Introduction
Hepatic veno-occlusive disease (VOD), also termed
sinusoidal obstruction syndrome (SOS), is a potentially
life-threatening complication observed following hematopoietic stem
cell transplantation (1).
Endothelial damage is recognized as the first event of hepatic VOD,
which is hypothesized to be caused by glutathione-consuming agents,
such as sirolimus, Bu, BCNU or TBI, or otherwise previous liver
diseases (2-4).
Microscopy analysis of hepatic VOD animal models showed that the
events following sinusoidal endothelial cell injury included the
activation of the coagulation and fibrinolytic pathway (3), which subsequently reduces the flow of
sinusoidal blood leading to the formation of microthrombi that
obstruct the sinusoidal pores (3).
As a result, the development of venule thrombosis and ischemia are
observed, ultimately resulting in perivascular hepatocyte necrosis
and fibrosis of sinusoids (4,5).
However, the mechanism by which endothelial injury induces
thrombosis in hepatic VOD is still not clear.
Currently, there are no effective preventative
treatment regimens for hepatic VOD (3,4).
Defibrotide is the only drug approved for the treatment of VOD in
the European Union, but its effect is limited (5). In an international multicenter
compassionate-use program performed between December 1998 and March
2016, the estimated overall 100+ day survival in 710 patients with
hepatic VOD treated with defibrotide was only 54%; however, adverse
events were reported in 53% of the patients receiving the drug
(6). Recently, a retrospective study
evaluated the effect of recombinant human tissue plasminogen
activator combined with heparin for the treatment of hepatic VOD,
and found that 29% of patients responded, but the treatment was
associated with a significant risk of life-threatening hemorrhage
(7). Thus, novel agents with high
efficiency and low toxicity are urgently required.
Ginsenoside Rb1 is a major constituent and effective
ingredient of Panax ginseng, and is one of the most widely used
traditional Chinese herbal medicines, which has been reported to
possess several purported effects on the cardiovascular, endocrine,
immune and nervous systems, apparently with low side effects
(8,9). Ginsenoside Rb1 was also found to
effectively prevent vascular endothelial dysfunction through the
upregulation of ghrelin secretion, nitric oxide production and
endothelial nitric oxide synthase protein expression (10-12).
It has been shown that ginsenoside Rb1 can effectively block
homocysteine-induced endothelial dysfunction in porcine coronary
arteries (13). According to these
findings, it was hypothesized that ginsenoside Rb1 may help
prohibit the development of hepatic VOD with a favorable safety
profile by protecting against endothelial injury.
Endothelial microparticles (EMPs), characterized by
the surface expression of endothelial antigens such as CD62E (also
termed E-selectin), are submicron vesicles released from
endothelial cells in response to cell activation, injury or
apoptosis and have emerged as new markers of endothelial injury
(14-17).
It has been reported that under certain pathological conditions
such as antiphospholipid syndrome, lupus and several types of
cancer, the concentration of EMPs in the blood may increase and
contribute to blood coagulation, angiogenesis and inflammation
(18-20).
In our previous study, an increased presence of EMPs was shown to
serve as a marker of endothelial activation in patients with graft
vs. host disease (GVHD) following hematopoietic stem cell
transplantation (17). Expression of
Fas/FasL on EMPs was involved in the development of GVHD, and
miRNA155 in EMPs may promote GVHD progression by regulating T cell
function (17). Therefore, it was
hypothesized that EMPs may participate in GVHD development by
regulating coagulation.
Monocrotaline (MCT) is a pyrrolizidine alkaloid
phytotoxin that is well established to cause hepatic toxicity in
both animals and humans (21-23).
Rats treated with MCT have been widely used as an in vivo
model of hepatic VOD (24,25). Therefore, in the present study, MCT
was used to induce endothelial cell injury in EA.hy926 cells to
imitate the endothelial damage caused by hepatic VOD in
vitro. Furthermore, the effect of ginsenoside Rb1 on MCT
treated EA.hy926 cells and CD62E+-EMPs were assessed, to
investigate the molecular mechanisms underlying endothelial
protection mediated by ginsenoside Rb1. In addition, the expression
of tissue factor (TF) protein on endothelial cells and EMPs was
studied, to determine the process by which coagulation
abnormalities were induced by endothelial cell injury in hepatic
VOD and to determine the value of ginsenoside Rb1 administration in
hepatic VOD/SOS by blocking endothelial injury and thrombosis
simultaneously.
Materials and methods
Cell culture and treatments
The human umbilical vein endothelial cell line
EA.hy926 was obtained from The Cell Bank of Type Culture Collection
of the Chinese Academy of Sciences. Ginsenoside Rb1 was purchased
from The China Food and Drug Administration. Mycoplasma testing was
performed on the cells to ensure they were not contaminated. Cells
were cultured in DMEM supplemented with 10% FBS and incubated with
5% CO2 at 37˚C. Adherent cells were harvested by
digestion with trypsin and centrifuged (1,000 x g for 5 min at
37˚C).
The experiments were divided into the following
groups: Control group, ginsenoside Rb1 control group, EA.hy926
cells treated with 160 mg/l ginsenoside Rb1 alone for 48 h; MCT
group, cells treated with 7.5 mM MCT for 48 h; and ginsenoside
Rb1+MCT group, treated with 40, 80 or 160 mg/l ginsenoside Rb1 for
2 h prior to treatment with 7.5 mM MCT for 48 h. All cell culture
results shown are based on at least three individual
experiments.
