Introduction
Sarcopenia is a harmful condition in patients with
chronic liver disease (1). The
definition of sarcopenia varies based on the criteria used. The
European Working Group on Sarcopenia in Older People (EWGSOP)
defined sarcopenia as encompassing a low handgrip strength (GS),
slow walking speed and low skeletal muscle (SM) mass in
2010(2). The International Working
Group on Sarcopenia suggested criteria similar to the criteria
defined by the EWGSOP, but with a different walking speed in
2011(3). The criteria for Asian
people of small builds was developed by the Asian Working Group for
Sarcopenia in 2014(4). In 2019, the
EWGSOP revised its criteria (5) to
include an algorithm for case-finding, diagnosing and quantifying
the severity of sarcopenia, and a simple questionnaire (SARC-F)
(6). In 2020, the AWGS also revised
its criteria to include use of the calf circumference (CC), SARC-F,
or SARC-F and CC together for identifying cases in primary health
care settings (7). SARC-F has been
suggested to be a possible rapid diagnostic test for diagnosing
sarcopenia and includes only five areas of consideration: Strength,
assistance with working, rising from a chair, climbing stairs and
falls (6). Several reports have
previously described the potential of SARC-F for screening of
sarcopenia in patients with chronic liver disease (8). In our previous study, it was reported
that the calculated body muscle mass (CBMM) is a useful screening
marker for discerning low SM mass and sarcopenia in chronic liver
disease (9). CBMM was calculated
using body weight in kg, serum creatinine (Cr) and serum cystatin C
(CysC), and the approximated body muscle mass was measured using
dual-energy X-ray absorptiometry in both derivation and validation
cohorts (10).
The Japan Society of Hepatology (JSH) decided to
establish its own criteria for the assessment of sarcopenia in
liver disease in 2015 due to a high number of patients with liver
disease and sarcopenia (11). Based
on the JSH criteria, if the GS is <26 kg in men or <18 kg in
women, muscle volume should be evaluated using computed tomography
(CT) or bioelectrical impedance analysis. The JSH criteria was used
for diagnosing sarcopenia in the present study. Hiraoka et
al (12) reported the value of
the finger-ring test as an effective screening method for
predicting early-stage muscle atrophy in patients with chronic
liver disease. Additionally, Hirota et al (13) reported that the liver frailty index
predicted muscle atrophy with high sensitivity, even in patients
with normal GS. As the value of SARC-F and CBMM have both been
evaluated in assessing liver disease, the abilities of these
indices in screening for sarcopenia according to the JSH criteria
was assessed in the present study. Additionally, a simple
diagnostic tool for screening sarcopenia in chronic liver disease
was established.
Patients and methods
Patients
A series of 482 patients with chronic liver disease
were admitted to Nagasaki Harbor Medical Center between October
2019 and April 2020. In the outpatient department, patients were
evaluated for the cause of their liver disease (for example,
hepatitis C virus, hepatitis B virus, autoimmune hepatitis, primary
biliary cholangitis and other causes); degree of liver damage
[using the Child-Pugh score (14),
albumin-bilirubin score (15), model
for end-stage liver disease (16)
and fibrosis-4(17)]; renal function
[measuring, serum Cr, CysC, Cr-glomerular filtration rate (GFR) and
CysC-GFR]; body mass index (kg/m2); GS (kg); and SARC-F
score (Table I). Diabetes mellitus
status was evaluated based on the patients' history and prescribed
medication at recruitment in the present study. Of the 482
patients, 273 were screened for hepatocellular carcinoma using CT.
Informed consent was obtained from each patient included in the
study, and the patients were guaranteed the option to leave the
study at any point. The study protocol conformed to the Ethical
Guidelines of the 1975 Declaration of Helsinki, and was approved by
the Human Research Ethics Committee of Nagasaki Harbor Medical
Center (approval no. H30-031).
 | Table IClinicopathological characteristics of
the patients. |
Table I
Clinicopathological characteristics of
the patients.
