Introduction
Obesity has been classified as an epidemic in the
United States for >30 years. Recent data (2020) from the Centers
for Disease Control (CDC) place the prevalence of obesity in US
adults at 42.4% in 2017-2018, up from 30.5% in 1999-2000, while the
prevalence of severe obesity has increased from 4.7 to 9.2% during
the same period (1). Worldwide,
>1.9 billion adults are overweight, and >600 million adults
are obese (2). In the USA, childhood
obesity affects ~12.5 million children and teens. Data in a
nationally representative study of US children and adolescents aged
2-19 years showed that the prevalence of obesity in 2011-2014 was
17.0% and that of extreme obesity was 5.8% (3,4). Obesity
has been associated with increased morbidities and mortality rates,
including diabetes, cardiovascular disease, several types of cancer
and liver steatosis (5).
Importantly, the incidence of these life-threatening comorbidities
increases with the duration of obesity, and therefore, age.
Non-alcoholic fatty liver disease (NAFLD), the major
cause of abnormal liver function in the US and worldwide, is often
associated with obesity and diabetes (5). The mortality rates in individuals with
NAFLD is significantly higher than in the general population, with
liver-related complications being a common cause of death (6). An estimated 70 million adults and 7
million US children have NAFLD. Amongst the children with obesity,
NAFLD is present in 33-58% of cases, and it is now the most common
cause of chronic liver disease in the pediatric population
(7). Data from our laboratory using
the obese Zucker rat model suggest that obesity serves an important
role in promotion of liver steatosis (NAFLD) (8,9).
Metformin is a first line oral anti-hyperglycemic
agent approved by the FDA in 1994 to treat type 2 diabetes in
adults and children >10 years of age. Although it has been
proven to be safe after decades of use, its exact mechanisms of
action remains unclear and contested (10). It is not metabolized and it is
excreted by the kidneys and bile (11). In the liver, it has been shown to
inhibit complex I of the mitochondrial respiratory chain, and to
activate AMP-activated protein kinase, processes that have been
related to its ability to inhibit hepatic lipogenesis and
gluconeogenesis, increasing hepatic insulin sensitivity, indirectly
lowering circulating glucose and insulin levels (10,12,13).
These findings have encouraged the research of metformin as a
pharmacological treatment for NAFLD. Several investigators have
used different animal models and doses of metformin to study its
effect on liver steatosis. There have been positive reports of
metformin reducing liver steatosis (14-16),
but these are not conclusive, and additionally negative studies
have also been published (17-19).
There is insufficient evidence regarding the effects of metformin
in pediatric obesity. There are very few published data on the
effects of metformin on liver steatosis in the adolescent
population; therefore, the role of metformin on protection from
NAFLD in an adolescent model was investigated. The major objectives
of this study were to investigate the effects of obesity and
short-term metformin treatment on i) body weight, ii) liver
steatosis score and iii) serum aspartate aminotransferase (AST),
alanine aminotransferase (ALT) and leptin and adiponectin levels.
Lean and obese female Zucker rats were placed on a control diet for
8 weeks to induce NAFLD, and then both lean and obese rats were
randomly placed on a diet with or without metformin (1 g metformin
per kg of food) for 10 weeks. Obese Zucker rats were used as the
model for early adolescent obesity related diseases (20).
Materials and methods
Experimental design
The animal protocols used in the present study were
approved by the Institutional Animal Care and Use Committee (IACUC)
at the University of Arkansas for Medical Sciences in 2018
(approval no. 3882).
A total of 32, 5-week-old female Zucker rats (16
obese fa/fa and 16 lean) were purchased from Envigo. The rats were
genotypically identified fa/fa and lean/lean rats at 24 days of
age. Upon receipt, the rats were housed 1 per cage with ad
libitum access to water and a semi-purified diet similar to
AIN-93G diet, containing casein (20% w/w/protein) a dietary source
of protein (Envigo) for 8 weeks to induce NAFLD (8,9). After 8
weeks, lean and obese rats were randomly assigned to one of the
following four groups (8 rats/group): i) lean without metformin
(LC), ii) lean with metformin (LM), iii) obese without metformin
(OC), and iv) obese with metformin (OM). Metformin was mixed with
the AIN-93G diet at 1 g metformin per kg of food. Rats were weighed
twice per week. All rats were sacrificed 10 weeks post-metformin
treatment, using CO2 (30%) prior to decapitation. Livers
and blood samples were collected following euthanasia. Liver
samples and serum were stored at -80˚C for subsequent
experiments.