In the preliminary experiments, the protective
effect of different concentrations of ginsenoside Rb1 (40, 80, 160
or 200 mg/l) against MCT-induced damage of umbilical vein
endothelial cells were assessed, and the results showed that there
was no statistically significant difference between 160 and 200
mg/l ginsenoside Rb1 (data not shown); thus, the maximum effective
concentration of ginsenoside Rb1 was deemed to be 160 mg/l. When
deciding on the concentration of ginsenoside for the Rb1 control
group, the maximum concentration of 160 mg/l was used in order to
exclude the harmful effect of ginsenoside Rb1 on umbilical vein
endothelial cells.
Hoechst 33258 staining
In order to visually show that MCT induced cell
apoptosis, and that ginsenoside Rb1 protected against MCT-induced
apoptosis, Hoechst 33258 staining was used. The cells were cultured
in 6-well plates until they were 80% confluent and then incubated
with the aforementioned drugs with 5% CO2 at 37˚C. The
cells were washed with PBS, then fixed with 4% formaldehyde for 10
min at room temperature, and subsequently stained with 10 µg/ml
Hoechst 33258 at room temperature for 10 min. Finally, after the
cells were washed with PBS, morphological changes were observed
under a fluorescence microscope.
Flow cytometry analysis of
apoptosis
Annexin V-FITC-propidium iodide (PI) double staining
assay was used to quantify apoptosis in EA.hy926 cells using
FACScan flow cytometer (BD Biosciences). After 48 h of drug
treatment, cells were harvested, washed with ice-cold PBS,
resuspended in 250 µl binding buffer and incubated with 5 µl
Annexin V-FITC for 10 min at room temperature in the dark.
Subsequently, samples were washed with binding buffer, resuspended
in PBS, counterstained with 5 µg/l PI for 10 min at room
temperature in the dark and analyzed by flow cytometry to identify
apoptotic cells. The extent of early apoptosis was determined as
the percentage of Annexin V+/PI- cells.
Collection of EMPs
EMPs were isolated as described previously (17,26).
Cell culture supernatants were collected and centrifuged for 10 min
at 3,000 x g at 4˚C to remove any detached cells and fragments. The
supernatants were then ultracentrifuged at 16,000 x g for 1 h at
4˚C. The EMP-rich pellets were resuspended in PBS and
ultracentrifuged again at 16,000 x g for 1 h at 4˚C, and then
resuspended in 50 µl PBS for use in subsequent experiments.
Detection of EMPs by confocal laser
scanning microscopy
First, 50-µl aliquots of EMP suspensions were
incubated with 20 µl phycoerythrin-labeled anti-CD62E (BD
Pharmingen; BD Biosciences) at room temperature in the dark for 30
min, and the reaction was stopped with 1.5 ml PBS, then
ultracentrifuged at 16,000 x g for 1 h at 4˚C. The samples were
resuspended in 50 µl PBS, mixed with 2 µl fluorescent beads
(Sigma-Aldrich; Merck KGaA) 1-µm in diameter, then added to the
microscope slide and immediately analyzed using confocal laser
scanning microscopy (magnification, x300; Olympus FV500; Olympus
Corporation) with excitation and emission wavelengths of 549 and
565 nm, respectively. Differential interference contrast images and
fluorescent confocal images were obtained simultaneously.
Flow cytometry analysis for
quantification of EMPs
As described above, EMP-rich pellets were suspended
in 250 µl PBS and analyzed on a FACScan flow cytometer. EMPs were
defined as particles ≤1.0 µm in size positive for the endothelial
cell antigen CD62E. Fluorescent beads, 1-µm in diameter, were used
as the internal standard and for gating the particles.
Western blotting of apoptosis related
proteins and TF in cells and EMPs
Detection of apoptosis related proteins was detected
in whole-cell lysates. Following the collection of EMPs, the TF
bound to EMPs and the soluble TF were separated and preserved in
EMP suspensions or the supernatant, respectively. The cells and
EMPs were homogenized and lysed separately, in order to further
detect the TF proteins bound to each. Subsequently, proteins were
loaded on an 8 or 12% SDS-gel, resolved using SDS-PAGE and
transferred to nitrocellulose membranes. The blots were probed with
mouse anti-TF (cat. no. 553014; 1:500; BD Pharmingen), and
anti-β-actin (cat. no. sc-8432; 1:1,000; Santa Cruz Biotechnology,
Inc.) at 4˚C overnight. Immunoblots were washed and then incubated
with horseradish peroxidase-conjugated secondary antibodies (cat.
no. 016-030-084; 1:4,000; Pierce; Thermo Fisher Scientific, Inc.)
at room temperature for 2 h, and signals were visualized using
SuperSignal™ enhanced chemiluminescence detection (Thermo Fisher
Scientific, Inc.) and detected using a chemiluminescence detection
system (Bio-Rad Laboratories, Inc.).
ELISA for soluble TF
A commercial ELISA kit (cat. no. RPN1231; Imubind
Tissue Factor, American Diagnostica Inc.) was used to detect
soluble TF protein in the supernatant medium, according to the
manufacturer's protocol. TF protein levels are expressed as pg/ml
using a reference curve created with TF standards provided with the
kit.
