Introduction
Liver fibrosis is a dynamic wound-healing process in
which scar tissue replaces liver parenchyma as a result of
repetitive liver injuries (1).
Persisting liver injury due to various factors, such as chronic
inflammation and progressive fibrogenesis, may lead to cirrhosis
(2). The liver is supplied by poorly
oxygenated venous blood via the portal vein, whereas the well
oxygenated blood is supplied by the hepatic artery (3). Hepatic microvascular structures consist
of two types of vessels: The larger vessels (such as the hepatic
portal venule and hepatic arterioles) are lined by continuous
endothelium, whereas the smaller sinusoids are lined by fenestrated
endothelium. The central venules drain the sinusoids and, in turn,
combine to form the hepatic veins (4). Fibrous tissue hinders the flow of blood
through the liver, which in turn results in abnormal liver function
(5-7).
Angiogenesis is the process of formation of new
blood vessels from pre-existing vessels. Vessel formation can occur
by sprouting angiogenesis or a process of vessel splitting known as
intussusceptive angiogenesis (IA) (8). Angiogenesis is crucial in both normal
development and pathological conditions, such as wound healing
(9).
Cirrhosis is one of the most common diseases in
humans (10,11) and animals, such as dogs (12-14)
and horses (15). This disease is
not yet curable, and treatments are usually focused on preventing
its progress (16). Only few
established antifibrotic drugs are available, which are primarily
used for fibrosis in the lungs or skin (17). Some natural products may however
possess hepatoprotective effects. The bark of Butea
monosperma can at least partially reverse changes in markers
associated with fibrosis to normal and inhibit thioacetamide
(TAA)-induced expression of phosphorylated PI3K, Akt and mTOR in
hepatocytes (18). Justicia
tranquebariesis extract enhances the activities of antioxidant
enzymes in TAA-induced liver fibrosis in rats (19). Curcumin extract attenuates fibrosis
and decreases inflammation in rats (20).
Extracts of purple mangosteen (Garcinia
mangostana L.) are rich in xanthones, which inhibit some of the
CytP450 isoenzymes and possess antifungal activity (21,22).
Furthermore, such extracts inhibit tumor growth in vitro
(23). Previously, α-mangostin
[1,3,6-trihydroxy-7-methoxy-2,8-bis
(3-methyl-2-butenyl)-9H-xanthen-9-one;
C24H26O6; AM], which is the major
constituent in the polyphenolic xanthone fractions of Garcinia
mangostana L. extracts, have been investigated in vitro
for its antioxidant (24-27),
anti-bacterial (28,29), anti-inflammatory (30-32),
anti-fibrotic (33), anti-cancer
(34-40)
and anti-angiogenic activitie (41).
Whether AM has beneficial effects in vivo on fibrotic livers
has not yet been investigated.
The focus of the present study was to establish
whether AM possessed protective effects on the hepatic
micro-angio-architecture in TAA-induced fibrosis in rats in
vivo. Hepatic histomorphology and micro-angio-architecture were
assessed both qualitatively and quantitatively in vascular
corrosion casts (VCCs) using a scanning electron microscope (SEM)
and 3D morphometry of the VCCs. It was hypothesized that AM
treatment would prevent or mitigate the appearance of pathological
changes in the hepatic micro-angio-architecture of the TAA-treated
rats.
Materials and methods
Animals and reagents
A total of 40 male Wistar rats, aged between 4-5
weeks and weighing between 130-160 grams, were purchased from the
National Laboratory Animal Center, Mahidol University, Thailand.
Rats were provided ad libitum access to water and commercial
food pellets, and were housed in ordinary cages at room temperature
(25˚C), with a relative humidity of 50%, and a 12 h light/dark
cycle. Rats were treated in accordance with the guidelines
described in the Guide for the Care and Use of Laboratory Animals
(42). All of the experiments were
approved by the Institutional Animal Care and Use Committee at the
Faculty of Veterinary Medicine, Chiang Mai University (approval no.
A.20/2555). Animal behavior and welfare were monitored weekly, and
this included: i) Behavior (feeding, grooming and responsive
reactions); ii) rat grimace scale for assessing the occurrence or
severity of pain; and iii) weekly weight loss percentage.
AM (96% pure) was received from Dr Primchanien
Moongkarndi, Department of Microbiology, Faculty of Pharmacy,
Mahidol University. Mangosteen fruits, Garcinia mangostana
L. (Clusiaceae), were harvested in the Chanthaburi Province,
Thailand. The plant was authenticated by Dr Omboon Vallisuta,
Department of Pharmacognosy, Mahidol University, Thailand (voucher
specimen no. WGM0615). Preparation, extraction and purification of
AM were performed as described previously (43,44). TAA
(≥99.0% pure) was purchased from Sigma-Aldrich; Merck KGaA; cat.
no. 163678).
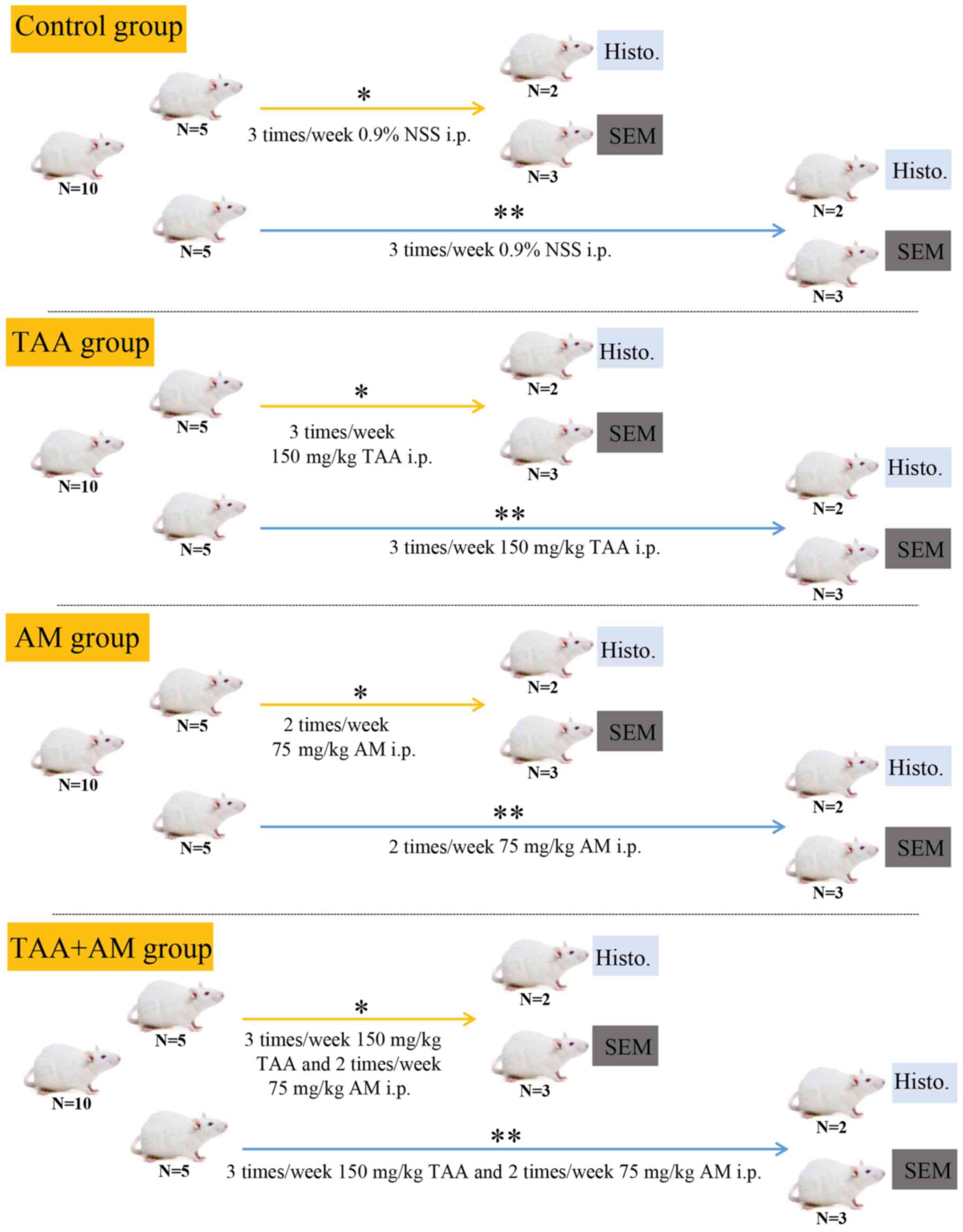
Experimental design
After 1 week of acclimatization, rats were randomly
divided into 4 groups (n=10 per group; Fig. 1): The control group (Ctrl) was
injected intraperitoneally with 0.9% normal saline solution (NSS) 3
times per week. The TAA group was injected with 150 mg TAA/kg body
weight (BW) intraperitoneally 3 times per week to induce liver
fibrosis (45). The AM group was
injected with 75 mg AM/kg BW intraperitoneally twice per week. The
TAA+AM group was injected with 150 mg TAA/kg BW intraperitoneally 3
times per week plus 75 mg AM/kg BW intraperitoneally twice per
week. A total of 5 rats per a group were treated for 30 days and
the other 5 were treated for 60 days. In each subgroup, 2 rats were
processed for light microscopy analysis and 3 rats for VCC. The
treatment regimen was adopted from Poonkhum et al (46), but the dosage of TAA and AM was 75%
of that administered by Poonkhum et al.
VCCs
Rats underwent non-recovery anesthesia by
intraperitoneal injection of 60 mg pentobarbital sodium/kg BW
(NEMBUTAL® Sodium Solution for injection). VCC was
performed as described previously (47). Briefly, after opening the thoracic
wall, a blunt needle (18 G) was inserted into the thoracic aorta
and fixed, then the blood samples were collected. After opening the
right atrium, circulating blood was rinsed out by perfusion with
200 ml NSS containing heparin (5,000 IU/l). Rats died shortly after
when a clear reflux emerged from the opened right atrium. Vital
signs were re-evaluated and death was confirmed by lack of vital
signs observed (no response to withdrawal reflex in all limbs, no
deep pain and lack of heart beat, heart rate and breathing). Next,
20 ml Mercox-Cl-2B (cat. no. 21246; Ladd Research, Inc.) mixed with
0.4 g Benzoyl Peroxide (catalyst for Mercox; cat. no. 21246A; Ladd
Research, Inc.) was injected at a constant flow rate of 4 ml/min
via an auto-syringe pump (Terumo model TE 311; Terumo Corporation).
After keeping the specimens for at least 30 min at room temperature
to allow polymerization, animals were transferred into a water bath
at 60˚C for 12 h to further harden the cast. Thereafter, animals
were transferred into a solution of 1.34 mol/l KOH for at least 12
h (40˚C), rinsed in up to three passages of distilled water, and
then submerged in a solution of 1.33 mol/l formic acid
(CH2O2; 5-10 min at room temperature) to
remove any tissue remnants adhering to the cast surfaces. Finally,
VCCs were rinsed in several passages of distilled water, and
air-dried. Dissected dry specimens comprising most of the liver
were mounted on copper stubs, using the conductive bridge method
(48), and coated with evaporated
carbon (using the electric arc method) and gold (by resistive
heating under a high vacuum of a minimum of 1x10-4 mbar)
using a vacuum evaporator (EPA 100; Leybold-Hereaus). Coated
specimens were assessed using a SEM (Phillips ESEM XL30 FEI;
Philips Medical Systems B.V.) at an accelerating voltage of 10 kV,
and images were captured using Orion version 6.60.4 (E.L.I.
s.p.r.l.) at magnifications of x150, x251, x1,000 and x1,500.
