Introduction
According to the Japanese guidelines for
gastroesophageal reflux disease (GERD) 2015, a proton pump
inhibitor (PPI) is the first-choice drug for the treatment of
patients with GERD (1). Although
PPIs have comparatively better acid inhibition properties than
histamine-2 receptor antagonists, ~45% of GERD patients treated
with PPI suffer from persistent GERD symptoms in an observational
study (2). In patients with
PPI-resistant GERD, weak acid reflux is observed even with
standard-dose PPI treatment (3), and
double-dose PPI cannot adequately control PPI-resistant GERD
(4). Therefore, more potent acid
inhibition is necessary to control symptomatic PPI-resistant
GERD.
Vonoprazan, a potassium-competitive acid blocker,
strongly suppresses gastric acid release by inhibiting
H+-K+ exchange in the gastric parietal cells.
The holding time ratios of gastric pH >4 when treated with
vonoprazan is significantly higher than treatment with PPIs
(5). Due to its strong and
sustainable acid inhibition, vonoprazan has become the first-choice
drug for GERD treatment and Helicobacter pylori (H.
pylori) eradication therapy (6,7).
Vonoprazan is useful in overcoming PPI-resistant GERD, and our
previous study was the first to report the short-term effects of
vonoprazan in patients with symptomatic PPI-resistant GERD
(8). However, previous reports
regarding the effect of vonoprazan on PPI-resistant GERD were
limited to healing of esophageal erosions and/or short-term
symptomatic improvement (9-12).
To the best of our knowledge, there are no reports regarding the
long-term effects of vonoprazan on symptomatic improvement in
patients with PPI-resistant GERD without esophageal erosions. The
aim of the present study was to evaluate the long-term outcomes of
patients with PPI-resistant GERD treated with vonoprazan.
Patients and methods
Patients
Consecutive patients with PPI-resistant symptomatic
GERD treated for 1 year with continuous vonoprazan therapy at the
Shinozaki Medical Clinic between February 2016 and October 2020
were included in the present study, and their medical records were
retrospectively reviewed. The final cohort consisted of 7 males and
23 females with a median age of 69 (age range, 35-86).
Gastrointestinal symptoms were routinely evaluated using the Izumo
scale, a self-reporting questionnaire (13). All patients underwent
esophagogastroduodenoscopy (EGD) prior to starting vonoprazan
therapy. The patients were asked to complete the Izumo scale
questionnaire 0, 1, 3, 6, 9 and 12 months after starting therapy as
previously reported (14). All
patients visited the clinic, and vonoprazan was prescribed monthly.
The treating physician confirmed the nature of abdominal symptoms
at each visit. If patients reported changes in abdominal symptoms,
the Izumo scale was immediately administered and the next treatment
strategy considered. If GERD symptoms were not sufficiently
improved, the dose was increased from 10 to 20 mg or the addition
of acotiamide was considered. For patients taking acotiamide at
baseline, vonoprazan was added without ceasing acotiamide. After 1
year of vonoprazan therapy, a follow-up EGD was used to evaluate
the grade of erosive esophagitis. Based on the Los Angeles
classification of reflux esophagitis, erosive esophagitis was
defined as grade A or higher (15).
The degree of gastric atrophy was evaluated based on
Kimura-Takemoto classification (16). H. pylori infection status was
evaluated using the serum H. pylori IgG kit (E-plate; cat.
no. I-DQ77; Eiken Chemical, Co., Ltd.) according to the
manufacturer's protocol, and serum IgG values of <3, 3-9, ≥10
were considered negative, undetermined and positive, respectively.
In case of an indeterminate result, a 13C-urea breath test (UBIT;
Otsuka Pharmaceutical, Co., Ltd.) or stool antigen test (Testmate
rapid pylori antigen; Wakamoto Pharmaceutical, Co., Ltd.) were
used. H. pylori eradication history was determined based on
the medical record or based on the patient's recollection. The
Institutional Review Board of the Shinozaki Medical Clinic approved
this retrospective review. The need for informed consent was waived
due to the retrospective nature of the study.
Izumo scale
The Izumo scale was developed to evaluate various
abdominal symptoms simultaneously (13). The Izumo scale has been validated and
is a widely used self-reporting questionnaire with five domains:
GERD (Q1-3), epigastric pain syndrome (Q4-6), postprandial distress
syndrome (Q7-9), constipation (Q10-12) and diarrhea (Q13-15)
(17-20).
Each domain has three items and each item is scored from 0-5 on a
Likert scale as follows: 0, not bothered; 1, not so bothered; 2,
slightly bothered; 3, bothered; 4, strongly bothered; and 5,
intolerably bothered. Higher scores indicate more severe
gastrointestinal symptoms. The domain-specific score is calculated
as a total score of three items ranging from 0 to 15. A
domain-specific score of ≥4 is considered a ‘significant symptom’
(21).
