Introduction
Rheumatoid arthritis (RA) is an autoimmune disease
characterized by chronic inflammation of the synovial lining of the
joint (1), with progressive joint
destruction and systemic complications being commonly observed
(2). The prevalence rate of RA has
been estimated to range between 0.5 and 1.0% (3). As an autoimmune disease, 70-80% of the
patients with RA possess auto-antibodies, such as rheumatoid factor
or anti-citrullinated protein antibodies (ACPA) (4). Early aggressive disease-modifying
antirheumatic drug therapy can improve the clinical outcomes of
patients with RA, including a decrease in long-term radiographic
progression, and anti-TNF therapy can alter the natural progression
of RA (5). Therefore, identifying
patients at high risk of severe RA is important, such that more
suitable treatments can be offered earlier on, thereby improving
their disease prognosis (6).
Genetic polymorphisms may serve as useful markers of
RA disease prognosis (7,8). To date, polygenic risk scores have
largely been used to predict RA disease progression in humans
(7), although other genomic
predictive approaches using animal models or statistical methods
have also been described (9,10). However, the International HapMap
Project, which defines variations in the map of the human genome
(11), has allowed genome-wide
association screening (GWAS) of genetic variants, including single
nucleotide polymorphisms (SNPs) (12). As an example, through GWAS,
PADI4 was identified as a non-major-histocompatibility
complex genomic locus predictive of RA in a Japanese population
(13). GWAS meta-analyses provided
further evidence of a shared genetic background amongst patients
with RA across different populations (14,15). A
large-scale GWAS meta-analysis in a Japanese population of patients
with RA identified the following new RA risk loci: B3GNT2,
ANXA3, CSF2, CD83, NFKBIE, ARID5B, PDE2A-i PLD4 and
PTPN2 (16).
Clinically, radiological damage provides an
objective measure of RA severity; with joint destruction quantified
using radiographic scores, such as the Sharp/van der Heijde score
(SHS), which reflects the inflammatory status of the joint
(17). Only a few studies to date
have evaluated the association between radiographic joint
destruction and genetic analysis. Rodriguez-Rodriguez et al
(18) reported a probable
association between the prostaglandin E receptor 4 variant,
rs76523431 and radiographic joint destruction in Caucasian patients
with RA. Suzuki et al (19)
reported an association between the PADI4 risk allele and
radiographic joint destruction amongst Japanese patients with RA.
However, GWAS has not previously been used to identify risk factors
of radiographic joint destruction. Therefore, the purpose of the
present study was to identify genomic factors predictive of
susceptibility to joint destruction in patients with RA by
performing a GWAS of genetic variants, including SNPs.
Patients and methods
Ethics statement and patient
consent
The methods used in the present study were approved
by the Research Institute of Joint Disease Kobe on June 11, 2008.
All patients included in the study satisfied the American College
of Rheumatology 1987 revised criteria for RA (20) and completed the genetic analysis in
the Patient Registry. A total of 240 patients were recruited, all
of whom provided written informed consent in Matsubara Mayflower
Hospital between October 2008 and December 2012. Additionally,
another group of 228 patients (median age, 55 years old; age range,
23-84 years; 49 males and 179 females) were recruited between
January 2013 and September 2017. Data collection, including blood
examination and x-rays of the 240 patients (median age 60 years
old; age range 21-83 years; 45 males and 195 females), was
performed in the Matsubara Mayflower Hospital between January 2009
and December 2012. In the second group of 228 patients, data
collection was performed between January 2013 and December 2017.
All DNA samples were included in the Japanese GWAS analysis.
Radiographic evaluation
Radiographs of the hands and feet were scored
according to the Sharp method (17).
