Introduction
Breast cancer is the most common malignancy and
leading cause of cancer-associated death in women between the ages
of 35 and 50. Obesity is a well-established risk factor for
development of breast cancer, exerting its effect via several
different biological mechanisms (1,2). As an
endocrine organ, adipose tissue secretes molecules called
adipocytokines that act in an endocrine, paracrine and autocrine
manner, and may promote the malignant progression of breast cancer
(3). Several studies have indicated
that adipocytokines mediate the survival, growth, invasion and
metastasis of breast cancer cells (4-7).
Apelin (APLN) and retinol-binding protein 4 (RBP4) are
adipocytokines that may serve a role in carcinogenesis (8-10).
Evaluation of their role may be useful in predicting survival times
and cancer recurrence (11-13).
APLN was isolated as an endogenous ligand from
bovine stomach epithelial cells in 1998(14). Further studies have demonstrated that
APLN is also expressed in heart muscles, brain, kidneys, liver,
lungs and spleen, as well as in mammary glands, placenta and
gastric mucosa (15). The small
peptide is involved in several vital physiological processes, such
as angiogenesis, fluid homeostasis and glucose metabolism (16). Growing evidence has suggested that
APLN induces the maturation of tumor blood capillaries and prompts
tumor vascularization (17).
Moreover, APLN also shows lymph angiogenic potential in relation to
tumor growth and lymph node metastasis (18). Upregulated expression of APLN has
been found in various types of cancer, including breast cancer,
where its levels have been shown to be correlated with shorter
survival times and a higher incidence of cancer recurrence
(19-21).
RBP4 is a more recently identified adipokine that
transports retinol (vitamin A) from the liver to peripheral tissues
(11). In adipose tissues, RBP4 is
expressed in mature, lipid-laden adipocytes (22). It has been observed that increased
RBP4 is positively correlated with obesity-linked complications,
including impaired glucose tolerance, insulin resistance, type 2
diabetes mellitus, dyslipidemia, hypertension and cardiovascular
disease (23). Studies have also
indicated that upregulated expression of RBP4 is associated with
colorectal, ovarian and endometrial cancer (24-26).
Recently Jiao et al (11)
reported that elevated RBP4 concentrations were associated with an
increased risk of breast cancer independent of BMI, lipid levels
and other potential risk factors (11). The specific role of RBP4 in
carcinogenesis is not understood, to the best of our knowledge. It
has been shown to potentiate migration and proliferation of tumor
cells by stimulating the synthesis of MMP-2 and MMP-9, thus
facilitating tumor cell infiltration in surrounding tissues
(25).
It is well documented that carcinogenesis is
associated with the overproduction of reactive oxygen species (ROS)
(27-29).
ROS can cause oxidative damage to lipids, proteins and DNA.
Oxidized and damaged DNA (resulting in genetic mutations) is
involved in malignant transformation. 8-hydroxydeoxyguanosine
(8-oxo-dG) is a specific marker of 2-deoxyguanosine damage
following ROS-mediated damage to DNA. High levels of 8-oxo-dG in
tumors, blood samples or urine have been found in patients with
various types of cancer (30,31).
Moreover, 8-oxo-dG may be useful for predicting prognosis in
different types of cancer (32,33). ROS
levels are maintained within narrow limits under physiological
conditions by an antioxidant defense system consisting of multiple
independent components (34).
However, there is evidence of impaired antioxidant status in
patients with various types of cancer, including breast cancer, due
to an imbalance between ROS production and elimination, resulting
in oxidative damage to key biomolecules (35). Furthermore, recent studies have
indicated a close correlation between adipocytokines and oxidative
stress, where certain adipocytokines were shown to increase the
production of free radicals, whereas others inhibited this process
(31,36-38).
Although alterations in the levels of adipocytokines
and markers of oxidative stress in patients with breast cancer are
well documented, relatively little is known regarding the degree to
which they vary in different breast cancer subtypes or in relation
to the aggressiveness of the breast cancer (39,40).
Breast cancer is a complex and heterogeneous disease, and its
prognosis may vary based on the combined characteristics of the
tumor itself and any underlying conditions a patient may have
(41). The role of obesity-related
markers and oxidative stress in tumor growth and metastasis may be
associated with distinct subtypes of breast cancer. Therefore, in
the present study, the concentrations of APLN, RBP4 and 8-oxo-dG in
breast cancer patients with different clinicopathological features,
including tumor grade and size, HER-2/neu expression, hormone
receptor status and lymph node status were investigated. Due to the
fact that there is a great interest in identifying biomarkers that
may be used to predict chemotherapy treatment responses, the second
aim of the present study was to analyze the effects of 6-week
adjuvant chemotherapy on APLN, RBP4 and 8-oxo-dG levels.
Materials and methods
Patients and methods
The study cohort was formed of women with breast
cancer treated at the Greater Poland Cancer Center in Poznan
between December 2016 to November 2017 who qualified for adjuvant
chemotherapy. The study was performed according to principles of
the Declaration of Helsinki (42).
The protocol used in the present study was approved by the Local
Bioethical Committee of Poznan University of Medical Sciences
(approval no. 1016/16). The study included 60 women and written
informed consent was obtained from all participants. The median age
was 58 years old (range, 31-76). Selected clinical criteria
collected in the pre-operative period included tumor histological
grade and size, HER-2/neu expression, and the presence/absence of
regional lymph node metastases, and this data was used to divide
patients into different categories. The histological grading of
breast tumors was based on the Modified Bloom-Richardson Grading
Scheme (43). The status the
HER2-neu, progesterone and estrogen receptor expression was
assessed by immunohistochemistry (IHC) analysis at the Department
of Cancer Pathology, Greater Poland Cancer Center, as a routine
diagnostic procedure, using the EnVision™ + HRP complex
(DakoCytomation; Agilent Technologies, Inc.). HER2 status
(HercepTest™; Agilent Technologies, Inc.) was scored as follows: 0,
no staining; 1, weakly visible staining in >10% of neoplastic
cells; 2, weak or moderate staining in >10% of neoplastic cells;
and 3, strong staining in >10% of neoplastic cells. In the case
of moderate expression (2+), a FISH test was performed to assess
HER2-neu gene amplification using HER2 IQFISH pharmDx™ kit (Dako
Omnis; Agilent Technologies, Inc.). HER2 IQFISH pharmDx™ reagent
(Dako Omnis; Agilent Technologies, Inc.) was used as a reference
standard for HER2. Assessment of HER2 gene amplification with the
HER2 IQFISH pharmDx™ test (Dako Omnis) was fully automated and was
performed on the Dako Omnis device according tot the manufacturer's
protocol. For FISH analysis, the slides were deparaffinized by
immersing them in Clearify™ for 10 min, unmasking the antigen using
ISH Pre-Treatment Solution (Dako Omnis; Agilent Technologies, Inc.)
for 15 min, followed by dehydration in 96% ISH Ethanol Solution
(Dako Omnis; Agilent Technologies, Inc.) at 32˚C twice for 3 min.
Subsequently, slides were treated with pepsin for 15 min and
finally air dried at 45˚C for 15 min. Next, the slides were
subjected to denaturation by immersing them in a denaturing
solution at 66˚C for 10 min. Slides underwent hybridization by
applying HER2 IQFISH pharmDx™ (Dako Omnis; Agilent Technologies,
Inc.). HER2 IQFISH pharmDx™ reagent (Dako Omnis; Agilent
Technologies, Inc.) is a mix of IQISH probes consisting of a mix of
Texas red labeled DNA probes (218 kb long region) containing the
HER2 gene on chromosome 17, and a mix of fluorescein labeled probes
peptide nucleic acid directed against the centromeric region of
chromosome 17 (CEN-17). Specific hybridization with target regions
results in a distinct red fluorescent signal for each HER2 gene
locus and a green fluorescent signal for each centromere of
chromosome 17. The slides were hybridized at 45˚C for 75 min. A
deep rinse was performed with ISH Stringent Wash Buffer (Dako
Omnis™; Agilent Technologies, Inc.) at 61˚C for 10 min. After
staining in the Dako Omnis machine, sections were mounted on a
slide with Fluorescence Mounting Medium (Dako Omnis™; Agilent
Technologies, Inc.) containing DAPI and then covered with a
coverslip. Using a fluorescence microscope equipped with
appropriate filters, the position of tumor cells was determined and
the red (HER2) and green (CEN-17) signals were counted, and the
HER2/CEN-17 ratio was calculated. Healthy cells in the analyzed
tissue sections served as the internal control for positive
staining. IHC scores of 3+ and 2+ with positive HER2 amplification
was considered to be indicative of positive HER2-neu receptor
activity. IHC 0, 1+ and 2+ with negative HER2 amplification was
taken to be negative HER2-neu receptor activity. To assess the
activity of estrogen and progesterone receptors, monoclonal
antibodies against estrogen (cat. no. 1D5; 1:50; DakoCytomation;
Agilent Technologies, Inc.) and progesterone receptors (cat. no.