Statistical analysis
All data were analyzed using SPSS version 22.0 (IBM
Corp.). All results are expressed as the mean ± standard deviation.
A Student's t-test or a one-way ANOVA followed by a Tukey's
post-hoc test were used to compare quantitative data populations
with normal distributions and equal variances. P<0.05 was
considered to indicate a statistically significant difference.
Results
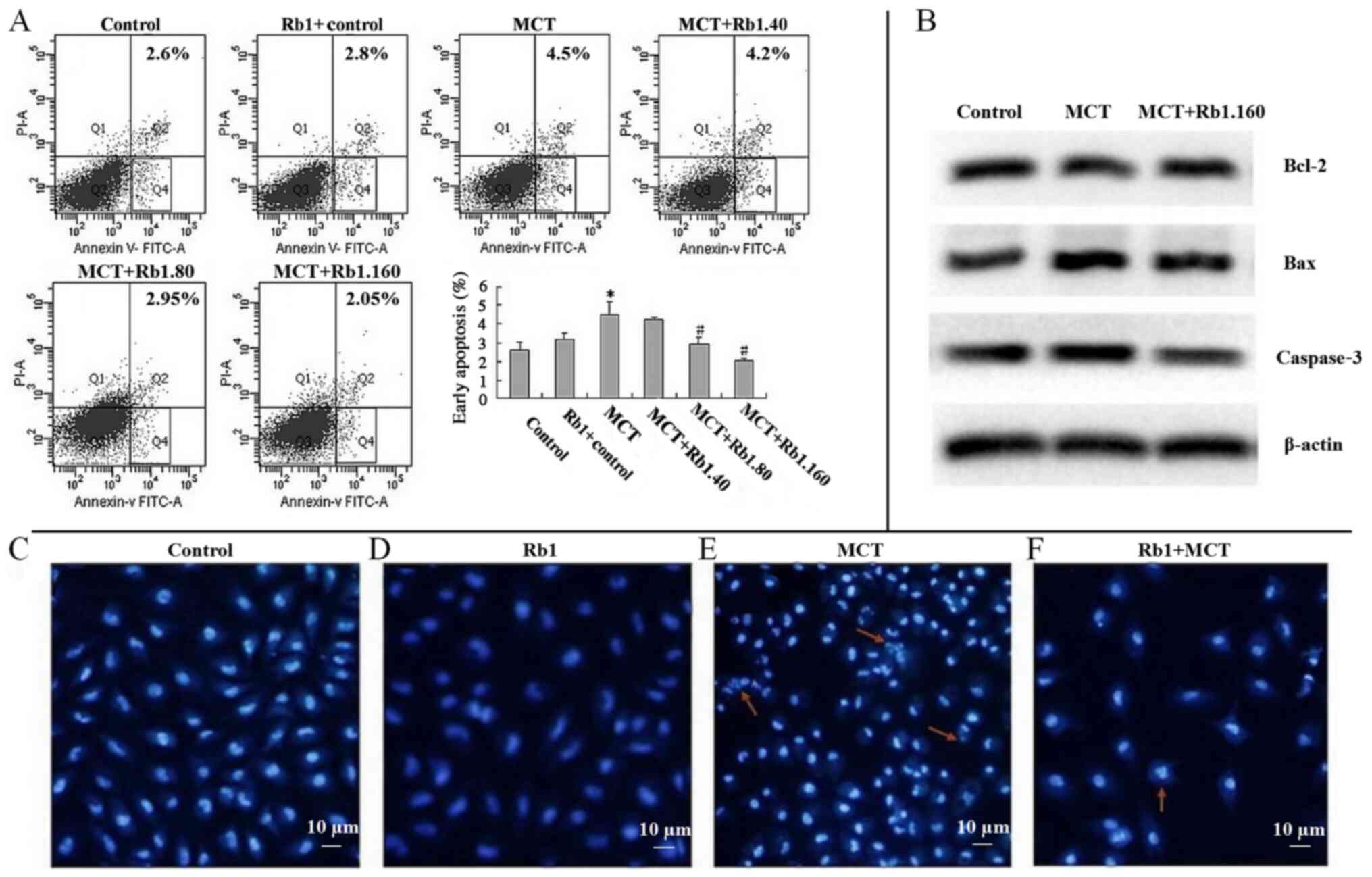
Ginsenoside Rb1 inhibits MCT-induced
apoptosis in EA.hy926 cells
As shown in Fig. 1A,
after EA.hy926 cells were treated with 7.5 mM MCT for 48 h, the
percentage of early apoptotic cells was significantly increased
compared with the control group (4.5±0.71 vs. 2.6±0.42%;
P<0.05). There was no significant difference between the
percentage of apoptotic cells in the Rb1 control group and the
control group, showing the low toxicity of ginsenoside Rb1.
However, the percentage of early apoptotic cells was significantly
lower in 80 (2.95±0.35%) or 160 mg/l (2.05±0.07%) ginsenoside
Rb1+MCT groups compared with the MCT group (4.5±0.71%; both
P<0.05; Fig. 1A).