All radiographic data included in the analysis were obtained within
5 years of RA diagnosis, and clinical data and blood samples were
collected on the same day as the diagnosis. In total, 228
radiographs (228 patients) were scored by a single experienced
examiner who was blinded with respect to the clinical and genetic
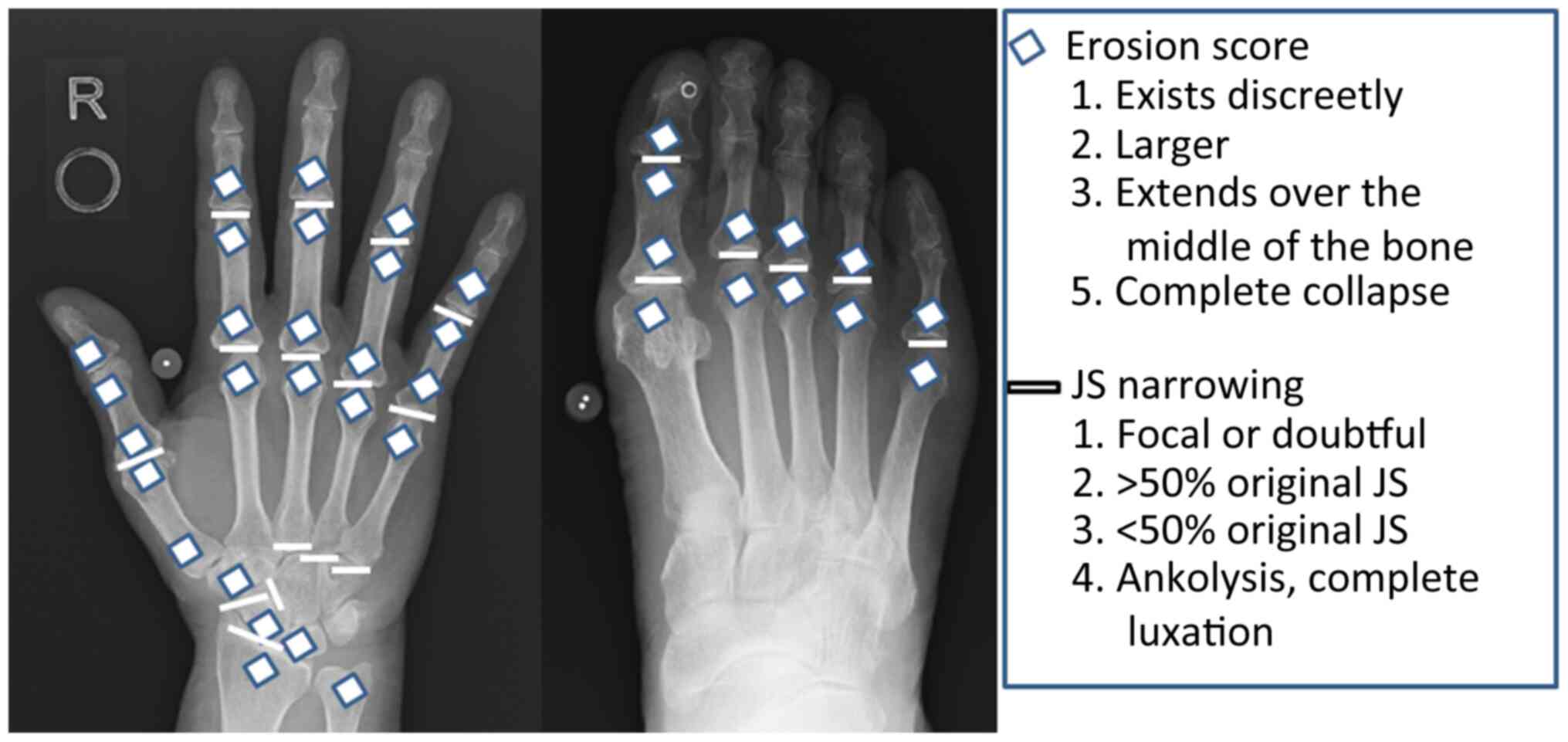
data. A total of 16 and 6 areas were considered for assessing
erosions and joint space narrowing (JSN) for the hands and feet,
respectively (Fig. 1). Erosions were
scored as follows: 1, discrete but clearly present; 2 or 3, larger,
dependent on the surface area of the joint involved. A score of 3
was given if the erosion was large and extended over the imaginary
middle of the bone. A score of 5 was given if a complete collapse
of the joint was observed or if the entirety of the joint was
affected. In each joint, individual erosions were totaled, up to a
maximum of 5 (Fig. 1). For JSN, a
normal joint space was scored 0. A score of 2 indicated focal
narrowing of the joint. A score of 1 was not used when the reviewer
was unsure whether there was joint space narrowing. A generalized
narrowing leaving >50% of the original joint space present was
scored as 2. A generalized narrowing leaving <50% of the
original joint space present was scored as 3. A subluxation of the
joint was also scored as 3. A bony ankylosis or a complete luxation
of the joint was scored as 4 (Fig.
1). The maximum erosion score of the hands and wrists was 160
and that of the feet was 120 (maximum total erosion score, 280).
Accordingly, the maximum JSN score of the hands and wrists was 120
and that of the feet was 48 (maximum total JSN score, 168). The sum
of the erosion and JSN scores was the total SHS (maximum, 448).
Radiographic joint destruction was quantified as the total SHS
score divided by the duration of RA.
To avoid bias in the RA duration, radiographic joint
destruction was quantified as the total SHS score divided by the
duration of RA. A previous study defined the remission of joint
destruction as changes from baseline of <0.5 points per year and
rapid joint destruction as changes from baseline to >5 points
per year (21). However, the SHS
score change from baseline because was not determined as
radiographical data was collected at the time of blood sample
collection. The ATTRACT trial showed that an SHS of >90 points
indicated a high degree of joint destruction within 3 years of RA
diagnosis (22). Furthermore, the
patient populations in the present study with high disease activity
based on DAS 28 (C-reactive protein) >5.1 (15.6%) and
populations with over 50 points SHS/year (13.2%) were similar to
that observed in the ATTRACT trial. Therefore, >50 points of
SHS/year was defined as rapid joint destruction. Patients were
assigned to either the rapid joint destruction group (defined as
total SHS/year ≥50) or the slow joint destruction group (defined by
a total SHS/year <50).