Specimens were repeatedly trimmed manually using micro-dissection
forceps and scissors to expose interesting vascular
territories.
Tissue preparation for
histomorphology
Preparatory steps and blood sample collection were
performed as described in the VCCs section above. Vascular
perfusions from the thoracic aorta were performed once the
perfusate from the opened right atrium did not contain
erythrocytes. Then, 20 ml 4% formaldehyde in PBS was injected with
an infusion pump (40 ml/h). After fixing for 1 h at 25˚C, the
abdominal cavity was opened, the liver was excised and fixed for a
further 12-24 h in fresh fixative at 4˚C. The entire liver was then
dehydrated in an ascending series of graded ethanol solutions and
embedded in paraplast (Surgipath™ Paraplast™; Leica Microsystems,
Inc.). The embedded livers were sectioned into 7 µm thick sections,
which were stained with hematoxylin and eosin (H&E). Tissues
were first stained in hematoxylin solution for 6 min then
counterstained in eosin solution for 1 min at 25˚C. The presence of
collagen fibers (fibrosis) was visualized by staining with Sirius
red for 1 h at 25˚C (Direct Red 80; cat. no. 365548; Sigma-Aldrich;
Merck KGaA). The Ishak scoring system was used to measure activity
and fibrosis, rating the different elements of activity as either
present or absent, ranging from 0 to 6, with a higher score
reflecting increased scarring (49),
by consensus between 2 experts.
All slides were scanned with a digital slide scanner
(Panoramic SCAN II; 3DHISTECH, Ltd.). Micrographs were captured and
exported to CaseViewer version 2.4. (3DHISTECH, Ltd.)
Liver enzyme markers
Following induction of deep anesthesia, blood
samples collected from the thoracic aorta during VCCs and tissue
preparation for histomorphology steps at day 30 and day 60 were
submitted to the Animal Health Diagnostic Laboratory, Faculty of
Veterinary Medicine, Chiang Mai University. Serum alanine
aminotransferase (ALT) and aspartate aminotransferase (AST)
activities were assessed using optimized UV-tests (50,51),
alkaline phosphatase (ALP) activities were assessed using a kinetic
photometric test (52) as described
by the International Federation of Clinical Chemistry and
Laboratory Medicine.
Microstructure description and
quantitative analyses
Differences in the microstructure of the liver
between groups were analyzed in a descriptive manner by consensus
between 2 experts. Information regarding dimensional changes of the
hepatic microvascular network, including diameters of venous
vessels and hepatic sinusoids, and branching angles of venous
vessels and sinusoids were measured from stereo-paired
SEM-micrographs of VCC of the liver by 3D-morphometry (53,54)
using the M3 software (Microstructure Morphometry Measurement Tool
version 2.2 (ComServ). The geometry of microvascular trees in terms
of spatial coordinates and derived distance, as well as angular
measurements in 3D space were calculated from two sets of planar
coordinates obtained from the stereopairs using the parallax and
considering the type of projection in the SEM by trigonometric
vector equation-based algorithms; for further details on
dimensional and angular measurement calculations please see Minnich
et al (54).
Statistical analysis
Quantitative data (diameters of venous vessels and
hepatic sinusoids, and branching angles of venous vessels and
sinusoids) were analyzed using a one-way ANOVA. A two-way ANOVA was
used to analyze the differences between the different groups
(control, AM, TAA, and TAA+AM) and time period (at day 30 and day
60), including the interaction between the different groups and
time periods. Post hoc analyses were performed following one and
two-way ANOVA using Bonferroni corrections. Data were analyzed
using SPSS version 26.0 (IBM Corp.). P<0.05 was considered to
indicate a statistically significant difference.
Results
Body weight
Differences in body weight were observed. After 30
days of exposure, there was a decreased body weight in the AM, TAA
and TAA+AM-treated rats compared with the control rats and an
increase in the liver-to-body weight ratio in the TAA-treated rats.
After 60 days of exposure, there was a decrease in body weight in
the TAA and TAA+AM-treated rats compared with the control rats.
There was a statistically significant difference in the mean body
weight between day 30 and day 60 in all treatment groups (Table I). All rats behaved normally and
showed no noticeable undesired effects following the TAA or AM
treatment.
 | Table IBody weight, liver weight and
liver-to-body weight ratio in the control in the treated
ratsa. |
Table I
Body weight, liver weight and
liver-to-body weight ratio in the control in the treated
ratsa.
| A, Day 30 |
|---|
| | Groups |
|---|
| Parameter | Control | AM | TAA | TAA+AM |
|---|
| Body weight, g | 487.5±15.54 |
427.7±13.55c |
401.0±6.83b |
401.8±6.37c |
| Liver weight,
g | 20.1±0.8 | 19.5±0.4 | 18.7±0.6 | 18.0±0.70 |
| Liver-to-body
weight ratio, % | 3.99±0.03 | 4.29±0.09 |
4.58±0.08b | 4.34±0.06 |
| B, Day 60 |
| | Groups |
| Parameter | Control | AM | TAA | TAA+AM |
| Body weight, g |
581.1±1.2e |
578.2±1.0e |
456.0±9.7c,e |
501.1±13.14c,e |
| Liver weight,
g | 23.0±0.07 |
23.1±0.25e |
25.0±0.7e |
21.2±0.3d |
| Liver-to-body
weight ratio, % | 4.10±0.01 | 4.47±0.48 | 5.25±0.07 | 4.13±0.03 |
Liver enzyme markers
The serum transaminases levels were similar in the
control and AM-treated rats, except for an increased ALP
concentration in the AM-treated rats after 60 days of treatment
compared with the control rats. The serum levels of AST, ALT and
ALP in the TAA-treated rats after 60 days of treatment were
significantly increased compared with the control rats, but
additional treatment with AM (TAA+AM-treated rats) normalized these
values after both 30 and 60 days of treatment (Table II).
 | Table IISerum concentrations of AST, ALT and
ALP in the treated ratsa. |
Table II
Serum concentrations of AST, ALT and
ALP in the treated ratsa.
| A, Day 30 |
|---|
| | Groups |
|---|
| Parameter | Control | AM | TAA | TAA+AM |
|---|
| AST, U/l | 100.50±4.50 | 115.50±20.50 | 207.50±10.50 | 121.50±5.50 |
| ALT, U/l | 40.00±2.00 | 26.00±43.00 | 39.00±14.00 | 28.50±2.50 |
| ALP, U/l | 74.50±11.50 | 83.50±1.50 |
160.00±3.00d |
104.50±5.50e |
| B, Day 60 |
| | Groups |
| Parameter | Control | AM | TAA | TAA+AM |
| AST, U/l | 117.50±22.50 | 191.50±5.50 |
364.00±53.00b |
154.50±6.36e |
| ALT, U/l | 41.00±12.00 | 32.00±50.50 |
198.50±6.0b |
31.00±12.00e |
| ALP, U/l | 80.00±5.00 |
153.50±12.50c |
233.00±9.00d |
105.50±10.50f |
VVCs
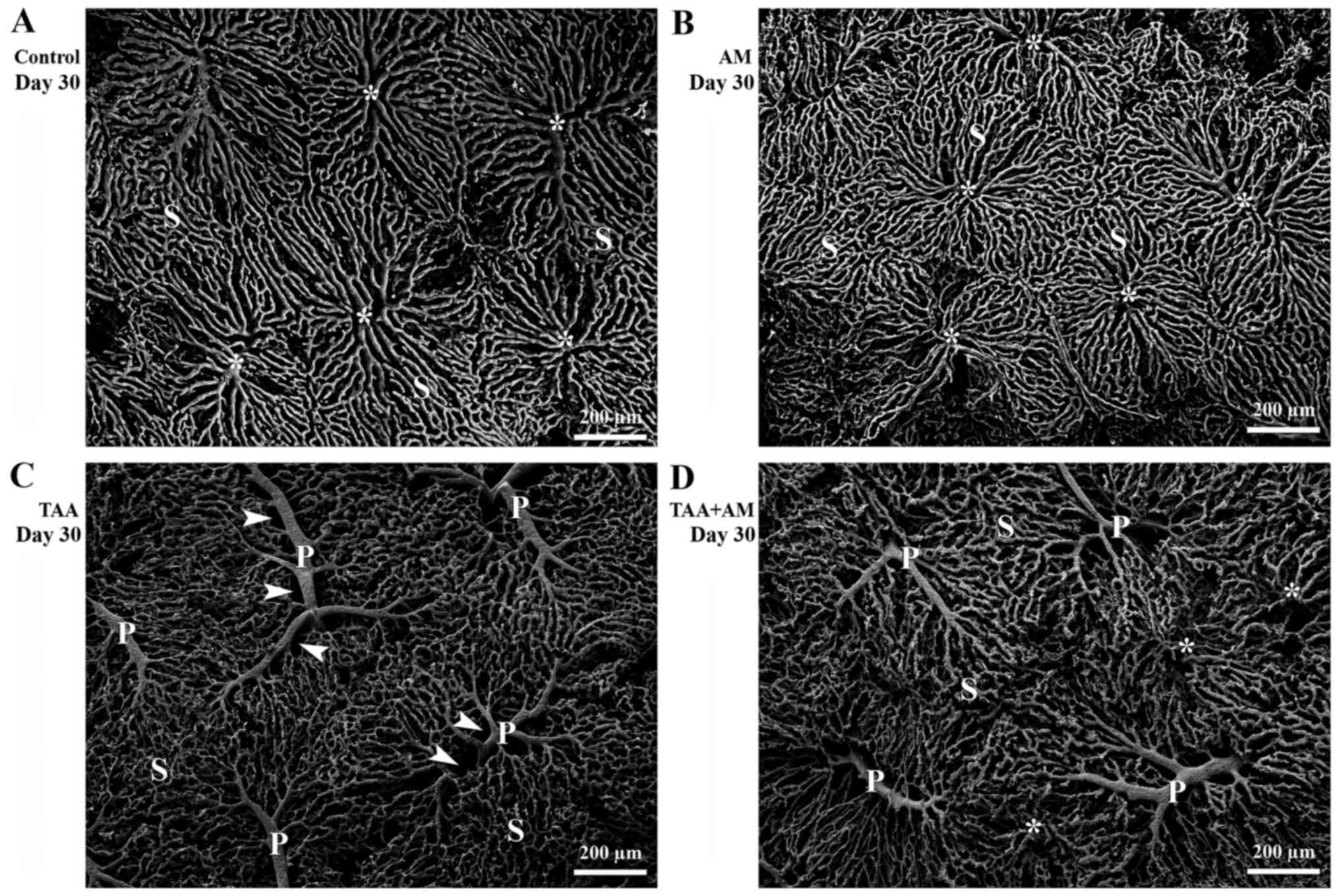
After 30 days of treatment, the surface of the VCCs
of the control rats (Fig. 2A)
revealed a normal hepatic microvascular architecture. The sinusoids
(denoted with an S) were arranged in a distinct continuous pattern,
had a constant diameter, and were typically arranged into lobular
units. The sinusoidal blood drained into central veins (denoted
with asterisks). The sinusoidal network of AM-treated rats
(Fig. 2B) resembled that of the
control rats. The livers of the TAA-treated rats (Fig. 2C) displayed a modified sinusoidal
arrangement, with replacement of the lobular structures with acinar
structures and considerable space (arrowhead) around the portal
vessels (denoted with a P). The livers of the TAA+AM-treated rats
showed changes in the portal vessels and sinusoidal patterns that
resembled that of the TAA-treated rats (Fig. 2D).