Livers were removed and weighed individually. Per
each lobe of the liver, two 3-mm sections were fixed in 10%
buffered formalin at room temperature for 2 days for histological
examination. Liver sections were cut (5 µm) and stained with
hematoxylin and eosin (H&E) for 45 min at room temperature. A
board-certified anatomic pathologist evaluated the H&E stained
sections of the livers, and they were blinded to the conditions.
The presence and extent of microvesicular and macrovesicular
steatosis was examined. Steatosis was semi-quantitatively scored
between 0 and 4 as follows: 0, no steatosis; 1, steatosis in
<25% of the hepatocytes; 2, steatosis in 25-50% of the
hepatocytes; 3, steatosis in 51-75% of the hepatocytes; and 4,
steatosis in >75% of the hepatocytes as previously reported
(8,9).
Serum analysis
Blood (2 ml) was collected immediately after
decapitation into 50 ml centrifuge tubes, allowed to clot, and
centrifuged at 2,000 x g for 10 min at room temperature to separate
serum. Serum was aliquoted and stored at -80˚C for further
analyses. Leptin and adiponectin levels were measured using ELISA
kits (cat. nos. EZRL-83K and EZRADP-62K, respectively; EMD
Millipore) according to the manufacturer's protocol. Serum AST and
ALT concentrations were analyzed on an RX Daytona Clinical Analyzer
(Randox).
Statistical analysis
Data on all outcome variables were assessed for
normality using the Shapiro-Wilk test and box-and-whisker plots.
The assumption of equal variance was verified using a Levene's
test. In cases where the assumption of normality or equal variance
was violated, the Welch's test statistic was used. Data are
presented as the mean ± standard deviation. To determine if outcome
variables differed between lean and obese rats and with or without
metformin, a general linear model procedure was employed with
treatment as the primary effector. If there was a significant
primary effect of treatment, the statistical differences among the
treatments were analyzed using contrast statements in the SAS GLM