Genome-wide SNP analysis and
association study
Samples of 7 ml venous blood were drawn into glass
tubes and stored at 4˚C until required for DNA extraction at
Mitsubishi BCL, Inc. DNA samples were prepared using a Gentra
Puregene DNA isolation kit according to the manufacturer's protocol
(cat. no. 158489; Qiagen, GmbH). DNA integrity was assessed using a
Nanodrop 2000 spectrophotometer (Thermo Fisher Scientific, Inc.) to
determinate the OD260/280 ratio (acceptable range, 1.82-1.92) and
the concentration of the total DNA (acceptable range, 8-28 µg/ml),
and all the samples were deemed to be of sufficient quality
(2.0>OD260/280>1.8; DNA concentration >5 µg/ml).
Furthermore, the integrity of the DNA was assessed by loading ~100
ng per sample on a 0.75% agarose gel and comparing separation of
the full length DNA band.
Genome-wide SNP genotyping was performed by deCode
Genetics Inc. using the Illumina HumanHap300K chip technology
(Illumina, Inc.). The genotyping of 317,503 SNPs was performed,
including a quality control analysis. SNP genotyping was performed
using an Infinium OmniExpressExome-8 Bead Chip kit (cat. no.
20024676; Illumina, Inc.) according to the manufacturer's protocol.
SNP genotyping calling and quality control for samples and SNPs
were performed using Illumina GenomeStudio version 2011 (Illumina,
Inc.) and a cluster file. Genotypes were scored using GenomeStudio
using a GenCall threshold of 0.15. Samples were accepted when their
call rates were >98%. SNPs were excluded if they: i) Had an R
mean value in at least 1 of 3 clusters <0.25; ii) Cluster Sep
values <0.4; or iii) the number of no calls value was >2 on
all chromosomes except for the Y chromosome.
After exclusion based on these criteria, 278,347
SNPs were retained in the case-control analysis.
Statistical analysis
Demographic and clinicopathological characteristics
of patients assigned to the rapid and slow joint destruction groups
were compared using a one-way ANOVA for continuous variables (such
as age and RA duration). Bonferroni's correction was used for
multiple comparisons. A contingency table for categorical data
(Steinbrocker stage and class) was analyzed using a χ2
test. The frequency of minor allele homo combinations of SNPs were
compared between the rapid and slow joint destruction groups for
277,339 SNPs using a Fisher's exact test in Plink (23). The frequency of the minor allele homo
combinations between the rapid and slow joint destruction groups
for another 240 samples using the genotyping data of 178,748 SNPs
to confirm the results obtained for the 228 samples using
genotyping data of 317,503 SNPs. P<0.05 was considered to
indicate a statistically significant difference.
Results
Patient characteristics
Among the 228 patients enrolled in the study, 30
patients were included in the rapid joint destruction group (group
A), and 198 patients were classified into the slow joint
destruction group (group B). There was no significant difference in
the distribution of sex, age, titer of IgM RF, titer of ACPA and
DAS28 (C-reactive protein) between groups A and B (Table I). The RA duration, number of
patients classed as Steinbrocker 3, and total SHS score/year (TSS)
were significantly higher in group A compared with group B. Amongst
the other 240 samples enrolled in the study, 32 patients were
included in the rapid joint destruction group (group C), and 204
patients were classified into the slow joint destruction group
(group D). The RA duration, number of patients classed as
Steinbrocker 2, 3 or 4, as well as TSS were significantly higher in
group C compared with group D (Table
II). The patient's characteristics were similar between the two
sets of patients.
 | Table IClinicopathological
characteristics. |
Table I
Clinicopathological
characteristics.
| Factor | TSS ≥50, n=30 | TSS <50,
n=198 | P-value |
|---|
| Sex, N (%) | | | |
|
Male | 4(13) | 45 (22.8) | |
|
Female | 26(87) | 153 (77.2) | 0.243 |
| Age, years | 58.1±12.5 | 54.1±13.0 | 0.227 |
| RA duration
(years) | 1.4±1.1 | 3.6±1.2 |
<0.001c |
| IgM RF titer,
IU/ml | 62.6±65.8 | 63.1±60.9 | 0.992 |
| ACPA titer,
IU/ml | 81.3±66.0 | 83.9±69.2 | 0.982 |
| Stage, N (%) | | | |
|
1 | 2 (6.7) | 35 (17.7) | 0.127 |
|
2 | 6 (20.0) | 72 (36.4) | 0.078 |
|
3 | 12 (40.0) | 56 (28.3) | 0.196 |
|
4 | 10 (33.3) | 35 (17.7) |
0.045a |
| Class (%) | | | |
|
1 | 4 (13.3) | 61 (16.7) | 0.143 |
|
2 | 17 (56.7) | 115 (58.0) | 0.884 |
|
3 | 9 (4.3) | 22 (10.1) |
0.005b |
|
4 | 0 (0.0) | 0 (0.0) | 1 |
| DAS 28, C-reactive
protein | 4.1±1.9 | 4.6±1.6 | 0.298 |
| Total sharp
score/year | 101.3±69.6 | 20.3±18.3 |
<0.001c |
 | Table IIPatients characteristics of the
second cohort of 240 samples. |
Table II
Patients characteristics of the
second cohort of 240 samples.