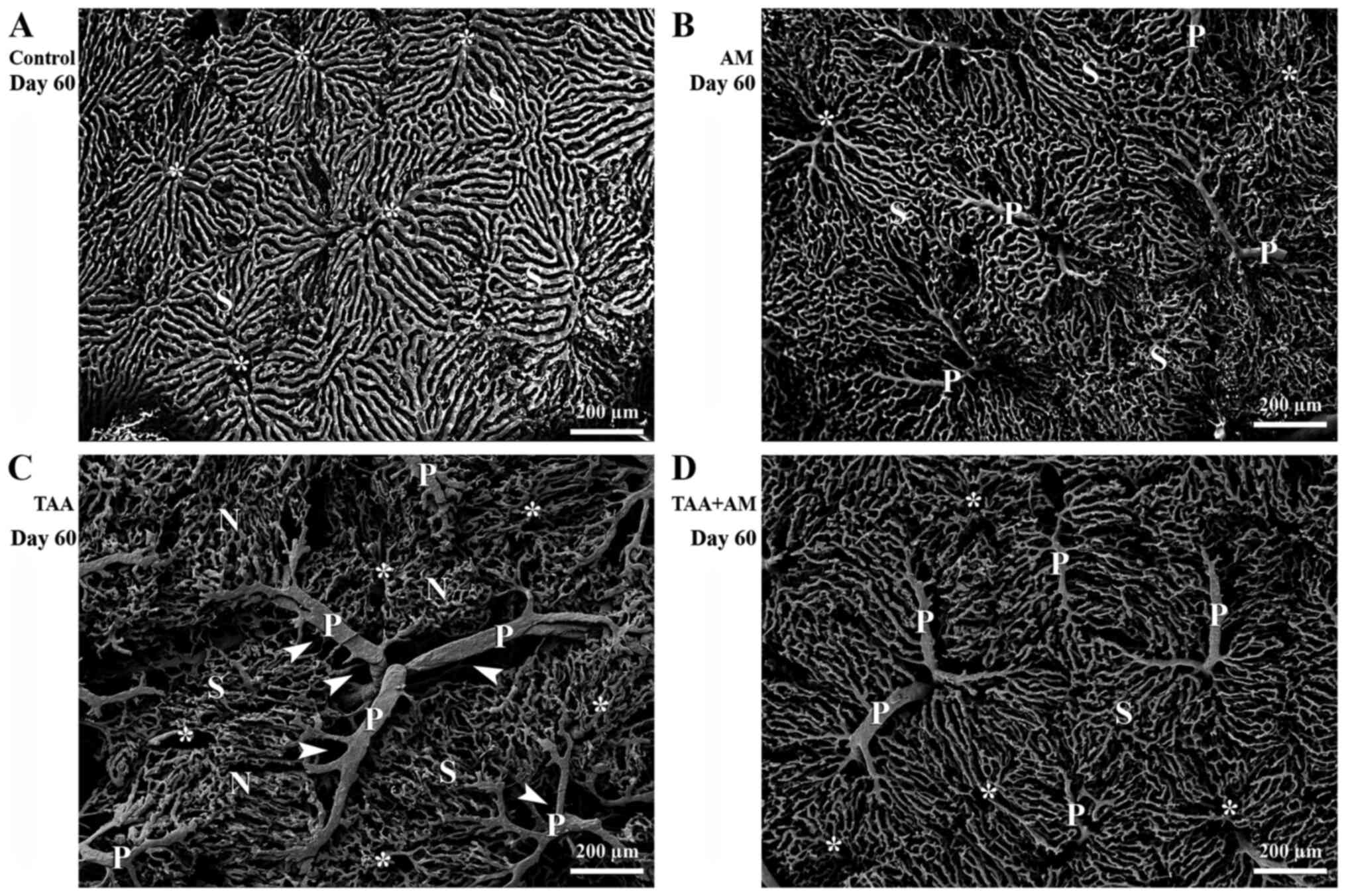
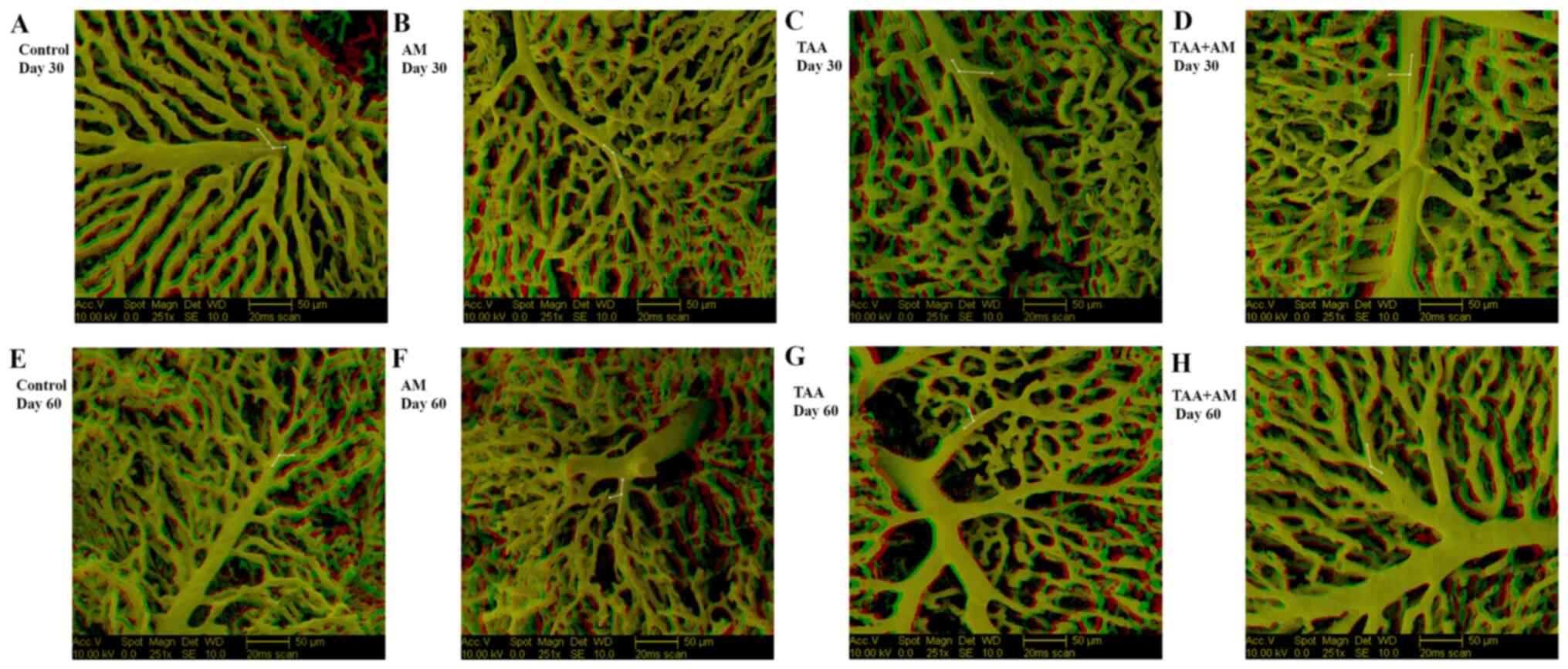
After 60 days of treatment, the superficial
microvascular pattern in the control rats (Fig. 3A) was similar to that of the controls
after 30 days. Portal vessels were well developed in the livers of
AM-treated rats and the microvascular architecture changed only
slightly (Fig. 3B). Significant and
progressive changes in the sinusoidal patterns were found in the
liver of the TAA-treated rats, with substantial space around the
portal vessels (arrowheads; Fig.
3C). Pericentral sinusoids were smaller and more closely
packed, which led to micro-nodule formation (denoted with an N). In
addition, tiny holes in the terminal portal venules near their
branching point were observed. In contrast, the livers of rats
treated with both TAA and AM had less space surrounding the portal
vessels (Fig. 3D), improved
preserved hepatic microvascular patterns, and minimally changed
sinusoidal patterns with few signs of terminal portal venule
remodeling. Thus, TAA and AM treatment partially preserved the
hepatic microvascular architecture. The terminal portal venules of
the TAA and TAA+AM-treated rats after 30 (Fig. 2C and D), and those in the AM, TAA and
TAA+AM-treated rats after 60 days of treatment (Fig. 3B-D) could be followed over a long
distance, whereas those in the control and AM-treated rats at day
30 (Fig. 2A and B) and control rats after day 60 (Fig. 3A) were covered entirely by
sinusoids.
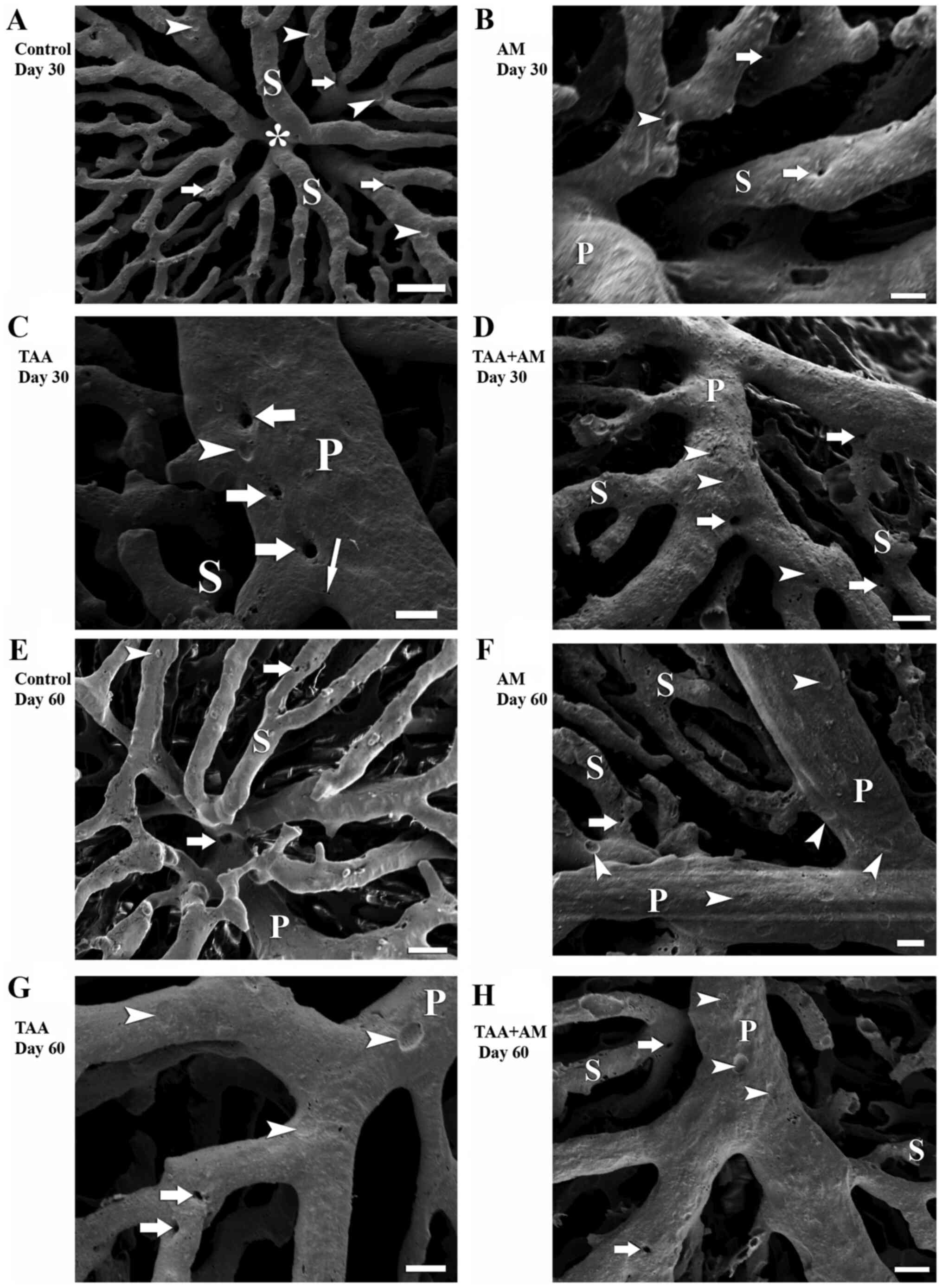
At higher magnifications, the VCCs clearly revealed
that new vessels formed from the original vessel by splitting,
which is termed IA. IA was visible as smooth-edged tiny holes with
a diameter of <5 µm in the VCCs. Tiny holes were frequently
observed in the pericentral area in the control rats (arrows;
Fig. 4A) and rats treated with AM
for 30 days (arrows; Fig. 4B), which
suggested ongoing sinusoidal formation. In addition, tiny holes
arranged in a row were seen on portal venules of TAA-treated rats
after 30 days (arrows; Fig. 4C),
which indicated that the vascular pruning process subsequently
finally led to splitting of an existing blood vessel. IA at the
branching angle of portal venules and sinusoids in the TAA+AM
treated livers (arrows; Fig. 4D).
