Introduction
Esophageal achalasia is an archetypal esophageal
motility disorder, which is characterized by impaired relaxation of
the lower esophageal sphincter (LES) and abnormal peristalsis of
the esophageal body. Esophageal achalasia can result in an impaired
ability to digest food and can thus reduce a patient's quality of
life (1-3).
Although this disease was first reported ~300 years
ago, its etiology remains unknown (4). Current treatment strategies aim to
reduce LES pressure. Methods of treatment include endoscopic
balloon dilation, botulinum toxin injection, laparoscopic Heller's
myotomy, and in serious advanced cases, surgical resection of the
affected esophagus (5). However, in
recent years, per-oral endoscopic myotomy (POEM) has been
established as an alternative minimally invasive method of treating
esophageal achalasia (6). This
treatment is effective and safe, even in elderly patients, allowing
for short and long-term prognoses (6,7). Sato
et al (8) performed per-oral
endoscopic biopsies from the muscle layer during POEM, called
POEM-b. According to their study, histopathological and
immunohistochemical analysis of POEM-b samples showed
neurodegenerative signatures rather than inflammatory infiltrates
in the muscular layer (8). There was
a tendency for preservation of interstitial cells of Cajal in
patients with type III achalasia, whereas more severe fibrosis was
observed in patients with type I achalasia, based on the Chicago
classification criteria high-resolution manometry (HRM) (8,9).
Currently, the proposed causal factors are varied
and multifactorial, and are hypothesized to involve complex
interactions between the autoimmune and inflammatory response, and
this may be initiated by viral infections in patients who are
genetically susceptible (2). Causal
viral agents include (but not limited to) herpes simplex virus,
which is a neurotropic virus that exhibits a predilection for
squamous epithelium, as well as varicella-zoster, measles and human
papillomavirus (4,5,7,10). Moreover, it has been reported that
HSV type 1 (HSV-1) DNA and RNA is detectable in all tissues from
patients with achalasia, but not in the control tissues (11). Therefore, HSV-1 infection may be
considered particularly relevant in the development and/or
progression of achalasia.
microRNAs (miRNAs/miRs) are single-stranded RNAs
that regulate gene expression and serve crucial roles in numerous
physiological and pathological processes, including viral
infections and antiviral response (12-15).
Certain viruses, particularly herpes viruses (including HSV-1),
express miRNAs, although their pathological roles are not
completely understood (12,15). In our previous study, it was shown
that expression of HSV1-miR-H1-3p in the esophageal mucosa of
patients with achalasia was increased (1). However, the analysis and understanding
of the miRNA expression profiles in the muscular layer of the LES
are still being elucidated. HSV1-miR-H1 can directly target E3
ubiquitin-protein ligase component n-recognition 1 in vitro
(UBR1) (14). This
miR-mediated downregulation of the ubiquitin-proteasome system
results in the accumulation of neurodegenerative-associated protein
fragment β amyloid (15).
Subsequently, the autophagy pathway is influenced, which is
essential for host defense against viral infection; in particular,
autophagy-independent antiviral functions of autophagy-related
genes (ATGs) have been reported to be activated (16-18).
For example, ATG 16-like 1 (ATG16L1), which is part of the
ATG5-ATG12-ATG16L1 complex, is activated following interferon-γ
treatment (18). Finally, autophagy
also increases interleukin-1β (IL-1β) secretion (19).
The aim of the present study was to analyze the mRNA
expression levels of UBR1, ATG16L1 and IL-1B
as potential targets for viral miRNAs, and to investigate the
mechanisms underlying onset of achalasia.
Materials and methods
Ethical considerations
Written informed consent was obtained from all
patients. The study protocol followed the ethical guidelines of the
Declaration of Helsinki and was approved by the Nagasaki University
Ethics Committee (approval no. 110328329).
Per-oral endoscopic muscular biopsy
sampling during POEM
The standard POEM procedure was performed as
previously described (6). Briefly,
the following steps were followed: Submucosal injection and mucosal
incision, submucosal tunneling, selective myotomy for the inner
circular muscle and then closing of the mucosal entry. All patients
who underwent POEM were under general anesthesia and endotracheal
intubation with positive pressure ventilation, and included
patients who underwent surgery between October 2011 and June 2012
at the Showa University Koto-Toyusu Hospital. Patients with any
severe underlying illnesses, such as cancer, or those who could not
tolerate general anesthesia due to other diseases were excluded.