Inclusion and exclusion criteria
The inclusion criteria were: i) Continuous
vonoprazan therapy for at least 1 year without cessation; ii) GERD
symptoms that had persisted for >8 weeks whilst taking a
standard dose of PPI; and iii) total score of GERD domain of ≥4
prior to initiation of vonoprazan therapy. The exclusion criteria
were: i) discontinuation of vonoprazan therapy or lost to follow-up
within 1 year; ii) lack of critical clinicopathological data; iii)
H. pylori positive status; and iv) status-post distal
gastrectomy. Safety analysis included all consecutively enrolled
patients regardless of these criteria.
Statistical analysis
Changes in domain-specific scores of the Izumo scale
during 1 year of vonoprazan therapy were compared using a Friedman
test. Categorical data and continuous variables were compared
between the erosive and non-erosive groups using a Fischer's exact
test and a Mann-Whitney U-Test, respectively. Statistical analysis
was performed using StatFlex version 7.0 (Artech). P<0.05 was
considered to indicate a statistically significant difference.
Results
Characteristics and outcome of
patients
Between February 2016 and October 2020, 47 patients
received vonoprazan therapy for PPI-resistant symptomatic GERD, and
17 patients were excluded for the following reasons: Cessation of
vonoprazan therapy due to improved GERD symptoms (n=6); lack of
critical clinicopathological data (n=4); lost to follow (n=3);
cessation of vonoprazan therapy due to adverse events (n=2);
cessation of vonoprazan therapy due to lack of effectiveness (n=1);
and H. pylori positive status (n=1). The remaining 30
patients were included in the final analysis.
Safety analysis was performed using all 47 patients,
including the 30 study patients and 17 excluded patients. Adverse
events occurred in two patients (4%). The first patient was a
71-year-old female who suffered from a dry mouth 3 months after
initiation of vonoprazan and ceased therapy. The second patient was
a 59-year-old female who suffered from constipation just after
starting vonoprazan therapy, and thus ceased therapy.
Baseline characteristics and outcomes of the 30
patients are shown in Table I.
First, patients were divided into two groups, a non-erosive (n=22)
and erosive group (n=8), and these two groups were compared. At
baseline, the Izumo scale score for postprandial distress symptoms
was significantly higher in the non-erosive group compared with the
erosive group (P=0.013). A total of 8 patients (8/22; 36%) in the
non-erosive group had been treated with acotiamide prior to
initiation of vonoprazan, and vonoprazan was prescribed in addition
to acotiamide. The initial dose of vonoprazan was 20 mg once daily
prior to April 2017 and subsequently lowered to 10 mg once daily.
As a result, 17 patients started with a 10 mg initial dose and were
maintained at that level. The remaining 13 patients received an
initial dose of 20 mg and underwent dose reduction to 10 mg after 1
month.
 | Table ICharacteristics and treatment regimens
of the 30 patients. |
Table I
Characteristics and treatment regimens
of the 30 patients.
| Characteristics | Non-erosive,
n=22 | Erosive, n=8 | P-value |
|---|
| Age, years, median
(IQR) | 69 (67-79) | 70 (65-77) | 0.605 |
| Sex, male, n (%) | 6 (27%) | 1 (13%) | 0.376 |
| Body mass index,
median (IQR) | 23.5 (22.6-25.3) | 26.7 (23.8-28.8) | 0.11 |
| Current Smoker, n
(%) | 3 (14%) | 0 (0%) | 0.379 |
| Alcohol use, >20
g/day, n (%) | 2 (9%) | 0 (0%) | 0.531 |
| Severity of GERD,
Izumo scale score, median (IQR) | 5.5 (4.0-7.0) | 6.0 (4.8-6.3) | 0.865 |
| Severity of
epigastric pain symptoms, median (IQR) | 2.5 (1.0-5.0) | 3.0 (0.0-5.0) | 0.849 |
| Severity of
postprandial distress symptoms, median (IQR) | 4.0 (1.3-6.0) | 1.0 (0.0-2.3) |
0.013a |
| Severity of
constipation, median (IQR) | 3.0 (0.0-5.5) | 0.5 (0.0-1.3) | 0.083 |
| Severity of
diarrhea, median (IQR) | 1.0 (0.0-3.0) | 0.5 (0.0-3.0) | 0.783 |
| History of H.