After 60 days, IA was also observed in the periportal sinusoids of
the control and AM-treated rats (Fig.
4E and F). Tiny holes in the
terminal portal venules near their branching points suggested that
an active branching and remodeling process eventually modified the
branching angle and resulted in sinusoidal hemodynamic changes
(Fig. 4G and H). The comparison of findings after 30 and
60 days of treatment revealed that TAA-induced fibrotic changes
were progressive in time, and that the beneficial effects of AM
only became visible after prolonged treatment, as is shown by
comparison of Figs. 2D and 3D.
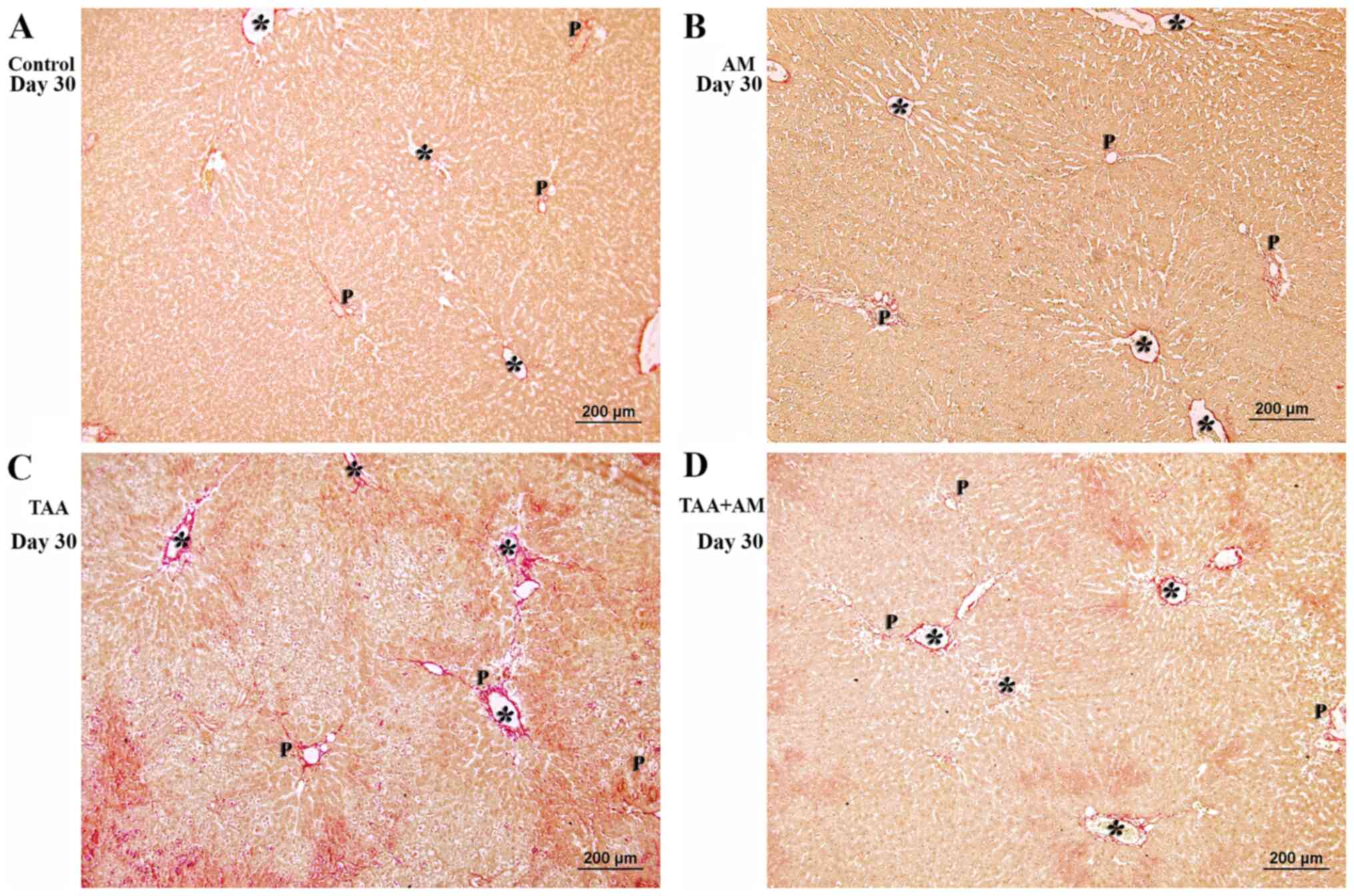
Histomorphology
Fig. 5A shows an
H&E-stained section of control liver parenchyma, which
exhibited a normal lobular architecture containing central or
terminal hepatic venules (denoted by asterisks) and portal tracts.
The livers of the AM-treated rats (Fig.
5B) did not differ from the control livers, with normal hepatic
cords and intact portal structures. Livers of the TAA-treated rats
showed acute hepatocellular injury, lobular necrosis with minimal
to absent necroinflammatory changes (Fig. 5C). Cellular injury and inflammatory
changes in zones 2 and 3, but preserved portal structures were
observed in the livers of the TAA+AM-treated rats (Fig. 5D). Fig.
5E and F show higher
magnifications of the portal tract, with a hepatic artery (denoted
by A), a bile duct (denoted by Bd), a lymph vessel (denoted by Lv)
and a portal venule (denoted by P) in a control and an AM-treated
animal. The periportal area of the TAA-treated rats (Fig. 5G) showed hepatocyte destruction,
whereas various degrees of hydropic changes were also seen in
several hepatocytes. Fig. 5H showed
only minimal periportal hepatocyte injury in the TAA+AM-treated
rats. Fibrosis was noticeable in periportal and perivenular areas
of the TAA-treated rats (Fig. 6C),
but was markedly attenuated in the TAA+AM-treated rats (Fig. 6D).
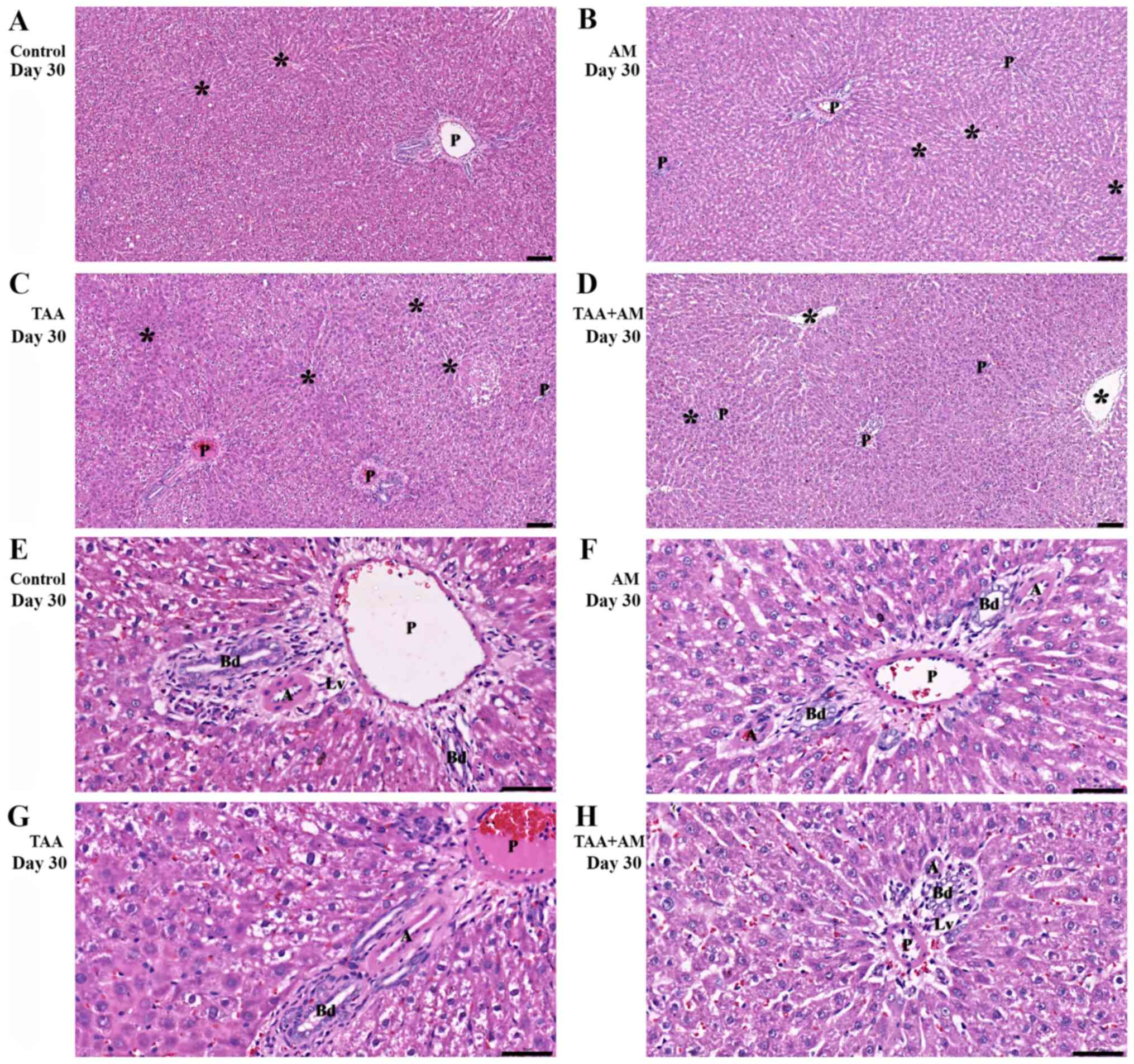 | Figure 5Histomorphology of rat livers after
30 days of treatment. Asterisks mark terminal hepatic venules. (A)
Control and (B) AM-treated rats, with hepatic cords and intact
portal structures. (C) Acute hepatocellular injury with periportal
and perivenular inflammation was observed in the TAA-treated rats.
(D) perivenular hepatocyte injury and intact portal structures in
the TAA+AM-treated rats. Scale bar, 100 μm. An intact portal tract
structure was observed in the (E) control and (F) AM-treated rats.