PgR636; 1:50; DakoCytomation; Agilent Technologies, Inc.) and a
polyclonal antibody against estrogen β receptors (Chemicon) were
used. The presence of estrogen and/or progesterone receptors on the
tumor surface defined the tumor as hormone dependent. The
characteristics of the study group are presented in Table I. All patients underwent four cycles
of adjuvant chemotherapy in a regimen involving doxorubicin and
cyclophosphamide (AC). HER2-positive patients received trastuzumab
in addition to AC.
 | Table IClinicopathological characteristics
of the study group. |
Table I
Clinicopathological characteristics
of the study group.
| Clinicopathological
feature | N (%) |
|---|
| Histopathological
grade | |
|
I/II | 31 (51.7) |
|
III | 29 (48.3) |
| HER-2/neu
expression | |
|
+ | 20 (33.3) |
|
- | 40 (66.7) |
| Tumor size | |
|
<2
cm | 33(55) |
|
>2
cm | 27(45) |
| Regional lymph node
metastases | |
|
Present | 32 (53.3) |
|
Absent | 28 (46.7) |
| Hormonal
sensitivity | |
|
Hormonal-positive | 44 (73.3) |
|
Hormonal-negative | 16 (26.7) |
Blood samples for biochemical analyses were taken
from the antecubital vein 1 day prior to chemotherapy after an
overnight fast, and 6 weeks later when the first and second cycles
of chemotherapy were completed. Samples were collected in EDTA
anticoagulant serum tubes. After 30 min, the tubes were centrifuged
at 1,000 x g for 15 min. Serum and plasma samples were stored at a
temperature of -80˚C until required for assay. The concentrations
of RBP4, APLN and 8-oxo-dG were measured using ELISA kits according
to the manufacturer's protocol (AssayPro LLC, cat. no. ER3005-1;
Phoenix Pharmaceuticals, cat. no. EKE-057-15, Inc, EIAab, cat. no.
E0660Ge). Total antioxidant capacity (TAC) was determined using an
Antioxidant assay kit (Cayman Chemical Company) and expressed in
Trolox equivalents. Trolox is a water-soluble analog of tocopherol
used to assess the antioxidant potential of a mixture containing
several antioxidants.
Women with diabetes mellitus, cardiovascular
diseases, hypertension, and inflammatory diseases were not included
in the present study.
Statistical analysis
Statistical analysis was performed using Statistica
version 12.0 (StatSoft Inc.). The normality of quantitative
variables was assessed using a Kolmogorov-Smirnov or Shapiro-Wilk
test. Normally distributed, continuous variables were presented as
the mean ± standard deviation. Parameters that were not normally
distributed are presented as a median (range). Comparisons between
appropriate categories of patients were made using an unpaired
Student's t-test or a Mann-Whitney U test depending on the
distribution of the data. A comparison between analyzed parameters
before and after chemotherapy was performed using a paired
Student's t-test or a Wilcoxon test, depending on the distribution
of the data. Pearson's or Spearman's correlation coefficient
analysis was used to assess the strength of any association between
different variables. Multivariate logistical regression analysis
was performed using APLN as an independent variable and HER-2/neu
status as a dependent variable after adjusting for age, BMI,
adipose tissue content, TAC value, 8-oxo-dG levels, lymph node
status, hormone receptor status, tumor grade and size. P<0.05
was considered to indicate a statistically significant
difference.
Results
Baseline concentrations of
adipocytokines and markers of oxidative stress in patients with
breast cancer
First, patients were divided into subgroups
according to selected clinical features, including histological
tumor grade and size, HER-2/neu expression, hormone receptor
status, the presence/absence of metastases in regional lymph nodes.
The concentrations of APLN, RBP4 and 8-oxo-dG, as well as the TAC
levels were assessed. All parameters were evaluated before
administration of any chemotherapy.
No differences in adipose tissue content and BMI
values were observed between the groups. RBP4 and 8-oxo-dG levels
also did not differ between the groups. RBP4 and 8-oxo-dG
concentrations did not differ amongst patients based on tumor size,
tumor grade, HER-2/neu status, presence of lymph node metastases or
hormone receptor status (Table II).
Only APLN levels were found to be significantly higher in HER-2/neu
positive cases compared with HER-2/neu negative patients (Table IIC). In addition, only TAC levels
were significantly higher in women with hormone-positive breast
cancer (Table IIE).
 | Table IIConcentration of adipocytokines and
selected markers of oxidative stress in women with breast
cancer. |
Table II
Concentration of adipocytokines and
selected markers of oxidative stress in women with breast
cancer.
| A, Tumor size |
|---|
| Analyzed
parameter | <2 cm | >2 cm | P-value |
|---|
| RBP4, µg/ml | 67.14
(55.07-103.7) | 69.02
(42.85-105.4) | 0.741 |
| APLN, ng/ml | 1.31±0.39 | 1.26±0.47 | 0.721 |
| 8-oxo-dG,
ng/ml | 10.1
(5.80-26.54) | 8.97
(3.68-31.61) | 0.886 |
| TAC, µg/ml | 2.85
(0.62-12.13) | 3.32
(2.02-9.20) | 0.156 |
| BMI,
kg/m2 | 25.7
(19.70-41.80) | 27.2
(17.70-37.40) | 0.950 |
| Adipose tissue
content, % | 33.5
(18.80-44.30) | 31.8
(15.30-43.40) | 0.223 |
| Age | 58.36±9.30 | 57.48±10.71 | 0.734 |
| B,
Histopathological grade |
| Analyzed
parameter | I/II | III | P-value |
| RBP4, µg/ml | 66.96
(55.07-103.4) | 69.02
(42.85-105.4) | 0.457 |
| APLN, ng/ml | 1.25±0.40 | 1.33±0.45 | 0.558 |
| 8-oxo-dG,
ng/ml | 9.28
(5.80-30.94) | 9.19
(3.68-31.61) | 0.392 |
| TAC, µg/ml | 2.98
(1.26-12.13) | 2.91
(0.62-9.20) | 0.968 |
| BMI,
kg/m2 | 25.3
(18.30-36.50) | 26.8
(17.70-41.80) | 0.142 |
| Adipose tissue
content, % | 31.6
(15.30-43.0) | 35.4
(18.50-44.30) | 0.095 |
| Age | 58.52±8.69 | 57.38±11.14 | 0.659 |
| C, HER-2/neu
expression |
| Analyzed
parameter | + | - | P-value |
| RBP4, µg/ml | 79.41
(42.85-103.7) | 67.14
(55.38-105.4) | 0.780 |
| APLN, ng/ml | 1.58±0.37 | 1.16±1.00 |
0.002b |
| 8-oxo-dG,
ng/ml | 9.96
(4.99-31.61) | 9.23
(3.68-30.94) | 0.893 |
| TAC, µg/ml | 3.57
(0.62-12.13) | 2.91
(1.26-9.20) | 0.408 |
| BMI,
kg/m2 | 25.2
(19.70-41.80) | 27
(17.70-37.40) | 0.541 |
| Adipose tissue
content, % | 32
(18.80-44.00) | 33.4
(15.30-44.30) | 0.632 |
| Age | 59.50±8.86 | 57.20±10.37 | 0.399 |
| D, Regional lymph
node metastases |
| Analyzed
parameter | Present | Absent | P-value |
| RBP4, µg/ml | 66.21
(42.85-105.4) | 72.92
(55.07-103.7) | 0.268 |
| APLN, ng/ml | 1.24±0.47 | 1.33±0.37 | 0.479 |
| 8-oxo-dG,
ng/ml | 10.15
(4.51-30.94) | 9.05
(3.68-31.61) | 0.545 |
| TAC, µg/ml | 3.48
(1.26-12.13) | 2.72
(0.62-9.80) | 0.321 |
| BMI,
kg/m2 | 25.5
(17.70-37.40) | 26.2
(21.20-41.80) | 0.51 |
| Adipose tissue
content, % | 31.8
(15.30-43.40) | 33.55
(21.90-44.30) | 0.219 |
| Age | 58.75±10.14 | 57.07±9.68 | 0.516 |
| E, Hormone receptor
status |
| Analyzed
parameter | Positive | Negative | P-value |
| RBP4, µg/ml | 66.95
(42.85-105.4) | 72.92
(57.61-103.4) | 0.277 |
| APLN, ng/ml | 1.22±1.07 | 0.87±0.55 | 0.265 |
| 8-oxo-dG,
ng/ml | 8.65
(3.68-30.94) | 14.07
(5.80-31.61) | 0.086 |
| TAC, µg/ml | 3.18
(0.62-12.13) | 2.07
(1.26-5.99) |
0.043a |
| BMI,
kg/m2 | 26.6
(17.70-37.40) | 24.85
(19.50-41.80) | 0.640 |
| Adipose tissue
content, % | 32.95
(15.30-44.30) | 33.25
(19.50-44.00) | 0.676 |
| Age | 57.8±8.83 | 58.44±12.66 | 0.826 |
Logistical regression showed that higher APLN levels
were significantly associated with positive HER-2/neu status
(Table III, Model 1), even after
adjusting for potential confounding factors, such as age, BMI,
adipose tissue content, markers of oxidative stress (Table III, Model 2), tumor grade and size,
hormone receptor status and lymph node metastasis (Table III, Model 3).