pylori eradication, n (%) | 7 (32%) | 4 (50%) | 0.309 |
| Previously treated
with acotiamide, n (%) | 8 (36%) | 0 (0%) | 0.054 |
| Reflux esophagitis,
n | | | |
|
Non-erosive | 22 | 0 | |
|
Erosive, Los
Angeles grade A/B/C/D | 0 | 2/5/1/0 | |
| Type of PPI before
starting vonoprazan, n (%) | | | |
|
Esomeprazole | 11 (50%) | 4 (50%) | 1 |
|
Lansoprazole | 3 (14%) | 3 (37%) | 0.174 |
|
Omeprazole | 3 (14%) | 0 (%) | 0.379 |
|
Rabeprazole | 5 (22%) | 1 (13%) | 0.480 |
| Grade of gastric
atrophy, n (%) | | | |
|
None | 10 (45%) | 2 (25%) | 0.282 |
|
Closed
type | 5 (23%) | 5 (62%) | 0.056 |
|
Open
type | 7 (32%) | 1 (13%) | 0.287 |
| Starting dose
(10/20 mg), n | 13/9 | 4/4 | 0.485 |
| Additional
treatment during 1y therapy, n (%) | 10 (45%) | 1 (13%) | 0.107 |
|
Dose
escalation of vonoprazan | 8 | 1 | |
|
Addition of
acotiamide | 2 | 0 | |
During the 1 year of treatment, the proportion of
patients requiring additional treatments or another sort of
intervention, including dose escalation of vonoprazan or addition
of acotiamide to control refractory GERD symptoms was higher in the
non-erosive group compared with the erosive group (45 vs. 13%),
although the difference was not significant (P=0.107; Table I). All but 1 patient in the erosive
group showed mucosal healing during follow-up EGD after 1 year; the
1 patient refused follow-up EGD.
Changes in GERD symptoms
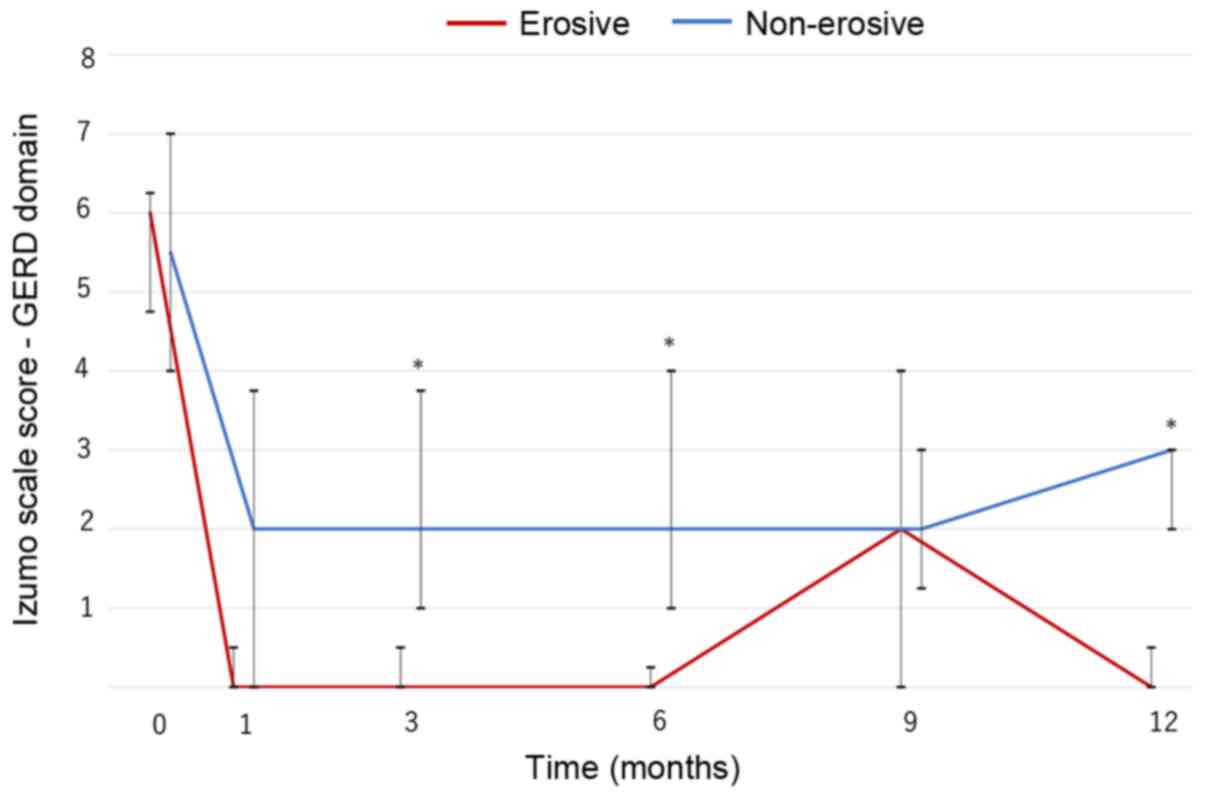
In both groups, GERD symptoms significantly improved
from baseline and were maintained for 1 year (P<0.001; Fig. 1). The non-erosive group had a higher
score than the erosive group except after 9 months, and significant
differences between the two groups were observed at 3, 6 and 12
months (Fig. 1). Nevertheless,
continuous vonoprazan therapy improved symptoms and provided good
control of GERD symptoms in patients with PPI-resistant non-erosive
GERD for 1 year, although the symptoms were more refractory than in
patients with PPI-resistant erosive GERD.