| Factor | TSS ≥50, n=32 | TSS <50,
n=208 | P-value |
|---|
| Sex, N (%) | | | |
|
Male | 7 (21.9) | 38 (18.3) | |
|
Female | 25 (78.1) | 170 (81.7) | 0.306 |
| Age, years | 57.5±16.0 | 61.0±12.4 | 0.316 |
| RA duration
(years) | 1.4±1.1 | 2.2±1.6 |
0.014a |
| IgM RF titer,
IU/ml | 60.1±70.1 | 68.3±64.2 | 0.919 |
| ACPA titer,
IU/ml | 86.1±60.9 | 79.1±61.8 | 0.816 |
| Stage, N (%) | | | |
|
1 | 2 (6.2) | 36, 17.3% | 0.111 |
|
2 | 4 (12.5) | 80, 38.5% |
0.004b |
|
3 | 15 (46.9) | 61, 29.3% |
0.047a |
|
4 | 11 (34.4) | 31, 14.9% |
0.007b |
| Class (%) | | | |
|
1 | 5 (15.6) | 22 (10.6) | 0.143 |
|
2 | 20 (62.5) | 149 (71.6) | 0.15 |
|
3 | 5 (15.6) | 37 (17.8) | 0.982 |
|
4 | 0 (0.0) | 0 (0.0) | 1 |
| DAS 28, C-reactive
protein | 4.1±1.9 | 4.7±1.6 | 0.251 |
| Total sharp
score/year | 115.6±59.1 | 19.4±11.4 |
<0.001c |
Analysis of genetic association with
joint destruction
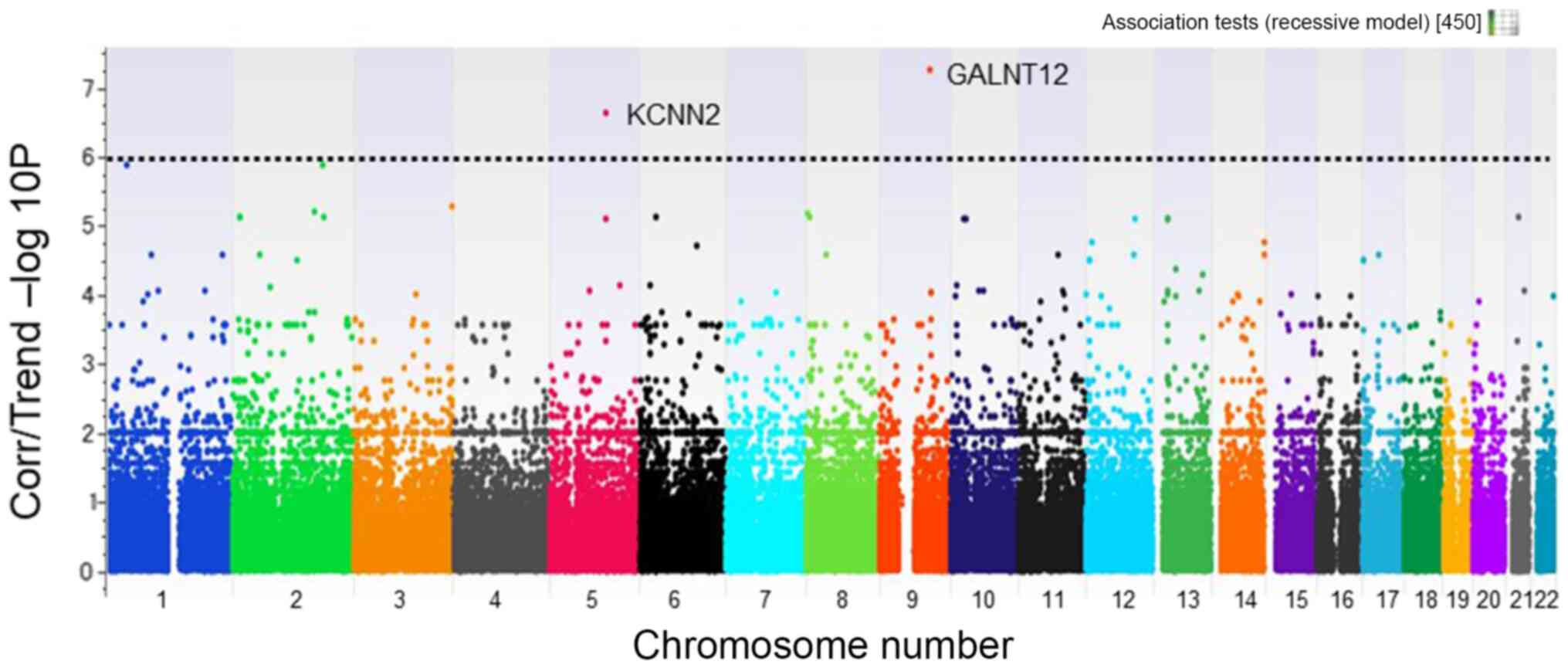
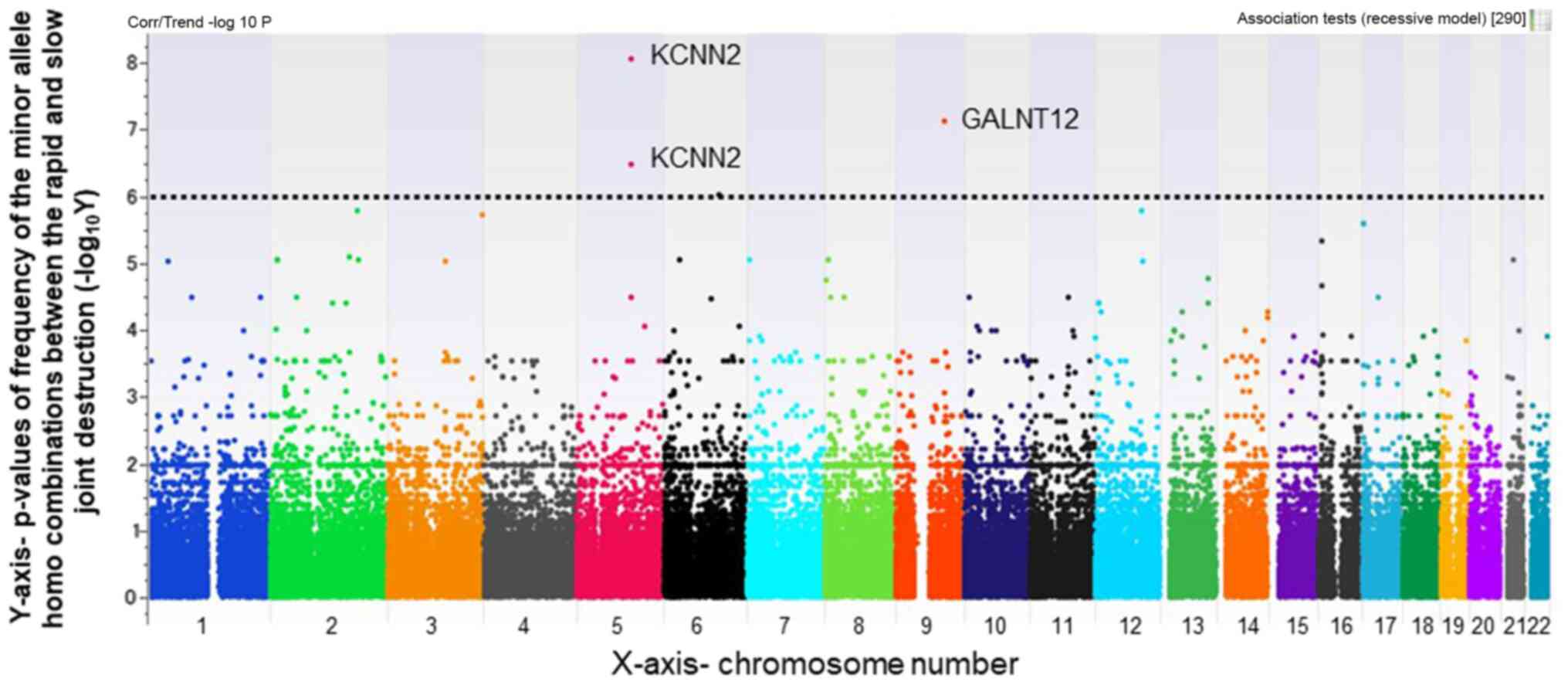
Several SNPs were identified that were strongly
associated with rapid radiographic joint destruction (Figs. 2 and 3). Using a Manhattan plot. SNPs that were
significantly different between the rapid and slow joint
destruction groups were identified; all of which had a different
chromosomal locus (Figs. 2 and
3). A focus was placed on SNPs with
smaller P-values <1x10-6. The selected SNPs, shown in
Table III, were as follows: one
minor allele homo SNP with a P-value difference between groups A
and B of 1x10-7.24 (rs2295926 located on chromosome 9;
gene symbol name, GALNT12); gene location, intron), and
another minor allele homo SNP with a P-value difference of
1x10-6.64 (rs11958855 located on chromosome 5; gene
symbol name, KCNN2; gene location, intron). The same minor
allele homo SNPs rs2295926 (GALNT12) and two SNPs of
rs11958855 and rs36963 in KCNN2 gene were detected between
the groups C and D (rs2295926; P=1x10-7.11, rs11958855;
P=1x10-8.05, rs36963; P=1x10-6.46) in the
genotyping results of 178,748 SNPs (Table IV).
 | Table IIISingle nucleotide polymorphisms
associated with rapid joint destruction in the first cohort of 228
patients. |
Table III
Single nucleotide polymorphisms
associated with rapid joint destruction in the first cohort of 228
patients.