 | Table IIIMultivariate logistical regression
analysis of the association between APLN and HER-2/neu status. |
Table III
Multivariate logistical regression
analysis of the association between APLN and HER-2/neu status.
| Variable | Odds ratio | 95% confidence
interval | P-value |
|---|
| Model 1 | 2.14 | 0.483-10.73 |
0.038a |
| Model 2 | 26.84 | 1.627-1363 |
0.045a |
| Model 3 | 91.15 | 3.142-30162 |
0.043a |
Univariate analysis performed on the levels before
chemotherapy demonstrated that neither APLN nor RBP4 concentrations
were correlated with patient age, BMI or the parameters of
oxidative stress (Table IV). RBP4
concentrations were only positively associated with adipose tissue
content (Table IV). 8-oxo-dG levels
were not correlated with age, adipose tissue content or TAC.
However, there was a significant negative association between
8-oxo-dG levels and BMI (Table
IV).
 | Table IVUnivariate analysis for association
between parameters before and after treatment. |
Table IV
Univariate analysis for association
between parameters before and after treatment.
| A, Before
chemotherapy |
|---|
| | APLN | RBP4 | 8-oxo-dG |
|---|
| Correlated
parameter | r | P-value | r | P-value | r | P-value |
|---|
| Age | 0.057 |
0.728b | 0.118 |
0.456c | 0.030 |
0.864c |
| BMI | -0.084 |
0.613b | 0.271 |
0.082c | -0.343 |
0.040a,c |
| Adipose tissue
content | -0.237 |
0.147b | 0.343 |
0.026a,c | -0.225 |
0.186c |
| TAC | -0.18 |
0.293c | -0.005 |
0.974c | 0.204 |
0.232c |
| 8-oxo-dG | -0.01 |
0.955c | 0.004 |
0.981c | - | - |
| B, After 6-weeks of
chemotherapy |
| | APLN | RBP4 | 8-oxo-dG |
| Correlated
parameter | r | P-value | r | P-value | r | P-value |
| Age | -0.028 |
0.868b | 0.162 |
0.305b | 0.039 |
0.822c |
| BMI | 0.104 |
0.530b | 0.230 |
0.143b | -0.262 |
0.123c |
| Adipose tissue
content | 0.295 |
0.069b | 0.318 |
0.040a,b | -0.202 |
0.236c |
| TAC | 0.161 |
0.348c | -0.160 |
0.317c | 0.365 |
0.029a,c |
| 8-oxo-dG | -0.240 |
0.194c | -0.096 |
0.579c | - | - |
Concentration of adipocytokines and
markers of oxidative stress after 6-weeks of chemotherapy
The effects of 6-week chemotherapy on APLN, RBP4,
8-oxo-dG and TAC levels in the entire group, as well as within the
different subgroups was next addressed.
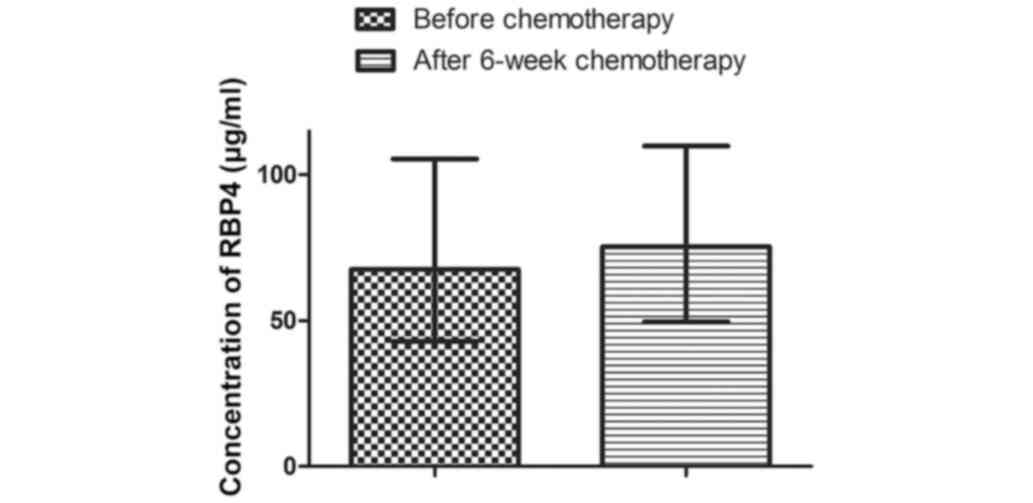
Levels of RBP4 in the total group of women increased
from an initial value of 67.51 µg/ml (42.85-105.4 µg/ml) to 75.27
µg/ml (49.56-109.8 µg/ml) after 6-weeks of chemotherapy (Fig. 1). However, this increase was not
statistically significant (P=0.2066).
No change in RBP4 concentration were observed after
6-weeks of chemotherapy in any of the sub-categories based on tumor
histological grade and size, HER-2/neu expression, hormone receptor
status, and the presence/absence of regional lymph node metastases
(Table V).
 | Table VEffect of 6-weeks of chemotherapy on
retinol-binding protein 4 concentration in women with breast
cancer. |
Table V
Effect of 6-weeks of chemotherapy on
retinol-binding protein 4 concentration in women with breast
cancer.
| Clinicopathological
feature | Before
chemotherapy, µg/mla | After 6-weeks of
chemotherapy, µg/mla | P-value |
|---|
| Histopathological
grade | | | |
|
I/II | 66.96
(55.07-103.4) | 72.03
(49.56-99.82) | 0.537 |
|
III | 69.02
(42.85-105.4) | 80.48
(53.68-105.4) | 0.254 |
| HER-2/neu
expression | | | |
|
+ | 79.41
(42.85-103.7) | 79.82
(49.56-109.8) | 0.791 |
|
- | 67.14
(55.38-105.4) | 73.63
(56.48-102.6) | 0.174 |
| Tumor size | | | |
|
<2
cm | 67.14
(55.07-103.7) | 72.93
(49.56-109.8) | 0.558 |
|
>2
cm | 69.02
(42.85-105.4) | 77.78
(53.68-99.82) | 0.239 |
| Regional lymph node
metastases | | | |
|
Present | 66.21
(42.85-105.4) | 67.69
(53.68-99.82) | 0.424 |
|
Absent | 72.92
(55.07-103.7) | 81.58
(49.56-109.8) | 0.348 |
| Hormonal
sensitivity | | | |
|
Hormonal-positive | 66.95
(42.85-105.4) | 76.4
(49.56-99.82) | 0.213 |
|
Hormonal-negative | 72.92
(57.61-103.4) | 70.94
(56.48-109.8) | 0.7 |
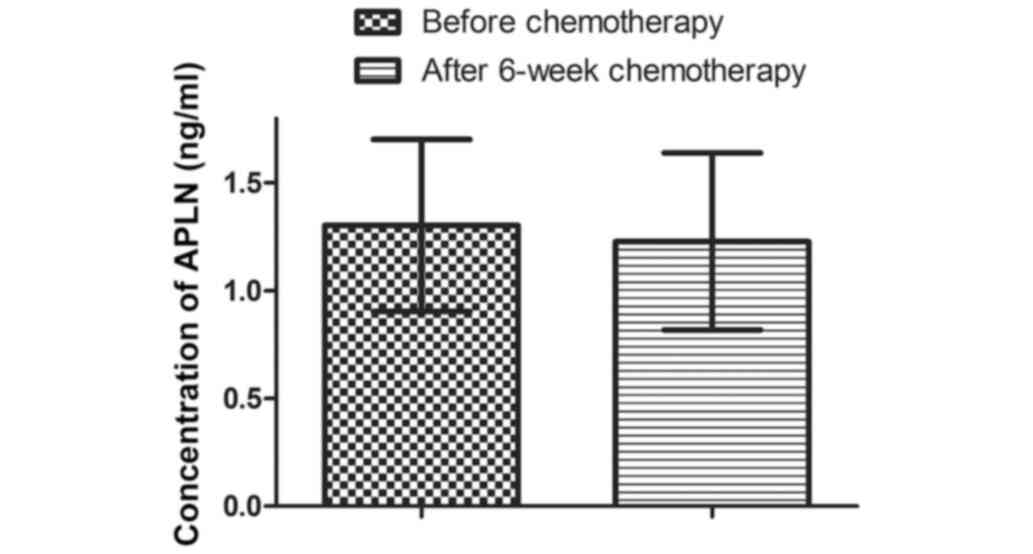
The concentrations of APLN prior to chemotherapy and
6 weeks after were 1.30±0.40 and 1.23±0.41 ng/ml, respectively.