| A, All cases,
n=482 |
|---|
| Characteristic | Number/mean (SD) |
|---|
| Sex, female/male | |
|
Female | 281 |
|
Male | 201 |
| Age, years | 66.29 (14.3) |
| Height, m | 1.584 (0.096) |
| Body weight,
kg | 60.003
(14.142) |
| BMI,
kg/m2 | 23.79 (4.6) |
| Liver disease | |
|
AIH | 24 |
|
AL | 31 |
|
HBV | 97 |
|
HCV | 18 |
| Complicated
malignancy disease | |
|
CCC | 1 |
|
HCC | 12 |
|
Gastric
cancer | 2 |
|
Pancreatic
cancer | 5 |
|
RCC | 1 |
| Diabetes
mellitus | 80 |
| Total bilirubin,
mg/dla | 1.068 (3.589) |
| Albumin,
g/dlb | 4.324 (3.145) |
| Prothrombin time,
%c | 102.65 (17.8) |
| Prothrombin time,
INRd | 1.007 (0.159) |
| Hepatic
encephalopathyi | |
|
1 | 477 |
|
2 | 5 |
|
3 | 0 |
|
Ascitesi | |
|
1 | 467 |
|
2 | 14 |
|
3 | 1 |
| Cr,
mg/le | 0.93 (0.996) |
| Cr-eGFR,
ml/min/1.73 m2 | 67.51 (20.636) |
| CysC,
mg/f | 1.188 (0.875) |
| CysC-eGFR,
ml/min/1.73 m2 | 68.05 (25.731) |
| Platelets,
x104/µlg | 18.66 (7.1) |
| AST,
U/lh | 42.317 (57.25) |
| AL, U/l | 38.8 (52.6) |
| CPS | 5.158 (0.642) |
| CPG | |
|
A | 463 |
|
B | 17 |
|
C | 2 |
| MELD | 7.574 (2.428) |
| FIB-4 | 2.901 (2.597) |
| ALBI | -2.923 (2.671) |
| ALBIG | 370/103/9 |
|
1 | |
|
2 | |
|
3 | |
| GS, kg | 19.76 (9.57) |
| GS low/normal | |
|
Low | 285 |
|
Normal | 197 |
| Sarcopenia
Index | 77.02 (30.7) |
| CBMM | 35.54 (8.39) |
| Sarcopenia/normal,
CBMM | 168/310 |
| deGFR | -0.109 (18.55) |
| SARC-F | 1.589 (2.05) |
| Sarcopenia/normal,
SARC-F | 85/397 |
| B, Patients who
underwent an evaluation of body composition, n=273 |
| Factors | Number/mean
(SD) |
| SM,
cm2 | 104.4 (27.08) |
| IMAT,
cm2 | 7.356 (0.445) |
| VAT,
cm2 | 112.86 (88.68) |
| SAT,
cm2 | 129.92 (82.32) |
| MA, HU | 30.21 (7.491) |
| SMI,
cm2/m2 | 41.42 (8.167) |
| Low SMI/normal | 120/153 |
| Sarcopenia | 96 |
Measurements
Laboratory and anthropometric measurement data were
obtained for each patient during the initial hospital visit as
standard procedure. Laboratory examinations included the assessment
of total bilirubin (mg/dl), albumin (mg/dl), alanine
aminotransferase (U/l), aspartate aminotransferase (U/l), platelet
counts (104/µl), prothrombin time (percentage), Cr
(mg/dl) and CysC (mg/l). Cr- and CysC-based estimated GFRs (eGFRs)
(ml/min/1.73 m2) in females and males were calculated
using the Japanese Society of Nephrology for Japanese patients
equation guidelines (18). Chronic
kidney disease (CKD) was staged based on the levels of Cr-based
eGFRs in ml/min/1.73 m2 (18). The difference in GFR was calculated
as follows (19): Cr-based eGFR -
CysC-based eGFR. The sarcopenia index was calculated as follows
(20): Cr/CysC x 100. CBMM was
calculated as follows (10): CBMM =
[body weight (kg) x Cr]/[(K x body weight (kg) x CysC) + Cr], where
K=0.00675 for men and K=0.01006 for women. Cutoff CBMM values for
sarcopenia were 27.903 in females and 39.731 in males (9). The cutoff SARC-F score for sarcopenia
was ≥4 points (6).
GS was measured using a dynamometer (Smedlay Dynamo
Meter; TTM) with participants standing in an erect position with
both arms at their sides. The maximum results of two tests were
used for further analysis. Using the JSH criteria, female patients
with a maximum GS <18 kg and male patients with a maximum GS
<26 kg were categorized as the low GS group (11).
CT analysis of body composition
Cross-sectional CT images of the third lumbar
vertebrae were analyzed using Slice-O-Matic version 5.0
(Tomovision) to determine the SM mass in 273 patients. Muscle areas
of interest included the psoas, erector spinae, quadratus lumborum,
transversus abdominis, external and internal obliques and rectus
abdominis. Tissue Hounsfield unit (HU) thresholds ranging from -29
to 150 HU for SMs (21) were used.
The SMs were normalized for height using m2 and
expressed as cm2/m2 to determine the SM index
(SMI). Patients with an SMI <39 cm2/m2 for
women and <42 cm2/m2 for men were
categorized into the low SMI group. Sarcopenia was diagnosed as low
GS and low SMI based on the JSH guidelines for sarcopenia (11).
Statistical analysis
Data were analyzed using StatFlex version 6.0
(Artech Co., Ltd.) and are presented as mean ± standard deviation.