`In addition, similar protective effects of
ginsenoside Rb1 were observed on MCT-induced apoptosis in EA.hy926
cells using fluorescent Hoechst 33358 staining (Fig. 1C-F). Cells treated with 7.5 mM MCT
for 48 h appeared shrunken and dark, and chromatin condensation,
marginalization or nuclear beading was observed in the nuclei.
Fragmentation of apoptotic nuclei dividing into smaller structures
was observed frequently as well (Fig.
1E). However, in the Rb1 control group, only a few typical
morphological features of apoptotic nuclei, including pyknotic
nuclei and formation of apoptotic bodies, were observed (Fig. 1F).
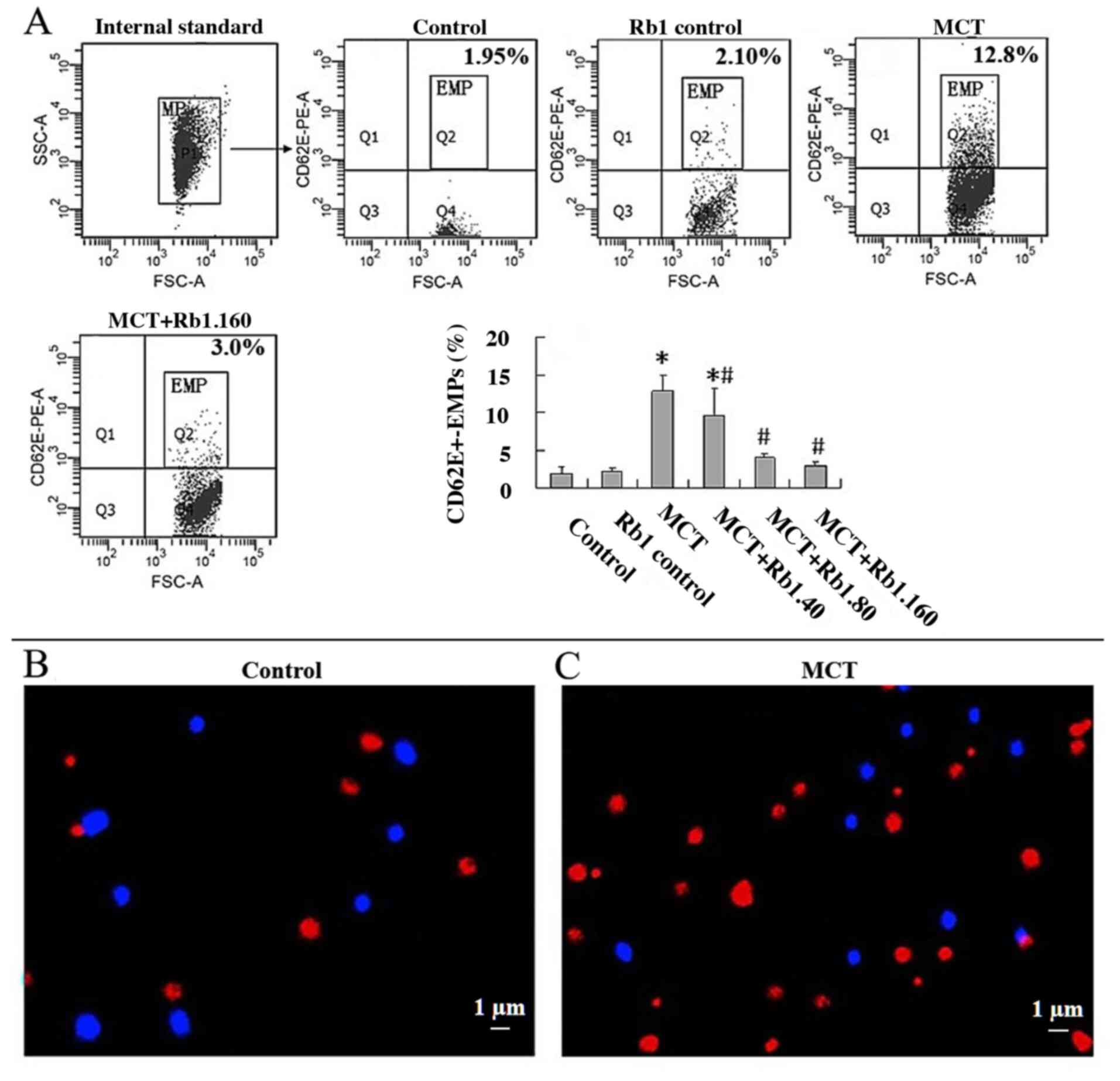
Ginsenoside Rb1 decreases MCT-induced
EMP secretion from EA.hy926 cells
EMPs are cell membrane vesicles derived from
endothelial cells. The morphological features of EMPs were
confirmed using confocal microscopy. Fluorescent beads 1 µm in
diameter were used as an internal standard and appeared as blue
particles. EMPs appeared as red, rounded vesicular structures with
a diameter of ~1 µm and were positive for CD62E. The proportion of
EMPs in the MCT group was significantly higher compared with
control group (Fig. 2B and C).
To further investigate the effect of ginsenoside Rb1
on MCT-induced EMP secretion, the percentage of
CD62E+-EMPs in culture medium in different groups using
flow cytometry was determined (Fig.