However, this reduction was not significantly different (P=0.2058;
Fig. 2).
APLN concentration was not significantly altered in
any of the subgroups after 6-weeks of chemotherapy (Table VI).
 | Table VIEffect of 6-weeks of chemotherapy on
apelin concentration in women with breast cancer. |
Table VI
Effect of 6-weeks of chemotherapy on
apelin concentration in women with breast cancer.
| Clinicopathological
feature | Before
chemotherapy, ng/mla | After 6-weeks of
chemotherapy, ng/mla | P-value |
|---|
| Histopathological
grade | | | |
|
I/II | 1.25±0.40 | 1.21±0.47 | 0.661 |
|
III | 1.33±0.45 | 1.23±0.38 | 0.223 |
| HER-2/neu
expression | | | |
|
+ | 1.58±0.37 | 1.46±0.44 | 0.415 |
|
- | 1.16±1.00 | 1.11±0.38 | 0.459 |
| Tumor size | | | |
|
<2
cm | 1.31±0.39 | 1.26±0.43 | 0.561 |
|
>2
cm | 1.26±0.47 | 1.17±0.43 | 0.306 |
| Regional lymph node
metastases | | | |
|
Present | 1.24±0.47 | 1.11±0.40 | 0.101 |
|
Absent | 1.33±0.37 | 1.33±0.42 | 0.937 |
| Hormonal
sensitivity | | | |
|
Hormonal-positive | 1.22±1.07 | 1.08±0.55 | 0.290 |
|
Hormonal-negative | 0.87±0.55 | 0.74±0.42 | 0.095 |
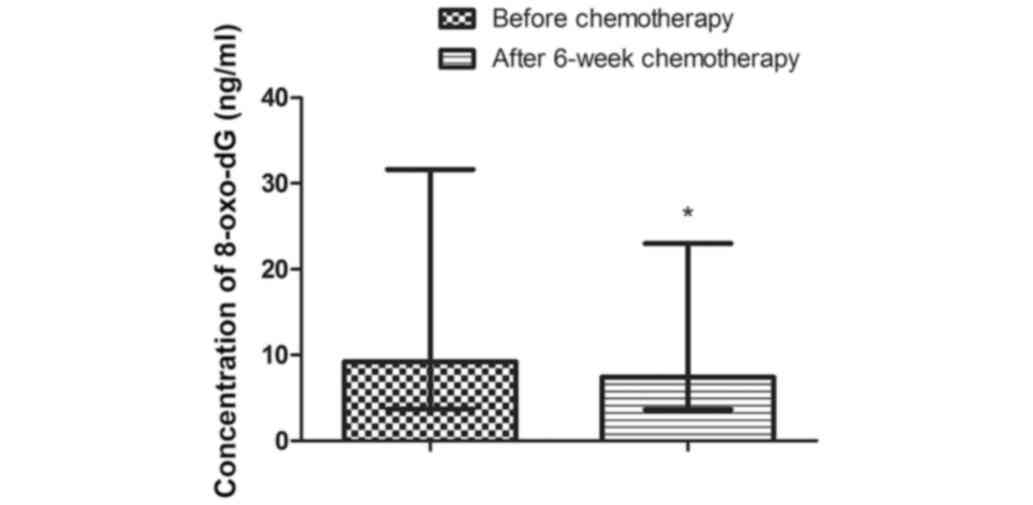
The concentration of 8-oxo-dG decreased
significantly when comparing all the data together, from 9.23 ng/ml
(3.68-31.61 ng/ml) to 7.42 ng/ml (3.64-22.97 ng/ml) after 6-weeks
of chemotherapy; P=0.0009 (Fig. 3).
A similar observation was seen when comparing patients with tumors
>2 cm (P=0.007), with metastases in the regional lymph nodes
(P=0.004), irrespective of the HER-2/neu status (HER2/neu positive
P=0.021; HER2/neu negative P=0.017) (Table VII). A decrease in 8-oxo-dG levels
was observed regardless of tumor grade (I/II P=0.007; III P=0.041)
and hormone receptor status (hormone-positive P=0.038;
hormone-negative P=0.004) (Table
VII).
 | Table VIIEffect of 6-weeks of chemotherapy on
8-hydroxydeoxyguanosine concentration in women with breast
cancer. |
Table VII
Effect of 6-weeks of chemotherapy on
8-hydroxydeoxyguanosine concentration in women with breast
cancer.
| Clinicopathological
feature | Before
chemotherapy, ng/mlc | After 6-weeks of
chemotherapy, ng/mlc | P-value |
|---|
| Histopathological
grade | | | |
|
I/II | 9.28
(5.80-30.94) | 7.26
(3.90-22.97) |
0.007b |
|
III | 9.19
(3.68-31.61) | 7.44
(3.64-14.68) |
0.041a |
| HER-2/neu
expression | | | |
|
+ | 9.96
(4.99-31.61) | 7.5
(3.64-13.30) |
0.021a |
|
- | 9.23
(3.68-30.94) | 7.05
(4.62-22.97) |
0.017a |
| Tumor size | | | |
|
<2
cm | 10.1
(5.80-26.54) | 7.98
(3.64-22.97) | 0.061 |
|
>2
cm | 8.97
(3.68-31.61) | 6.81
(4.30-12.52) |
0.007b |
| Regional lymph node
metastases | | | |
|
Present | 10.15
(4.51-30.94) | 7.33
(4.30-22.97) |
0.004b |
|
Absent | 9.05
(3.68-31.61) | 7.52
(3.64-14.68) | 0.059 |
| Hormonal
sensitivity | | | |
|
Hormonal-positive | 8.65
(3.68-30.94) | 6.85
(3.90-22.97) |
0.038a |
|
Hormonal-negative | 14.07
(5.80-31.61) | 7.56
(3.64-10.68) |
0.004b |
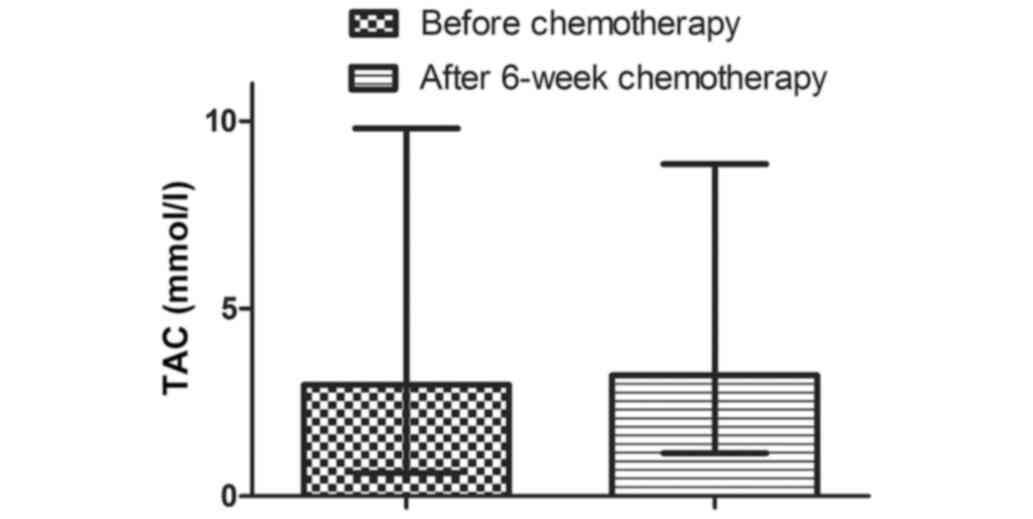
TAC values increased after 6 weeks of chemotherapy
in the entire group of women from an initial value of 2.97 mmol/l
(0.62-9.80 mmol/l) to 3.22 mmol/l (1.15-8.85 mmol/l) (Fig. 4). However, this increase was not
significant (P=0.8984). There was no statistically significant
change in TAC in any of the subgroups after 6-weeks of chemotherapy
(Table VIII).