Each patient was diagnosed with sporadic and classic achalasia by
routine analysis, including barium follow through, upper
gastrointestinal endoscopy and manometry. An incision was
subsequently made in the circular muscle bundle from the entrance
to the LES, where the two muscular biopsies were performed using
both ends of the biopsy forceps. As controls, biopsy samples were
collected from the LES of patients whose excised esophagogastric
junction (EGJ) was used. The control group consisted of patients
with esophageal cancer requiring surgical resection whose cancer
lesions did not reach the LES. Patients were successfully treated
with esophagectomy in all control patients, and immediately after
removal of the esophagus, including the LES, they deployed the
resected specimens in a longitudinal direction. Positional
identification of the EGJ in the macroscopic findings was
uncomplicated. Following confirmation from a physician endoscopist
with extensive experience in the POEM procedure, ~2 mm of tissue
was collected from the inner circular muscle at the position where
the LES appeared to be directly above the EGJ from the mucosal side
using a pointed blade. Patients in the control group did not
undergo evaluation of esophageal peristalsis by HRM, but medical
examinations, including barium follow-through, did not show any
symptoms or signs suggestive of abnormal esophageal motility. All
samples were immediately placed in 1 ml RNAlater®
reagent (Ambion; Thermo Fisher Scientific, Inc.) and stored at
-80˚C until subsequent RNA isolation. Reverse
transcription-quantitative (RT-q)PCR was performed on samples from
6 control (5 males and 1 female; age range 35-69 years; median age,
66) and 11 achalasia cases (7 males and 4 females; age range 27-78
years old; median age, 40, which included 6 smokers). Based on the
Descriptive Rules for Achalasia of the Esophagus (20), there were 8 straight-types and 3
sigmoid-types; and one patient with grade I achalasia and 10
patients with grade II achalasia.
RT-qPCR
cDNAs were prepared from total RNA using a
High-Capacity cDNA Reverse Transcription kit (cat. no. 4374966,
Thermo Fisher Scientific Inc.). Reverse transcription reactions
were performed in reactions containing 5 µl total RNA, 1x RT
buffer, 4 mM dNTP mix, 1x RT random primers, 50 units MultiScribe™
reverse transcriptase, 20 units RNase inhibitor and nuclease-free
water added up to 20 µl. Reactions were performed at 25˚C for 10
min, followed by 37˚C for 120 min and 85˚C for 5 min. Primer
sequences for quantitative PCR were as follows: UBR1
forward, 5'-CTTCGCTGTGCTGCATTGTT-3' and reverse,
5'-TCTAGGGTACCTGACCACGG-3'; ATG16L1 forward,
5'-CAGGCACGAGATAAGTCCCG-3' and reverse,
5'-AACTCCCCACGTTTCTTGTGT-3'; IL-1β forward,
5'-CAGCTACGAATCTCCGACCAC-3' and reverse,
5'-GGCAGGGAACCAGCATCTTC-3'; and β-actin forward,
5'-GCATCCTCACCCTGAAGTA-3' and reverse, 5'-TGTGGTGCCAGATTTTCTCC-3'.
qPCR reactions were performed in 20 µl aliquots containing 1 µl RT
product with 4 µl LightCycler® FastStart DNA MasterPLUS
SYBR Green I (cat. no. 03515869001, Roche Diagnostics,), 0.5 µM of
each primer and 14.6 µl nuclease-free water. The reactions were
carried out in a Real Time PCR LightCycler 1.5 Complete system
(Roche Diagnostics). The thermocycling conditions were:
Denaturation at 95˚C for 10 min; followed by 45 cycles of 95˚C for
10 sec, 60˚C for 10 sec and 72˚C for 10 sec. The quantification
cycle (Cq) was recorded for mRNA amplification using LightCycler
Software version 3.5.28 (Roche Diagnostics), and β-actin was
used as an endogenous control for data normalization. Relative
expression was calculated using the following formula
2-ΔΔCq=2-(ΔCq,
reagent treatment-ΔCq,
control).