procedure. Multiple comparisons amongst means were adjusted using
Tukey's honestly significant difference tests. P<0.05 was
considered to indicate a statistically significant difference.
Statistical analysis was performed in SAS version 9.4 (SAS
Institute).
Results
Body weight
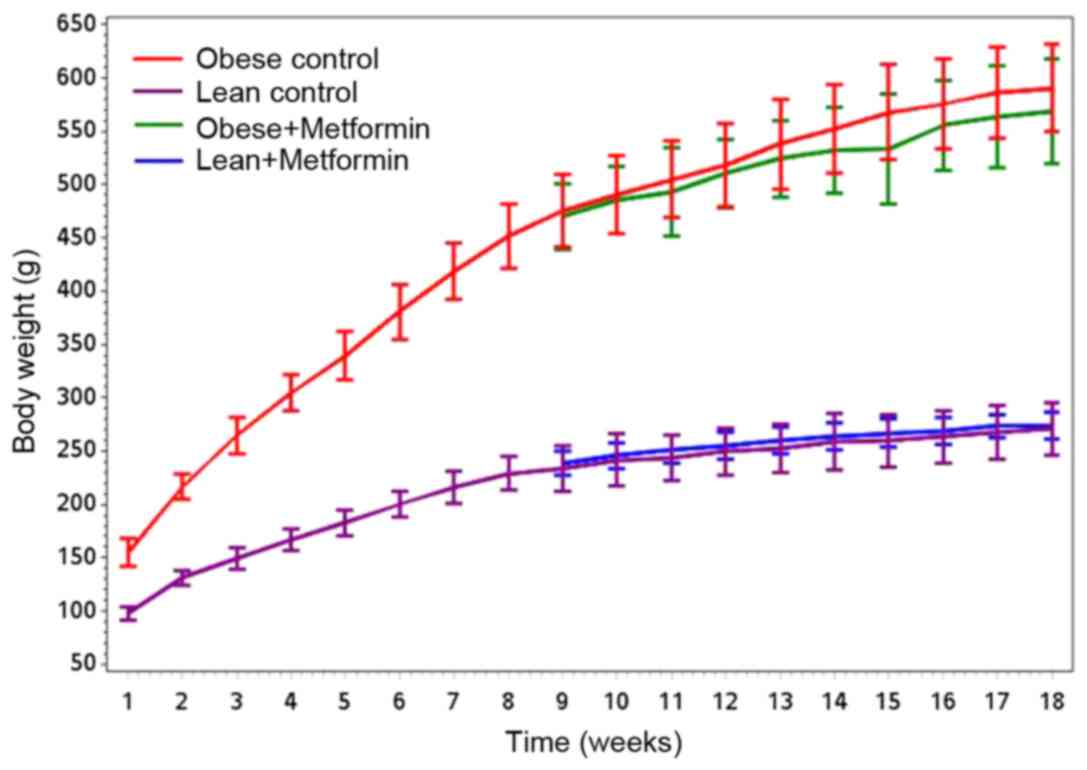
Mean body weight in grams at the beginning of the
experiment was 97.8±6.5 and 154.8±13.3 for LC and OC rats,
respectively. Table I presents the
body weights at the end of the 18-week experiment. Fig. 1 shows that obese rats gained
significantly more weight (P<0.001) compared with the lean rats
for both control and metformin treatment groups, and there was no
significant difference between OC vs. OM groups (P=0.20). Final
body weights differed significantly between the LC and OC rats as
well as between the LM and OM rats (P<0.0001; Table I). There was no significant
difference in the final body weights between LC and LM rats or
between OC and OM rats. The liver weights in g and as a percentage
of final body weight are presented in Table I.
 | Table IEffects of obesity and metformin
treatment on the final BW, liver weight and liver weight as a
percentage of BW. |
Table I
Effects of obesity and metformin
treatment on the final BW, liver weight and liver weight as a
percentage of BW.
| | Mean standard ±
deviation | P-value |
|---|
| Parameter | LC | LM | OC | OM | LC vs. LM | LC vs. OC | LM vs. OM | OC vs. OM |
|---|
| Final BW | 268.0±26.3 | 278.0±13.8 | 598.0±41.4 | 573.0±48.1 | 0.521 |
<0.001a |
<0.001a | 0.207 |
| Liver weight, g | 8.1±1.3 | 8.8±1.0 | 35.5±4.6 | 34.9±4.3 | 0.652 |
<0.001a |
<0.001a | 0.743 |
| Liver weight,
%BW | 3.0±0.3 | 3.1±0.3 | 3.0±1.0 | 6.1±0.6 | 0.618 |
<0.001a |
<0.001a | 0.710 |
Liver weights and histological
analysis
The liver weights in g and as a percentage of final
body weight is presented in Table I.
Liver weights in g and as a percentage of final body weight were
significantly higher in obese rats compared with the lean rats in
both the control and metformin treated groups (P<0.0001).