Multivariate analyses was performed using logistic regression
analyses. Correlations were evaluated based on Pearson's
correlation coefficient (R). Receiver operating characteristic
(ROC) curve analyses were used to evaluate associations between
groups and factors, with the cutoff points being equal values for
sensitivity and specificity. P<0.05 was considered to indicate a
statistically significant difference.
Results
Correlation between SARC-F, CBMM and
SMI
First, the relationship between SARC-F, CBMM, SMI
and GS was evaluated (Fig. 1). GS
was moderately correlated with SARC-F and CBMM (Fig. 1A and 1D), whereas SMI was moderately correlated
with CBMM for both sexes (Fig. 1E),
but not with SARC-F in females (Fig.
1B). The association between CBMM and SARC-F was weak (Fig. 1C). Second, the ROC curves in relation
to SARC-F, CBMM and sarcopenia were analyzed (Fig. 2). The AUC for CBMM against low GS was
larger than that for SARC-F, but the difference was not significant
(Fig. 2A and E). Separately, the AUCs for CBMM against
low SMI were significantly larger than those for SARC-F for both
sexes (Fig. 2B and F), whereas that for CBMM against sarcopenia
was significantly larger than that for SARC-F in males (Fig. 2C), but not so amongst females
(Fig. 2G). The discrimination
efficacy of CBMM for sarcopenia was higher than that of SARC-F in
males.
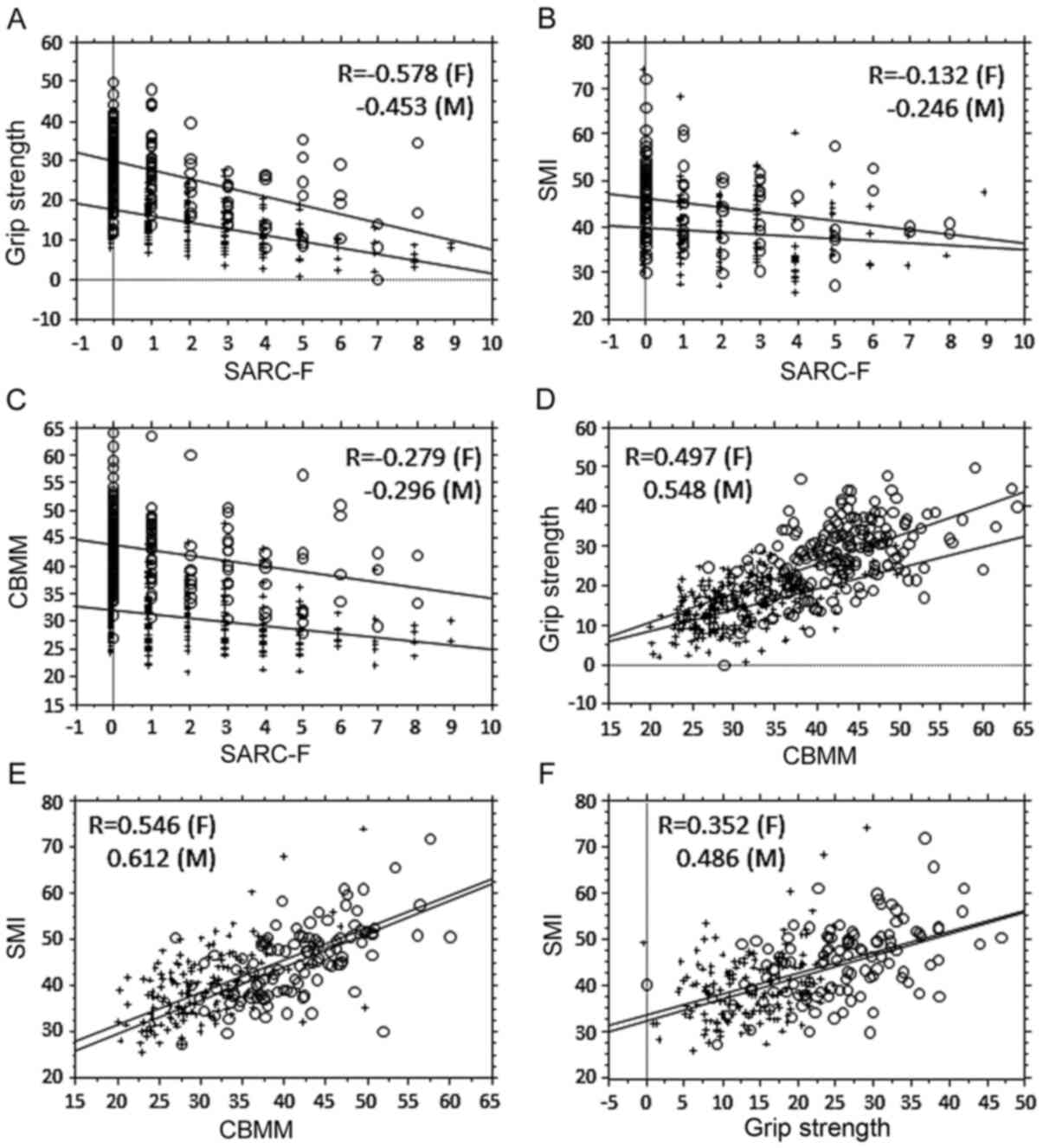 | Figure 1Relationship between SARC-F score,
CBMM and muscle factors. (A) Correlation analysis between SARC-F
and GS. Females, R=-0.578, P<0.0001; males, R=-0.453,
P<0.0001. Regression line for female GS based on
SARC-F=17.51-1.567x SARC-F; regression line for male GS based on
SARC-F=29.567-2.193x SARC-F. Females, R2=0.334; males
R2=0.205. (B) Correlation analysis between SARC-F and
SMI. Females, R=-0.132, P=0.0983; males R=-0.246, P=0.0076.