 | Table VIIIEffect of 6-weeks of chemotherapy on
total antioxidant capacity concentration in women with breast
cancer. |
Table VIII
Effect of 6-weeks of chemotherapy on
total antioxidant capacity concentration in women with breast
cancer.
| Clinicopathological
feature | Before
chemotherapy, mmol/l | After 6-weeks of
chemotherapy, mmol/l | P-value |
|---|
| Histopathological
grade | | | |
|
I/II | 2.98
(1.26-12.13) | 3.2
(1.15-8.85) | 0.626 |
|
III | 2.91
(0.62-9.20) | 3.66
(1.42-8.72) | 0.481 |
| HER-2/neu
expression | | | |
|
+ | 3.57
(0.62-12.13) | 3.5
(1.42-7.47) | 0.684 |
|
- | 2.91
(1.26-9.20) | 3.22
(1.15-8.85) | 0.700 |
| Tumor size | | | |
|
<2
cm | 2.85
(0.62-12.13) | 3.5
(1.15-8.72) | 0.284 |
|
>2
cm | 3.32
(2.02-9.20) | 3.22
(1.52-8.85) | 0.316 |
| Regional lymph node
metastases | | | |
|
Present | 3.48
(1.26-12.13) | 3.27
(1.52-8.85) | 0.889 |
|
Absent | 2.72
(0.62-9.80) | 3.19
(1.15-8.72) | 0.983 |
| Hormonal
sensitivity | | | |
|
Hormonal-positive | 3.18
(0.62-12.13) | 3.39
(1.15-8.72) | 0.627 |
|
Hormonal-negative | 2.07
(1.26-5.99) | 2.54
(1.59-8.85) | 0.313 |
In the post-treatment period, neither APLN, RBP4 or
8-oxo-dG were shown to be correlated with the age of patients or
their BMI. A positive correlation was observed between RBP4 and
adipose tissue content, and between 8-oxo-dG and TAC values
(Table IV).
Discussion
Breast cancer is the most common type of cancer in
women worldwide, and is the second-leading cause of
cancer-associated morbidity and mortality in women (44). The mechanisms and factors
contributing to development and progression of breast cancer have
been studied extensively. Adipocytokines and oxidative stress
appear to serve a significant role in carcinogenesis (45,46).
Moreover, findings from several studies have suggested that
adipocytokines and markers of oxidative stress may be promising
tools for identifying advanced stage caner, lymph node metastases
and adverse prognoses among cancer patients, including breast
cancer patients (47,48). Evaluation of these markers to predict
the efficiency of anti-cancer treatments and survival outcomes in
breast cancer patients is now becoming a subject of intense study
(49,50).
The aim of the present study was to evaluate the
concentrations of APLN, RBP4 and 8-oxo-dG, as well as the TAC
values, taking into account select clinicopathological
characteristics of breast cancer, such as tumor histological grade
and size, HER-2/neu expression, hormone receptor status, and the
presence/absence of regional lymph node metastases. The evaluations
were performed twice: before and after the second cycle of
chemotherapy administration.
Increased APLN levels were found to be a significant
and independent predictor of HER-2/neu expression. Women with a
higher APLN concentrations appeared to be more likely to develop a
positive HER-2/neu breast cancer phenotype. In general, HER-2/neu
was overexpressed in ~30% of breast carcinomas, and was associated
with aggressive tumor behavior and a poor prognosis. Interestingly,
HER-2 expression and functions have been shown to be modified by
obesity and/or lipid-related components (51). A growing number of studies indicate
the ability of adipocyte-secreted factors, namely leptin, to
activate signaling pathways involved in the upregulation of
HER-2/neu expression (52-54).
An independent association between APLN and positive HER-2/neu
status was demonstrated for the first time in the present study,
and this may suggest the involvement of APLN in the development of
this breast cancer subtype. The association between APLN and
HER-2/neu expression should thus be investigated further.
In contrast, RBP4 levels were not associated with
any clinicopathological features of breast cancer in the
pre-treatment period. Its concentration was found to be similar
amongst patients regardless of tumor size and histological grade,
HER-2/neu status, hormone receptor status and the presence/absence
of regional lymph node metastases. A limited number of studies have
investigated the association between adipocytokine levels and
clinicopathological features of breast cancer, with differing
results (55). Significant
differences in the serum concentrations of adipocytokines with
regard to histological subtype, clinical stage and metastasis
status were reported in some studies, but not others (56). Earlier observations supported by the
present study suggest that secretion patterns of adipocytokines are
specific to individuals, and are strictly dependent on the type of
cancer and its characteristic features. Certain adipocytokines may
rise or fall based on the particular type or subtype of cancer,
whereas others may stay constant. Disease progression may also
affect their levels differently. Therefore, the use of multiple
adipocytokines in a panel may be more informative and allow for
better prognostic prediction.
In the present study, no association was found
between increased breast tumor aggressiveness and invasiveness with
increased 8-oxo-dG concentrations or reduced antioxidant status.
Similar findings were reported by Himmetoglu et al (48), who found that 8-oxo-dG levels did not
change between breast cancer patients divided into different groups
based on tumor grade, tumor stage and the presence/absence of
metastases. In addition, Zowczak-Drabarczyk et al (57) demonstrated that the mean TAC levels
did not differ significantly in relation to lymph node or HER-2/neu
expression status in patients with newly diagnosed breast cancer.
These results may suggest that 8-oxo-dG and TAC values are not
useful for subtyping breast cancer and assessing its aggressiveness
or metastatic invasiveness.
It is well established that obesity significantly
increases the risk of breast cancer and is associated with
increased tumor burden, histopathological grade and a higher
incidence of lymph node metastasis (58). The mechanisms by which obesity
contributes to breast cancer are complex and have not yet been
fully elucidated. However, several reports have shown that
adipocytokines may be major contributing factors to
obesity-associated breast cancer (59). Their altered secretion patterns in
obese adipose tissues may modulate tumor cell behavior, such as
proliferation and migration (45,55). In
the present study, a significant association with adipose tissue
content was observed only for RBP4. In contrast, APLN was not
correlated with adipose tissue content nor with BMI. Previous
reports comparing the levels of adipocytokines between obese and
non-obese breast cancer patients have yielded inconsistent results
(55). Some findings indicate that
obese breast cancer patients (BMI >25 kg/m2) had
lower adiponectin and higher leptin levels compared with non-obese
patients (BMI <25 kg/m2) (60). However, concentrations of other major
adipocytokines, such as resistin and visfatin were found to not be
altered, regardless of BMI values (60). El-Benhawy et al (55) observed a higher level of vistafin in
women with breast cancer compared with the control group. However,
there was no significant difference in visfatin concentrations
between obese and non-obese subjects in the same study group of
patients (55). Moreover, visfatin
concentration were not correlated with BMI (55). The authors hypothesized that there
may be other significant sources of this adipocytokine other than
adipose tissue. Lymphocytes, neutrophils and other immune system
cells can secrete certain adipocytokines as inflammatory phase
proteins based on the inflammatory status of breast cancer.
Therefore, they are suspected to be an alternative source of
adipocytokines. The lack of any relationship between APLN and BMI
with adipose tissue content in the present study may suggest that
other factors, which are not necessarily connected with adipose
tissue, may influence its levels. Unexpectedly, there was a
significant negative association between 8-oxo-dG levels and BMI
values. Although previous cross-sectional studies have reported a
similar correlation in certain types of cancer, the exact
relationship between these two factors remains unclear (61,62).