(G) Periportal and perivenular hepatocyte injury was observed in
the TAA-treated rats. (H) Attenuation of periportal hepatocyte
injury in the TAA and AM-treated rats. Panels E-H are higher
magnifications of panels A-D, respectively. Scale bar, 50 µm. A,
hepatic arteries; Bd, bile ducts; Lv, lymphatic vessels; P, portal
venules; AM, α-mangostin; TAA, thioacetamide. |
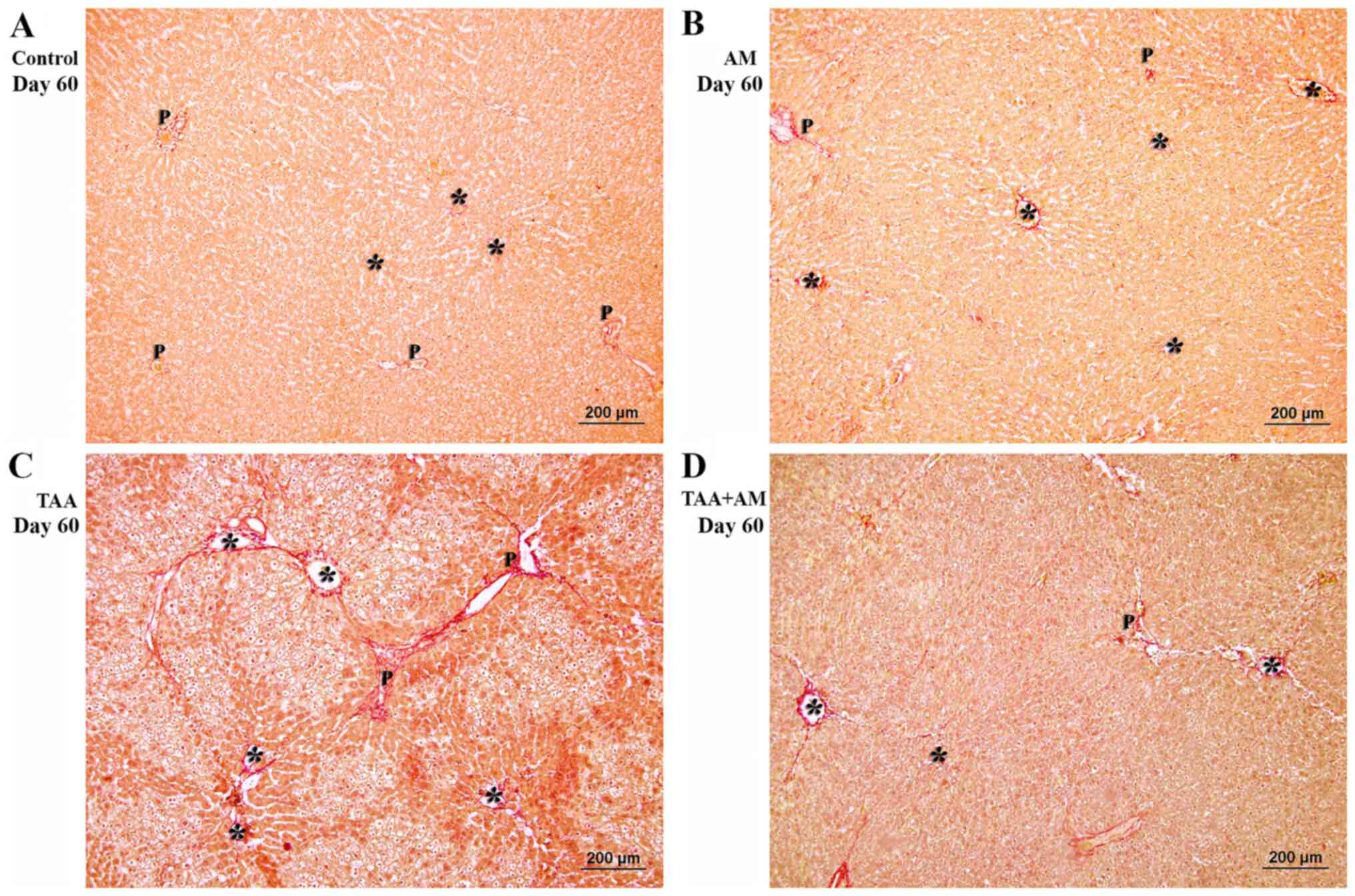
Liver parenchyma of the control rats at 60 days
(Fig. 7A) was similar to that of the
control rats at 30 days. AM-treatment (Fig. 7B) did not affect liver morphology and
showed normal hepatic plates and portal structures. TAA treatment
for 60 days caused fibrotic hepatocellular necroinflammatory
changes (Fig. 7C). By comparison,
the livers of the TAA+AM-treated rats revealed only mild
hepatocellular injury (Fig. 7D). At
higher magnifications, the portal tract of the control and
AM-treated rats (Fig. 7E and
F) showed a normal configuration.
Fibrosis with prominent periportal and perivenular inflammatory
activity and hepatocytes with microvesicular steatosis and
cholestasis (arrowhead; Fig. 7G) was
present in the livers of rats treated with TAA for 60 days.
Additionally, there was no evidence of steatosis or cholestasis,
but perivenular fibrosis was prevalent (Fig. 7H). Fibrous tissue formation in the
periportal areas differed from that in the perivenular areas
(Fig. 8C). This finding is
consistent with the spaces that were observed around the terminal
portal venules in the VCCs of the livers of rats treated with TAA
for 60 days. The periportal structures and perivenular areas in the
livers of rats treated with TAA+AM for 60 days were better
preserved and contained less fibrotic tissue than livers treated
with TAA only (Fig. 8D).
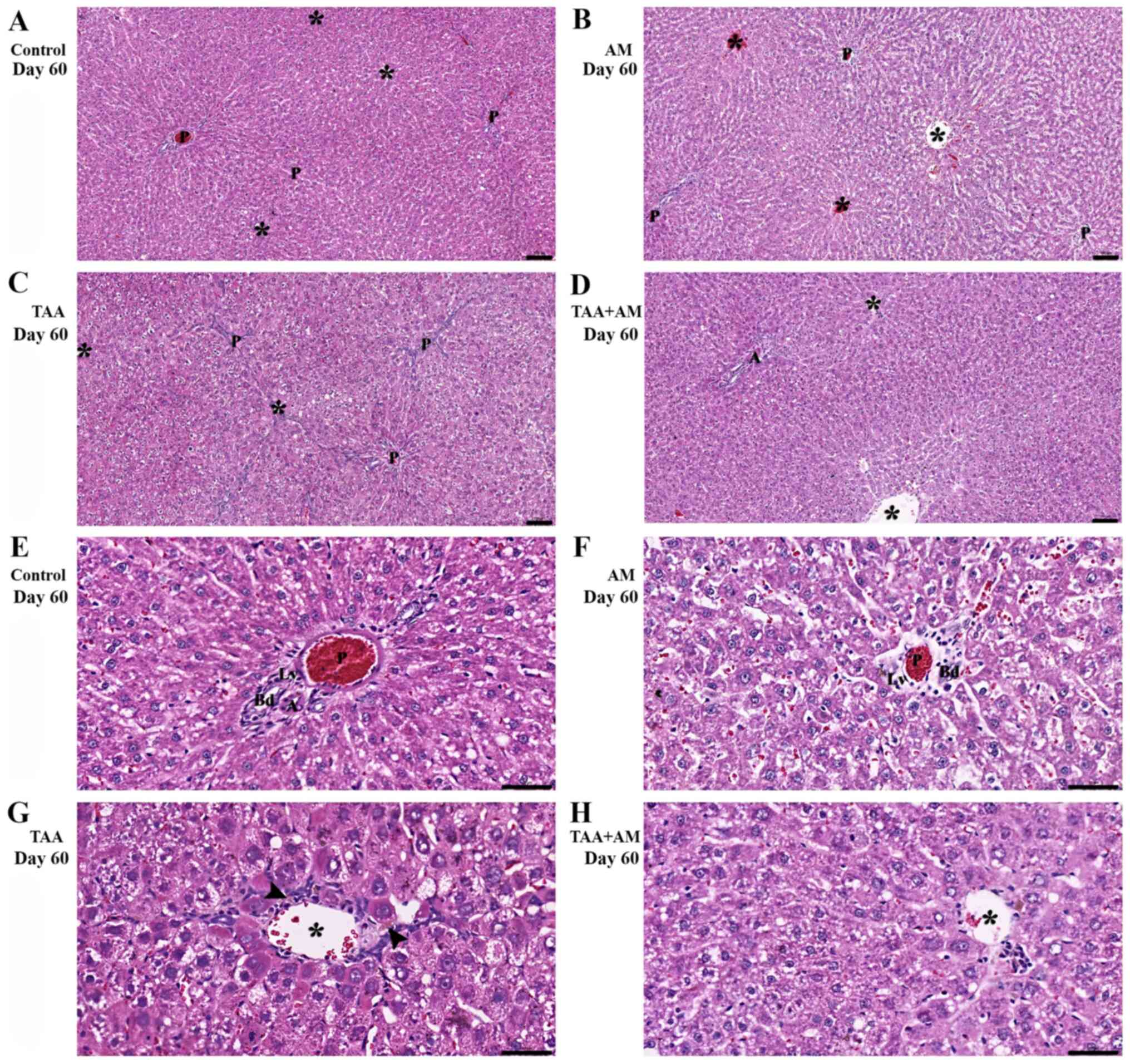 | Figure 7Histomorphology of the rat livers
after 60 days of treatment. Terminal hepatic venules are marked by
asterisks, whereas the arrowheads mark steatosis and cholestasis.
(A, B, E and F) Show normal hepatic cords and intact portal
structures in the control and AM-treated rats. (C) Periportal
fibrosis and perivenular formation of fibrous tissue (arrow) was
observed in the TAA-treated rats. (D) Perivenular fibrosis was also
observed in the rats treated with both TAA and AM. Scale bar, 100
µm. (G) Periportal microvesicular steatosis, cholestasis and
fibrosis, and necroinflammatory was observed in the TAA-treated
rats. (H) Attenuation of the perivenular fibrosis, as well as
absent steatosis and cholestasis was observed in the rats treated
with both TAA and AM. Panels E-H are higher magnifications of
panels A-D, respectively. Scale bar, 50 µm. A, hepatic arteries;
Bd, bile ducts; LV, lymphatic vessels; P, portal venules; AM,
α-mangostin; TAA, thioacetamide. |
After both day 30 and day 60, there was no fibrosis
in the control and AM-treated rats. In rats treated with TAA for 30
days, collagen fibers had expanded into several portal areas,
whereas distinct bridging fibrosis (portal to portal or portal to
central area) had developed after 60 days of TAA treatment.