Statistical analysis
Differences between two groups were compared using
an unpaired one-tailed Student's t-test. Data are presented as the
mean ± standard error of the mean. Correlations were calculated
using Pearson's correlation coefficient analysis. Statistical
analysis was performed using StatFlex version 7 (Artec Co., Ltd.).
P<0.05 was considered to indicate a statistically significant
difference.
Results
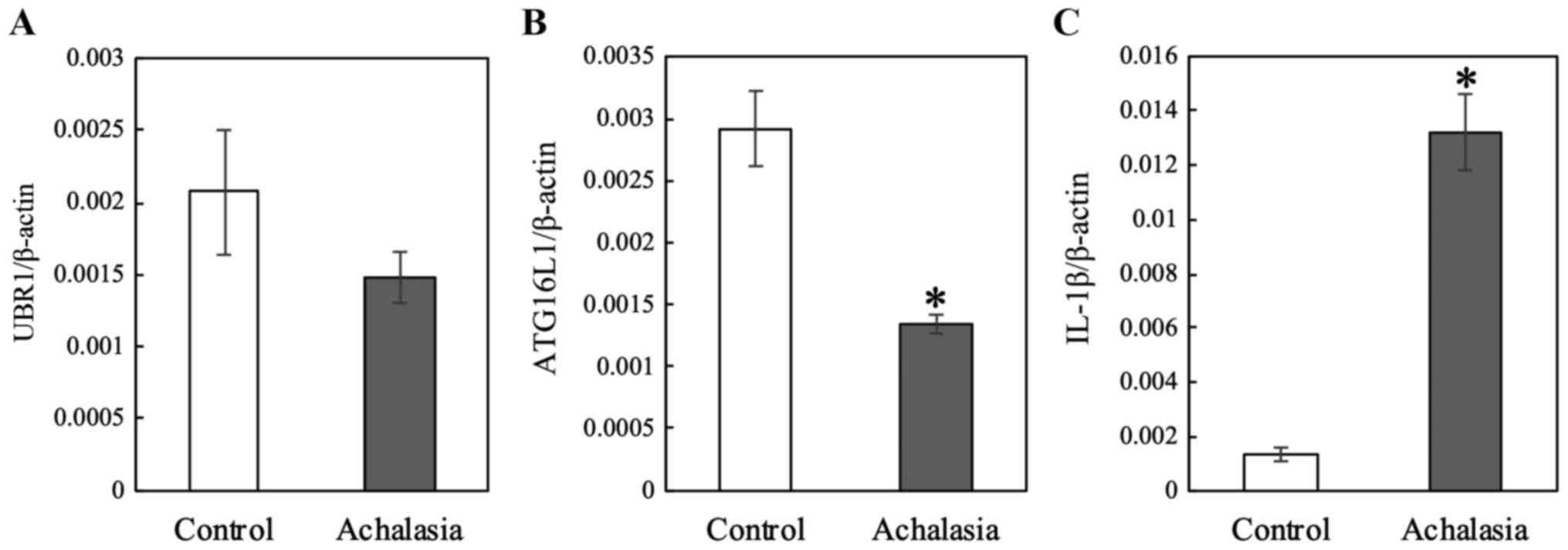
RT-qPCR was performed to assess the
mRNA expression levels of UBR1, ATG16L1 and IL-1β in samples from 6
controls and 11 achalasia patients
The duration of disease in the achalasia patients
was 3-240 months; mean, 100 months and 3 patients complained of
chest pains. Based on the Descriptive Rules for Achalasia of the
Esophagus (20), there were 8
straight-types and 3 sigmoid-types; and one patient with grade I
achalasia and 10 patients with grade II achalasia. UBR1 mRNA
levels were decreased, although not significant, (P=0.1561), and
the expression levels of ATG16L1 were significantly