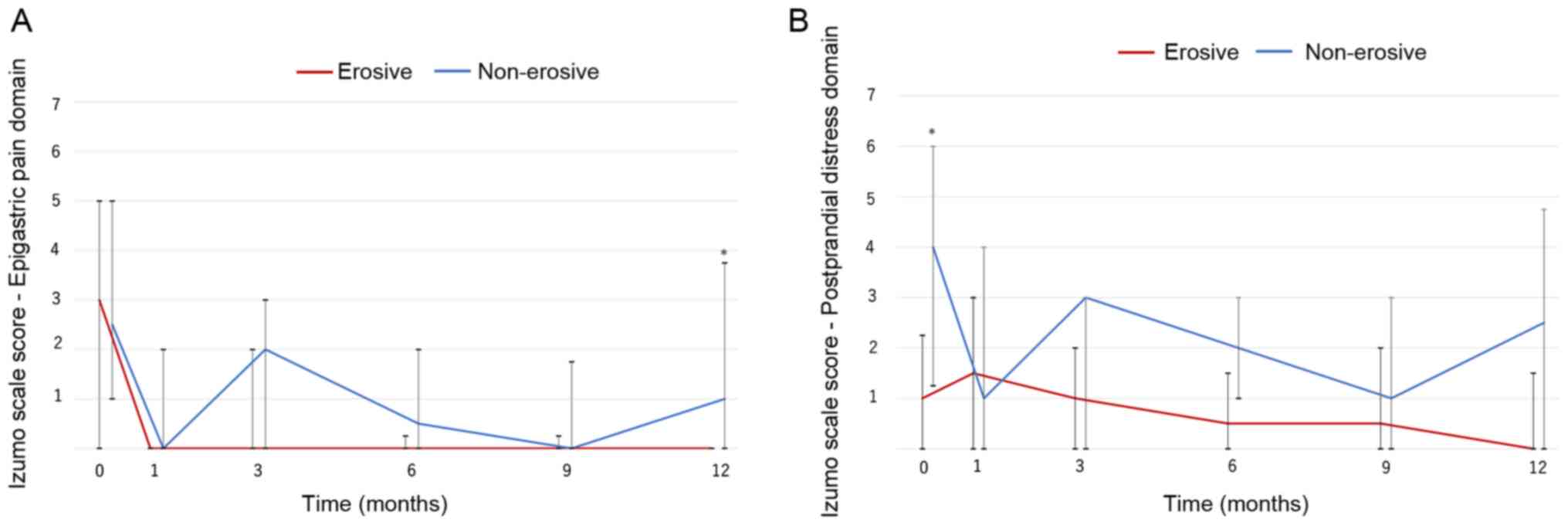
Changes in dyspepsia symptoms
Changes in dyspepsia symptoms were also investigated
(Fig. 2). The score for the
epigastric pain domain significantly improved over the study period
in both groups (non-erosive group, P=0.001; erosive group P=0.018;
Fig. 2A). The non-erosive group had
higher scores than the erosive group, and a significant difference
was observed at 12 months.
Throughout the 1 year treatment period, the Izumo
scale score for the postprandial distress domain significantly
decreased in the non-erosive group (P=0.023). Although it was
slightly higher than the erosive group, the difference was not
significant (Fig. 2B). Vonoprazan
therapy improved and controlled dyspepsia symptoms effectively in
patients with PPI-resistant non-erosive GERD.