Fibrosis was significantly decreased in TAA+AM-treated rats
compared with the TAA-treated rats after 30 and 60 days of
treatment (Table III).
 | Table IIIIshak scores of liver sections in the
treated ratsa. |
Table III
Ishak scores of liver sections in the
treated ratsa.
| | Groups |
|---|
| Ishak score | Control | AM | TAA | TAA+AM |
|---|
| Day 30 | 0.0±0.0 | 0.0±0.00 |
3.0±0.0b |
1.25±0.25c |
| Day 60 | 0.0±0.0 | 0.0±0.00 |
4.25±0.25b,d |
1.75±0.25c,d |
3D morphometry
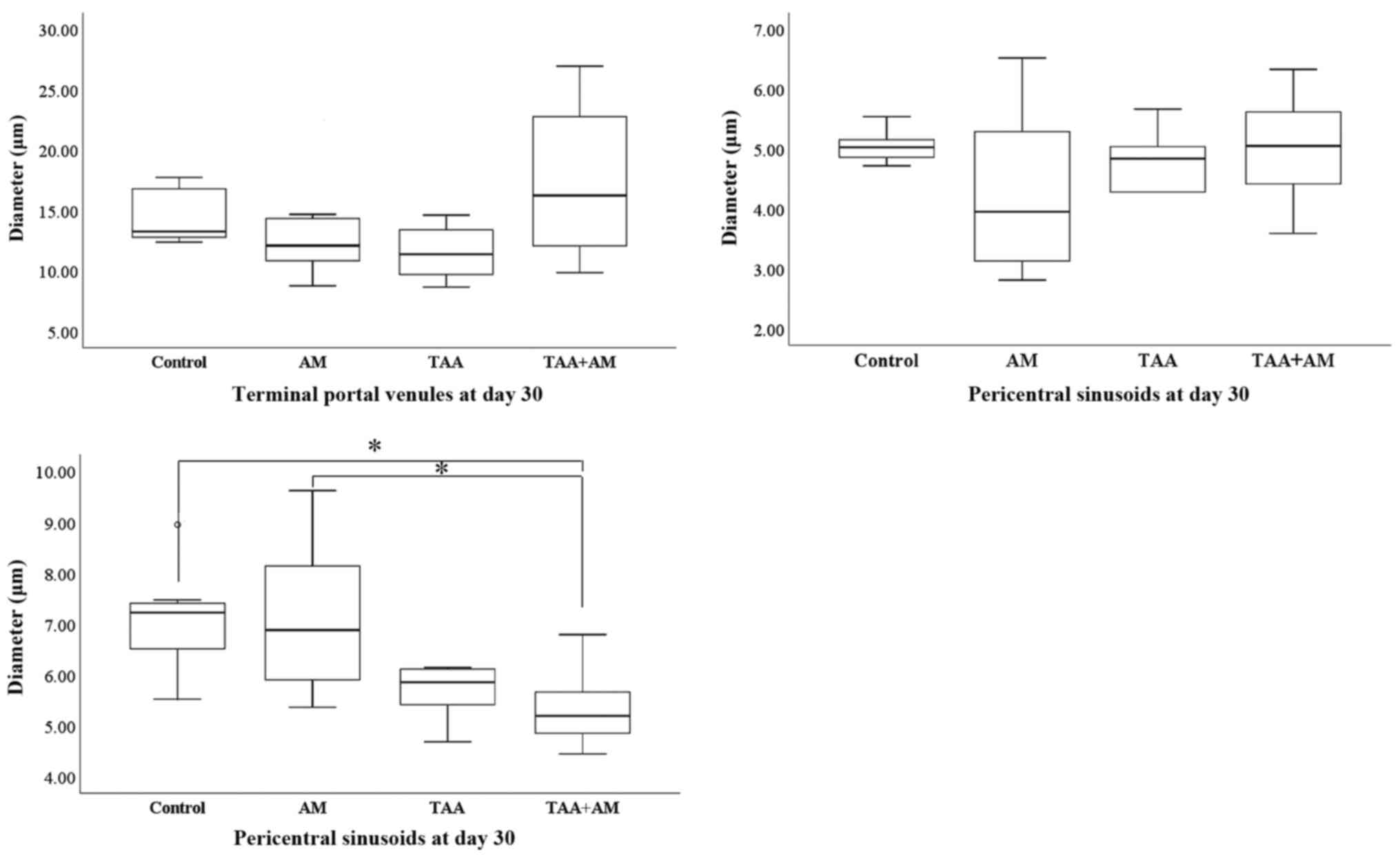
At day 30 of the experiment, the terminal portal
venules and the pericentral sinusoids did not differ between the
controls and any of the experimental groups. However, the diameter
of the periportal sinusoids was significantly smaller in the
TAA+AM-treated rats compared with the control or AM-treated rats
(Fig. 9).
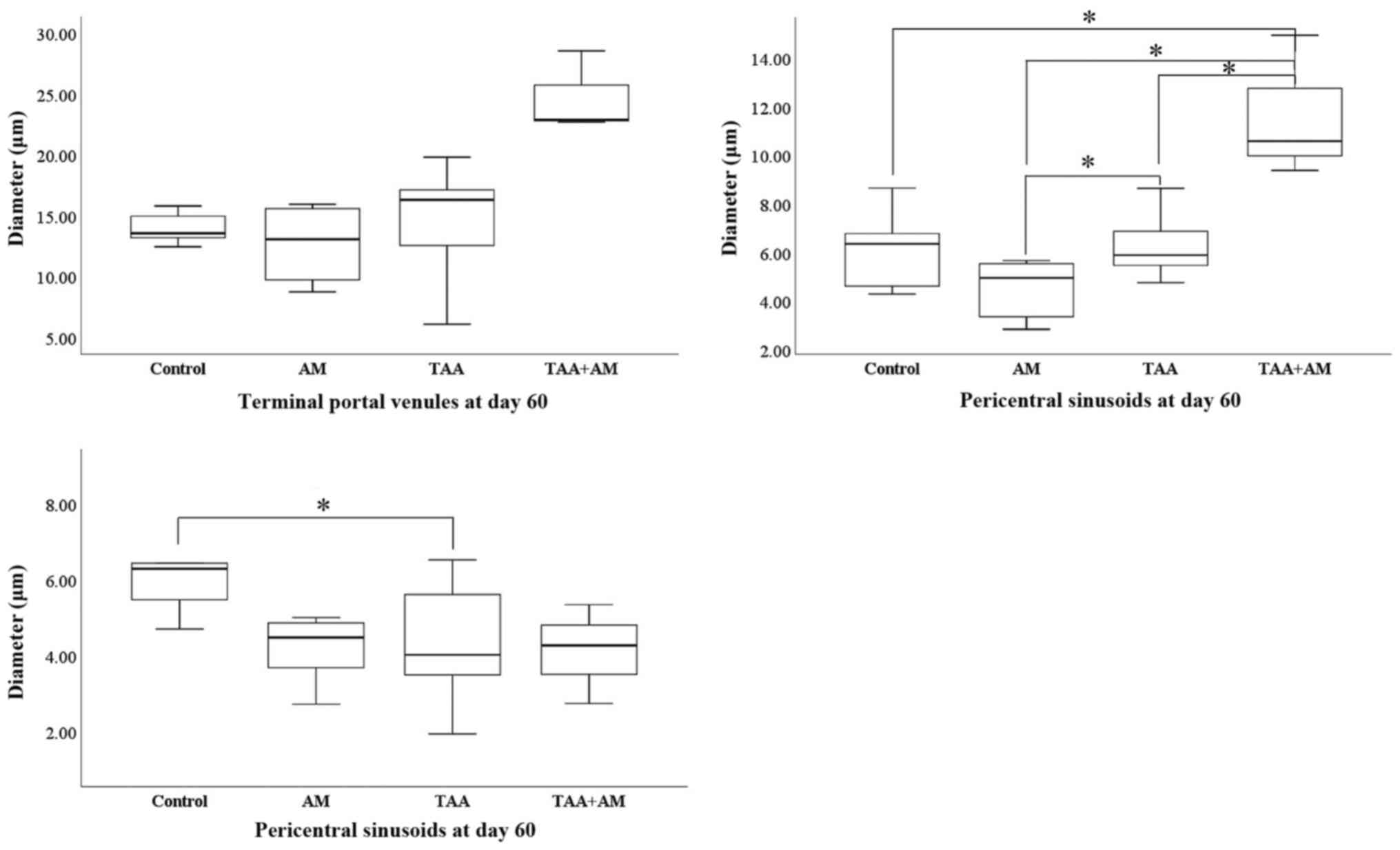
At day 60 of the experiment, none of the treatments
had affected the diameter of the portal venules. However,
TAA-treatment decreased the diameter of the periportal sinusoids
and increased the diameter of the pericentral sinusoids
significantly. Although AM treatment itself did not affect the
diameter of the pericentral or periportal sinusoids, it tended to
restore the diameter of the periportal venules to control values
when added to the TAA treatment, although this effect was not
significant. The TAA+AM treatment did however, further increase the
diameter of the pericentral sinusoids (Fig. 10). At day 30 of the experiment, the
branching angles between portal venules and sinusoids were acute
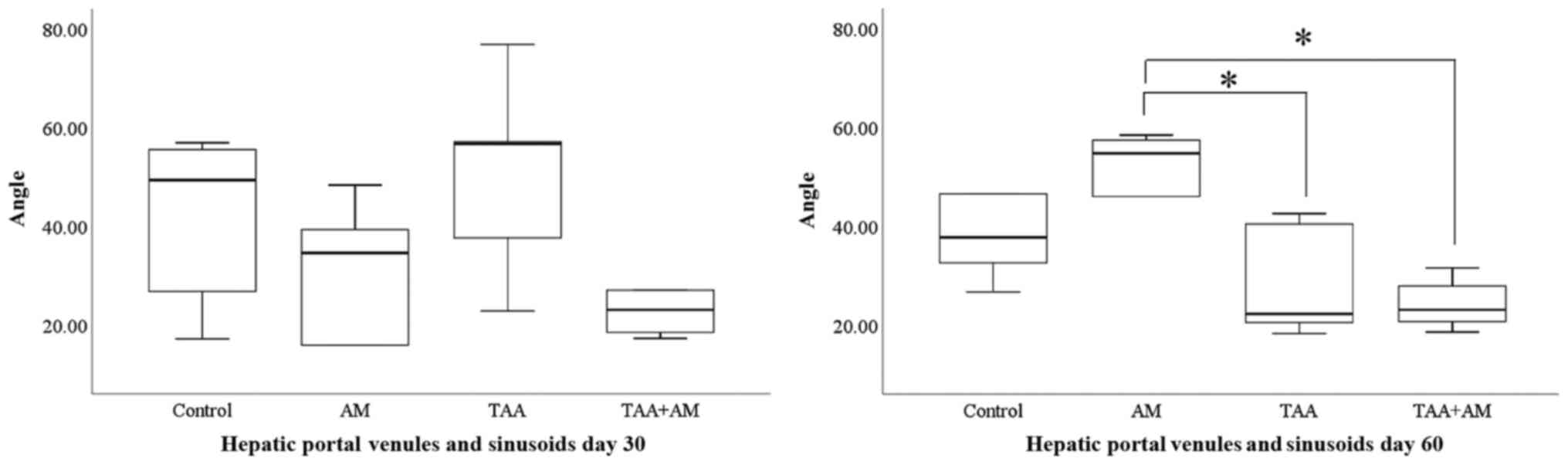
and similar in all groups. At day 60 of the experiment, the
branching angles between the hepatic portal venules and sinusoids
were significantly different between the AM-treated groups compared
with the TAA or TAA+AM-treated groups (Fig. 11). The representative anaglyphic
red-green images of 3D morphometry of the branching angle between
portal venules and sinusoids at day 30 and 60 in each treatment
group are displayed in Fig. 12.
In summary, 3D morphometry confirmed that terminal
portal venules were not affected in any of the groups. Pericentral
sinusoids became wider in the TAA-treated rats during the second
month of toxin administration, particularly if AM was added to the
TAA treatment. Periportal sinusoids, by contrast, became smaller in
the second month of toxin treatment. This effect was already
visible after 1 month if AM was present in the treatment mix.
Discussion
In the present study, it was demonstrated that AM
preserved sinusoidal microvascular architecture and ameliorated
TAA-induced hepatocellular injuries at day 60, but the effects were
not significant after 30 days of treatment. The cause of this
beneficial effect was likely the inhibition of fibrosis, which lead
to preservation of periportal structures and a delay in the
formation of periportal and pericentral fibrosis.
AM may induce morbidity and mortality in animals
with increased dosages (55-57).