decreased (P=0.0028) in the LES of patients compared with the
control (Fig. 1). In contrast,
IL-1β expression was significantly increased (P<0.0001)
in the LES of patients compared with the control (Fig. 1). In our previous study, it was shown
that relative HSV1-miR-H1-3p expression levels were significantly
higher in the LES samples of patients with achalasia compared with
the controls (1). The cohort used in
the present study was the same as that used in our previous study
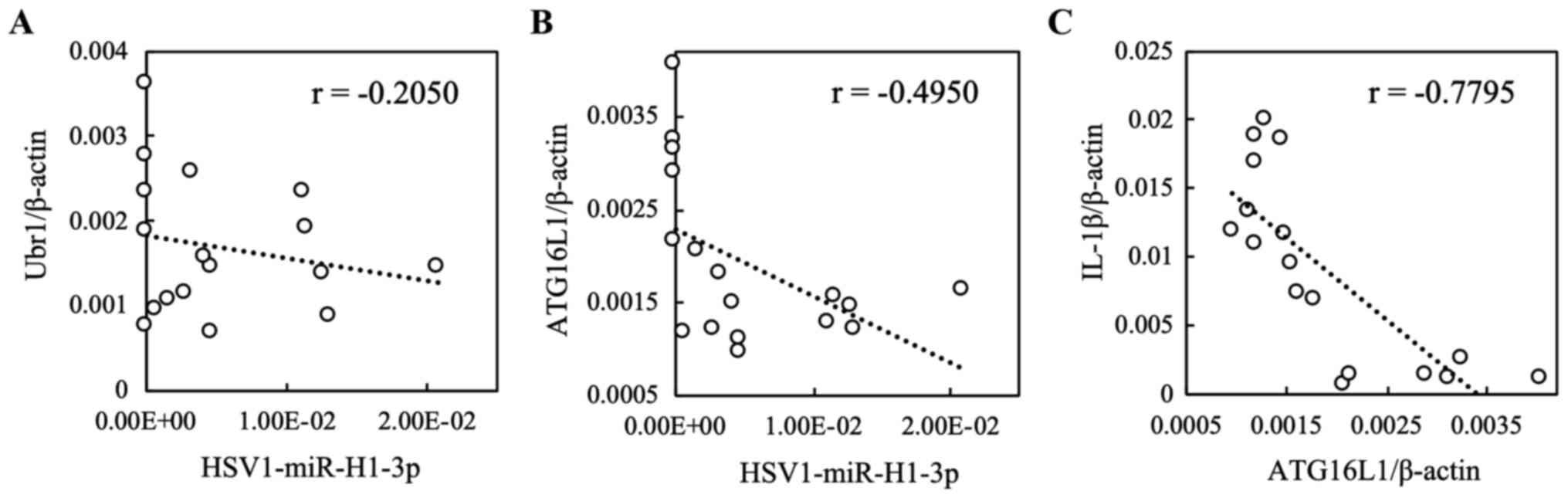
(1). As shown in Fig. 2, the correlation between
hsv-miR-H1-3p and UBR1 was not observed (r=-0.2050;
P=0.4300). However, a weak correlation was observed between
HSV1-miR-H1-3p and ATG16L1 (r=-0.4950; P=0.0434).
Furthermore, a strong correlation was observed between
ATG16L1 and IL-1β (r=-0.7795; P=0.0002; Fig. 2).
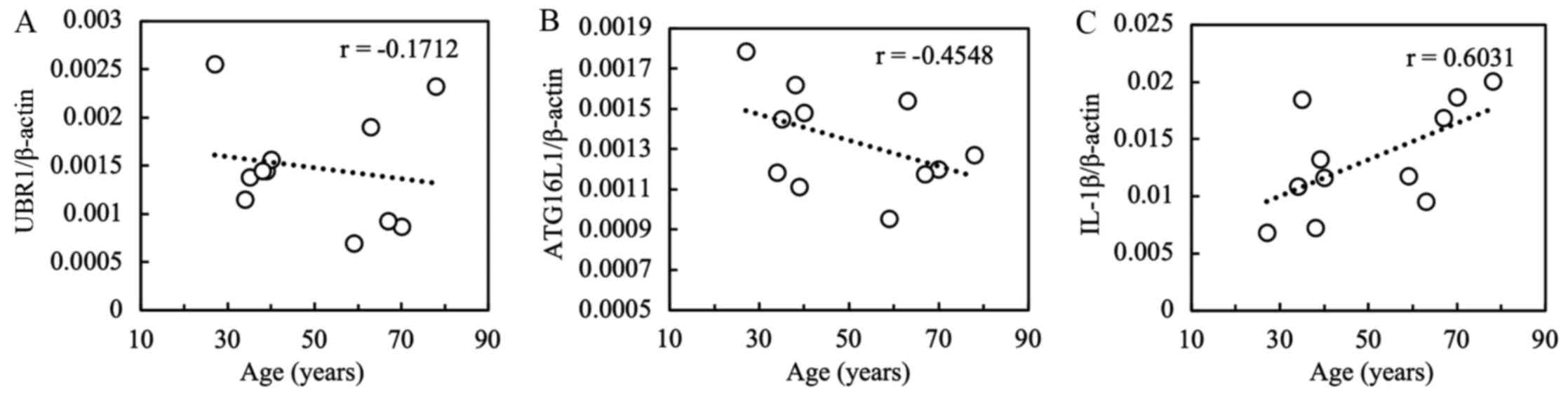
The relationship between the mRNA expression levels
and the patients' clinical parameters were assessed. A positive
correlation was found between patient age and IL-1β
expression (r=0.6031; P=0.0495; Fig.