|
-Log10(P-value) | Name | Chromosome | Gene symbol | Gene
description | Gene location |
|---|
| 7.24 | rs2295926 | 9 | GALNT12 | Polypeptide
N-acetylgalactosaminyltransferase 12 | Intron |
| 6.64 | rs11958855 | 5 | KCNN2 | Potassium
calcium-activated channel subfamily N member 2 | Intron |
| 5.88 | rs4266846 | 1 | LOC105378657 | Uncharacterized
LOC105378657 | Non-coding RNA |
| 5.88 | rs13029379 | 2 | SCHLAP1 | SWI/SNF complex
antagonist associated with prostate cancer 1 | Intron |
| 5.28 | rs7629215 | 3 | LOC105374287 | Uncharacterized
LOC105374287 | Non-coding RNA |
| 5.19 | rs355808 | 2 | COBLL1 | Cordon-blue WH2
repeat protein like 1 | Intron |
| 5.18 | rs7357519 | 8 | CSMD1 | CUB and Sushi
multiple domains 1 | Intron |
| 5.11 | rs4669995 | 2 | TRIB2/FAM84A | Tribbles homolog 2
(Drosophila)/ family with sequence similarity 84, member A | Intergenic |
| 5.11 | rs10181834 | 2 | TRIB2/FAM84A | Tribbles homolog 2
(Drosophila)/ family with sequence similarity 84, member A | Intergenic |
| 5.11 | rs833126 | 2 | PDE1A | Phosphodiesterase
1A | Intron |
 | Table IVSingle nucleotide polymorphisms
associated with rapid joint destruction in the second cohort of 240
patients. |
Table IV
Single nucleotide polymorphisms
associated with rapid joint destruction in the second cohort of 240
patients.
|
-Log10(P-value) | Name | Chromosome | Gene symbol | Gene
description | Gene location |
|---|
| 8.05 | rs11958855 | 5 | KCNN2 | Potassium
calcium-activated channel subfamily N member 2 | Intron |
| 7.11 | rs2295926 | 9 | GALNT12 | Polypeptide
N-acetylgalactosaminyltransferase 12 | Intron |
| 6.46 | rs36963 | 5 | KCNN2 | Potassium
calcium-activated channel subfamily N member 2 | Intron |
| 6.02 | rs1539403 | 6 | LOC100128588/
RFPL4B | Uncharacterized
LOC100128588/ PIN2/TERF1 interacting, telomerase inhibitor 1
pseudogene/ret finger protein-like 4B | Intergenic |
| 5.77 | rs13029379 | 2 | SCHLAP1 | SWI/SNF complex
antagonist associated with prostate cancer 1 | Intron |
| 5.77 | rs1582341 | 12 | NEDD1/RMST | Neural precursor
cell expressed, developmentally down-regulated 1/ rhabdomyosarcoma
2 associated transcript (non-protein coding) | Intergenic |
| 5.71 | rs7629215 | 3 | LOC105374287 | Uncharacterized
LOC105374287 | Non-coding RNA |
| 5.57 | rs2644714 | 17 | VPS53/FAM57A | Vacuolar protein
sorting 53 homolog (S. cerevisiae)/ family with sequence
similarity 57, member A | Intergenic |
| 5.57 | rs2295479 | 17 | TLCD3A | TLC domain
containing 3A | Intron |
| 5.33 | rs12447219 | 16 | RBFOX1 | RNA binding fox-1
homolog 1 | Intron |
Discussion
In the present study, various SNPs were first
identified as novel risk loci which may be used to predict
susceptibility to joint destruction in patients with RA. The risk
loci identified were rs2295926, an intronic SNP of the
GALNT12 gene located on chromosome 9, and rs11958855, an
intronic SNP of the KCNN2 gene located on chromosome 5.
GALNT12 belongs to a family of
hexosyltransferases, which are involved in the initial steps of the
mucin-type O-glycosylation (24).
Activity of these enzymes can lead to aberrant glycosylation, and
this is associated with alterations in cell growth,
differentiation, transformation, adhesion, metastasis and immune
surveillance in several types of cancer (25). GALNT12 expression is upregulated in
the digestive tract (24), and is
frequently downregulated in colorectal cancer (26). Glycosaminoglycans are proteoglycans
that are involved in the regulation of the diffusion of growth
factors, and can also bind different functional proteins, such as
fibroblast growth factors and hedgehog proteins, thereby modulating
various signaling pathways (27,28).
Mucin-type O-glycosylation consists of glycans attached via
O-linked N-acetyl-D-galactosamine to serine and threonine
residues, and it is one of the most abundant types of protein
glycosylation in animals, where it is controlled by a large family
of GALNT genes (29). GALNT12 and
GALNT3 regulate mucin-type O-glycosylation (24). Therefore, GALNT12 may serve a role in
cartilage homeostasis, and the rs2295926 SNP may serve a
significant role in rapid joint destruction in patients with
RA.
The SNP rs11958855 was identified as having a
genome-wide significant association with rapid joint destruction in
RA. rs11958855 is an intergenic SNP of the KCNN2 gene
located on chromosome 5. KCNN2 encodes an integral membrane protein
that forms small conductance calcium-activated potassium (SK)
channels. SK2 channels are voltage-independent
Ca2+-activated K+ channels. In neurons of the
central nervous system, activation of these channels modulates
neuronal excitability by hyperpolarizing the membrane (30). Regarding cartilage homeostasis, low
conductance-potassium calcium-activated channel transcript subtype
SK1 (KCNN1, KCa2.1) and SK3 (KCNN3, KCa2.3) and intermediate
potassium calcium-activated channel transcript subtypes (IK, KCNN4,
SK4 and KCa3.1) have been shown to be expressed in OUMS-27 cells (a
chondrosarcoma cell line). SK channels have been proposed to be
involved in the response to osmotic change in chondrocytes
(31). SK channels also provide a
direct link between inflammation and chondrocyte function (31). SK2 was not expressed in normal
cartilage tissues (31), but it may
be expressed and have a function related to inflammation in
cartilage tissues of patients with RA.