AM induced mortality after 72 h of intraperitoneal administration
with a 50% lethal concentration of 150 mg/kg in a mouse model
(57). To minimize the use and
potentially adverse effects of AM, a dosage of TAA and AM that was
75% of that administered by Poonkhum et al (46) was used. In this previous study, TAA
was administered at 200 mg/kg BW and AM at 100 mg/kg BW
(intraperitoneally), where the AM dose was 20x higher than in a
study by Rodniem et al (45),
where TAA was also administered at 200 mg/kg BW, but AM was
administered only at 5 mg/kg BW intraperitoneally. Thus, a 15-fold
higher AM concentration was used comparted with the study by
Rodniem et al (45). In a
pilot study, it was observed that treatment with AM
intraperitoneally at 75 mg/kg BW did improve the blood vessel
architecture in fibrotic liver without visible effects (data not
shown). AM was clearly effective when 75 mg/kg BW was administered
twice weekly. The primary effect of AM appeared to be an
anti-fibrotic effect, as it showed anti-fibrogenic action in
histological sections. Anti-fibrotic effects of AM preserved the
vascular architecture under pro-oxidative conditions, such as TAA
treatment. These findings underscore earlier studies that
demonstrated that AM lowers the liver area occupied by type I
collagen (46). At a dose of 100
mg/kg BW twice weekly, AM also reduced the risk of liver fibrosis
through a decrease in p53 expression when examining induction of
cirrhosis by TAA (58). Even a low
dose of AM (5 mg/kg BW) prevented TAA from inducing hepatocyte
damage based on the circulating hepatic enzyme concentrations and
fibrotic changes in the liver (45).
Relatively little information exists regarding the pharmacokinetics
and pharmacodynamics of AM in rats, as only a few dosages and
routes or intervals of administration have been studied previously
(45,59-62).
Further experiments along these lines are therefore warranted to
determine the optimal anti-fibrogenic and anti-angiogenetic
outcomes.
In the present study, it was shown that TAA-induced
fibrosis at 150 mg/kg BW when administered 3 times weekly, and this
caused tissue damage in the liver. In support of these findings, a
previous study demonstrated that liver sinusoidal endothelial cell
differentiation activated hepatic stellate cells and the associated
fibrotic processes (63), which
resulted in perivascular fibrosis (such as periportal and
perivenular fibrosis), and this finally led to cirrhosis. A
short-term low dose of TAA (50 mg/kg BW) can induce inflammation
(64). TAA is metabolized by
cytochrome P4502E1 into thioacetamides sulfoxide and
thioacetamide-S, S-dioxide (65).
These metabolites, whether they are further oxidized or not, form
species that are toxic to hepatocytes (66,67).
IA is a relatively more recently discovered novel
means of blood vessel formation and provides a mechanism for the
expansion of an existing microvascular network and vascular branch
remodeling in both normal and pathological conditions (68). The presence of tiny holes with a
diameter of 2-5 µm, which indicate signs of IA, are identifiable by
microvascular corrosion casting and SEM. Fibrotic livers exhibit
growth of numerous vessels through IA. The corrosion casts
confirmed these data, showing the presence of numerous tiny holes
within the hepatic vessels of fibrotic livers. It is speculated
that the greater degree of fibrosis may contribute to the relative
frequency of IA observed. Thus, microvascular alterations by IA may
be a pivotal pathogenetic mechanisms in the progression of
fibrosis. Interestingly, a recent study showed that IA was observed
in the lungs of patients with COVID-19 and also in the lungs of
patients with influenza (69). It
would therefore be tempting to speculate that AM may exert
anti-angiogenetic activity on continuous endothelium lined
intrahepatic vessels, and this may be the result of diminished IA
processes.
The progression of hepatocyte injuries is associated
with intrahepatic angiogenesis and fibrosis, which ultimately leads
to cirrhosis. The results showing that AM preserves the hepatic
microvascular pattern, periportal structure and perivenular
sinusoids underscores the hepatoprotective effect of AM on liver
fibrosis. AM extracts, therefore, appear to exert a mitigating
effect on liver fibrosis and angiogenesis. Further studies should
address the mechanism underlying the anti-angiogenic and
anti-fibrogenic effects of AM on intrahepatic vessels and
sinusoids.
Acknowledgements
We would like to thank Dr Terdsak Yano (Department
of Food Animal Clinic) and Dr Kannika Na Lampang (Department of
Veterinary Biosciences and Public Health, Faculty of Veterinary
Medicine, Chiang Mai University) for their assistance with the
statistical analysis; Associate Professor Primchanien Mongkarndi
(Department of Microbiology, Faculty of Pharmacy, Mahidol
University) for assisting with α-Mangostin extraction; Professor
Wouter H. Lamers (Department of Anatomy and Embryology, Maastricht
University) for manuscript corrections, and Associate Professor
Korakot Nganvongpanit (Department of Veterinary Biosciences and
Public Health, Faculty of Veterinary Medicine, Chiang Mai
University) for the valuable comments provided.
Funding
Funding: This study was supported by the Faculty of Veterinary
Medicine, Chiang Mai University (grant no. R000009358) and
Ernst-Mach-Stipendien (Ernst-Mach weltweit TSO; grant no.
ICM-02001) Austrian Agency for International Cooperation in
Education and Research OeAD-GmbH, Center for international
Cooperation and Mobility (ICM).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Author's contributions
WT and WP conceived and designed the study, and also
performed the histological examination of the liver. AL assisted
with the vascular corrosion casting technique and SEM and 3D
morphometry, and qualitatively analyzed the vascular corrosion
casts. WT performed the experiments, analyzed and interpreted the
quantitative 3D morphometry data, and was a major contributor in
writing the manuscript with support from WP. All authors have read
and approved the final manuscript. WP and AL confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
All of the experiments were approved by the
Institutional Animal Care and Use Committee at the Faculty of
Veterinary Medicine, Chiang Mai University (approval no.
A.20/2555)
Patient consent for publication
Not applicable.
Competing interests
The authors agree that they have no competing
interests.
References
|
1
|
Aydın MM and Akçalı KC: Liver fibrosis.
Turk J Gastroenterol. 29:14–21. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Parola M and Pinzani M: Liver fibrosis:
Pathophysiology, pathogenetic targets and clinical issues. Mol
Aspects Med. 65:37–55. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Vollmar B and Menger MD: The hepatic
microcirculation: Mechanistic contributions and therapeutic targets
in liver injury and repair. Physiol Rev. 89:1269–1339.
2009.PubMed/NCBI View Article : Google Scholar
|
|
4
|
McCuskey RS: The hepatic microvascular
system in health and its response to toxicants. Anat Rec (Hoboken).
291:661–671. 2008.PubMed/NCBI View
Article : Google Scholar
|
|
5
|
Lee UE and Friedman SL: Mechanisms of
hepatic fibrogenesis. Best Pract Res Clin Gastroenterol.
25:195–206. 2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wallace K, Burt AD and Wright MC: Liver
fibrosis. Biochem J. 411:1–18. 2008.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lamireau T, Desmoulière A, Bioulac-Sage P
and Rosenbaum J: Mechanisms of hepatic fibrogenesis. Arch Pediatr.
9:392–405. 2002.PubMed/NCBI View Article : Google Scholar : (In French).
|
|
8
|
Makanya AN, Hlushchuk R and Djonov VG:
Intussusceptive angiogenesis and its role in vascular
morphogenesis, patterning, and remodeling. Angiogenesis.
12:113–123. 2009.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Carmeliet P and Jain RK: Molecular
mechanisms and clinical applications of angiogenesis. Nature.
473:298–307. 2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Caballería L, Pera G, Arteaga I, Rodríguez
L, Alumà A, Morillas RM, de la Ossa N, Díaz A, Expósito C, Miranda
D, et al: high prevalence of liver fibrosis among european adults
with unknown liver disease: A population-based study. Clin
Gastroenterol Hepatol. 16:1138–1145.e5. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ginès P, Graupera I, Lammert F, Angeli P,
Caballeria L, Krag A, Guha IN, Murad SD and Castera L: Screening
for liver fibrosis in the general population: A call for action.
Lancet Gastroenterol Hepatol. 1:256–260. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Raghu C, Ekena J, Cullen JM, Webb CB and
Trepanier LA: Evaluation of potential serum biomarkers of hepatic
fibrosis and necroinflammatory activity in dogs with liver disease.
J Vet Intern Med. 32:1009–1018. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Eulenberg VM and Lidbury JA: Hepatic
fibrosis in dogs. J Vet Intern Med. 32:26–41. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Watson P: Canine breed-specific
hepatopathies. Vet Clin North Am Small Anim Pract. 47:665–682.
2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Verhoef JNC, Allen AL, Harding JCS and
Al-Dissi AN: Metallothionein expression in horses with chronic
liver disease and its correlation with Ki-67 immunoreactivity. Vet
Pathol. 55:703–710. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Perricone G, Vangeli M and Belli LS:
Treatment of patients with cirrhosis. N Engl J Med.
375(2103)2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Li X, Zhu L, Wang B, Yuan M and Zhu R:
Drugs and targets in fibrosis. Front Pharmacol. 8:855.
2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kaur V, Kumar M, Kaur P and Kaur S, Singh
AP and Kaur S: Hepatoprotective activity of Butea monosperma
bark against thioacetamide-induced liver injury in rats. Biomed
Pharmacother. 89:332–341. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Sukalingam K, Ganesan K and Xu B:
Protective effect of aqueous extract from the leaves of Justicia
tranquebariesis against thioacetamide-induced oxidative stress
and hepatic fibrosis in rats. Antioxidants. 7(7)2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kyung EJ, Kim HB, Hwang ES, Lee S, Choi
BK, Kim JW, Kim HJ, Lim SM, Kwon OI and Woo EJ: Evaluation of
Hepatoprotective Effect of curcumin on liver cirrhosis using a
combination of biochemical analysis and magnetic resonance-based
electrical conductivity imaging. Mediators Inflamm.
2018(5491797)2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Foti RS, Pearson JT, Rock DA, Wahlstrom JL
and Wienkers LC: In vitro inhibition of multiple cytochrome P450
isoforms by xanthone derivatives from mangosteen extract. Drug
Metab Dispos. 37:1848–1855. 2009.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Gopalakrishnan G, Banumathi B and Suresh
G: Evaluation of the antifungal activity of natural xanthones from
Garcinia mangostana and their synthetic derivatives. J Nat
Prod. 60:519–524. 1997.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Obolskiy D, Pischel I, Siriwatanametanon N
and Heinrich M: Garcinia mangostana L.: A phytochemical and
pharmacological review. Phytother Res. 23:1047–1065.
2009.PubMed/NCBI View
Article : Google Scholar
|
|
24
|
Martínez A, Galano A and Vargas R: Free
radical scavenger properties of α-mangostin:. Thermodynamics and
kinetics of HAT and RAF mechanisms. J Phys Chem B. 115:12591–12598.
2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Buelna-Chontal M, Correa F,
Hernández-Reséndiz S, Zazueta C and Pedraza-Chaverri J: Protective
effect of α-mangostin on cardiac reperfusion damage by attenuation
of oxidative stress. J Med Food. 14:1370–1374. 2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ngawhirunpat T, Opanasopi P, Sukma M,
Sittisombut C, Kat A and Adachi I: Antioxidant, free
radical-scavenging activity and cytotoxicity of different solvent
extracts and their phenolic constituents from the fruit hull of
mangosteen (Garcinia mangostana). Pharm Biol. 48:55–62.
2010.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Pedraza-Chaverrí J, Reyes-Fermín LM,
Nolasco-Amaya EG, Orozco-Ibarra M, Medina-Campos ON,
González-Cuahutencos O, Rivero-Cruz I and Mata R: ROS scavenging
capacity and neuroprotective effect of alpha-mangostin against
3-nitropropionic acid in cerebellar granule neurons. Exp Toxicol
Pathol. 61:491–501. 2009.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Koh JJ, Qiu S, Zou H, Lakshminarayanan R,
Li J, Zhou X, Tang C, Saraswathi P, Verma C, Tan DT, et al: Rapid
bactericidal action of alpha-mangostin against MRSA as an outcome
of membrane targeting. Biochim Biophys Acta. 1828:834–844.