3). However, UBR1 and ATG16L1 levels were not
significantly associated with age; there were no significant
associations between the expression levels of the three mRNAs
assessed with sex, smoking status, type of achalasia or duration of
the disease (Fig. S1).
Discussion
Esophageal primary achalasia is characterized by
aganglionosis or loss of myenteric neurons (2). These features are predominantly caused
by the degeneration of inhibitory neurons in the Auerbach's plexus
(20). At present, causal factors of
idiopathic achalasia are diverse and multifactorial, and they may
be involved in complex interactions of autoimmune responses,
degenerative neuronal processes, viral infections and the genetic
susceptibility of individuals (1,4).
HSV-1 is a member of the Herpesviridae
family, and has been proposed as the most likely candidate as a
primary target for treatment or management of esophageal primary
achalasia, taking into account its neural cell tropism and
predilection for squamous epithelium (5,12,15).
HSV-1 has a life cycle with two distinct programs consisting of
productive and latent phases (13).
Certain viral miRNAs can downregulate specific targets or promote
viral genome stability, translation and RNA accumulation (13). In our previous study, it was shown
that expression of HSV1-miR-H1, an HSV-1 miRNA, was increased in
the LES of achalasia patients (1).
Hence, the muscular layer of LES may act as a reservoir for HSV-1
and serve as a region for expression of viral miRNAs in patients
with achalasia. Additionally, HSV1-miR-H1 is a latency-associated
transcript (LAT) that is a non-coding viral miRNA. LAT-derived
miRNAs interfere with viral metabolites and regulate the host
immune response (13,15). In the present study, it was
hypothesized that UBR1 and ATG16L1 were the direct
targets of HSV1-miR-H1, as the downregulation of the
ubiquitin-proteasome system results in the accumulation of
neurodegenerative-associated protein fragment β-amyloid (15), and the downregulation of
autophagy-mediated viral clearance is advantageous for the survival
of viruses (16-18).
In the present study, UBR1 showed decreased expression
(although not significant), whereas ATG16L1 was
significantly downregulated at the site of LES. Furthermore, there
was a weak correlation between hsv-miR-H1-3p and ATG16L1
levels, but not UBR1. These data suggest that ATG16L1
is the target of HSV1-miR-H1. In contrast, IL-1β expression was
upregulated in the LES, and the inflammatory pathway may have been
influenced by viral miRNAs. A correlation between IL-1B mRNA
expression levels and patient age was also observed. However,
additional studies with larger cohorts are required to determine if
these observations are generalizable. Additionally, the studied
mRNAs are only part of a complex of virus-host interactions, and
further studies on the involvement of other transcripts are
required. The construction of a achalasia mouse model is also
important in obtaining a deeper understanding of the pathogenesis
in vivo.