2A). Cells in the Rb1 control group showed similar levels of
CD62E+-EMPs secretion when compared with the control
group (P>0.05). MCT significantly increased
CD62E+-EMPs production compared with the control group
(12.8±2.18 vs. 1.93±0.86; P<0.05). However, exposure of EA.hy926
cells to 40, 80 or 160 mg/l ginsenoside Rb1 prior to treating with
MCT resulted in a significant decrease in CD62E+-EMPs
production in a dose-dependent manner compared with the MCT group
(40 mg/l, 9.67±3.57; 80 mg/l, 4.08±0.46; 160 mg/l, 3.00±0.40; MCT
group, 12.8±2.18; all P<0.05; Fig.
2A).
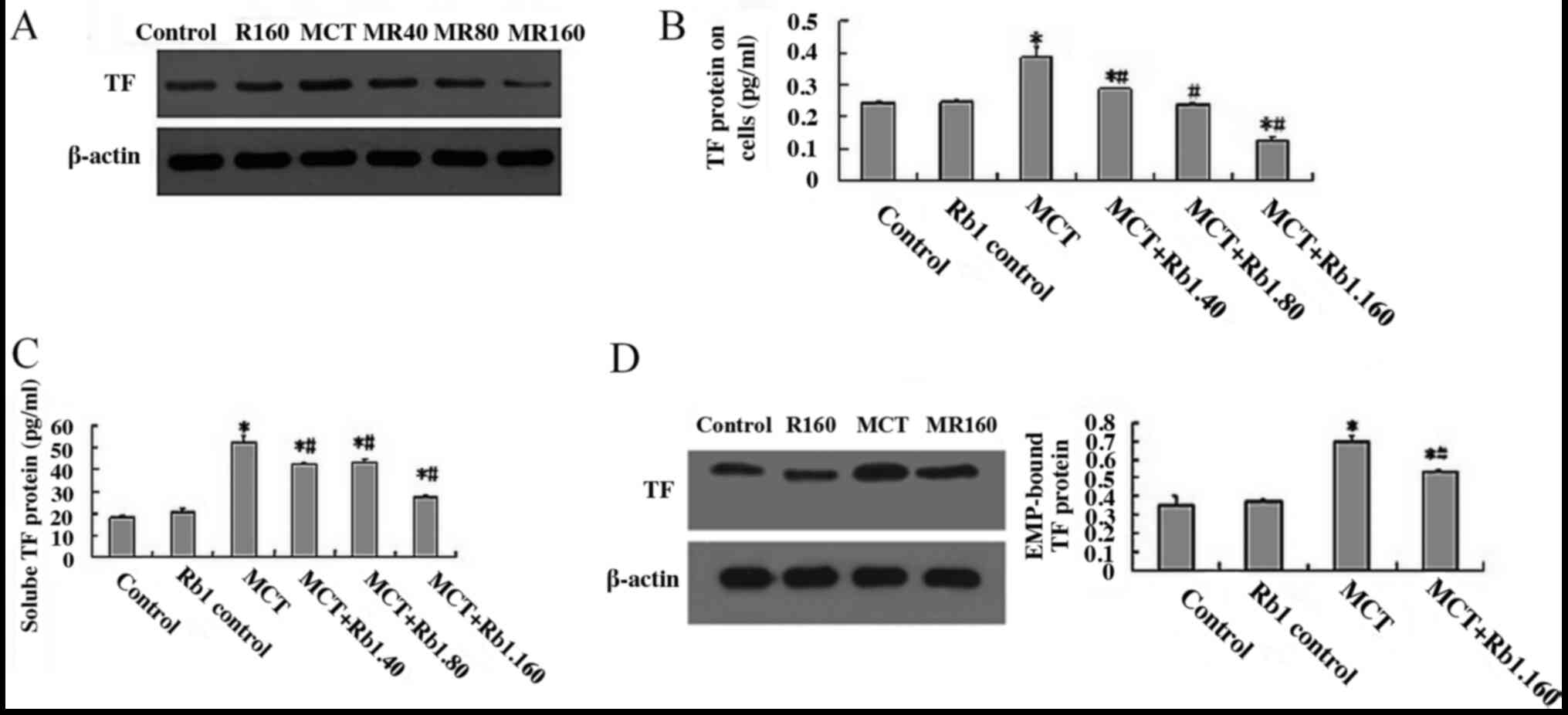
Ginsenoside Rb1 decreases MCT-induced
alterations in TF protein expression levels in EA.hy926 cells
TF is considered an integral membrane protein
expressed at the plasma membrane of endothelial cells that are not
exposed to blood. In the present study, the TF protein expression
levels on cells were determined by western blotting. The results
showed that the levels of TF protein in the MCT group were
significantly higher compared with the control group (0.387±0.03
vs. 0.243±0.09; P<0.05; Fig. 3A
and B). When cells were treated with
ginsenoside Rb1 (40, 80 and 160 mg/l) and MCT, the protein
expression levels of TF protein were significantly decreased in a
dose-dependent manner compared with the MCT group (40 mg/l,
0.288±0.04; 80 mg/l, 0.238±0.06; 160 mg/l, 0.128±0.07; MCT group,
0.387±0.03; all P<0.05; Fig. 3A
and B).