2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Dharmaratne HR, Sakagami Y, Piyasena KG
and Thevanesam V: Antibacterial activity of xanthones from
Garcinia mangostana (L.) and their structure-activity
relationship studies. Nat Prod Res. 27:938–941. 2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Liu SH, Lee LT, Hu NY, Huange KK, Shih YC,
Munekazu I, Li JM, Chou TY, Wang WH and Chen TS: Effects of
alpha-mangostin on the expression of anti-inflammatory genes in
U937 cells. Chin Med. 7(19)2012.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Chen LG, Yang LL and Wang CC:
Anti-inflammatory activity of mangostins from Garcinia
mangostana. Food Chem Toxicol. 46:688–693. 2008.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Chomnawang MT, Surassmo S, Nukoolkarn VS
and Gritsanapan W: Effect of Garcinia mangostana on
inflammation caused by Propionibacterium acnes. Fitoterapia.
78:401–408. 2007.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Chin YW, Shin E, Hwang BY and Lee MK:
Antifibrotic constituents from Garcinia mangostana. Nat Prod
Commun. 6:1267–1268. 2011.PubMed/NCBI
|
|
34
|
Wang JJ, Shi QH, Zhang W and Sanderson BJ:
Anti-skin cancer properties of phenolic-rich extract from the
pericarp of mangosteen (Garcinia mangostana Linn.). Food
Chem Toxicol. 50:3004–3013. 2012.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kurose H, Shibata MA, Iinuma M and Otsuki
Y: Alterations in cell cycle and induction of apoptotic cell death
in breast cancer cells treated with α-mangostin extracted from
mangosteen pericarp. J Biomed Biotechnol.
2012(672428)2012.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Krajarng A, Nilwarankoon S, Suksamrarn S
and Watanapokasin R: Antiproliferative effect of α-mangostin on
canine osteosarcoma cells. Res Vet Sci. 93:788–794. 2012.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Kaomongkolgit R: Alpha-mangostin
suppresses MMP-2 and MMP-9 expression in head and neck squamous
carcinoma cells. Odontology. 101:227–232. 2013.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Johnson JJ, Petiwala SM, Syed DN,
Rasmussen JT, Adhami VM, Siddiqui IA, Kohl AM and Mukhtar H:
α-Mangostin, a xanthone from mangosteen fruit, promotes cell cycle
arrest in prostate cancer and decreases xenograft tumor growth.
Carcinogenesis. 33:413–419. 2012.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Aisha AF, Abu-Salah KM, Ismail Z and Majid
AM: alpha-Mangostin enhances betulinic acid cytotoxicity and
inhibits cisplatin cytotoxicity on HCT 116 colorectal carcinoma
cells. Molecules. 17:2939–2954. 2012.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Chao AC, Hsu YL, Liu CK and Kuo PL:
α-Mangostin, a dietary xanthone, induces autophagic cell death by
activating the AMP-activated protein kinase pathway in glioblastoma
cells. J Agric Food Chem. 59:2086–2096. 2011.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Shiozaki T, Fukai M, Hermawati E,
Juliawaty LD, Syah YM, Hakim EH, Puthongking P, Suzuki T, Kinoshita
K, Takahashi K, et al: Anti-angiogenic effect of alpha-mangostin. J
Nat Med. 67:202–206. 2013.PubMed/NCBI View Article : Google Scholar
|
|
42
|
National Research Council Committee for
the Update of the Guide for the Care and Use of Laboratory A: The
National Academies Collection: Reports funded by National
Institutes of Health. In: Guide for the Care and Use of Laboratory
Animals. National Academies Press (US). National Academy of
Sciences, Washington, DC, 2011.
|
|
43
|
Sattayasai J, Chaonapan P, Arkaravichie T,
Soi-Ampornkul R, Junnu S, Charoensilp P, Samer J, Jantaravinid J,
Masaratana P, Suktitipat B, et al: Protective effects of mangosteen
extract on H2O2-induced cytotoxicity in
SK-N-SH cells and scopolamine-induced memory impairment in mice.
PLoS One. 8(e85053)2013.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Moongkarndi P, Srisawat C, Saetun P,
Jantaravinid J, Peerapittayamongkol C, Soi-ampornkul R, Junnu S,
Sinchaikul S, Chen ST, Charoensilp P, et al: Protective effect of
mangosteen extract against beta-amyloid-induced cytotoxicity,
oxidative stress and altered proteome in SK-N-SH cells. J Proteome
Res. 9:2076–2086. 2010.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Rodniem S, Tiyao V, Nilbu-Nga C, Poonkhum
R, Pongmayteegul S and Pradidarcheep W: Protective effect of
alpha-mangostin on thioacetamide-induced liver fibrosis in rats as
revealed by morpho-functional analysis. Histol Histopathol.
34:419–430. 2019.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Poonkhum R, Watanapokasin R and
Pradidarcheep W: Protective effect of alpha-mangostin against
type-I collagen formation in thioacetamide-induced cirrhotic rat. J
Med Assoc Thai. 95 (Suppl 12):S93–S98. 2012.PubMed/NCBI
|
|
47
|
Lametschwandtner A, Lametschwandtner U and
Weiger T: Scanning electron microscopy of vascular corrosion casts
- technique and applications: Updated review. Scanning Microsc.
4:889–940; discussion 941. 1990.PubMed/NCBI
|
|
48
|
Lametschwandtner A, Miodonski A and
Simonsberger P: On the prevention of specimen charging in scanning
electron microscopy of vascular corrosion casts by attaching
conductive bridges. Mikroskopie. 36:270–273. 1980.PubMed/NCBI
|
|
49
|
Standish RA, Cholongitas E, Dhillon A,
Burroughs AK and Dhillon AP: An appraisal of the histopathological
assessment of liver fibrosis. Gut. 55:569–578. 2006.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Schumann G, Bonora R, Ceriotti F, Férard
G, Ferrero CA, Franck PF, Gella FJ, Hoelzel W, Jørgensen PJ, Kanno
T, et al: International Federation of Clinical Chemistry and
Laboratory Medicine: IFCC primary reference procedures for the
measurement of catalytic activity concentrations of enzymes at 37
degrees C. International Federation of Clinical Chemistry and
Laboratory Medicine. Part 4. Reference procedure for the
measurement of catalytic concentration of alanine aminotransferase.
Clin Chem Lab Med. 40:718–724. 2002.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Schumann G, Bonora R, Ceriotti F, Férard
G, Ferrero CA, Franck PF, Gella FJ, Hoelzel W, Jørgensen PJ, Kanno
T, et al: International Federation of Clinical Chemistry and
Laboratory Medicine: IFCC primary reference procedures for the
measurement of catalytic activity concentrations of enzymes at 37
degrees C. International Federation of Clinical Chemistry and
Laboratory Medicine. Part 5. Reference procedure for the
measurement of catalytic concentration of aspartate
aminotransferase. Clin Chem Lab Med. 40:725–733. 2002.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Schumann G, Klauke R, Canalias F,
Bossert-Reuther S, Franck PF, Gella FJ, Jørgensen PJ, Kang D,
Lessinger JM, Panteghini M, et al: IFCC primary reference
procedures for the measurement of catalytic activity concentrations
of enzymes at 37˚C. Part 9: Reference procedure for the measurement
of catalytic concentration of alkaline phosphatase International
Federation of Clinical Chemistry and Laboratory Medicine (IFCC)
Scientific Division, Committee on Reference Systems of Enzymes
(C-RSE) (1)). Clin Chem Lab Med. 49:1439–1446. 2011.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Minnich B and Lametschwandtner A: Lengths
measurements in microvascular corrosion castings: Two-dimensional
versus three-dimensional morphometry. Scanning. 22:173–177.
2000.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Minnich B, Leeb H, Bernroider EW and
Lametschwandtner A: Three-dimensional morphometry in scanning
electron microscopy: A technique for accurate dimensional and
angular measurements of microstructures using stereopaired
digitized images and digital image analysis. J Microsc. 195:23–33.
1999.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Do HTT and Cho J: Mangosteen pericarp and
its bioactive xanthones: Potential therapeutic value in Alzheimer's
disease, Parkinson's disease, and depression with pharmacokinetic
and safety profiles. Int J Mol Sci. 21(21)2020.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Kittipaspallop W, Taepavarapruk P,
Chanchao C and Pimtong W: Acute toxicity and teratogenicity of
α-mangostin in zebrafish embryos. Exp Biol Med (Maywood).
243:1212–1219. 2018.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Choi YH, Han SY, Kim YJ, Kim YM and Chin
YW: Absorption, tissue distribution, tissue metabolism and safety
of α-mangostin in mangosteen extract using mouse models. Food Chem
Toxicol. 66:140–146. 2014.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Supawadee S, Thanet S, Wisut P, Somneuk N,
Sirinun N and Ramida W: Investigation of therapeutic effects of
α-mangostin on thioacetamide-induced cirrhosis in rats. J Med Assoc
Thai. 98 (Suppl 9):S91–S97. 2015.PubMed/NCBI
|
|
59
|
Ghasemzadeh Rahbardar M, Razavi BM and
Hosseinzadeh H: Investigating the ameliorative effect of
alpha-mangostin on development and existing pain in a rat model of
neuropathic pain. Phytother Res. 34:3211–3225. 2020.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Ibrahim MY, Hashim NM, Mariod AA, Mohan S,
Abdulla MA, Abdelwahab SI and Arbab IA: α-Mangostin from
Garcinia mangostana Linn: An updated review of its
pharmacological properties. Arab J Chem. 9:317–329. 2016.
|
|
61
|
Upegui Y, Robledo SM, Gil Romero JF,
Quiñones W, Archbold R, Torres F, Escobar G, Nariño B and Echeverri
F: In vivo Antimalarial Activity of α-Mangostin and the New
Xanthone δ-Mangostin. Phytother Res. 29:1195–1201. 2015.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Petiwala SM, Li G, Ramaiya A, Kumar A,
Gill RK, Saksena S and Johnson JJ: Pharmacokinetic characterization
of mangosteen (Garcinia mangostana) fruit extract
standardized to α-mangostin in C57BL/6 mice. Nutr Res. 34:336–345.
2014.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Xie G, Wang X, Wang L, Wang L, Atkinson
RD, Kanel GC, Gaarde WA and Deleve LD: Role of differentiation of
liver sinusoidal endothelial cells in progression and regression of
hepatic fibrosis in rats. Gastroenterology. 142:918–927.e6.
2012.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Schyman P, Printz RL, Estes SK, Boyd KL,
Shiota M and Wallqvist A: Identification of the toxicity pathways
associated with thioacetamide-induced injuries in rat liver and
kidney. Front Pharmacol. 9(1272)2018.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Porter WR and Neal RA: Metabolism of
thioacetamide and thioacetamide S-oxide by rat liver microsomes.
Drug Metab Dispos. 6:379–388. 1978.PubMed/NCBI
|
|
66
|
Spira B and Raw I: The effect of
thioacetamide on the activity and expression of cytosolic rat liver
glutathione-S-transferase. Mol Cell Biochem. 211:103–110.
2000.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Dyroff MC and Neal RA: Studies of the
mechanism of metabolism of thioacetamide s-oxide by rat liver
microsomes. Mol Pharmacol. 23:219–227. 1983.PubMed/NCBI
|
|
68
|
Mentzer SJ and Konerding MA:
Intussusceptive angiogenesis: Expansion and remodeling of
microvascular networks. Angiogenesis. 17:499–509. 2014.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Ackermann M, Verleden SE, Kuehnel M,
Haverich A, Welte T, Laenger F, Vanstapel A, Werlein C, Stark H,
Tzankov A, et al: Pulmonary Vascular Endothelialitis, Thrombosis,
and Angiogenesis in Covid-19. N Engl J Med. 383:120–128.
2020.PubMed/NCBI View Article : Google Scholar
|