A strong correlation was verified between
ATG16L1 and IL-1β in the present study. The
relationship between HSV1 and autophagy (21-24)
and between autophagy and IL-1β production (25-28)
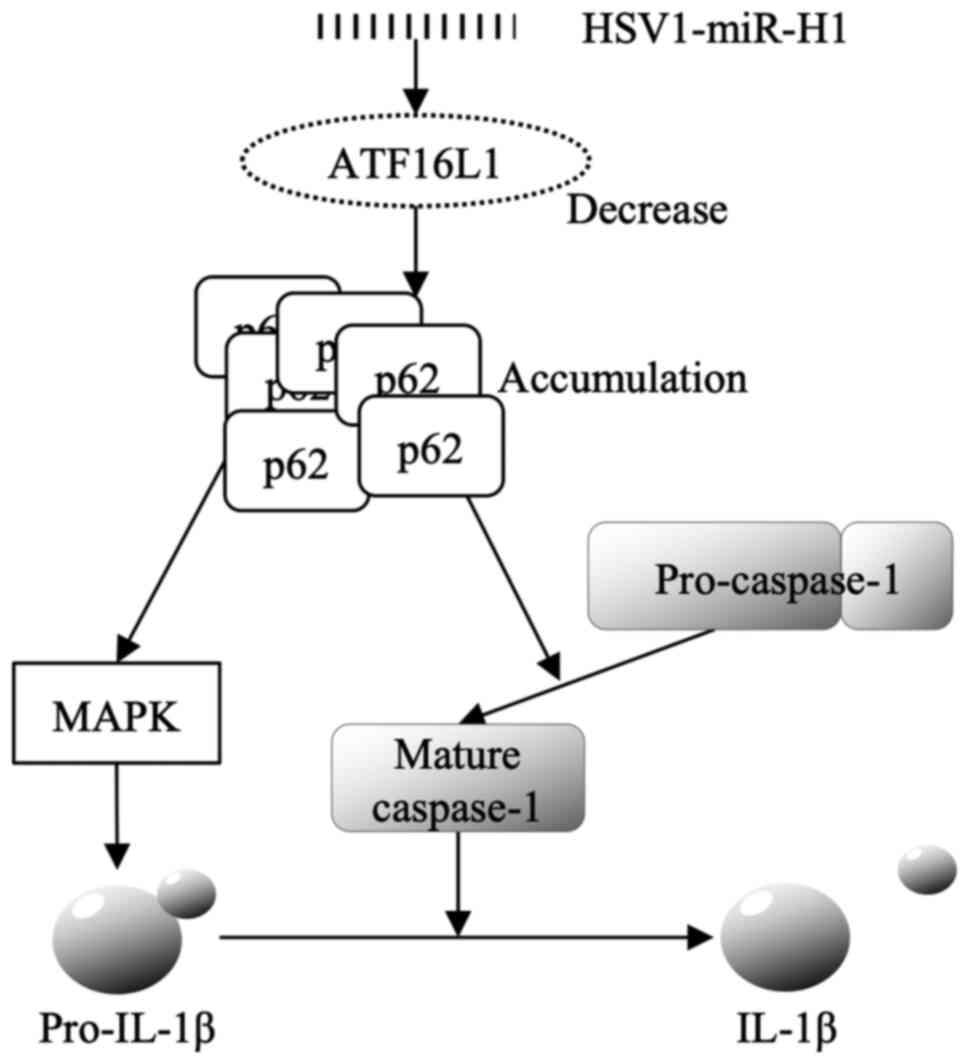
have been reported previously. Notably, p62, a selective autophagy
receptor, accumulates in ATG16L1 deficient cells, and p62 activates
MAPK and Caspase-1, in-turn increasing IL-1β production (29). Based on these previous studies, the
mechanism underlying upregulation of IL-1β expression through
ATG16L1 via HSV1-miR-H1 hypothesized in the present study is
presented in Fig. 4. HSV1-miR-H1
decreases ATG16L1, and the accumulation of p62 is induced by the
decrease in ATG16L1. The abundance p62 induces pro-IL-1β via MAPK
activation and activates Caspase-1 simultaneously. IL-1β secretion
in induced by MAPK and Caspase-1 (Fig.
4).
There are several limitations to this pilot study.
As the controls, tissues from non-motility patients with upper
gastrointestinal carcinomas not affecting the EGJ, including LES
were used. However, the ideal samples would be tissues from healthy
individuals, although there are ethical issues related to obtaining
such muscular samples. The number of samples was small and each
sample in this study was from a patient diagnosed by classical
criteria, primarily based on typical findings using barium.
Additionally, all samples were used for RNA extraction due to the
small sample size; thus protein expression analysis could not be
performed. Finally, whether the influence of ATG16L1 was direct or
indirect was not determined. To overcome these problems, a
prospective study with a larger sample size and detailed
pathological analysis is required.