Ginsenoside Rb1 decreases MCT-induced
secretion of soluble (s)TF from EA.hy926 cells
Recently, significantly higher levels of soluble TF
proteins were observed in several endothelial related diseases, and
these soluble TF proteins possess functions in numerous
pathological processes, including hemostasis, thrombosis and
inflammation (27,28). In the present study, the supernatant
medium of EA.hy926 cells treated with ginsenoside Rb1 and MCT were
collected, and the sTF levels were detected using ELISA. The
results showed that the levels of sTF in the MCT group were
significantly higher compared with the control groups (52.574±2.571
vs. 18.121±0.969 pg/ml, respectively; P<0.05; Fig. 3C). When cells were treated with 40,
80 or 160 mg/l ginsenoside Rb1 combined with MCT, and the levels of
sTF were significantly decreased in each group compared with the
MCT group (MCT+Rb1.40 group, 42.549±0.954 pg/ml; MCT+Rb1.80 group,
43.368±1.712 pg/ml; MCT+Rb1.160 group 27.538±1.152 pg/ml; MCT
group, 52.574±2.571 pg/ml; all P<0.05; Fig. 3C).
Ginsenoside Rb1 decreases EMP-bound TF
protein levels induced by MCT in EA.hy926 cells
To clarify the molecular mechanism underlying the
biological effects of EMPs in endothelial damage, the expression of
EMP-bound TF proteins were determined by western blotting analysis
(Fig. 3D). The results showed that
in cells treated with 7.5 mM MCT for 48 h, the expression of
EMP-bound TF proteins was significantly increased compared with the
control groups (0.704±0.024 vs. 0.355±0.0495; P<0.05; Fig. 3D). However, when cells were treated
with 160 mg/l ginsenoside Rb1 for 2 h prior to exposing cells to
7.5 mM MCT for 48 h, the levels of EMP-bound TF protein were
significantly lower compared with the MCT group (0.536±0.01 vs.
0.704±0.024; P<0.05; Fig.
3D).
Discussion
The present study is the first to report that
ginsenoside Rb1 prevents endothelial injury through the EMP
pathway. Ginsenoside Rb1 significantly decreased MCT-induced EMP
levels in EA.hy926 cells and reduced apoptosis. In addition,
ginsenoside Rb1 decreased the secretion of soluble TF from EA.hy926
cells and EMP-bound TF protein induced by MCT, and thereby may
prevent the development of microthrombosis resulting from
endothelial injury.
Ginsenoside Rb1 is an active compound of ginseng,
which has been shown to exhibit several pharmacological properties,
including anticarcinogenic, immunomodulatory, anti-inflammatory,
antiallergic, antiatherosclerotic, antihypertensive and
antidiabetic effects, as well as anti-stress activity and effects
on the central nervous system (12).
Recently, studies have shown that ginsenoside Rb1 may prevent
hyperhomocysteine-induced endothelial injury and dysfunction and
enhance nitric oxide release from endothelial cells (29). The results of the present study
showed that ginsenoside Rb1 inhibited MCT-induced apoptosis in
EA.hy926 cells in a dose-dependent manner. Additionally, the
expression of Bax and caspase-3 proteins, that are promoters of
apoptosis, were increased in the MCT group, whereas the expression
of Bcl-2 was decreased. However, pretreatment with ginsenoside Rb1
reversed these alterations; the expression of Bcl-2 was increased,
whereas the levels of Bax and caspase-3 were decreased. Bcl-2 is an
important apoptosis-inhibiting protein (30). Therefore it was hypothesized that
ginsenoside Rb1 may protect endothelial cells by regulating the
expression of apoptosis-related proteins.
In addition, it was also shown that ginsenoside Rb1
reduced the levels of EMPs which can be used to reflect the level
of endothelial injury. A variety of prolonged stimuli are able to
induce EMP vesiculation from cultured endothelial cells (16). At low concentrations, EMP generation
may serve as an early adaptive response to activated or injured
endothelial cells, which protects the endothelium. However, when
the number of circulating EMPs exceeds a certain threshold, the
EMPs become an important factor exacerbating the pathophysiology of
the disease, by directly damaging the endothelium or significantly
impairing endothelium-dependent relaxation of macrovessels in
vitro (18). The results of the
present study showed that ginsenoside Rb1 significantly decreased
MCT-induced EMP levels in EA.hy926 cells, suggesting that
ginsenoside Rb1 may prevent endothelial injury via an EMP
associated pathway.
Compared with the parental cells, cell derived
microparticles can exert more potent effects on target organs as
they can deliver highly concentrated biological messages including
protein, DNA, mRNA and microRNA. In the case of acute injury,
endothelial cells may respond by dislodgement-induced EMP
generation and EMPs are capable of inducing a procoagulant and
thrombotic state (31-33).
Coagulant TF has been shown to be an important pathway in these
events (34,35). As an essential enzyme activator, TF
forms a catalytic complex with FVIIa, and initiates coagulation by
activating FIX and FX, ultimately resulting in the formation of
thrombin (36). Biologically active
TF has been detected in the adventitia of blood vessels, in the
circulating blood and in the lipid cores of atherosclerotic plaques
(37). Thus, it was hypothesized
that EMPs may participate in coagulation via TF. In the present
study, TF encapsulation was first detected in EMPs, indicating that
TF may circulate in the blood in a stable EMP-bound form, and EMPs
may promote inflammation and initiate thrombosis through EMP-bound
TF proteins. In addition, it was shown that MCT promoted TF release
from endothelial cells, whereas ginsenoside Rb1 significantly
decreased the levels of TF and EMP-bound TF protein induced by MCT.