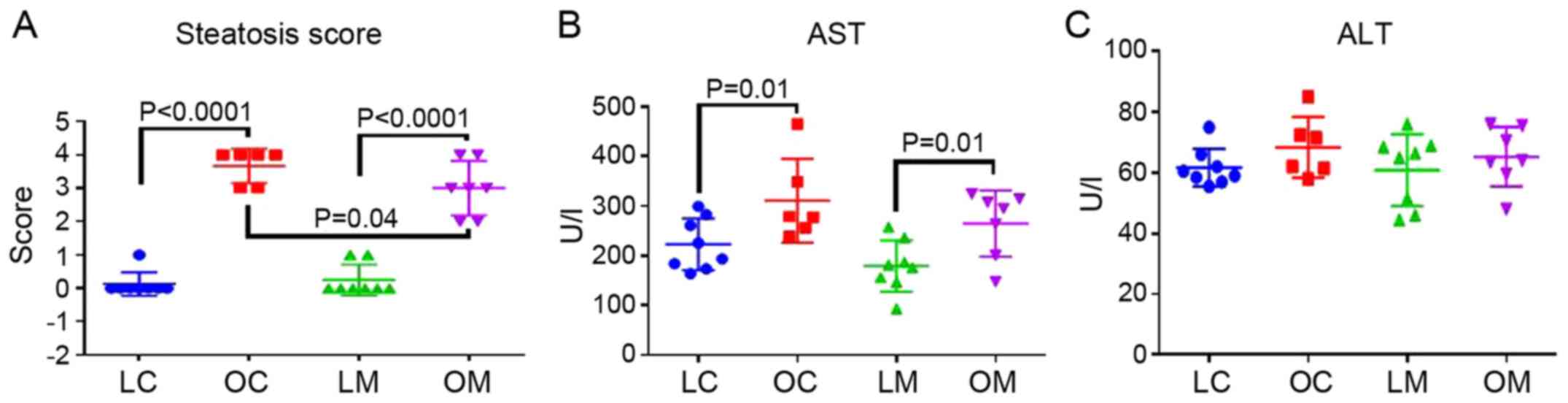
Steatosis scores are presented in Fig.
2A. Steatosis scores were significantly elevated in obese rats
compared with the lean rats in both control (OC) and metformin (OM)
treated groups (P<0.0001). In addition, rats in the OM group had
lower levels of liver steatosis compared to the OC group
(P<0.04; Fig. 2A). Representative
photomicrographs of liver parenchyma of lean and obese rats with
and without metformin treatment are shown in Fig. 3.
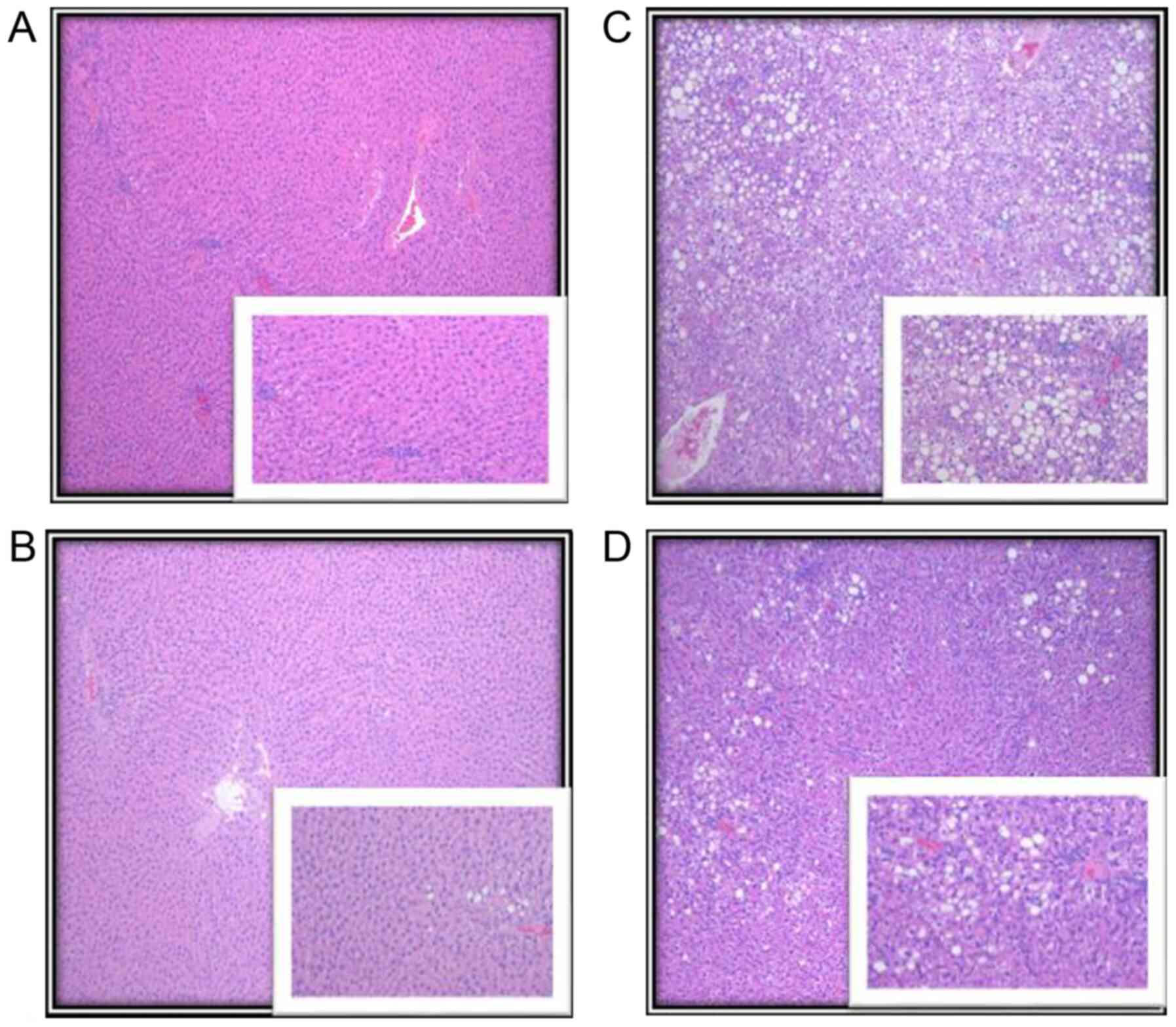 | Figure 3Representative images of liver
parenchyma of the lean and obese rats with and without metformin
treatment. (A) LC, showing complete preservation of the
architecture with no evidence of fatty changes, as shown in the
higher magnification insert. (B) LM, showing complete preservation
of the architecture with minimal steatosis seen in <2% of the
hepatocytes, predominantly within zone 3 (periportal region). (C)
OC, preservation of overall architecture with macrosteatosis and
microsteatosis seen in >75% of hepatocytes, which involved all
