 | P-value |
|---|
| Age, years, mean ±
standard deviation | 44.35±17.44 | 51.39±18.15 | 0.009b |
| Sex, n (%) | | | 0.98 |
|
Male | 65 (56.5) | 40 (56.3) | |
|
Female | 50 (43.5) | 31 (43.7) | |
| Onset-hospitalization
interval, days (IQR) | 4 (2-7) | 7 (3-11) | <0.001 |
| Comorbid chronic
disease, n (%) | | | 0.005b |
|
Yes | 25 (21.7) | 29 (40.8) | |
|
No | 90 (78.3) | 42 (59.2) | |
| Ribavirin use, n
(%) | | | 0.002b |
|
Yes | 5 (4.3) | 13 (18.3) | |
|
No | 110 (95.7) | 58 (81.7) | |
| Lopinavir/ritonavir
use, n (%) | | | <0.001 |
|
Yes | 106 (92.2) | 52 (73.2) | |
|
No | 9 (7.8) | 19 (26.8) | |
| Umifenovir use, n
(%) | | | 0.028a |
|
Yes | 21 (18.3) | 23 (32.4) | |
|
No | 94 (81.7) | 48 (67.6) | |
| Corticosteroid use, n
(%) | | | 0.007b |
|
Yes | 12 (10.4) | 18 (25.4) | |
|
No | 103 (89.6) | 53 (74.6) | |
Analysis of influencing factors on the
duration of viral RNA shedding
As the median duration of SARS-CoV-2 shedding was 13
days, a 14-day cut-off was used. A 12 or 13-day cut-off was not
appropriate as the 12 or 13-day cut-off was not statistically
significant, although there was a difference. This study focused on
analysing the factors influencing viral shedding duration (≤14 and
>14 days). With the duration of viral shedding as a dependent
variable, univariate analysis of sex, age, onset-hospitalization
interval, whether the patient had a comorbid chronic disease,
ribavirin use, lopinavir/ritonavir use, Umifenovir use and
corticosteroid use were defined as independent variables and
logistic stepwise multiple regression analysis was performed.
Univariate analysis showed that the factors
significantly associated with prolonged viral shedding were age,
onset-hospitalization interval, comorbid chronic disease, use of
LPV/r, use of ribavirin, use of Umifenovir and use of a
corticosteroid. However, sex was not associated with prolonged
viral shedding.
For the multivariate analysis, four parameters were
included in the final logistic regression model:
Onset-hospitalization interval, comorbid chronic disease, ribavirin
use and LPV/r use. Multiple regression analysis suggested that the
onset-hospitalization interval (OR, 1.27, 95% CI, 1.15-1.41;
P<0.001), comorbid chronic disease (OR, 2.43, 95% CI, 1.14-5.17;
P=0.021) and ribavirin use (OR, 5.97, 95% CI, 1.77-20.09; P=0.004)
were independent risk factors for prolonged viral shedding. LPV/r
was an independent protective factor (OR, 0.28, 95% CI, 0.11-0.75;
P=0.011) that could shorten the duration of viral shedding
(Table II).
 | Table IIMultivariate analysis of factors
associated with the duration of viral shedding of COVID-19. |
Table II
Multivariate analysis of factors
associated with the duration of viral shedding of COVID-19.