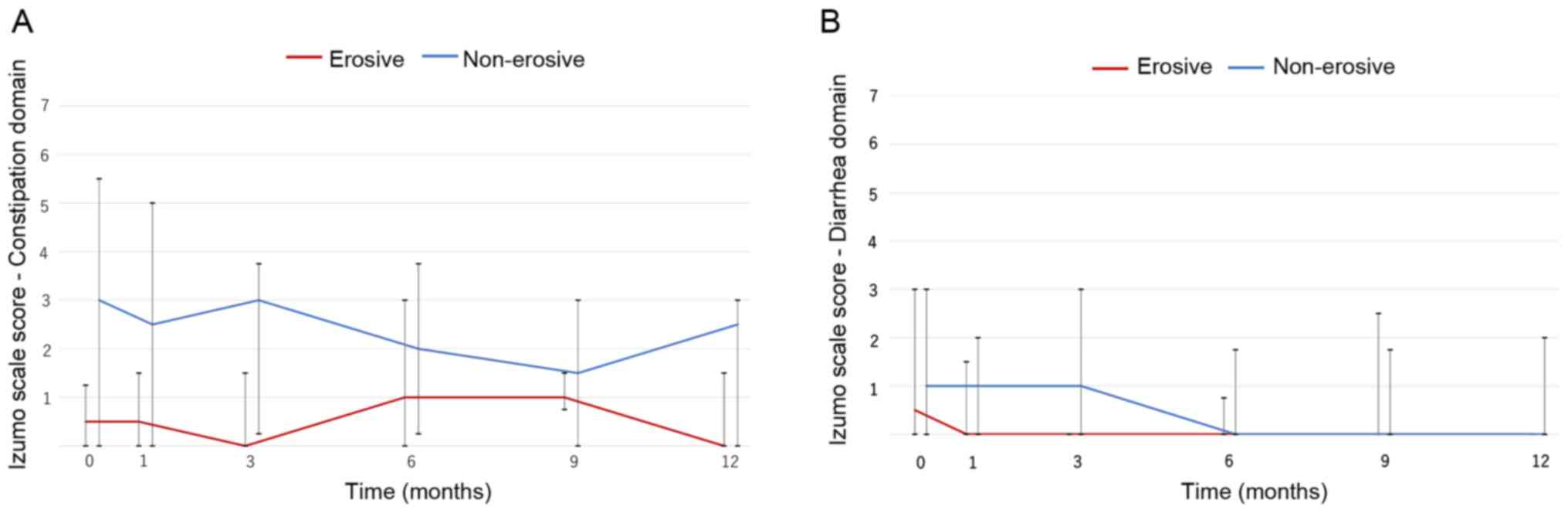
Changes in lower gastrointestinal
symptoms
Lower gastrointestinal symptoms were evaluated using
the Izumo scale. Vonoprazan did not largely affect constipation.
The non-erosive group had a slightly higher score compared with the
erosive group, but the difference was not significant (Fig. 3A). Similarly, vonoprazan did not
notably affect diarrhea (Fig.
3B).
Discussion
This retrospective study of the long-term
effectiveness of vonoprazan on PPI-resistant GERD showed
symptomatic improvement in patients with non-erosive GERD was more
refractory than in patients with erosive GERD, and that patients
with non-erosive GERD were more likely to require additional
treatments to control the refractory symptoms. Nevertheless, 1 year
of treatment with vonoprazan resulted in significantly sustained
improvement of GERD symptoms in both non-erosive and erosive GERD
patients. This regimen also improved epigastric pain and
postprandial distress symptoms, that frequently complicate
non-erosive GERD (20). Although
there are some studies showing the effect of vonoprazan on
PPI-resistant GERD, they primarily report the outcomes of patients
with PPI-resistant erosive GERD (10,12,22). The
present study demonstrated the long-term effectiveness of
vonoprazan on PPI-resistant non-erosive GERD as well as erosive
GERD.
GERD remains symptomatic in ~50% of patients treated
with standard doses of PPI, particularly in patients with
non-erosive GERD (23). Resolution
of these symptoms is important to improve the quality of life and
sleep of patients regardless of the presence of esophageal
erosions. In patients with PPI-resistant GERD, acid reflux is still
present in 57% of patients and serves a more important determining
role in nocturnal GERD symptoms than bile reflux (24). In patients with PPI-resistant
non-erosive GERD, 96% of liquid reflux episodes were acidic, and
acidic reflux is the major cause of persistent GERD symptoms
(4). However, the number of acid
reflux events is almost zero in patients treated with vonoprazan
based on data from 24 h pH monitoring (25). The present study suggests that the
strong acid suppression provided by vonoprazan contributes to the
improvements in GERD symptoms.
Controlling PPI-resistant non-erosive GERD is more
difficult than erosive GERD. In patients with naïve symptomatic
GERD, erosive GERD is more easily treatable than non-erosive GERD
(23). Unlike symptomatic erosive
GERD, symptoms in patients with non-erosive GERD are largely
influenced by visceral hypersensitivity, impaired intestinal
motility coordination, gastric accommodation disorders and
psychiatric disorders (1). In the
present study, postprandial distress symptoms and constipation were
more common in the non-erosive group compared with the erosive
group at baseline. This suggests that acid reflux serves a lesser
role in causing these symptoms in patients with non-erosive GERD
than in patients with erosive GERD. Additional therapies such as
prokinetics, dietary manipulation and psychiatric medications may
be necessary to treat patients with PPI-resistant non-erosive GERD
(1).
The effectiveness of PPI on dyspepsia symptoms is
well demonstrated based on a meta-analysis, and is slightly
superior to that of prokinetics (26). Direct introduction of hydrochloric
acid into the stomach induces dysmotility-like predominant
dyspeptic symptoms, including a heavy feeling, bloating and
belching (27). Introducing
hydrochloric acid directly into the duodenum also induces dyspeptic
symptoms (28). Therefore, gastric
acid surely influences dyspeptic symptoms, such as epigastric pain
and postprandial distress. Suppression of gastric acid by
vonoprazan partially improved dyspepsia symptoms in the present
study.