The present study has some limitations. First, the
actual effects of these genetic variants were not assessed in
vitro or in vivo. Further studies are necessary to fully
elucidate the effects of these and other SNPs. Second, SHS was
assessed by a single examiner, which may have led to a potential
bias. The cohorts were comprised entirely of Japanese patients;
SNPs may differ in subjects of different ethnicities. Finally, the
SHS score change from the baseline was not evaluated as only
radiographical data was collected at the time of blood sample
collection; however, previous reports have defined rapid joint
destruction as changes from baseline of >5 points per year.
In conclusion, the rs2295926 SNP variant in the
GALNT12 gene and rs11958855 in the KCNN2 gene may be
associated with rapid joint destruction amongst Japanese patients
with RA. At present, there are no means of managing RA with the aim
of reducing joint destruction. Therefore, the identification of
genetic predictors of rapid joint destruction in RA (GALNT12
and KCNN2) may highlight potentially novel therapeutic
targets to improve management of disease progression, thereby
improving the quality of life of patients.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets generated and/or analyzed during the
present study are not publicly available due to an application for
a patent in Japan (patent no. 4869834, registration date Nov 25,
2011) but are available from the corresponding author upon
reasonable request.
Authors' contributions
SH, RK and TsM designed the study. All authors were
involved in drafting and revising the manuscript. KoF, SH, KeF,
ToM, TK, MH and YT participated in data collection. SH, TsM and KeF
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The methods used in the present study were approved
by the Research Institute of Joint Disease Kobe on June 11, 2008.
All patients provided written informed consent for
participation.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Klareskog L, Catrina AI and Paget S:
Rheumatoid arthritis. Lancet. 373:659–672. 2009.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Radner H, Smolen JS and Aletaha D:
Comorbidity affects all domains of physical function and quality of
life in patients with rheumatoid arthritis. Rheumatology (Oxford).
50:381–388. 2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Myasoedova E, Crowson CS, Kremers HM,
Therneau TM and Gabriel SE: Is the incidence of rheumatoid
arthritis rising?: Results from Olmsted County, Minnesota,
1955-2007. Arthritis Rheum. 62:1576–1582. 2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Smolen JS, Aletaha D, Barton A, Burmester
GR, Emery P, Firestein GS, Kavanaugh A, McInnes IB, Solomon DH,
Strand V, et al: Rheumatoid arthritis. Nat Rev Dis Primers.
4(18001)2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Breedveld F: The value of early
intervention in RA - a window of opportunity. Clin Rheumatol. 30
(Suppl 1):S33–S39. 2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
van Nies JA, Krabben A, Schoones JW,
Huizinga TW, Kloppenburg M and van der Helm-van Mil AH: What is the
evidence for the presence of a therapeutic window of opportunity in
rheumatoid arthritis? A systematic literature review. Ann Rheum
Dis. 73:861–870. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Dudbridge F: Power and predictive accuracy
of polygenic risk scores. PLoS Genet. 9(e1003348)2013.Erratum in:
PLoS Genet: doi:
10.1371/annotation/b91ba224-10be-409d-93f4-7423d502cba0.
|
|
8
|
Spiliopoulou A, Nagy R, Bermingham ML,
Huffman JE, Hayward C, Vitart V, Rudan I, Campbell H, Wright AF,
Wilson JF, et al: Genomic prediction of complex human traits:
Relatedness, trait architecture and predictive meta-models. Hum Mol
Genet. 24:4167–4182. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Abraham G, Kowalczyk A, Zobel J and Inouye
M: Performance and robustness of penalized and unpenalized methods
for genetic prediction of complex human disease. Genet Epidemiol.
37:184–195. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Warren H, Casas JP, Hingorani A, Dudbridge
F and Whittaker J: Genetic prediction of quantitative lipid traits:
Comparing shrinkage models to gene scores. Genet Epidemiol.
38:72–83. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
International HapMap Consortium. The
International HapMap Project. Nature. 426:789–796. 2003.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ozaki K, Ohnishi Y, Iida A, Sekine A,
Yamada R, Tsunoda T, Sato H, Sato H, Hori M, Nakamura Y, et al:
Functional SNPs in the lymphotoxin-alpha gene that are associated
with susceptibility to myocardial infarction. Nat Genet.
32:650–654. 2002.PubMed/NCBI View
Article : Google Scholar
|
|
13
|
Suzuki A, Yamada R, Chang X, Tokuhiro S,
Sawada T, Suzuki M, Nagasaki M, Nakayama-Hamada M, Kawaida R, Ono
M, et al: Functional haplotypes of PADI4, encoding citrullinating
enzyme peptidylarginine deiminase 4, are associated with rheumatoid
arthritis. Nat Genet. 34:395–402. 2003.PubMed/NCBI View
Article : Google Scholar
|
|
14
|
Traylor M, Knevel R, Cui J, Taylor J,
Harm-Jan W, Conaghan PG, Cope AP, Curtis C, Emery P, Newhouse S, et
al: Genetic associations with radiological damage in rheumatoid
arthritis: Meta-analysis of seven genome-wide association studies
of 2,775 cases. PLoS One. 14(e0223246)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zeng Z, Zhang W, Qian Y, Huang H, Wu DJH,
He Z, Ye D, Mao Y and Wen C: Association of telomere length with
risk of rheumatoid arthritis: A meta-analysis and Mendelian
randomization. Rheumatology (Oxford). 59:940–947. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Okada Y, Terao C, Ikari K, Kochi Y, Ohmura
K, Suzuki A, Kawaguchi T, Stahl EA, Kurreeman FA, Nishida N, et al:
Meta-analysis identifies nine new loci associated with rheumatoid
arthritis in the Japanese population. Nat Genet. 44:511–516.