Mizoue et al (62) postulated
that weight loss induced by increased energy expenditure that
accompanies certain types of cancer, leading to elevation of
mitochondrial ROS production, may be reflected in the rise of
8-oxo-dG levels (63). Numerous
studies have confirmed the relationship between the secretion of
adipocytokines by adipose tissue, and the stimulation or inhibition
of ROS production. Than et al (64) showed that APLN inhibits the
production and release of ROS in adipocytes by increasing the
expression of antioxidant enzymes such as catalase, superoxide
dismutase 1 and glutathione peroxidase, and also inhibits the
expression of enzymes with pro-oxidative properties. Conversely,
Wang et al (65) demonstrated
the effects of RBP4 on the stimulation of anion radical production
by mitochondria in the aortic vascular endothelium. However, in the
present study, a correlation between the concentrations of the
selected adipocytokines and indicators of oxidative stress was not
found.
Chemotherapy is the most frequently used treatment
for breast cancer patients, contributing to the reduction in
cause-specific mortality by lowering the risk of recurrence and
metastasis (66). Responses to
chemotherapy vary greatly amongst individuals, making it difficult
to accurately predict the outcomes of the treatment. Chemotherapy
affects biochemical processes and as a result, alters the levels of
various molecules, including those detectable in the blood
(67). Understanding the
relationship between changes in the concentration profiles of
multiple circulating markers following different chemotherapeutic
regimens may help predict their effectiveness.
The present study is the first to evaluate the
effects of adjuvant chemotherapy on circulating levels of APLN and
RBP4 in women with breast cancer. Previously, Słomian et al
(67) assessed the adipocytokine
levels in patients with colorectal cancer who received palliative
chemotherapy. They observed increased plasma levels of the
anti-inflammatory protein adiponectin, and decreased plasma levels
of visfatin and resistin. Coskun et al (68) performed a study in which they
enrolled patients with breast cancer who underwent tumor resection
and then received adjuvant chemotherapy and/or radiotherapy. The
authors did not observe altered serum levels of visfatin,
adiponectin or leptin in the patients. However, resistin levels
were found to be increased. In the present study, there were no
significant differences in levels of APLN and RBP4 regardless of
the clinicopathological features of the tumor during administration
of chemotherapy. The constant and unchanged concentrations of APLN
and RBP4 were observed during chemotherapy likely exclude them as
candidates for monitoring the effects of treatments in breast
cancer patients.
The mechanism of action of certain chemotherapeutic
agents have been definitively linked to the generation of free
radicals which, in-turn, induce tumor-cell apoptosis (69). However, the majority of the drugs act
in a non-specific manner, harming both malignant and normal cells
to a similar degree. This is evident by the elevation of key
markers of oxidative stress, and reduced plasma levels of
antioxidants that are observed during chemotherapy (70). Free radicals produced during
chemotherapy cause oxidative damage to important biomolecules
including DNA. One of the most frequently studied markers of
oxidative DNA damage is the production of 8-oxo-dG, when ROS reacts
with guanine bases in DNA (71).
Several studies have shown that oxidative stress levels, based on
8-oxo-dG levels in urine or blood serum, may be a useful biomarker
for determining the response to radiotherapy and chemotherapy in
cancer patients (33). In most
cases, chemotherapy accompanied by ROS overproduction results in
elevated levels of 8-oxo-dG in patients with various types of
cancer (72,73).
In contrast to previous reports, in the present
study, decreased levels of 8-oxo-dG were observed during
chemotherapy in almost all groups of breast cancer patients. One
exception was women with tumor sizes <2 cm and without lymph
node metastases. Pour Khavari et al (33) investigated the serum levels of
8-oxo-dG following chemotherapy in patients with upper
gastrointestinal tumors, and found that a decrease in its
concentration was associated with worse response to treatment and
shorter progression-free survival. Thus, it is hypothesized that
the low serum levels of 8-oxo-dG observed in the present study
during chemotherapy may reflect enhanced systemic antioxidant
defense in response to ROS production induced by chemotherapy. The
increased production of antioxidants may assist in the prevention
of ROS-induced DNA damage, leading to decreased formation of
8-oxo-dG. It has been suggested that this mechanism may also occur
in tumor cells and lead to chemoresistance by offering these cells
a growth advantage through evasion of apoptosis and necrosis caused
by ROS (74). Thus, chemoresistance
may explain why patients with the worst chemotherapy responses have
lower serum levels of 8-oxo-dG (33).
Contrary to previous reports, which demonstrated
depletion in antioxidant status caused by chemotherapy (70), in the present study, TAC in breast
cancer patients remained stable or increased slightly. Similar
results were found by Hewala and Abo Elsoud (75), who observed that chemotherapy in
breast cancer patients had no significant effect on serum TAC
values. Moreover, Subramanyam et al (76) demonstrated that chemotherapy resulted
in a significant increase in blood serum TAC values in cervical
cancer patients. These contrasting observations may be important
evidence of adaptation of the antioxidant system to enhanced
production of ROS induced by chemotherapy. A positive correlation
between TAC value and rising 8-oxo-dG levels during chemotherapy
observed in the present study further confirms this hypothesis.
The present study has several limitations. First,
the number of participants was small. Due to the size of the group,
no division into molecular subtypes of breast cancer was performed
and no correlation between them and adipocytokines and markers of
oxidative stress was investigated. An analysis of the correlation
between such small groups of patients would give unreliable and
possibly unrepresentative results. Therefore, these preliminary
observations serve as a proof-of-concept and basis for future
clinical investigations involving larger cohorts of patients. The
study did not take into account the division into histological
subtypes of breast cancer, as it may wrongly suggest that only
women with a specific histological subtype of breast cancer
participated in the present study. Factors that may influence the
concentrations of the analyzed parameters, such as menopause status
and smoking habit were not considered. Additionally, the effects of
the complete chemotherapeutic regimen was not assessed, only the
effects of the first phase on the concentration of adipocytokines
and markers of oxidative stress were investigated. To confirm the
clinical usefulness of 8-oxo-dG in women with breast cancer, a
prospective cohort study is required to determine 8-oxo-dG levels
after completion of chemotherapy to investigate its association
with outcomes.
In conclusion, the present preliminary study
demonstrated that high APLN levels were independently associated
with HER/neu expression, and may therefore be useful in subtyping
this aggressive type of breast cancer. Moreover, 8-oxo-dG, which
decreased during chemotherapy, may act as a serum marker for
monitoring treatment effects. However, further studies are required
to validate the clinical potential of these parameters. This
includes confirming these results in a larger study group and
considering important aspects such as other health status measures
not included in the present study, as well as duration of
chemotherapy, including after completion of the full regimen vs.
analysis at different timepoints during the course of
treatment.
Acknowledgements
Not applicable.
Funding
This work was supported by Poznan University of
Medical Sciences (grant no. 502-01-22283-700-03102).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JG, JJB, MB, BG, MPK, WK, EL and MI conceived the
study. JG, JJB, EL and WK collected the data. JG, MB, BG, and MPK
analyzed the data. JG, JJB, EL and MI performed the experiments. JG
and MB wrote the manuscript. MI reviewed and edited the manuscript.
All authors read and approved the final manuscript. JG and MB
confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
This study was performed in line with the principles
of the Declaration of Helsinki. Approval was granted by the Ethics
Committee of Poznan University of Medical Sciences (approval no.
1016/16).
Patient consent for publication
Informed consent was obtained from all individual
participants included in the study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gershuni VM, Ahima RS and Tchou J: Obesity
and breast cancer: A complex relationship. Curr Surg Rep.
4(14)2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Rigby AJ, Ray M and Basu TK: Biochemical
status of vitamin A in patients with malignant and benign breast
disease. J Clin Biochem Nutr. 13:53–61. 1992.
|
|
3
|
De Pergola G and Silvestris F: Obesity as
a major risk factor for cancer. J Obes. 2013(291546)2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wu Q, Li B, Li Z, Li J and Sun S and Sun
S: Cancer-associated adipocytes: Key players in breast cancer
progression. J Hematol Oncol. 12(95)2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Chu DT, Phuong TNT, Tien NLB, Tran DK,
Nguyen TT, Thanh VV, Quang TL, Minh LB, Pham VH, Ngoc VTN, et al:
The effects of adipocytes on the regulation of breast cancer in the
tumor microenvironment: An update. Cells. 8(857)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Li J and Han X: Adipocytokines and breast
cancer. Curr Probl Cancer. 42:208–214. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Hou WK, Xu YX, Yu T, Zhang L, Zhang WW, Fu
CL, Sun Y, Wu Q and Chen L: Adipocytokines and breast cancer risk.