These results suggest that ginsenoside Rb1 may prevent endothelial
injury by decreasing the expression of TF and therefore reduce
microthrombosis and ischemic injury, thus protecting endothelial
cells.
The present study has some limitation, including a
lack of 3D reconstruction from the confocal images. Additionally,
the mechanisms by which ginsenoside Rb1 exerts its protective
effects on endothelial injury require further study.
In conclusion, Ginsenoside Rb1 may serve as a potent
agent for the protection of endothelial injury by reducing cell
apoptosis and preventing endothelial injury-induced microthrombosis
through an EMP-mediated pathway.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural
Sciences Foundation of China (grant no. 30973835).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
QW and LX conceived and designed the study as well
as wrote and revised the manuscript. MZ and JX performed
experiments, analyzed the data as well as wrote and revised the
manuscript. HS and XW assisted in performing experiments. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Johnson DB and Savani BN: How can we
reduce hepatic veno-occlusive disease-related deaths after
allogeneic stem cell transplantation? Exp Hematol. 40:513–517.
2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Roeker LE, Kim HT, Glotzbecker B,
Nageshwar P, Nikiforow S, Koreth J, Armand P, Cutler C, Alyea EP,
Antin JH, et al: Early clinical predictors of hepatic
Veno-occlusive Disease/sinusoidal obstruction syndrome after
myeloablative stem cell transplantation. Biol Blood Marrow
Transplant. 25:137–144. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Dalle JH and Giralt SA: Hepatic
Veno-occlusive disease after hematopoietic stem cell
transplantation: Risk factors and stratification, prophylaxis, and
treatment. Biol Blood Marrow Transplant. 22:400–409.
2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Corbacioglu S, Jabbour EJ and Mohty M:
Risk Factors for development of and progression of hepatic
Veno-occlusive Disease/sinusoidal obstruction syndrome. Biol Blood
Marrow Transplant. 25:1271–1280. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Dignan FL, Wynn RF, Hadzic N, Karani J,
Quaglia A, Pagliuca A, Veys P and Potter MN: Haemato-oncology Task
Force of British Committee for Standards in Haematology; British
Society for Blood and Marrow Transplantation. BCSH/BSBMT guideline:
Diagnosis and management of veno-occlusive disease (sinusoidal
obstruction syndrome) following haematopoietic stem cell
transplantation. Br J Haematol. 163:444–457. 2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Corbacioglu S, Carreras E, Mohty M,
Pagliuca A, Boelens JJ, Damaj G, Iacobelli M, Niederwieser D,
Olavarría E, Suarez F, et al: Defibrotide for the treatment of
hepatic Veno-occlusive disease: Final results from the
international compassionate-use program. Biol Blood Marrow
Transplant. 22:1874–1882. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Bagal B, Chandrasekharan A, Chougle A and
Khattry N: Low, fixed dose defibrotide in management of hepatic
Veno-occlusive disease post stem cell transplantation. Hematol
Oncol Stem Cell Ther. 11:47–51. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Cui YC, Pan CS, Yan L, Li L, Hu BH, Chang
X, Liu YY, Fan JY, Sun K, Li Q and Han JY: Ginsenoside Rb1 protects
against Ischemia/reperfusion-induced myocardial injury via energy
metabolism regulation mediated by RhoA signaling pathway. Sci Rep.
7(44579)2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ren S, Leng J, Xu XY, Jiang S, Wang YP,
Yan XT, Liu Z, Chen C, Wang Z and Li W: Ginsenoside Rb1, A major
saponin from panax ginseng, exerts protective effects against
acetaminophen-induced hepatotoxicity in mice. Am J Chin Med.
47:1815–1831. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wang J, Qiao L, Li Y and Yang G:
Ginsenoside Rb1 attenuates intestinal ischemia-reperfusion-induced
liver injury by inhibiting NF-kappaB activation. Exp Mol Med.
40:686–698. 2008.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ke L, Guo W, Xu J, Zhang G, Wang W and
Huang W: Ginsenoside Rb1 attenuates activated microglia-induced
neuronal damage. Neural Regen Res. 9:252–259. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Xu Z, Lan T, Wu W and Wu Y: The effects of
ginsenoside Rb1 on endothelial damage and ghrelin expression
induced by hyperhomocysteine. J Vasc Surg. 53:156–164.
2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zheng X, Wang S, Zou X, Jing Y, Yang R, Li
S and Wang F: Ginsenoside Rb1 improves cardiac function and
remodeling in heart failure. Exp Anim. 66:217–228. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Valencia-Nuñez DM, Kreutler W,
Moya-Gonzalez J, Alados-Arboledas P, Muñoz-Carvajal I, Carmona A,
Ramirez-Chamond R and Carracedo-Añon J: Endothelial vascular
markers in coronary surgery. Heart Vessels. 32:1390–1399.
2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Santilli F, Marchisio M, Lanuti P,
Boccatonda A, Miscia S and Davi G: Microparticles as new markers of
cardiovascular risk in diabetes and beyond. Thromb Haemost.
116:220–234. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sierko E, Sokół M and Wojtukiewicz MZ:
Endothelial microparticles (EMP) in physiology and pathology.