2012.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Sharp JT, Young DY, Bluhm GB, Brook A,
Brower AC, Corbett M, Decker JL, Genant HK, Gofton JP, Goodman N,
et al: How many joints in the hands and wrists should be included
in a score of radiologic abnormalities used to assess rheumatoid
arthritis? Arthritis Rheum. 28:1326–1335. 1985.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Rodriguez-Rodriguez L, Ivorra-Cortes J,
Carmona FD, Martín J, Balsa A, van Steenbergen HW, van der Helm-van
Mil AH, González-Álvaro I and Fernandez-Gutiérrez B: PTGER4 gene
variant rs76523431 is a candidate risk factor for radiological
joint damage in rheumatoid arthritis patients: A genetic study of
six cohorts. Arthritis Res Ther. 17(306)2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Suzuki T, Ikari K, Yano K, Inoue E, Toyama
Y, Taniguchi A, Yamanaka H and Momohara S: PADI4 and HLA-DRB1 are
genetic risks for radiographic progression in RA patients,
independent of ACPA status: Results from the IORRA cohort study.
PLoS One. 8(e61045)2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Arnett FC, Edworthy SM, Bloch DA, McShane
DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS,
et al: The American Rheumatism Association 1987 revised criteria
for the classification of rheumatoid arthritis. Arthritis Rheum.
31:315–324. 1988.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Vastesaeger N, Xu S, Aletaha D, St Clair
EW and Smolen JS: A pilot risk model for the prediction of rapid
radiographic progression in rheumatoid arthritis. Rheumatology
(Oxford). 48:1114–1121. 2009.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lipsky PE, van der Heijde DM, St Clair EW,
Furst DE, Breedveld FC, Kalden JR, Smolen JS, Weisman M, Emery P,
Feldmann M, et al: Anti-Tumor Necrosis Factor Trial in Rheumatoid
Arthritis with Concomitant Therapy Study Group: Infliximab and
methotrexate in the treatment of rheumatoid arthritis. N Engl J
Med. 343:1594–1602. 2000.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Purcell S, Neale B, Todd-Brown K, Thomas
L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ,
et al: PLINK: A tool set for whole-genome association and
population-based linkage analyses. Am J Hum Genet. 81:559–575.
2007.PubMed/NCBI View
Article : Google Scholar
|
|
24
|
Guo JM, Zhang Y, Cheng L, Iwasaki H, Wang
H, Kubota T, Tachibana K and Narimatsu H: Molecular cloning and
characterization of a novel member of the UDP-GalNAc:polypeptide
N-acetylgalactosaminyltransferase family, pp-GalNAc-T12. FEBS Lett.
524:211–218. 2002.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Brockhausen I: Mucin-type O-glycans in
human colon and breast cancer: Glycodynamics and functions. EMBO
Rep. 7:599–604. 2006.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Guo JM, Chen HL, Wang GM, Zhang YK and
Narimatsu H: Expression of UDP-GalNAc:polypeptide
N-acetylgalactosaminyltransferase-12 in gastric and colonic cancer
cell lines and in human colorectal cancer. Oncology. 67:271–276.
2004.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Chuang CY, Lord MS, Melrose J, Rees MD,
Knox SM, Freeman C, Iozzo RV and Whitelock JM: Heparan
sulfate-dependent signaling of fibroblast growth factor 18 by
chondrocyte-derived perlecan. Biochemistry. 49:5524–5532.
2010.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Cortes M, Baria AT and Schwartz NB:
Sulfation of chondroitin sulfate proteoglycans is necessary for
proper Indian hedgehog signaling in the developing growth plate.
Development. 136:1697–1706. 2009.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Bennett EP, Mandel U, Clausen H, Gerken
TA, Fritz TA and Tabak LA: Control of mucin-type O-glycosylation: A
classification of the polypeptide GalNAc-transferase gene family.
Glycobiology. 22:736–756. 2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Lin MT, Luján R, Watanabe M, Adelman JP
and Maylie J: SK2 channel plasticity contributes to LTP at Schaffer
collateral-CA1 synapses. Nat Neurosci. 11:170–177. 2008.PubMed/NCBI View
Article : Google Scholar
|
|
31
|
Funabashi K, Ohya S, Yamamura H, Hatano N,
Muraki K, Giles W and Imaizumi Y: Accelerated Ca2+ entry
by membrane hyperpolarization due to Ca2+-activated
K+ channel activation in response to histamine in
chondrocytes. Am J Physiol Cell Physiol. 298:C786–C797.
2010.PubMed/NCBI View Article : Google Scholar
|