Chin Med J (Engl). 120:1592–1596. 2007.PubMed/NCBI
|
|
8
|
Christodoulatos GS, Spyrou N, Kadillari J,
Psallida S and Dalamaga M: The role of in breast cancer: Current
evidence and perspectives. Curr Obes Rep. 8:413–433.
2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Cabia B, Andrade S, Carreira MC, Casanueva
FF and Crujeiras AB: A role for novel adipose tissue-secreted
factors in obesity-related carcinogenesis. Obes Rev. 17:361–376.
2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Luo Y, Yang C, Ye M, Jin C, Abbruzzese JL,
Lee MH, Yeung SC and McKeehan WL: Deficiency of metabolic regulator
FGFR4 delays breast cancer progression through systemic and
microenvironmental metabolic alterations. Cancer Metab.
1(21)2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Jiao C, Cui L, Ma A, Li N and Si H:
Elevated serum levels of retinol-binding protein 4 are associated
with breast cancer risk: A case-control study. PLoS One.
12(e0167498)2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Salman T, Demir L, Varol U, Akyol M,
Oflazoglu U, Yildiz Y, Taskaynatan H, Cengiz H, Guvendi G,
Kucukzeybek Y, et al: Serum apelin levels and body composition
changes in breast cancer patients treated with an aromatase
inhibitor. J Buon. 21:1419–1424. 2016.PubMed/NCBI
|
|
13
|
Uribesalgo I, Hoffmann D, Zhang Y,
Kavirayani A, Lazovic J, Berta J, Novatchkova M, Pai TP, Wimmer RA,
László V, et al: Apelin inhibition prevents resistance and
metastasis associated with anti-angiogenic therapy. EMBO Mol Med.
11(e9266)2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Tatemoto K, Hosoya M, Habata Y, Fujii R,
Kakegawa T, Zou MX, Kawamata Y, Fukusumi S, Hinuma S, Kitada C, et
al: Isolation and characterization of a novel endogenous peptide
ligand for the human APJ receptor. Biochem Biophys Res Commun.
251:471–476. 1998.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kleinz MJ and Davenport AP: Emerging roles
of apelin in biology and medicine. Pharmacol Ther. 107:198–211.
2005.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Mughal A and O'Rourke ST: Vasculareffects
of apelin: Mechanisms and therapeutic potential. Pharmacol Ther.
190:139–147. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Muto J, Shirabe K, Yoshizumi T, Ikegami T,
Aishima S, Ishigami K, Yonemitsu Y, Ikeda T, Soejima Y and Maehara
Y: The apelin-APJ system induces tumor arteriogenesis in
hepatocellular carcinoma. Anticancer Res. 34:5313–5320.
2014.PubMed/NCBI
|
|
18
|
Berta J, Hoda MA, Laszlo V, Rozsas A,
Garay T, Torok S, Grusch M, Berger W, Paku S, Renyi-Vamos F, et al:
Apelin promotes lymphangiogenesis and lymph node metastasis.
Oncotarget. 12:4426–4437. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yang Y, Lv SY, Ye W and Zhang L:
Apelin/APJ system and cancer. Clin Chim Acta. 457:112–116.
2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lacquaniti A, Altavilla G, Picone A,
Donato V, Chirico V, Mondello Aloisi C, Marabello G, Loddo S, Buemi
A, et al: Apelin beyond kidney failure and hyponatremia: A useful
biomarker for cancer disease progression evaluation. Clin Exp Med.
15:97–105. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Feng M, Yao G, Yu H, Qing Y and Wang K:
Tumor apelin, not serum apelin, is associated with the clinical
features and prognosis of gastric cancer. BMC Cancer.
16(794)2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kotnik P, Fischer-Posovszky P and Wabitsch
M: RBP4: A controversial adipokine. Eur J Endocrinol. 165:703–711.
2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Graham TE, Yang Q, Bluher M, Hammarstedt
A, Ciaraldi TP, Henry RR, Wason CJ, Oberbach A, Jansson PA, Smith U
and Kahn BB: Retinol-binding protein 4 and insulin resistance in
lean, obese, and diabetic subjects. N Engl J Med. 354:2552–2563.
2006.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Fei W, Chen L, Chen J, Shi Q, Zhang L, Liu
S, Li L, Zheng L and Hu X: RBP4 and THBS2 are serum biomarkers for
diagnosis of colorectal cancer. Oncotarget. 54:92254–92264.
2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wang Y, Wong Y and Zhang Z: Adipokine RBP4
drives ovarian cancer cell migration. J Ovarian Res.
11(29)2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Uzan J, Laas E, Alsamad IA, Skalli D,
Mansouri D, Haddad B and Touboul C: Supervised clustering of
adipokines and hormonal receptors predict prognosis in a population
of obese women with type 1 endometrial cancer. Int J Mol Sci.
18(1055)2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kruk J and Aboul-Enein HY: Reactive oxygen
and nitrogen species in carcinogenesis: Implications of oxidative
stress on the progression and development of several cancer types.
Mini Rev Med Chem. 17:904–919. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Waris G and Ahsan H: Reactive oxygen
species: Role in the development of cancer and various chronic
conditions. J Carcinog. 5(14)2006.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ivanova D, Zhelev Z, Aoki I, Bakalova R
and Higashi T: Overproduction of reactive oxygen species-obligatory
or not for induction of apoptosis by anticancer drugs. Chin J
Cancer Res. 28:383–396. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Berstein LM, Poroshina TE, Kovalenko IM
and Vasilyev DA: Serum levels of 8-hydroxy-2'-deoxyguanosine DNA in
patients with breast cancer and endometrial cancer with and without
diabetes mellitus. Bull Exp Biol Med. 161:547–549. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Opanuraks J, Boonla C, Saelim C,
Kittikiwit W, Sumpatanukul P, Honglertsakul C and Tosukhowong P:
Elevated urinary total sialic acid and increased oxidative stress
in patients with bladder cancer. Asian Biomed. 4:703–710. 2010.
|
|
32
|
Roszkowski K, Jozwicki W, Blaszczyk P,
Mucha-Malecka A and Siomek A: Oxidative damage DNA: 8-oxoGua and
8-oxodG as molecular markers of cancer. Med Sci Monit.
17:CR329–CR333. 2011.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Pour Khavari A, Liu Y, He E, Skog S and
Haghdoost S: Serum 8-Oxo-dG as a predictor of sensitivity and
outcome of radiotherapy and chemotherapy of upper gastrointestinal
tumors. Oxid Med Cell Longev. 2018(4153574)2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Di Meo S, Reed TT, Venditti P and Victor
VM: Role of ROS and RNS sources in physiological and pathological
conditions. Oxid Med Cell Longev. 2016(1245049)2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Zowczak-Drabarczyk MM, Murawa D, Kaczmarek
L, Połom K and Litwiniuk M: Total antioxidant status in plasma of
breast cancer patients in relations to ERβ expression. Contemp
Oncol (Pozn). 17:499–503. 2013.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Dludla PV, Nkambule BB, Jack B, Mkandla Z,
Mutize T, Silvestri S, Orlando P, Tiano L, Louw J and
Mazibuko-Mbeje SE: Inflammation and oxidative stress in an obese
state and the protective effects of gallic acid. Nutrients.
11(23)2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Ren Y, Li Y, Yan J, Ma M, Zhou D, Xue Z,
Zhang Z, Liu H, Yang H, Jia L, et al: Adiponectin modulates
oxidative stress-induced mitophagy and protects C2C12 myoblasts
against apoptosis. Sci Rep. 7(3209)2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Frühbeck G, Catalán V, Rodríguez A,
Ramírez B, Becerril S, Salvador J, Portincasa P, Colina I and
Gómez-Ambrosi J: Involvement of the leptin-adiponectin axis in
inflammation and oxidative stress in the metabolic syndrome. Sci
Rep. 7(6619)2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Jakovcevic D, Dedic-Plavetic N, Vrbanec D,
Jakovcevic A and Jakic-Razumovic J: Breast cancer molecular
subtypes and oxidative DNA damage. Appl Immunohistochem Mol
Morphol. 23:696–703. 2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Li JC, Yi F, Diao S and Li JY: Association
between plasma adiponectin and risk of breast cancer by molecular
subtypes. Sichuan Da Xue Xue Bao Yi Xue Ban. 50:708–713.