Postepy Hig Med Dosw (Online). 69:925–932. 2015.PubMed/NCBI View Article : Google Scholar : (In Polish).
|
|
17
|
Wu Q, Chen H, Fang J, Xie W, Hong M and
Xia L: Elevated Fas/FasL system and endothelial cell microparticles
are involved in endothelial damage in acute graft-versus-host
disease: A clinical analysis. Leuk Res. 36:275–280. 2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Campello E, Spiezia L, Radu CM, Bulato C,
Gavasso S, Tormene D, Woodhams B, Dalla Valle F and Simioni P:
Circulating microparticles and the risk of thrombosis in inherited
deficiencies of antithrombin, protein C and protein S. Thromb
Haemost. 115:81–88. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Atehortúa L, Rojas M, Vásquez G,
Muñoz-Vahos CH, Vanegas-García A, Posada-Duque RA and Castaño D:
Endothelial activation and injury by microparticles in patients
with systemic lupus erythematosus and rheumatoid arthritis.
Arthritis Res Ther. 21(34)2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Mobarrez F, Svenungsson E and Pisetsky DS:
Microparticles as autoantigens in systemic lupus erythematosus. Eur
J Clin Invest. 48(e13010)2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Xia Q, Zhao Y, Lin G, Beland FA, Cai L and
Fu PP: Pyrrolizidine alkaloid-protein adducts: Potential
non-invasive biomarkers of pyrrolizidine alkaloid-induced liver
toxicity and exposure. Chem Res Toxicol. 29:1282–1292.
2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Stegelmeier BL, Colegate SM and Brown AW:
Dehydropyrrolizidine alkaloid toxicity, cytotoxicity, and
carcinogenicity. Toxins (Basel). 8(356)2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Tamariz J, Burgueno-Tapia E, Vázquez MA
and Delgado F: Pyrrolizidine Alkaloids. Alkaloids Chem Biol.
80:1–314. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kleiner DE: The histopathological
evaluation of drug-induced liver injury. Histopathology. 70:81–93.
2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Fang J, Zhang G, Teng X, Zhang Z, Pan J,
Shou Q and Chen M: Hematologic toxicity of Gynura segetum and
effects on vascular endothelium in a rat model of hepatic
veno-occlusive disease. Zhonghua Gan Zang Bing Za Zhi. 23:59–63.
2015.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
26
|
Wasinee K, Kunwadee P, Kittiphong P, Kovit
P, Pornthip C and Saovaros S: Microparticles From
β-thalassaemia/HbE patients induce endothelial cell dysfunction.
Sci Rep. 8(13033)2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
The composition and daily variation of
microparticles in whole blood in stable coronary artery disease. J
Physiol Pharmacol. 69:6–9. 2018.
|
|
28
|
Christersson C, Lindahl B and Siegbahn A:
The composition and daily variation of microparticles in whole
blood in stable coronary artery disease. Scand J Clin Lab Invest.
76:25–32. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Jia F, Mou L and Ge H: Protective effects
of ginsenoside Rb1 on H2O2-induced oxidative
injury in human endothelial cell line (EA. hy926) via miR-210. Int
J Immunopathol Pharmacol. 33(1681103269)2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Beumer TL, Roepers-Gajadien HL, Gademan
IS, Lock TM, Kal HB and De Rooij DG: Apoptosis regulation in the
testis: Involvement of Bcl-2 family members. Mol Reprod Dev.
56:353–359. 2000.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Sansone R, Baaken M, Horn P, Schuler D,
Westenfeld R, Amabile N, Kelm M and Heiss C: Release of endothelial
microparticles in patients with arterial hypertension, hypertensive
emergencies and catheter-related injury. Atherosclerosis.
273:67–74. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Jenkins NT, Padilla J, Boyle LJ, Credeur
DP, Laughlin MH and Fadel PJ: Disturbed blood flow acutely induces
activation and apoptosis of the human vascular endothelium.
Hypertension. 61:615–621. 2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Kou Y, Zou L, Liu R, Zhao X, Wang Y, Zhang
C, Dong Z, Kou J, Bi Y, Fu L and Shi J: Intravascular cells and
circulating microparticles induce procoagulant activity via
phosphatidylserine exposure in heart failure. J Thromb
Thrombolysis. 48:187–194. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Shustova ON, Antonova OA, Golubeva NV,
Khaspekova SG, Yakushkin VV, Aksuk SA, Alchinova IB, Karganov MY
and Mazurov AV: Differential procoagulant activity of
microparticles derived from monocytes, granulocytes, platelets and
endothelial cells: Impact of active tissue factor. Blood Coagul
Fibrinolysis. 28:373–382. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Geddings JE and Mackman N: Tumor-derived
tissue factor-positive microparticles and venous thrombosis in
cancer patients. Blood. 122:1873–1880. 2013.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Cheng X, Qiu X, Liu Y, Yuan C and Yang X:
Trimethylamine N-oxide promotes tissue factor expression and
activity in vascular endothelial cells: A new link between
trimethylamine N-oxide and atherosclerotic thrombosis. Thromb Res.
177:110–116. 2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Collier M, Akinmolayan A and Goodall AH:
Comparison of tissue factor expression and activity in foetal and
adult endothelial cells. Blood Coagul Fibrinolysis. 28:452–459.
2017.PubMed/NCBI View Article : Google Scholar
|