three zones (central, mid and periportal region). (D) OM,
preservation of overall architecture with steatosis seen in 25% of
hepatocytes, macrovesicular type, predominantly in the periportal
region. Lower right inserts show higher magnification of the zone
involved in steatosis. Original magnification, x40; insert, x100.
LC, lean control; LM, lean + metformin; OC, obese control; OM,
obese + metformin. |
Serum measurements
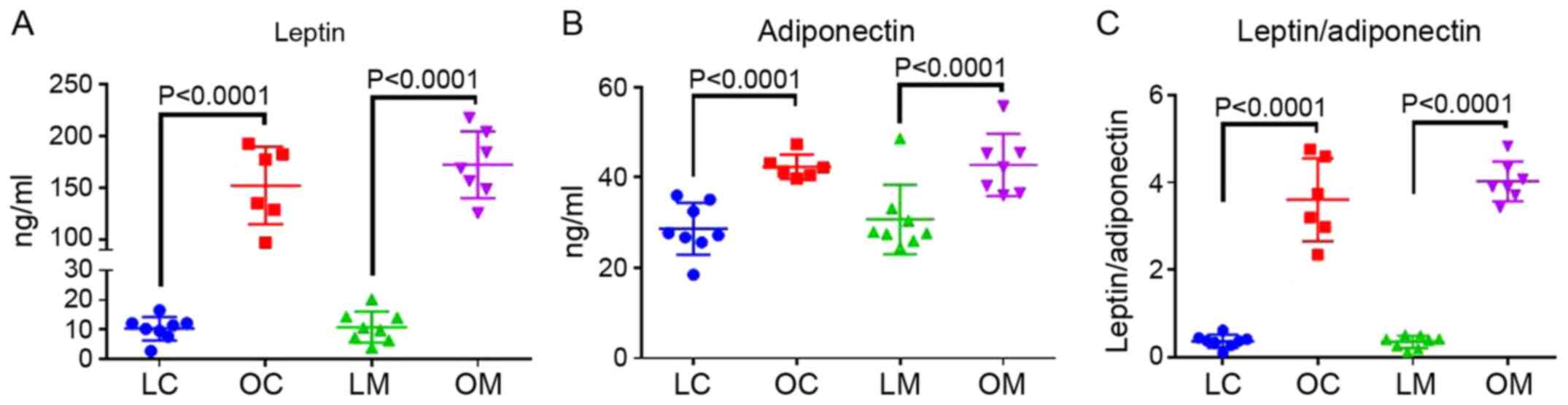
Figs. 2 and 4 show the serum levels of ALT, AST, leptin
and adiponectin. Serum AST levels were significantly elevated in
obese rats compared with the lean rats in both the control and
metformin treatment groups (P=0.01); however, serum ALT levels did
not differ between groups. Leptin (P<0.0001), adiponectin
(P=0.01) and the ratio of leptin to adiponectin (P<0.0001) were
all increased in obese rats compared with the lean rats in both the
control and metformin treatment groups. The leptin/adiponectin
ratio is an important marker of insulin resistance in obesity
(20). There were no effects of
metformin on any of the serum markers.