Regression line for female SMI based on SARC-F=39.781-0.491x
SARC-F; regression line for male SMI based on SARC-F=46.27-0.986x
SARC-F. Females, R2=0.018; males R2=0.06. (C)
Correlation analysis between SARC-F and CBMM. Females, R=-0.279,
P<0.0001; males R=-0.296, P<0.0001. Regression line for
female CBMM based on SARC-F=31.92-0.704x SARC-F; regression line
for male CBMM based on SARC-F=43.636-0.961x SARC-F. Females,
R2=0.078; males, R2=0.071. (D) Correlation
analysis between CBMM and GS. Females, R=0.497, P<0.0001; males,
R=0.548, P<0.0001. Regression line for female GS based on
CBMM=-1.843+0.533x CBMM; regression line for male GS based on
CBMM=-3.798+0.73x CBMM. Females, R2=0.247; males,
R2=0.3. (E) Correlation analysis between CBMM and SMI.
Females, R=0.546, P<0.0001; males, R=0.612, P<0.0001.
Regression line for female SMI based on CBMM=17.438+0.704x CBMM;
regression line for male SMI based on CBMM=15.052+0.728x CBMM.
Females, R2=0.298; males, R2=0.375 in males.
(F) Correlation analysis between SMI and GS. Females, R=0.352,
P<0.0001; males, R=0.486, P<0.0001. Regression line for
female SMI based on GS=32.251+0.465x GS; regression line for male
SMI based on GS=33.396+0.455x GS. Females, R2=0.131;
males, R2=0.236. O, males; +, females. CBMM, calculated
body muscle mass; GS, grip strength; SMI, skeletal muscle index; F,
females; M, males. |
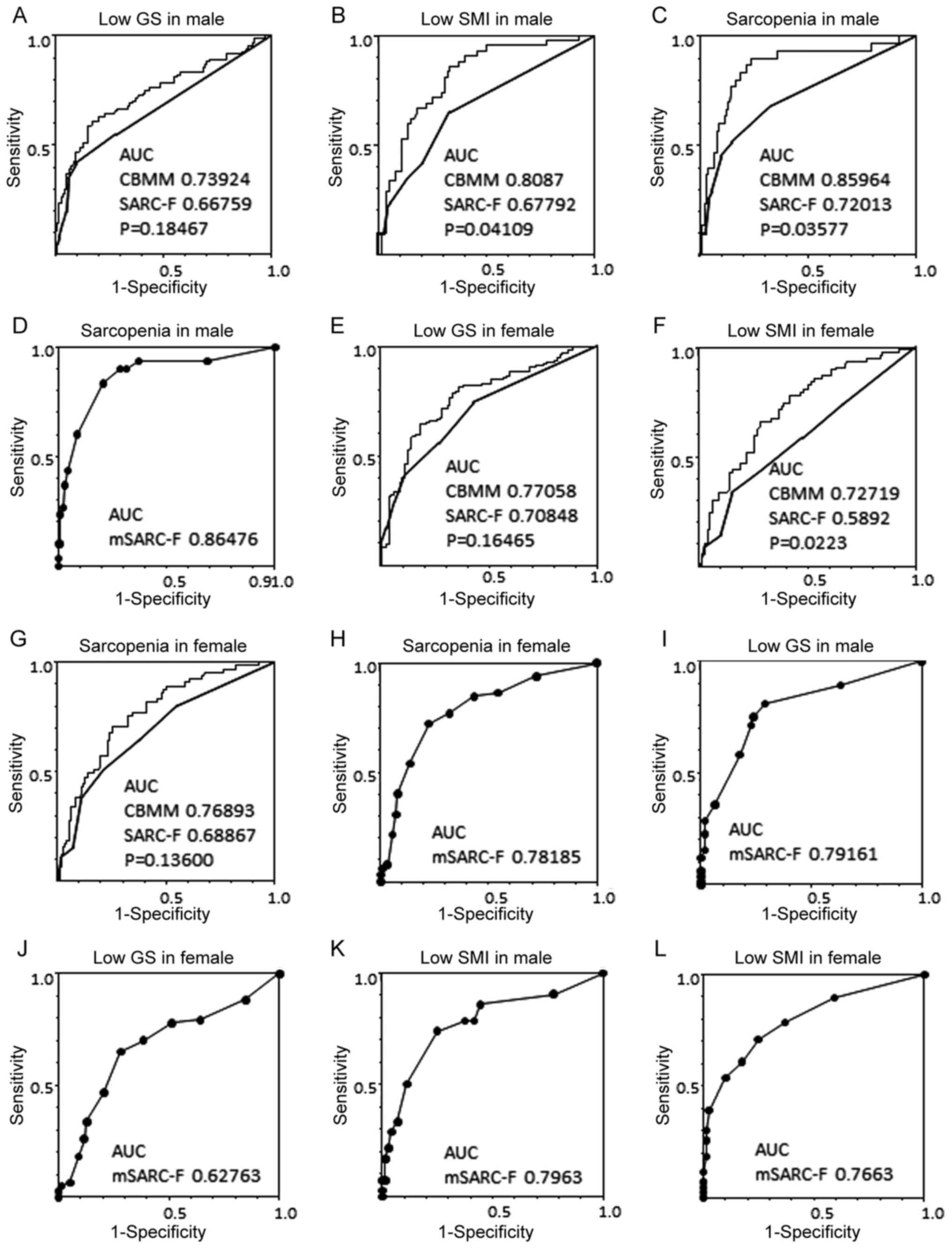 | Figure 2Receiver operating characteristic
curve analysis of CBMM, SARC-F score and sarcopenia. (A)
Association between low GS with CBMM or SARC-F in males. (B)
Association between low SMI with CBMM or SARC-F in males. (C)
Association between sarcopenia with CBMM or SARC-F in males. The
mSARC-F score was calculated as follows: mSARC-F = SARC-F + CBMM
(sarcopenia, 4 points; not sarcopenia, 0 points) + sex (female, 2
points; male, 0 points) + age (≥65 years, 1 point; <65 years, 0
points). (D) Association between sarcopenia and mSARC-F in males.
(E) Association between low GS with CBMM or SARC-F in females. (F)
Association between low SMI with CBMM or SARC-F in females. (G)