It is well-established that the acid suppressing
effect of vonoprazan is greater than that of PPIs, and this more
potent suppression contributes to its effectiveness in the
treatment of patients with PPI-resistant GERD (5). Patients with extensive metabolism of
the CYP2C19 genotype have a higher risk of PPI-resistant reflux
esophagitis than those with poor metabolism (29); the acid suppression effect of
vonoprazan is not influenced by the CYP2C19 genotype (5). An open-label cross-over study showed
significantly superior acid suppression by vonoprazan compared with
rabeprazole, and the pH4 holding time ratios were 88.4 and 53.8% in
patients treated with 20 mg vonoprazan and 20 mg rabeprazole,
respectively (30). A Japanese
randomized controlled trial showed improved pH4 holding times
following 8 weeks of 20 mg and 40 mg vonoprazan therapy for
PPI-resistant erosive GERD (9).
Another Japanese study reported that acid clearance time and the
number of reflux events were significantly reduced following
treatment with 20 mg/day vonoprazan (10). The nocturnal acid suppression effect
of vonoprazan is superior to that of PPI (31), and in another study it was shown that
changing therapy to vonoprazan from PPI resulted in resolution of
GERD symptoms within a few days (12). Vonoprazan exhibits a rapid onset,
long duration of action and strong acid suppression effects, and
thus successfully improved GERD symptoms in patients with
PPI-resistant GERD in the present study.
A phase III trial in naïve non-erosive GERD patients
did not show a significant difference between vonoprazan and
placebo groups regarding the proportion of days without heartburn
in the full-analysis-set. Additionally, the per-protocol-set
analysis showed a significant difference, and complete heartburn
resolution in the fourth week of treatment was higher in vonoprazan
group compared with the placebo group (P=0.002) (32). Unlike the phase III trial, all
patients included in the present study did not have naïve
symptomatic GERD, but instead PPI-resistant symptomatic GERD.
Therapeutic susceptibility to PPI-resistant GERD is different from
naïve GERD (1). In the present
study, the effectiveness of vonoprazan on long-term outcomes in
patients with PPI-resistant non-erosive GERD were determined.
Randomized controlled studies are necessary to confirm these
preliminary results.
A recent Japanese prospective study showed that
long-term maintenance therapy with vonoprazan for PPI-resistant
erosive GERD was effective (22).
All patients included in the present study had suffered from
persistent, recurrent and/or refractory GERD symptoms over a long
period of time. Therefore, vonoprazan therapy was not ceased just
after improvement of the GERD symptoms. Based on these results, it
is suggested that long-term maintenance of vonoprazan therapy
should be considered as a standard treatment for patients with
PPI-resistant symptomatic GERD.
The present study has some limitations. First, this
study was a retrospective observational study. Second, CYP3A4 or
CYP2C19 polymorphisms were not examined; vonoprazan and PPIs are
primarily metabolized by CYP3A4 and CYP2C19, respectively (1,33).
Third, the types of PPI used before starting vonoprazan differed
between patients. However, the observation period in the present
study is longer than previous studies, and patients were
continuously treated with vonoprazan. Additionally, patients with
active H. pylori infections that can influence gastric acid
secretion and motility were excluded.
In conclusion, vonoprazan therapy for 1 year
improved GERD symptoms in patients with PPI-resistant GERD.
Long-term vonoprazan therapy does not adversely affect lower
gastrointestinal symptoms. The present study is the first study of
long-term outcomes of patients with symptomatic PPI-resistant
non-erosive GERD treated with vonoprazan.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
SS conceived and designed the study, collected the
data, performed the data analysis and interpretation and drafted
the manuscript. HO and AKL drafted the manuscript, and performed
the data analysis and interpretation. YH, YM and HY performed the
data analysis and interpretation. All authors read and approved the
final manuscript. HO and YH confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board (approval no. ID#30-R001). The need for written
informed consent was waived due to the retrospective design of the
study.
Patient consent for publication
Not applicable.
Competing interests
SS, HO and YM have received honoraria from Takeda
and Otsuka Pharmaceuticals. HY has received honoraria from Takeda
Pharmaceutical. All the other authors declare that they have no
conflicts of interest.
References
|
1
|
Iwakiri K, Kinoshita Y, Habu Y, Oshima T,
Manabe N, Fujiwara Y, Nagahara A, Kawamura O, Iwakiri R, Ozawa S,
et al: Evidence-based clinical practice guidelines for
gastroesophageal reflux disease 2015. J Gastroenterol. 51:751–767.
2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
El-Serag H, Becher A and Jones R:
Systematic review: Persistent reflux symptoms on proton pump
inhibitor therapy in primary care and community studies. Aliment
Pharmacol Ther. 32:720–737. 2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Frazzoni M, Conigliaro R and Melotti G:
Weakly acidic refluxes have a major role in the pathogenesis of
proton pump inhibitor-resistant reflux oesophagitis. Aliment
Pharmacol Ther. 33:601–606. 2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Iwakiri K, Sano H, Tanaka Y, Kawami N,
Umezawa M, Futagami S, Hoshihara Y, Nomura T, Miyashita M and
Sakamoto C: Characteristics of symptomatic reflux episodes in
patients with non-erosive reflux disease who have a positive
symptom index on proton pump inhibitor therapy. Digestion.