| | Multivariable
analysis | Stepwise
analysis |
|---|
| Variable | OR | 95% CI | P-value | OR | 95% CI | P-value |
|---|
| Age | 1.02 | 1.01-1.04 | 0.010 | - | - | - |
| Sex | 1.01 | 0.56-1.83 | 0.980 | - | - | - |
|
Onset-hospitalization interval | 1.22 | 1.11-1.33 | <0.001 | 1.27 | 1.15-1.41 |
<0.001c |
| Comorbid chronic
disease | 2.49 | 1.30-4.75 | 0.006 | 2.43 | 1.14-5.17 | 0.021a |
| Using
ribavirin | 4.93 | 1.68-14.51 | 0.004 | 5.97 | 1.77-20.09 | 0.004b |
| Using
lopinavir/ritonavir | 0.23 | 0.10-0.55 | 0.001 | 0.28 | 0.11-0.75 | 0.011a |
| Using
Umifenovir | 2.15 | 1.08-4.26 | 0.029 | - | - | - |
| Using
corticosteroid | 2.92 | 1.31-6.50 | 0.009 | - | - | - |
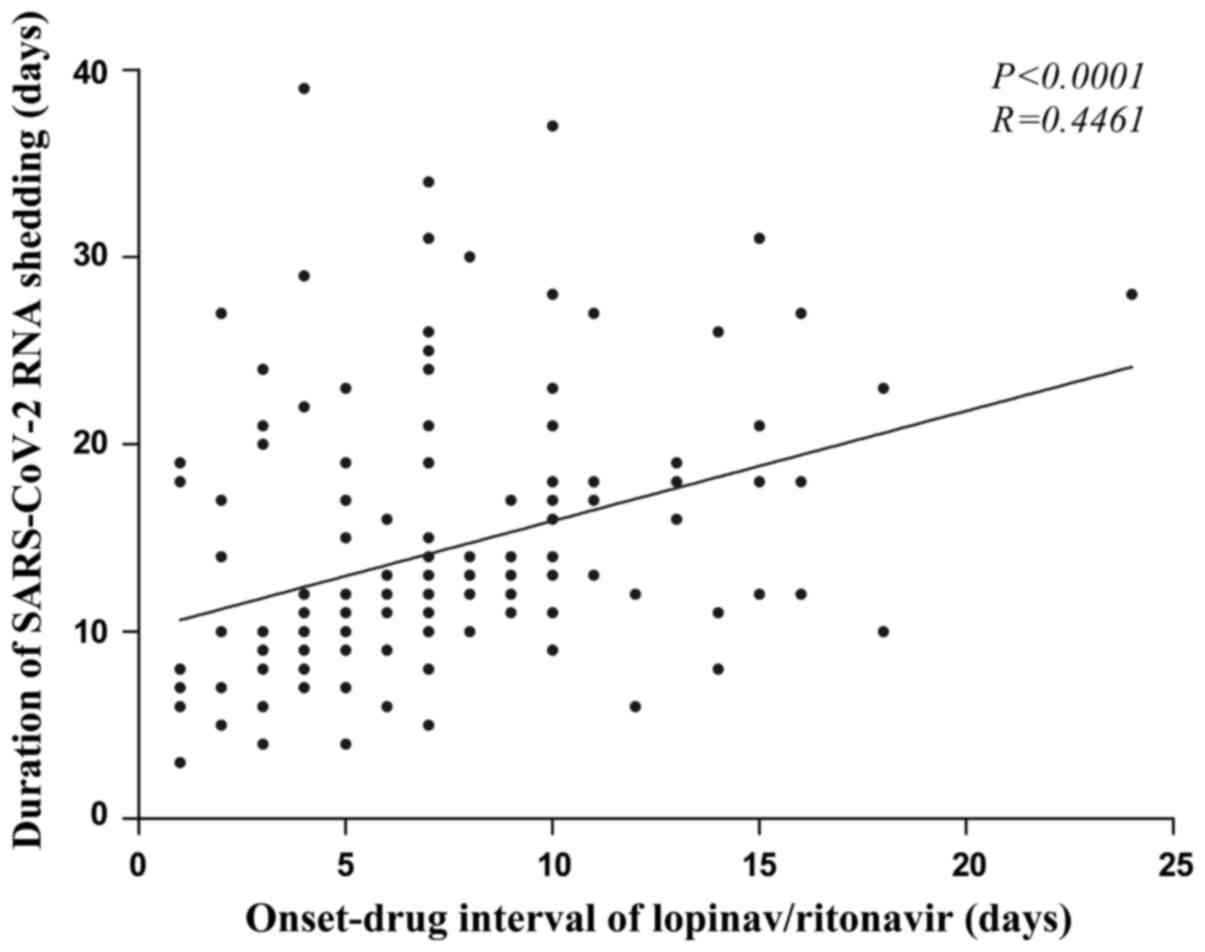
Relationship between duration of
SARS-CoV-2 shedding and onset-drug interval of LPV/r
A total of 140 patients were administered LPV/r, and
the onset-drug interval was positively correlated with the duration
of viral shedding (rank correlation test r=0.446; P<0.0001). The
longer the onset-drug interval of LPV/r was, the longer the
duration of viral shedding was (Fig.