Discussion
To investigate the role of metformin and obesity on
liver steatosis, the Zucker rat (fa/fa) model was used, which is
the most widely used model for obesity related research. Obesity in
the Zucker rat is inherited as an autosomal recessive trait caused
by a mutation in the leptin receptor gene, such that Zucker rats
become noticeably obese by the age of 3 to 5 weeks, and by 14
weeks, >40% of their body is composed of lipids (21). Obese Zucker rats develop
hyperinsulinemia and insulin resistance before they develop
obesity-associated, non-insulin-dependent diabetes mellitus in a
manner similar to that in humans, making them an excellent model
for investigating the relationship between obesity and liver
steatosis. Lean Zucker rats, in contrast, exhibit normal metabolic
function and are considered ideal controls (22,23). In
addition, this animal model develops hepatic steatosis due to
dysregulated metabolic gene expression in the liver.
NAFLD is the most commonly observed liver problem in
obese pediatric and adult populations in the US as well as
worldwide, and it is primarily managed through lifestyle changes,
as with obesity and type 2 diabetes. Success with lifestyle changes
is hampered by patient adherence, particularly in the pediatric
population, and alternative therapeutics are thus required. In our
previous study, it was shown that using the obese Zucker rat model,
obesity increased body weight, and this resulted in an increase in
liver steatosis compared with lean rats (8,9,21,24).
However, the effect of short-term metformin treatment on liver
steatosis and the related liver enzymes has not been previously
assessed, to the best of our knowledge.
In the present study, it was shown that obese rats
gained significantly more weight than lean rats, and metformin
treatment had no effect on weight gain. Furthermore, liver
steatosis was significantly higher in obese rats compared with the
lean rats, and metformin treatment reduced liver steatosis. This
result was further supported by the changes in serum AST levels.
The leptin to adiponectin ratio was increased in obese rats
compared with the lean rats, and metformin treatment had no effect
on the levels of these serum biomarkers. Metformin was previously
shown to reduce liver steatosis in ob/ob leptin deficient mice, and
to also reduce hepatic TNF expression (14). One of the first pilot studies in
humans to assess metformin treatment on liver steatosis showed a
promising increase in insulin sensitivity, reduction of ALT levels
and a reduction in the volume of the liver (15). In addition, metformin inhibits
inflammatory signaling, which in turn suppresses the production of
proinflammatory cytokines in the liver tissues (25). Cyclooxygenase-2 (COX-2) is considered
to be partly responsible for the obesity-related inflammation in
diabetes and fatty liver. A COX-2 inhibitor was found to exert a
synergistic beneficial effect with metformin on obesity-associated
metabolic and cardiovascular disorders in male Sprague-Dawley rats
fed a high-fat diet (26). Metformin
improved hepatic insulin receptor substrate 2 and PI3K/Akt
signaling in insulin-resistant rats of a non-alcoholic
steatohepatitis (NASH) and cirrhosis model, where the
pathophysiological appearance of the liver was largely improved by
treatment with metformin, and a decrease in lipid and collagen
accumulation was observed in the liver tissues (16).