82:156–161. 2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kagami T, Sahara S, Ichikawa H, Uotani T,
Yamade M, Sugimoto M, Hamaya Y, Iwaizumi M, Osawa S, Sugimoto K, et
al: Potent acid inhibition by vonoprazan in comparison with
esomeprazole, with reference to CYP2C19 genotype. Aliment Pharmacol
Ther. 43:1048–1059. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Mori H and Suzuki H: Role of acid
suppression in acid-related diseases: proton pump inhibitor and
potassium-competitive acid blocker. J Neurogastroenterol Motil.
25:6–14. 2019.PubMed/NCBI View
Article : Google Scholar
|
|
7
|
Shinozaki S, Kobayashi Y, Osawa H,
Sakamoto H, Hayashi Y, Lefor AK and Yamamoto H: Effectiveness and
safety of vonoprazan versus proton pump inhibitors for second-line
Helicobacter pylori eradication therapy: systematic review
and meta-analysis. Digestion. 1–7. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Shinozaki S, Osawa H, Hayashi Y, Sakamoto
H, Kobayashi Y, Lefor AK and Yamamoto H: Vonoprazan 10 mg daily is
effective for the treatment of patients with proton pump
inhibitor-resistant gastroesophageal reflux disease. Biomed Rep.
7:231–235. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Iwakiri K, Sakurai Y, Shiino M, Okamoto H,
Kudou K, Nishimura A, Hiramatsu N, Umegaki E and Ashida K: A
randomized, double-blind study to evaluate the acid-inhibitory
effect of vonoprazan (20 mg and 40 mg) in patients with proton-pump
inhibitor-resistant erosive esophagitis. Therap Adv Gastroenterol.
10:439–451. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Yamashita H, Kanamori A, Kano C, Hashimura
H, Matsumoto K, Tsujimae M, Yoshizaki T, Momose K, Obata D, Eguchi
T, et al: The Effects of switching to vonoprazan, a novel
potassium-competitive acid blocker, on gastric acidity and reflux
patterns in patients with erosive esophagitis refractory to proton
pump inhibitors. Digestion. 96:52–59. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Okuyama M, Nakahara K, Iwakura N, Hasegawa
T, Oyama M, Inoue A, Ishizu H, Satoh H and Fujiwara Y: Factors
associated with potassium-competitive acid blocker non-response in
patients with proton pump inhibitor-refractory gastroesophageal
reflux disease. Digestion. 95:281–287. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hoshino S, Kawami N, Takenouchi N, Umezawa
M, Hanada Y, Hoshikawa Y, Kawagoe T, Sano H, Hoshihara Y, Nomura T,
et al: Efficacy of vonoprazan for proton pump inhibitor-resistant
reflux esophagitis. Digestion. 95:156–161. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kakuta E, Yamashita N, Katsube T,
Kushiyama Y, Suetsugu H, Furuta K and Kinoshita Y: Abdominal
symptom-related QOL in individuals visiting an outpatient clinic
and those attending an annual health check. Intern Med.
50:1517–1522. 2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Shinozaki S, Osawa H, Kobayashi Y,
Sakamoto H, Hayashi Y, Miura Y, Kawarai Lefor A and Yamamoto H:
Long-term outcomes of patients with symptomatic gastroesophageal
reflux disease treated with vonoprazan. Scand J Gastroenterol.
53:897–904. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Lundell LR, Dent J, Bennett JR, Blum AL,
Armstrong D, Galmiche JP, Johnson F, Hongo M, Richter JE, Spechler
SJ, et al: Endoscopic assessment of oesophagitis: Clinical and
functional correlates and further validation of the Los Angeles
classification. Gut. 45:172–180. 1999.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kimura K and Takemoto T: An endoscopic
recognition of the atrophic border and its significance in chronic
gastritis. Endoscopy. 1:87–97. 1969.
|
|
17
|
Furuta K, Ishihara S, Sato S, Miyake T,
Ishimura N, Koshino K, Tobita H, Moriyama I, Amano Y, Adachi K, et
al: Development and verification of the Izumo Scale, new
questionnaire for quality of life assessment of patients with
gastrointestinal symptoms. Nihon Shokakibyo Gakkai Zasshi.