In conclusion, the levels of ATG16L1, a
target of HSV1-miR-H1, are reduced in the LES of achalasia
patients, and this reduction could be the cause of the esophageal
motility disorder.
Supplementary Material
Figure S1. Differences in mRNA
expression levels of UBR1, ATG16L1 and IL‑1β based on
various factors. Differences in mRNA expression levels of UBR1,
ATG16L1 and IL‑1β between the patients in the two groups
based on (A) sex, (B) type of achalasia and (C) smoking status. (D)
Correlation coefficients between mRNA expression levels and the
duration of disease were calculated. URB1, E3
ubiquitin‑protein ligase component n‑recognition 1; ATG16L1,
autophagy‑related 16‑like 1; IL‑1β, interleukin‑1β.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated and/or analyzed in the present
study are included in the published article.
Authors' contributions
TK and AY made substantial contributions to
acquisition of data, analysis and interpretation of data, and to
drafting of the manuscript. YI made substantial contributions to
analysis and interpretation of the data, and to drafting of the
manuscript. HIk, TS, SU and HM contributed to acquisition of the
data. KN and HIn contributed to conception and design of the study.
HIs made substantial contributions to conception and design of the
study, and to drafting of the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from all
patients. The study protocol followed the ethical guidelines of the
Declaration of Helsinki and was approved by the Nagasaki University
Ethics Committee (approval no. 110328329).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ikebuchi Y, Kanda T, Ikeda H, Yoshida A,
Sakaguchi T, Urabe S, Minami H, Nakao K, Kuwamoto S, Inoue H and
Isomoto H: Identification of human herpes virus 1 encoded microRNAs
in biopsy samples of lower esophageal sphincter muscle during
peroral endoscopic myotomy for esophageal achalasia. Dig Endosc.
32:136–142. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kahrilas PJ and Boeckxstaens G: The
spectrum of achalasia: Lessons from studies of pathophysiology and
high-resolution manometry. Gastroenterology. 145:954–965.
2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Minami H, Isomoto H, Miuma S, Kobayashi Y,
Yamaguchi N, Urabe S, Matsushima K, Akazawa Y, Ohnita K, Takeshima
F, et al: New endoscopic indicator of esophageal achalasia:
‘Pinstripe pattern’. PLoS One. 10(e0101833)2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ghoshal UC, Daschakraborty SB and Singh R:
Pathogenesis of achalasia cardia. World J Gastroenterol.
18:3050–3057. 2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Furuzawa-Carballeda J, Torres-Landa S,
Valdovinos MÁ, Coss-Adame E, Martín Del Campo LA and
Torres-Villalobos G: New insights into the pathophysiology of
Achalasia and implications for future treatment. World J
Gastroenterol. 22:7892–7907. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Inoue H, Shiwaku H, Iwakiri K, Onimaru M,
Kobayashi Y, Minami H, Sato H, Kitano S, Iwakiri R, Omura N, et al:
Clinical practice guidelines for peroral endoscopic myotomy. Dig
Endosc. 30:563–579. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Isomoto H and Ikebuchi Y: Japanese
guidelines for peroral endoscopic myotomy: 1st edition. Dig Endosc.
31:27–29. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Sato H, Inoue H, Ikeda H, Sato C, Santi E,
Phalanusitthepha C, Aoyagi Y and Kudo S: In vivo histopathological
assessment of the muscularis propria in achalasia by using
endocytoscopy (with video). Endosc Int Open. 2:E178–E182.
2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Nakajima N, Sato H, Takahashi K, Hasegawa
G, Mizuno K, Hashimoto S, Sato Y and Terai S: Muscle layer
histopathology and manometry pattern of primary esophageal motility
disorders including achalasia. Neurogastroenterol Motil.
29(e12968)2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Pressman A and Behar J: Etiology and
pathogenesis of idiopathic achalasia. J Clin Gastroenterol.
51:195–202. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Furuzawa-Carballeda J, Aguilar-León D,
Gamboa-Domínguez A, Valdovinos MA, Nuñez-Álvarez C,
Martín-del-Campo LA, Enríquez AB, Coss-Adame E, Svarch AE,
Flores-Nájera A, et al: Achalasia-An autoimmune inflammatory
disease: A cross-sectional study. J Immunol Res.