Association between sarcopenia with CBMM or SARC-F in females. (H)
Association between sarcopenia and mSARC-F in females. (I and J)
Association between low GS and mSARC-F in males and females,
respectively. (K and L) Association between low SMI and mSARC-F in
males and females, respectively. The x-axis is the sensitivity and
the y-axis is the specificity; P-values represent comparisons
between the AUCs of CBMM and SARC-F in each panel. The fine line
refers to CBMM and the bold line refers to SARC-F. AUC, area under
the curve; CBMM, calculated body muscle mass; GS, grip strength;
skeletal muscle index. |
Analysis of factors contributing to
sarcopenia and the modified SARC-F
To establish an optimized sarcopenia screening
method, the factors that contribute to sarcopenia were evaluated
(Table II). In the univariate
logistic regression analysis, age (≥65 years), sex (male),
albumin-bilirubin score (2-3 points), fibrosis-4 score (<3.25
points), CKD (stages 3-5), SARC-F (≥4 points) and CBMM (low) were
significant contributors to the presence of sarcopenia. In the
multivariate analysis, age, sex, SARC-F score and CBMM were found
to contribute to sarcopenia. As a result, the SARC-F questionnaire
was modified (mSARC-F questionnaire) as follows: mSARC-F=SARC-F
score + CBMM (sarcopenia, 4 points; not sarcopenia, 0 points) + sex
(female, 2 points; male, 0 points) + age (≥65 years, 1 point;
<65 years, 0 points). Weighted points for CBMM, SARC-F and older
age were decided based on the odds ratio, and 2 points was assigned
for female sex. AUCs for mSARC-F against sarcopenia were 0.864 in
males (Fig. 2D) and 0.78185 in
females (Fig. 2H). When the cutoff
mSARC-F for sarcopenia was set to 4 points, the sensitivity and
specificity were 0.76923 and 0.68 in males, and 0.8333 and 0.79286
in females, respectively (Table
III). AUC for mSARC-F against low GS was 0.79161 in males
(Fig. 2I) and 0.7663 in females
(Fig. 2J), and that against low SM
was 0.7963 in males (Fig. 2K) and
0.62763 in females (Fig. 2L).
 | Table IIFactors contributing to
sarcopenia. |
Table II
Factors contributing to
sarcopenia.