1).
Discussion
COVID-19 is a newly discovered infectious disease
with no specific drug treatments and asymptomatic patients have
been identified as a considerable source of infection (12). At present, several drugs are being
used based on previous experience in the treatment of similar
coronavirus epidemics such as SARS and MERS; however, the efficacy
of these drugs require urgent verification. Therefore, in the
present study, a multicentre retrospective analysis of 186 patients
was performed to explore the effects of therapeutic drugs and
clinicopathological factors on the duration of SARS-CoV-2 viral
shedding.
The duration of viral shedding is used as an index
to measure infectivity and evaluate the efficacy of antiviral drugs
(13-15).
The median duration of SARS-CoV-2 shedding was 13 days (IQR, 10-19
days), with a maximum of 40 days. The results showed that the
duration of SARS-CoV-2 shedding was generally long; this indicated
a long period where a person is potentially infectious, and thus
requires prolonged isolation, in which case, the course of
antiviral treatment should be extended accordingly. The median
viral shedding period for severe patients was 17 days (IQR, 12-23
days), which was significantly longer than the 12 days (IQR, 8-17
days) of non-severe patients. Since viral load is the initial
factor exhibiting persistent aggravation, early antiviral therapy
is very important in theory.
In the present study, 44 patients (23.7%) were
treated with Umifenovir (200 mg tid po), and the administration of
Umifenovir was relatively early. Multivariate analysis showed that
Umifenovir did not affect SARS-CoV-2 shedding. This finding
suggests that Umifenovir has no beneficial anti-SARS-CoV-2
effect.
The results of multivariate analysis showed that
ribavirin was a high-risk factor for prolonging the time of viral
shedding. This was an unexpected result that differed from a
previous study (16), and this may
have been caused by data bias. Additional clinical trials,
particularly randomized controlled clinical trials, are required to
further evaluate the effect of ribavirin on patients with
SARS-CoV-2.
As the most widely used anti-inflammatory and
immunosuppressive agents in clinical practice, corticosteroids
serve an important role in the treatment of critically ill patients
(17). However, whether and how to
use them for the treatment of COVID-19 remains controversial.
Previous studies have shown that corticosteroids may prolong the
duration of viral shedding and may facilitate adverse effects such
as secondary infection and delirium; however, corticosteroids has
been shown to also reduce mortality in patients with severe
COVID-19 (18-20).
The present study found no evidence suggesting that corticosteroid
treatment prolonged viral shedding time. In China, a low dose and
short course of corticosteroid therapy is generally used on severe
and critical COVID-19 patients (21). In the present study, 30 patients were
treated with 40-120 mg corticosteroids during hospitalization. The
dose is generally <80 mg and the course of treatment is ~7 days.
Therefore, for critically ill patients, small doses and short
courses of a corticosteroid may be considered under special
circumstances.
In the total 186 patients, the median time of
onset-hospitalization interval was 5 days (IQR, 2-8 days).
Multivariate regression analysis found that the
onset-hospitalization interval was an independent risk factor for
prolonged viral shedding time. Therefore, early hospitalization of
COVID-19 patients can shorten the duration of viral shedding and
improve the prognosis of patients, whilst also reducing the
infectivity of the patient.
Complications with a comorbid chronic disease was
also an independent risk factor for prolonged viral shedding.
COVID-19 patients with long-term comorbid chronic diseases may have
relatively low immunity; these patients were susceptible to severe
pneumonia and thus had a poor prognosis (22).
LPV has been used in combination with ritonavir (a
booster) in HIV infection therapy and prevention, and it functions
as an antiretroviral protease inhibitor (23). LPV/r has shown efficacy in the
treatment of SARS-CoV and MERS (24-26).