2015(729217)2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Piedade D and Azevedo-Pereira JM: The role
of microRNAs in the pathogenesis of herpesvirus infection. Viruses.
8(156)2016.PubMed/NCBI View
Article : Google Scholar
|
|
13
|
Brdovčak MC, Zubković A and Jurak I:
Herpes simplex virus 1 deregulation of host microRNAs. Noncoding
RNA. 4(36)2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zheng K, Liu Q, Wang S, Ren Z, Kitazato K,
Yang D and Wang Y: HSV-1-encoded microRNA miR-H1 targets Ubr1 to
promote accumulation of neurodegeneration-associated protein. Virus
Genes. 54:343–350. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Bernier A and Sagan SM: The diverse roles
of microRNAs at the host-virus interface. Viruses.
10(440)2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lussignol M and Esclatine A: Herpesvirus
and autophagy: ‘All right, everybody be cool, this is a robbery!’.
Viruses. 9(372)2017.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Cavignac Y and Esclatine A: Herpesviruses
and autophagy: Catch me if you can! Viruses. 2:314–333.
2010.PubMed/NCBI View
Article : Google Scholar
|
|
18
|
Dong X and Levine B: Autophagy and
viruses: Adversaries or allies? J Innate Immun. 5:480–493.
2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Harris J, Hartman M, Roche C, Zeng SG,
O'Shea A, Sharp FA, Lambe EM, Creagh EM, Golenbock DT, Tschopp J,
et al: Autophagy controls IL-1β secretion by targeting Pro-IL-1β
for degradation. J Biol Chem. 286:9587–9597. 2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Japan Esophageal Society: Descriptive
rules for achalasia of the esophagus, June 2012: 4th edition.
Esophagus 14: 275-289, 2017.
|
|
21
|
McFarlane S, Aitken J, Sutherland JS,
Nicholl MJ, Preston VG and Preston CM: Early induction of autophagy
in human fibroblasts after infection with human cytomegalovirus or
herpes simplex virus 1. J Virol. 85:4212–4221. 2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
O'Connell D and Liang C: Autophagy
interaction with herpes simplex virus type-1 infection. Autophagy.
12:451–459. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhang H, Zheng L, McGovern DP, Hamill AM,
Ichikawa R, Kanazawa Y, Luu J, Kumagai K, Cilluffo M, Fukata M, et
al: Myeloid ATG16L1 facilitates host-bacteria interactions in
maintaining intestinal homeostasis. J Immunol. 198:2133–2146.
2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yakoub AM and Shukla D: Autophagy
stimulation abrogates herpes simplex virus-1 infection. Sci Rep.
5(9730)2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Saitoh T, Fujita N, Jang MH, Uematsu S,
Yang BG, Satoh T, Omori H, Noda T, Yamamoto N, Komatsu M, et al:
Loss of the autophagy protein Atg16L1 enhances endotoxin-induced
IL-1β production. Nature. 456:264–268. 2008.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lassen KG, Kuballa P, Conway KL, Patel KK,
Becker CE, Peloquin JM, Villablanca EJ, Norman JM, Liu TC, Heath
RJ, et al: Atg16L1 T300A variant decreases selective autophagy
resulting in altered cytokine signaling and decreased antibacterial
defense. Proc Natl Acad Sci USA. 111:7741–7746. 2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Lee J, Kim HR, Quinley C, Kim J,
Gonzalez-Navajas J, Xavier R and Raz E: Autophagy suppresses
interleukin-1β (IL-1β) signaling by activation of p62 degradation
via lysosomal and proteasomal pathways. J Biol Chem. 287:4033–4040.
2012.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Saitoh T and Akira S: Regulation of
inflammasomes by autophagy. J Allergy Clin Immunol. 138:28–36.
2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Choe JY, Jung HY, Park KY and Kim SK:
Enhanced p62 expression through impaired proteasomal degradation is
involved in caspase-1 activation in monosodium urate
crystal-induced interleukin-1β expression. Rheumatology (Oxford).
53:1043–1053. 2014.PubMed/NCBI View Article : Google Scholar
|