| | Univariate | Multi-variate |
|---|
|
Characteristics | P-value | OR | 95% CI | P-value | OR | 95% CI |
|---|
| Age ≥65 years |
<0.0001c | 6.647 | 3.593-12.3 | 0.0006c | 3.461 | 1.702-7.04 |
| Male sex | 0.0007c | 0.427 | 0.261-0.696 | 0.0012b | 0.375 | 0.208-0.678 |
| High SARC-F
score |
<0.0001c | 6.157 | 3.364-11.267 | 0.0032b | 2.913 | 1.43-5.936 |
| Low CBMM | <0.0001 | 6.738 | 4.034-11.253 |
<0.0001c | 5.113 | 2.854-9.161 |
| ALBIG 2/3 | 0.0173a | 1.897 | 1.12-3.213 | 0.2657 | 1.471 | 0.746-2.902 |
| FIB-4 >3.25 | 0.0004c | 0.41 | 0.251-0.669 | 0.5768 | 0.836 | 0.446-1.567 |
| CKD 3/4/5 | 0.0161a | 1.827 | 1.118-2.985 | 0.281 | 1.403 | 0.758-2.598 |
 | Table IIIScreening using SARC-F, CBMM, and
mSARC-F. |
Table III
Screening using SARC-F, CBMM, and
mSARC-F.
| | Male | Female |
|---|
| Factor | SARC-F | CBMMa | mSARC-F | SARC-F | CBMMa | mSARC-F |
|---|
| Sarcopenia | | | | | | |
|
Sensitivity | 0.15116 | 0.58333 | 0.76923 | 0.17085 | 0.52261 | 0.83333 |
|
Specificity | 0.98261 | 0.85187 | 0.68 | 0.97561 | 0.97653 | 0.79286 |
| Low skeletal muscle
index | | | | | | |
|
Sensitivity | 0.2093 | 0.71429 | 0.7381 | 0.14286 | 0.57143 | 0.7013 |
|
Specificity | 0.9589 | 0.73611 | 0.75 | 0.9 | 0.74684 | 0.61538 |
| Low grip
strength | | | | | | |
|
Sensitivity | 0.15116 | 0.58333 | 0.58333 | 0.17085 | 0.51759 | 0.60606 |
|
Specificity | 0.96522 | 0.85088 | 0.82456 | 0.97561 | 0.87654 | 0.82716 |
Discussion
When compared with CBMM, the SARC-F showed high
specificity but reduced sensitivity for screening of sarcopenia. It
is hypothesized that the reasons for the reduced sensitivity
include the fact that SARC-F is related to GS but not to muscle
mass. As a screening method, the SARC-F questionnaire is less
useful than CBMM. However, when SARC-F was modified to encompass
CBMM, age and sex (mSARC-F), the AUC of mSARC-F was greater than
that of SARC-F and CBMM.
Previous reports have described that SARC-F is a
marker of muscle strength (22) and
exhibits low sensitivity for sarcopenia (22-24).
SARC-F combined with CC or finger-ring testing has been assessed in
patients with chronic liver disease as a method of sarcopenia
screening (22-25).
Additionally, it has been reported that SARC-F is an inadequate
screening method for community-dwelling older adults, but a useful
screening method in selected populations, such as adults in
hospital (26). Interestingly,
SARC-F appears suitable for detecting individuals at risk of
adverse outcomes from sarcopenia (27), whereas its use alone showed
sarcopenia was independently associated with the risk of mortality
compared with combination of SARC-F and CC (28). Based on previous reports, it is
hypothesized that SARC-F score is more suitable as a marker of
disease severity rather than a screening method in patients with
sarcopenia.
Separately, CBMM appeared to be a suitable screening
method for sarcopenia based on the results of the present study. In
our previous study, it was shown that the AUC for CBMM against
sarcopenia was 0.78504 in females and 0.85067 in males (9), in agreement with the results of the
present study. Since the study population was different between the
previous and present study, the efficacy of CBMM for sarcopenia
screening has been validated by both. The nature of the association
of SMI with CBMM and SARC-F was different. Since CBMM was
associated with GS and SMI, CBMM is a better screening tool for
sarcopenia than SARC-F. CBMM is simple and minimally invasive to
use, where low levels are indicative of sarcopenia in patients with
liver disease.
In the present study, sex and age also affected the
rate of sarcopenia, and a difference between the sexes was also
found in our previous study as well (9). Age is a well-established factor for
sarcopenia (29). According to the
multivariate analysis encompassing SARC-F, CBMM, sex and age into
the mSARC-F questionnaire for sarcopenia screening, the AUCs for
mSARC-F against sarcopenia were greater than the AUCs for CBMM.
However, the AUC for mSARC-F amongst females was less than that for
males. Thus, screening methods in females should be evaluated
independently from males.