SARS-CoV-2 is a coronavirus similar to SARS-CoV and MERS (27); however, the effects of LPV/r on
SARS-CoV-2 remains unclear. In a recent retrospective study of risk
factors associated with death in hospitalized COVID-19 patients in
Wuhan, Zhou et al (14) found
no reduction in the duration of viral shedding following LPV/r
treatment. In critically ill patients with COVID-19, Cao et
al (28) found that LPV/r did
not significantly accelerate clinical improvement, reduce
mortality, or reduce the viral RNA load detected in the throat.
These results may be due to the small sample sizes, single factor
retrospective analysis, or the severity of the patients' illness.
More recent studies have shown that hospital mortality rates were
lower in the LPV/r group (29), and
the lack of LPV/r treatment was independently associated with
prolonged SARS-CoV-2 RNA shedding (30). The multiple factor regression
analysis performed in the present study showed that LPV/r shortened
the duration of SARS-CoV-2 shedding. In the study by Cao et
al (28), all the patients had
severe symptoms, the number of cases was small and treatment with
LPV/r started relatively later compared with the present study. In
contrast, in the present study, the data on 158 patients who used
LPV/r and 28 patients who did not was collected, and the median
onset-drug interval was only 7 days (IQR, 4-10 days). The strengths
of the present study include a larger number of cases, which
consisted primarily of patients with non-severe patients and the
earlier administration of antivirals.
To further evaluate the effect of early antiviral
treatment with LPV/r on SARS-CoV-2 shedding, the correlation
between the onset-drug interval and the duration of viral shedding
was assessed, and this showed a significant positive correlation.
The longer the onset-drug interval of LPV/r, the longer the
duration of viral shedding; these findings suggested that early
antiviral treatment was important for favourable outcomes. A
previous study showed that the timing of LPV/r combined with
hydroxychloroquine treatment initiation did not seem to affect the
clinical course of COVID-19 patients (31), in contrast to the results of the
present study. Thus, additional studies with larger cohorts are
required to demonstrate the efficacy of LPV/r on COVID-19
infection.
The present study has limitations in that it was a
retrospective analysis, several factors were included, and the
sample size was limited. Although the research factors were
screened by single factor analysis and stepwise regression, the
results still require further verification in large-scale
prospective studies.
In conclusion, the results of the present study
showed that the duration of viral shedding of SARS-COV-2 based on
treatment, and prolonged viral shedding was an important factor
causing persistent aggravation of the patient's condition. The
onset-hospitalization interval and complication with comorbid
chronic diseases were independent risk factors for prolonged viral
shedding. LPV/r application shortened the duration of viral
shedding, and the earlier LPV/r was used, the shorter the duration
of viral shedding was. These results suggest that COVID-19 patients
should be hospitalized and receive antiviral treatment as early as
possible, and the use of LPV/r is a viable option.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
YP contributed to the conception and design of study
and the analysis and interpretation of data. QLi contributed to the
acquisition, analysis and interpretation of data and manuscript
review. XY contributed to the acquisition, analysis and
interpretation of data. QLuo contributed to the interpretation of
data and manuscript review. TQ, NX, QZhang, XL, XD, QZhao and LS
contributed to the acquisition of data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
This study was exempt from the need to obtain
patient consent due to the retrospective nature of the study, and
was approved by the Medical Ethics Committee of Zhengzhou
University (approval no. 2020-KY-162).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sohrabi C, Alsafi Z, O'Neill N, Khan M,
Kerwan A, Al-Jabir A, Iosifidis C and Agha R: World Health
Organization declares global emergency: A review of the 2019 Novel
Coronavirus (COVID-19). Int J Surg. 76:71–76. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Dong L, Hu S and Gao J: Discovering drugs
to treat coronavirus disease 2019 (COVID-19). Drug Discov Ther.
14:58–60. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Calina D, Docea AO, Petrakis D, Egorov AM,
Ishmukhametov AA, Gabibov AG, Shtilman MI, Kostoff R, Carvalho F,
Vinceti M, et al: Towards effective COVID-19 vaccines: Updates,
perspectives and challenges (Review). Int J Mol Med. 46:3–16.
2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zhou P, Yang XL, Wang XG, Hu B, Zhang L,
Zhang W, Si HR, Zhu Y, Li B, Huang CL, et al: A pneumonia outbreak
associated with a new coronavirus of probable bat origin. Nature.