There is insufficient evidence regarding the effects
of short-term metformin treatment on pediatric obesity and liver
steatosis. Several clinical trials have identified modest
improvements following metformin treatment in insulin sensitivity
in obese children with normal glucose tolerance (27-29),
as well as a decrease in the BMI of obese adolescents (30). In addition, metformin appears to
improve lipid profiles in obese children (31,32).
El-Lakkany et al (33) found
that the co-administration of metformin and N-acetylcysteine, the
precursor of the antioxidant glutathione, paired with dietary
control improved the biochemical and histological manifestations in
rats with NAFLD (33). Additionally,
the concomitant administration of fish oil with metformin regulates
the expression of genes involved in lipid metabolism in a diabetic
rat model, exerting potentially beneficial effects (34).
It has been reported that short-term metformin
treatment has beneficial effects on lowering blood lipid levels and
protecting hepatocytes from lipid accumulation (2,7-9);
however, several studies with long-term metformin treatment did not
show histological protection of hepatic tissue (21,22,24).
Studies have used the Zucker diabetic fatty (ZDF)
rat as a diabetic model to investigate the effects of long-term
metformin treatment. As well as different doses of metformin on
liver steatosis. Sui et al (35) placed ZDF rats on either vehicle or
metformin treatment (50 mg/kg body weight) for 6 months. They
reported that metformin treatment reduced blood glucose, but this
did not prevent the development of liver steatosis and dysregulated
blood lipid profiles. Chen et al (36) used Sprague Dawley rats on a high fat
diet to induce obesity and type 2 diabetes mellitus, and were
placed on either a low-dose (100 mg/kg) or high-dose (200 mg/kg)
metformin derivative (MD568), or metformin (200 mg/kg) for 8 weeks.
They reported that the new metformin derivative MD568 significantly
reduced plasma glucose, insulin, total cholesterol, triglyceride
and low-density lipoprotein cholesterol levels. Additionally, MD568
treatment also improved the insulin resistance of obese type 2
diabetes mellitus model rats.
There are potential limitations in the present
study, including the sample size and the length of the experiment.
A larger sample size and a longer period under treatment with
metformin may strengthen the weight/power of the data.
Nevertheless, the results show that metformin is a suitable
candidate for further study on its effects in reducing liver
steatosis in the pediatric population.
In conclusion, it was shown that 10 weeks metformin
treatment in obese rats reduced liver steatosis, but had no effects
on the levels of serum markers. It is hypothesized that a longer
treatment period may be required for the metformin treatment to
exert a significant effect on the levels of liver damage
markers.
Acknowledgements
We would like to thank Ms. Sheena Joyner (Department
of Dietetics and Nutrition at University of Arkansas for Medical
Sciences) for her valuable assistance in preparation of this
manuscript.
Funding
Funding: The present study was supported by funding from the
Arkansas Bioscience Institute for funding and the National
Institute of General Medical Sciences of the National Institutes of
Health (grant no. 5P20GM109096).
Availability of data and materials
The datasets used and/or analyzed in the present
study are all included in the published article.
Authors' contributions
RH designed the study, and participated in the
collection of data and writing of the manuscript. SR participated
in study design, performed the experiments, and participated in
collection of data and writing of the manuscript. BS participated
in the study design, statistical analysis and writing of the
manuscript. MK participated in study design, collection of data,
interpretation of the results and in writing the manuscript. SK
performed the experiments, interpretation of the study results, and
writing of the manuscript. All authors read and approved the final
manuscript. All authors confirm authenticity all of the raw
data.
Ethics approval and consent to
participate
The animal protocols used in the present study were
approved by the Institutional Animal Care and Use Committee (IACUC)
at the University of Arkansas for Medical Sciences (approval no.