The present study has some limitations that include
the small number of patients with advanced liver disease or CKD,
since CBMM is based on Cr and CysC. It is suggested that CBMM is
preferable as the screening method, as SARC-F shows less
sensitivity and has a lower AUC value for sarcopenia. However, the
newly established mSARC-F may be a useful method for screening
sarcopenia. SARC-F may instead be better as a marker of the
severity of sarcopenia.
In conclusion, CBMM is a more useful sarcopenia
screening method than SARC-F, and the newly developed mSARC-F may
exhibit better screening ability than both CBMM and mSARC-F.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
TI wrote the paper, analyzed the data and designed
the study. HM, SM, YM, MY, SY, MK, YN, TH, HY, RU, OM, YK, KK, NT
and KN collected the data. All authors read and approved the final
manuscript. TI and HM confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
Informed consent was obtained from each patient
included in the study, and the patients were guaranteed the option
to leave the study at any point. The study protocol conformed to
the Ethical Guidelines of the 1975 Declaration of Helsinki, and was
approved by the Human Research Ethics Committee of Nagasaki Harbor
Medical Center (approval no. H30-031).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
van Vugt JLA, Alferink LJM, Buettner S,
Gaspersz MP, Bot D, Darwish Murad S, Feshtali S, van Ooijen PMA,
Polak WG, Porte RJ, et al: A model including sarcopenia surpasses
the MELD score in predicting waiting list mortality in cirrhotic
liver transplant candidates: A competing risk analysis in a
national cohort. J Hepatol. 68:707–714. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Cruz-Jentoft AJ, Baeyens JP, Bauer JM,
Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y,
Schneider SM, et al: European Working Group on Sarcopenia in Older
People: Sarcopenia: European consensus on definition and diagnosis.
Age Ageing. 39:412–423. 2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
IWG on Sarcopenia. Sarcopenia: an
undiagnosed condition in older adults. Current consensus
definition: prevalence, etiology, and consequences. International
working group on sarcopenia. J Am Med Dir Assoc. 12:249–256.
2009.
|
|
4
|
Chen LK, Liu LK, Woo J, Assantachai P,
Auyeung TW, Bahyah KS, Chou MY, Chen LY, Hsu PS, Krairit O, et al:
Sarcopenia in Asia: Consensus report of the Asian working group for
sarcopenia. J Am Med Dir Assoc. 15:95–101. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie
Y, Bruyère O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA,
et al: Writing Group for the European Working Group on Sarcopenia
in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2:
Sarcopenia: Revised European consensus on definition and diagnosis.
Age Ageing. 48:16–31. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Malmstrom TK and Morley JE: Sarcopenia:
the target population. J Frailty Aging. 2:55–56. 2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chen LK, Woo J, Assantachai P, Auyeung TW,
Chou MY, Iijima K, Jang HC, Kang L, Kim M, Kim S, et al: Asian
Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia
Diagnosis and Treatment. J Am Med Dir Assoc. 21:300–307.e2.
2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ida S, Kojima Y, Hamaoka S, Urawa N, Araki
J, Kaneko R and Murata K: Validity of Japanese version of SARC-F
questionnaire in patients with chronic liver disease. J
Gastroenterol Hepatol. 34:947–953. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ichikawa T, Miyaaki H, Miuma S, Motoyoshi
Y, Yamashima M, Yamamichi S, Koike M, Honda T, Yajima H, Uehara R,
et al: Calculated body muscle mass as a useful screening marker for
low skeleton muscle mass and sarcopenia in chronic liver disease.
Hepatol Res. 50:704–714. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kim SW, Jung HW, Kim CH, Kim KI, Chin HJ
and Lee H: A new equation to estimate muscle mass from creatinine
and cystatin C. PLoS One. 11(e0148495)2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Nishikawa H, Shiraki M, Hiramatsu A,
Moriya K, Hino K and Nishiguchi S: Japan Society of Hepatology
guidelines for sarcopenia in liver disease (1st edition):
Recommendation from the working group for creation of sarcopenia
assessment criteria. Hepatol Res. 46:951–963. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hiraoka A, Izumoto H, Ueki H, Yoshino T,
Aibiki T, Okudaira T, Yamago H, Suga Y, Iwasaki R, Tomida H, et al:
Easy surveillance of muscle volume decline in chronic liver disease
patients using finger-circle (yubi-wakka) test. J Cachexia
Sarcopenia Muscle. 10:347–354. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hirota K, Kawaguchi T, Koya S, Nagamatsu
A, Tomita M, Hashida R, Nakano D, Niizeki T, Matsuse H, Shiba N, et
al: Clinical utility of the Liver Frailty Index for predicting
muscle atrophy in chronic liver disease patients with
hepatocellular carcinoma. Hepatol Res. 50:330–341. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Child CG and Turcotte JG: Surgery and
portal hypertension. Major Probl Clin Surg. 1:1–85. 1964.PubMed/NCBI
|
|
15
|
Johnson PJ, Berhane S, Kagebayashi C,
Satomura S, Teng M, Reeves HL, O'Beirne J, Fox R, Skowronska A,
Palmer D, et al: Assessment of liver function in patients with
hepatocellular carcinoma: A new evidence-based approach-the ALBI
grade. J Clin Oncol. 33:550–558. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kamath PS, Wiesner RH, Malinchoc M, Kremer
W, Therneau TM, Kosberg CL, D'Amico G, Dickson ER and Kim WR: A
model to predict survival in patients with end-stage liver disease.