579:270–273. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Sarzi-Puttini P, Giorgi V, Sirotti S,
Marotto D, Ardizzone S, Rizzardini G, Antinori S and Galli M:
COVID-19, cytokines and immunosuppression: What can we learn from
severe acute respiratory syndrome? Clin Exp Rheumatol. 38:337–342.
2020.PubMed/NCBI
|
|
6
|
Zhang Z, Wang S, Tu X, Peng X, Huang Y,
Wang L, Ju W, Rao J, Li X, Zhu D, et al: A comparative study on the
time to achieve negative nucleic acid testing and hospital stays
between danoprevir and Lopinavir/Ritonavir in the treatment of
patients with COVID-19. J Med Virol: Jun 5, 2020 (Epub ahead of
print).
|
|
7
|
Zuo Y, Liu Y, Zhong Q, Zhang K, Xu Y and
Wang Z: Lopinavir/ritonavir and interferon combination therapy may
help shorten the duration of viral shedding in patients with
COVID-19: A retrospective study in two designated hospitals in
Anhui, China. J Med Virol: Jun 3, 2020 (Epub ahead of print).
|
|
8
|
Nitulescu GM, Paunescu H, Moschos SA,
Petrakis D, Nitulescu G, Ion GND, Spandidos DA, Nikolouzakis TK,
Drakoulis N and Tsatsakis A: Comprehensive analysis of drugs to
treat SARS-CoV-2 infection: Mechanistic insights into current
COVID-19 therapies (Review). Int J Mol Med. 46:467–488.
2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Du X, Yu X, Li Q, Li X, Qin T, Luo Q, Wang
M, Jiang M, Bai L, Wang X and Pan Y: Duration for carrying
SARS-CoV-2 in COVID-19 patients. J Infect. 81:e78–e79.
2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Petrakis D, Margină D, Tsarouhas K, Tekos
F, Stan M, Nikitovic D, Kouretas D, Spandidos DA and Tsatsakis A:
Obesity-a risk factor for increased COVID-19 prevalence, severity
and lethality. Int J Mol Med. 22:9–19. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Skalny AV, Rink L, Ajsuvakova OP, Aschner
M, Gritsenko VA, Alekseenko SI, Svistunov AA, Petrakis D, Spandidos
DA, Aaseth J, et al: Zinc and respiratory tract infections:
Perspectives for COVID-19 (Review). Int J Mol Med. 46:17–26.
2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Pan Y, Yu X, Du X, Li Q, Li X, Qin T, Wang
M, Jiang M, Li J, Li W, et al: Epidemiological and clinical
characteristics of 26 asymptomatic severe acute respiratory
syndrome coronavirus 2 carriers. J Infect Dis. 221:1940–1947.
2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Young BE, Ong SWX, Kalimuddin S, Low JG,
Tan SY, Loh J, Ng OT, Marimuthu K, Ang LW, Mak TM, et al:
Epidemiologic features and clinical course of patients infected
With SARS-CoV-2 in Singapore. JAMA. 323:1488–1494. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z,
Xiang J, Wang Y, Song B, Gu X, et al: Clinical course and risk
factors for mortality of adult inpatients with COVID-19 in Wuhan,
China: A retrospective cohort study. Lancet. 395:1054–1062.
2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Tsang TK, Cowling BJ, Fang VJ, Chan KH, Ip
DK, Leung GM, Peiris JS and Cauchemez S: Influenza A virus shedding
and infectivity in households. J Infect Dis. 212:1420–1428.
2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Tong S, Su Y, Yu Y, Wu C, Chen J, Wang S
and Jiang J: Ribavirin therapy for severe COVID-19: A retrospective
cohort study. Int J Antimicrob Agents. 56(106114)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Sibila O, Agustí C and Torres A:
Corticosteroids in severe pneumonia. Eur Respir J. 32:259–264.