2019.PubMed/NCBI(In Chinese).
|
|
41
|
Januškevičienė I and Petrikaitė V:
Heterogeneity of breast cancer: The importance of interaction
between different tumor cell populations. Life Sci.
239(117009)2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
World Medical Association. World Medical
Association Declaration of Helsinki: Ethical Principles for Medical
Research Involving Human Subjects. JAMA. 310:2191–2194.
2013.PubMed/NCBI View Article : Google Scholar
|
|
43
|
American Joint Committee on Cancer. AJCC
Cancer Staging Manual. 7th edition. Springer, New York, NY,
2010.
|
|
44
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Divella R, De Luca R, Abbate I, Naglieri E
and Daniele A: Obesity and cancer: The role of adipose tissue and
adipocytokines-induced chronic inflammation. J Cancer.
15:2346–2359. 2016.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Klaunig JE and Kamendulis LM: The role of
oxidative stress in carcinogenesis. Annu Rev Pharmacol Toxicol.
44:239–267. 2004.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Han C, Zhang HT, Du L, Liu X, Jing J, Zhao
X, Yang X and Tian B: Serum levels of leptin, insulin, and lipids
in relation to breast cancer in China. Endocrine. 26:19–24.
2005.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Himmetoglu S, Dincer Y, Ersoy YE,
Bayraktar B, Celik V and Akcay T: DNA oxidation and antioxidant
status in breast cancer. J Investig Med. 57:720–723.
2009.PubMed/NCBI View Article : Google Scholar
|
|
49
|
De Rossi T, Panis C, Victorino VJ, Freitas
LF, Herrera1 ACSA, Cecchini AL and Cecchini R: Breast cancer and
oxidative stress in chemotherapy. Appl Cancer Res. 29:150–156.
2009.
|
|
50
|
Vera-Ramirez L, Sanchez-Rovira P,
Ramirez-Tortosa MC, Ramirez-Tortosa CL, Granados-Principal S,
Lorente JA and Quiles JL: Oxidative stress status in metastatic
breast cancer patients receiving palliative chemotherapy and its
impact on survival rates. Free Radic Res. 46:2–10. 2012.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Ray A: Tumor-linked HER2 expression:
Association with obesity and lipid-related microenvironment. Horm
Mol Biol Clin Investig: 32, 2017 doi: 10.1515/hmbci-2017-0020.
|
|
52
|
Giordano C, Vizza D, Panza S, Barone I,
Bonofiglio D, Lanzino M, Sisci D, De Amicis F, Fuqua SA, Catalano S
and Andò S: Leptin increases HER2 protein levels through a
STAT3-mediated up-regulation of Hsp90 in breast cancer cells. Mol
Oncol. 7:379–391. 2013.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Soma D, Kitayama J, Yamashita H, Miyato H,
Ishikawa M and Nagawa H: Leptin augments proliferation of breast
cancer cells via transactivation of HER2. J Surg Res. 149:9–14.
2008.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Fiorio E, Mercanti A, Terrasi M, Micciolo
R, Remo A, Auriemma A, Molino A, Parolin V, Di Stefano B, Bonetti
F, et al: Leptin/HER2 crosstalk in breast cancer: In vitro study
and preliminary in vivoanalysis. BMC Cancer. 8(305)2008.PubMed/NCBI View Article : Google Scholar
|
|
55
|
El-Benhawy SA, Abd El Moneim NA and Ebeid
SA: Serum adipocytokines (Visfatin and Resistin): New biomarkers of
breast carcinogenesis. Middle East J Cancer. 6:253–265. 2015.
|
|
56
|
Chen DC, Chung YF, Yeh YT, Chaung HC, Kuo
FC, Fu OY, Chen HY, Hou MF and Yuan SSF: Serum adiponectin and
leptin levels in Taiwanese breast cancer patients. Cancer Lett.
237:109–114. 2006.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Zowczak-Drabarczyk M, Murawa D, Połom K,
Szarszewska M, Nowakowski W and Mańczak M: Plasma total antioxidant
status in breast cancer women in relation to lymph node involvement
and HER-2/neu expression. Rep Pract Oncol Radiother. 12:319–322.
2007.
|
|
58
|
Picon-Ruiz M, Morata-Tarifa C,
Valle-Goffin JJ, Friedman ER and Slingerland JM: Obesity and
adverse breast cancer risk and outcome: Mechanistic insights and
strategies for intervention. CA Cancer J Clin. 6:378–397.
2017.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Lee CH, Woo YC, Wang Y, Yeung CY, Xu A and
Lam KSL: Obesity, adipokines and cancer: An update. Clin
Endocrinol. 83:147–156. 2015.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Gui Y, Pan Q, Chen X, Xu S, Luo X and Chen
L: The association between obesity related adipokines and risk of
breast cancer: A meta-analysis. Oncotarget. 8:75389–75399.
2017.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Loft S, Vistisen K, Ewertz M, Tjønneland
A, Overvad K and Poulsen HE: Oxidative DNA damage estimated by
8-hydroxydeoxyguanosine Excretion in humans: Influence of smoking,
gender and body mass index. Carcinogenesis. 13:2241–2247.
1992.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Mizoue T, Kasai H, Kubo T and Tokunaga S:
Leanness, smoking, and enhanced oxidative DNA damage. Cancer
Epidemiol Biomarkers Prev. 15:582–585. 2006.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Mizoue T, Tokunaga S, Kasai H, Kawai K,
Sato M and Kubo T: Body mass index and oxidative DNA damage: A
longitudinal study. Cancer Sci. 98:1254–1258. 2007.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Than A, Zhang X, Leow MK, Poh CL, Chong SK
and Chen P: Apelin attenuates oxidative stress in human adipocytes.
J Biol Chem. 289:3763–3774. 2014.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Wang J, Chen H, Liu Y, Zhou W, Sun R and
Xia M: Retinol binding protein 4 induces mitochondrial dysfunction
and vascular oxidative damage. Atherosclerosis. 240:335–344.
2015.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Nies YH, Ali AM, Abdullah N, Islahudin F
and Shah NM: A qualitative study among breast cancer patients on
chemotherapy: Experiences and side-effects. Patient Prefer
Adherence. 12:1955–1964. 2018.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Słomian G, Świętochowska E, Nowak G,
Pawlas K, Żelazko A and Nowak P: Chemotherapy and plasma adipokines
level in patients with colorectal cancer. Postepy Hig Med Dosw.
71:281–290. 2017.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Coskun T, Kosova F, Ari Z, Sakarya A and
Kaya Y: Effect of oncological treatment on serum adipocytokine
levels in patients with stage II-III breast cancer. Mol Clin Oncol.
4:893–897. 2016.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Conklin KA: Chemotherapy-associated
oxidative stress: Impact on chemotherapeutic effectiveness. Integr
Cancer Ther. 3(294)2014.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Abdel-Salam OME, Youness ER and Hafez HF:
The antioxidant status of plasma in patients with breast cancer
undergoing chemotherapy. Open J Mol Integr Physiol. 1:29–35.
2011.
|
|
71
|
Chernikov AV, Gudkov SV, Usacheva AM and
Bruskov VI: Exogenous 8-oxo-7,8-dihydro-2'-deoxyguanosine:
Biomedical properties, mechanisms of action, and therapeutic
potential. Biochemistry (Mosc). 82:1686–1701. 2017.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Roszkowski K, Filipiak J, Wisniewska M,
Mucha-Malecka A and Basta P: Potential survival markers in cancer
patients undergoing chemotherapy. Clin Exp Med. 15:381–387.
2015.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Crohns M, Saarelainen S, Erhola M, Alho H
and Kellokumpu-Lehtinen P: Impact of radiotherapy and chemotherapy
on biomarkers of oxidative DNA damage in lung cancer patients. Clin
Biochem. 42:1082–1090. 2009.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Sova H, Jukkola-Vuorinen A, Puistola U,
Kauppila S and Karihtala P: 8-hydroxydeoxyguanosine: A new
potential independent prognostic factor in breast cancer. Br J
Cancer. 102:1018–1023. 2010.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Hewala TI and Abo Elsoud MR: The clinical
significance of serum oxidative stress biomarkers in breast cancer
females. Med Res J. 4:1–7. 2019.
|
|
76
|
Subramanyam D, Subbaiah KCV, Rajendra W
and Lokanatha V: Serum selenium concentration and antioxidant
activity in cervical cancer patients before and after treatment.
Exp Oncol. 35:97–100. 2013.PubMed/NCBI
|