106:1478–1487. 2009.PubMed/NCBI(In Japanese).
|
|
18
|
Fujishiro M, Kushiyama A, Yamazaki H,
Kaneko S, Koketsu Y, Yamamotoya T, Kikuchi T, Sakoda H, Suzuki R
and Kadowaki T: Gastrointestinal symptom prevalence depends on
disease duration and gastrointestinal region in type 2 diabetes
mellitus. World J Gastroenterol. 23:6694–6704. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kinoshita Y and Chiba T: FUTURE Study
Group. Characteristics of Japanese patients with chronic gastritis
and comparison with functional dyspepsia defined by ROME III
criteria: Based on the large-scale survey, FUTURE study. Intern
Med. 50:2269–2276. 2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Okimoto E, Ishimura N, Morito Y, Mikami H,
Shimura S, Uno G, Tamagawa Y, Aimi M, Oshima N, Kawashima K, et al:
Prevalence of gastroesophageal reflux disease in children, adults,
and elderly in the same community. J Gastroenterol Hepatol.
30:1140–1146. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Shinozaki S, Osawa H, Sakamoto H, Hayashi
Y, Kawarai Lefor A and Yamamoto H: The effect of acotiamide on
epigastric pain syndrome and postprandial distress syndrome in
patients with functional dyspepsia. J Med Invest. 63:230–235.
2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Tanabe T, Hoshino S, Kawami N, Hoshikawa
Y, Hanada Y, Takenouchi N, Goto O, Kaise M and Iwakiri K: Efficacy
of long-term maintenance therapy with 10-mg vonoprazan for proton
pump inhibitor-resistant reflux esophagitis. Esophagus. 16:377–381.
2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Fass R and Sifrim D: Management of
heartburn not responding to proton pump inhibitors. Gut.
58:295–309. 2009.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Hershcovici T, Jha LK, Cui H, Powers J and
Fass R: Night-time intra-oesophageal bile and acid: A comparison
between gastro-oesophageal reflux disease patients who failed and
those who were treated successfully with a proton pump inhibitor.
Aliment Pharmacol Ther. 33:837–844. 2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Masaoka T, Kameyama H, Yamane T, Yamamoto
Y, Takeuchi H, Suzuki H, Kitagawa Y and Kanai T: Pathophysiology of
potassium-competitive acid blocker-refractory gastroesophageal
reflux and the potential of potassium-competitive acid blocker
test. J Neurogastroenterol Motil. 24:577–583. 2018.PubMed/NCBI View
Article : Google Scholar
|
|
26
|
Pinto-Sanchez MI, Yuan Y, Hassan A, Bercik
P and Moayyedi P: Proton pump inhibitors for functional dyspepsia.
Cochrane Database Syst Rev. 11(CD011194)2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Miwa H, Nakajima K, Yamaguchi K, Fujimoto
K, Veldhuyzen VAN, Zanten SJ, Kinoshita Y, Adachi K, Kusunoki H and
Haruma K: Generation of dyspeptic symptoms by direct acid infusion
into the stomach of healthy Japanese subjects. Aliment Pharmacol
Ther. 26:257–264. 2007.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ishii M, Manabe N, Kusunoki H, Kamada T,
Sato M, Imamura H, Shiotani A, Hata J and Haruma K: Real-time
evaluation of dyspeptic symptoms and gastric motility induced by
duodenal acidification using noninvasive transnasal endoscopy. J
Gastroenterol. 43:935–941. 2008.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ichikawa H, Sugimoto M, Sugimoto K, Andoh
A and Furuta T: Rapid metabolizer genotype of CYP2C19 is a risk
factor of being refractory to proton pump inhibitor therapy for
reflux esophagitis. J Gastroenterol Hepatol. 31:716–726.
2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Takeuchi T, Furuta T, Fujiwara Y, Sugimoto
M, Kasugai K, Kusano M, Okada H, Suzuki T, Higuchi T, Kagami T, et
al: Randomised trial of acid inhibition by vonoprazan 10/20 mg once
daily vs rabeprazole 10/20 mg twice daily in healthy Japanese
volunteers (SAMURAI pH study). Aliment Pharmacol Ther. 51:534–543.
2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Sakurai Y, Mori Y, Okamoto H, Nishimura A,
Komura E, Araki T and Shiramoto M: Acid-inhibitory effects of
vonoprazan 20 mg compared with esomeprazole 20 mg or rabeprazole 10
mg in healthy adult male subjects - a randomised open-label
cross-over study. Aliment Pharmacol Ther. 42:719–730.
2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kinoshita Y, Sakurai Y, Takabayashi N,
Kudou K, Araki T, Miyagi T, Iwakiri K and Ashida K: Efficacy and
safety of vonoprazan in patients with nonerosive gastroesophageal
reflux disease: a randomized, placebo-controlled, phase 3 study.
Clin Transl Gastroenterol. 10(e00101)2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Sugimoto M, Ban H, Hira D, Kamiya T,
Otsuka T, Inatomi O, Bamba S, Terada T and Andoh A: Letter:
CYP3A4/5 genotype status and outcome of vonoprazan-containing
Helicobacter pylori eradication therapy in Japan. Aliment
Pharmacol Ther. 45:1009–1010. 2017.PubMed/NCBI View Article : Google Scholar
|