Hepatology. 33:464–470. 2001.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Vallet-Pichard A, Mallet V, Nalpas B,
Verkarre V, Nalpas A, Dhalluin-Venier V, Fontaine H and Pol S:
FIB-4: An inexpensive and accurate marker of fibrosis in HCV
infection. comparison with liver biopsy and fibrotest. Hepatology.
46:32–36. 2007.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Matsuo S, Imai E, Horio M, Yasuda Y,
Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H and Hishida A:
Collaborators developing the Japanese equation for estimated GFR.
Revised equations for estimated GFR from serum creatinine in Japan.
Am J Kidney Dis. 53:982–992. 2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ichikawa T, Miyaaki H, Miuma S, Motoyoshi
Y, Yamashima M, Yamamichi S, Koike M, Takahashi Y, Honda T, Yajima
H, et al: Indices calculated by serum creatinine and cystatin C as
predictors of liver damage, muscle strength and sarcopenia in liver
disease. Biomed Rep. 12:89–98. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kashani KB, Frazee EN, Kukrálová L,
Sarvottam K, Herasevich V, Young PM, Kashyap R and Lieske JC:
Evaluating muscle mass by using markers of kidney function:
development of the sarcopenia index. Crit Care Med. 45:e23–e29.
2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Fujiwara N, Nakagawa H, Kudo Y, Tateishi
R, Taguri M, Watadani T, Nakagomi R, Kondo M, Nakatsuka T, Minami
T, et al: Sarcopenia, intramuscular fat deposition, and visceral
adiposity independently predict the outcomes of hepatocellular
carcinoma. J Hepatol. 63:131–140. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Barbosa-Silva TG, Menezes AMB, Bielemann
RM, Malmstrom TK and Gonzalez MC: Grupo de Estudos em Composição
Corporal e Nutrição (COCONUT): Enhancing SARC-F: Improving
sarcopenia screening in the clinical practice. J Am Med Dir Assoc.
17:1136–1141. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yang M, Hu X, Xie L, Zhang L, Zhou J, Lin
J, Wang Y, Li Y, Han Z, Zhang D, et al: Screening sarcopenia in
community-dwelling older adults: SARC-F vs SARC-F combined with
calf circumference (SARC-CalF). J Am Med Dir Assoc.
19:277.e1–277.e8. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ida S, Murata K, Nakadachi D, Ishihara Y,
Imataka K, Uchida A, Monguchi K, Kaneko R, Fujiwara R and Takahashi
H: Development of a Japanese version of the SARC-F for diabetic
patients: An examination of reliability and validity. Aging Clin
Exp Res. 29:935–942. 2017.PubMed/NCBI View Article : Google Scholar : Erratum in: Aging
Clin Exp Res 32: 2113, 2020.
|
|
25
|
Hiraoka A, Nagamatsu K, Izumoto H, Yoshino
T, Adachi T, Tsuruta M, Aibiki T, Okudaira T, Yamago H, Suga Y, et
al: SARC-F combined with a simple tool for assessment of muscle
abnormalities in outpatients with chronic liver disease. Hepatol
Res. 50:502–511. 2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kera T, Kawai H, Hirano H, Kojima M,
Watanabe Y, Motokawa K, Fujiwara Y, Osuka Y, Kojima N, Kim H, et
al: Limitations of SARC-F in the diagnosis of sarcopenia in
community-dwelling older adults. Arch Gerontol Geriatr.
87(103959)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Malmstrom TK, Miller DK, Simonsick EM,
Ferrucci L and Morley JE: SARC-F: A symptom score to predict
persons with sarcopenia at risk for poor functional outcomes. J
Cachexia Sarcopenia Muscle. 7:28–36. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yang M, Jiang J, Zeng Y and Tang H:
Sarcopenia for predicting mortality among elderly nursing home
residents: SARC-F versus SARC-CalF. Medicine (Baltimore).
98(e14546)2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Papadopoulou SK: Sarcopenia: a
contemporary health problem among older adult populations.
Nutrients. 12(1293)2020.PubMed/NCBI View Article : Google Scholar
|
