2008.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Prescott HC and Rice TW: Corticosteroids
in COVID-19 ARDS: Evidence and hope during the pandemic. JAMA: Sep
2, 2020 (Epub ahead of print).
|
|
19
|
WHO Rapid Evidence Appraisal for COVID-19
Therapies (REACT) Working Group. Sterne JAC, Murthy S, Diaz JV,
Slutsky AS, Villar J, Angus DC, Annane D, Azevedo LCP, Berwanger O,
et alAssociation between administration of systemic corticosteroids
and mortality among critically ill patients with COVID-19: A
meta-analysis. JAMA: Sep 2, 2020 (Epub ahead of print).
|
|
20
|
Lee N, Allen Chan KC, Hui DS, Ng EK, Wu A,
Chiu RW, Wong VW, Chan PK, Wong KT, Wong E, et al: Effects of early
corticosteroid treatment on plasma SARS-associated Coronavirus RNA
concentrations in adult patients. J Clin Virol. 31:304–309.
2004.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhao JP, Hu Y, Du RH, Chen ZS, Jin Y, Zhou
M, Zhang J, Qu JM and Cao B: Expert consensus on the use of
corticosteroid in patients with 2019-nCoV pneumonia. Zhonghua Jie
He Hu Xi Za Zhi. 43:183–184. 2020.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
22
|
Guan WJ, Liang WH, Zhao Y, Liang HR, Chen
ZS, Li YM, Liu XQ, Chen RC, Tang CL, Wang T, et al: Comorbidity and
its impact on 1590 patients with Covid-19 in China: A nationwide
analysis. Eur Respir J. 55(2000547)2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Meini S, Pagotto A, Longo B, Vendramin I,
Pecori D and Tascini C: Role of Lopinavir/ritonavir in the
treatment of Covid-19: A review of current evidence, guideline
recommendations, and perspectives. J Clin Med.
9(2050)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Chu CM, Cheng VC, Hung IF, Wong MM, Chan
KH, Chan KS, Kao RY, Poon LL, Wong CL, Guan Y, et al: Role of
lopinavir/ritonavir in the treatment of SARS: Initial virological
and clinical findings. Thorax. 59:252–256. 2004.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sheahan TP, Sims AC, Leist SR, Schäfer A,
Won J, Brown AJ, Montgomery SA, Hogg A, Babusis D, Clarke MO, et
al: Comparative therapeutic efficacy of remdesivir and combination
lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat
Commun. 11(222)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Chan JF, Yao Y, Yeung ML, Deng W, Bao L,
Jia L, Li F, Xiao C, Gao H, Yu P, et al: Treatment with
lopinavir/ritonavir or interferon-β1b improves outcome of MERS-CoV
infection in a nonhuman primate model of common marmoset. J Infect
Dis. 212:1904–1913. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhu N, Zhang D, Wang W, Li X, Yang B, Song
J, Zhao X, Huang B, Shi W, Lu R, et al: A novel coronavirus from
patients with pneumonia in China, 2019. N Engl J Med. 382:727–733.
2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Cao B, Wang Y, Wen D, Liu W, Wang J, Fan
G, Ruan L, Song B, Cai Y, Wei M, et al: A trial of
lopinavir-ritonavir in adults hospitalized with severe covid-19. N
Engl J Med. 382:1787–1799. 2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Karolyi M, Pawelka E, Mader T, Omid S,
Kelani H, Ely S, Jilma B, Baumgartner S, Laferl H, Ott C, et al:
Hydroxychloroquine versus lopinavir/ritonavir in severe COVID-19
patients: Results from a real-life patient cohort. Wien Klin
Wochenschr: Aug 10, 2020 (Epub ahead of print).
|
|
30
|
Yan D, Liu XY, Zhu YN, Huang L, Dan BT,
Zhang GJ and Gao YH: Factors associated with prolonged viral
shedding and impact of lopinavir/ritonavir treatment in
hospitalised non-critically ill patients with SARS-CoV-2 infection.
Eur Respir J. 56(2000799)2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Giacomelli A, Pagani G, Ridolfo AL, Oreni
L, Conti F, Pezzati L, Bradanini L, Casalini G, Bassoli C, Morena
V, et al: Early administration of lopinavir/ritonavir plus
hydroxychloroquine does not alter the clinical course of SARS-CoV-2
infection: A retrospective cohort study. J Med Virol: Aug 10, 2020
(Epub ahead of print).
|