3882).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Centers for Disease Control and
Prevention: Adult Obesity Facts. CDC, 2020.
|
|
2
|
World Health Oraganization: Overweight and
obesity. WHO, 2020.
|
|
3
|
Ogden CL, Carroll MD, Curtin LR, Lamb MM
and Flegal KM: Prevalence of high body mass index in US children
and adolescents, 2007-2008. JAMA. 303:242–249. 2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ogden CL, Carroll MD, Lawman HG, Fryar CD,
Kruszon-Moran D, Kit BK and Flegal KM: Trends in obesity prevalence
among children and adolescents in the United States, 1988-1994
through 2013-2014. JAMA. 315:2292–2299. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lazo M, Hernaez R, Eberhardt MS, Bonekamp
S, Kamel I, Guallar E, Koteish A, Brancati FL and Clark JM:
Prevalence of nonalcoholic fatty liver disease in the United
States: The Third National Health and Nutrition Examination Survey,
1988-1994. Am J Epidemiol. 178:38–45. 2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Angulo P: GI epidemiology: Nonalcoholic
fatty liver disease. Aliment Pharmacol Ther. 25:883–889.
2007.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Welsh JA, Karpen S and Vos MB: Increasing
prevalence of nonalcoholic fatty liver disease among United States
adolescents, 1988-1994 to 2007-2010. J Pediatr. 162:496–500.e1.
2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Hakkak R, Al-Dwairi A, Fuchs GJ, Korourian
S and Simmen FA: Dietary soy protein induces hepatic lipogenic
enzyme gene expression while suppressing hepatosteatosis in obese
female Zucker rats bearing DMBA-initiated mammary tumors. Genes
Nutr. 7:549–558. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hakkak R, Zeng H, Dhakal IB and Korourian
S: Short- and long-term soy diet versus casein protects liver
steatosis independent of the arginine content. J Med Food.
18:1274–1280. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Thomas I and Gregg B: Metformin; a review
of its history and future: From lilac to longevity. Pediatr
Diabetes. 18:10–16. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Khokhar A, Umpaichitra V, Chin VL and
Perez-Colon S: Metformin use in children and adolescents with
prediabetes. Pediatr Clin North Am. 64:1341–1353. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Luong DQ, Oster R and Ashraf AP: Metformin
treatment improves weight and dyslipidemia in children with
metabolic syndrome. J Pediatr Endocrinol Metab. 28:649–655.
2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Madiraju AK, Qiu Y, Perry RJ, Rahimi Y,
Zhang XM, Zhang D, Camporez JG, Cline GW, Butrico GM, Kemp BE, et
al: Metformin inhibits gluconeogenesis via a redox-dependent
mechanism in vivo. Nat Med. 24:1384–1394. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lin HZ, Yang SQ, Chuckaree C, Kuhajda F,
Ronnet G and Diehl AM: Metformin reverses fatty liver disease in
obese, leptin-deficient mice. Nat Med. 6:998–1003. 2000.PubMed/NCBI View
Article : Google Scholar
|
|
15
|
Marchesini G, Brizi M, Bianchi G,
Tomassetti S, Zoli M and Melchionda N: Metformin in non-alcoholic
steatohepatitis. Lancet. 358:893–894. 2001.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Xu H, Zhou Y, Liu Y, Ping J, Shou Q, Chen
F and Ruo R: Metformin improves hepatic IRS2/PI3K/Akt signaling in
insulin-resistant rats of NASH and cirrhosis. J Endocrinol.
229:133–144. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Tiikkainen M, Häkkinen AM, Korsheninnikova
E, Nyman T, Mäkimattila S and Yki-Järvinen H: Effects of
rosiglitazone and metformin on liver fat content, hepatic insulin
resistance, insulin clearance, and gene expression in adipose
tissue in patients with type 2 diabetes. Diabetes. 53:2169–2176.
2004.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Nair S, Diehl AM, Wiseman M, Farr GH Jr
and Perrillo RP: Metformin in the treatment of non-alcoholic
steatohepatitis: A pilot open label trial. Aliment Pharmacol Ther.
20:23–28. 2004.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Lavine JE, Schwimmer JB, Van Natta ML,
Molleston JP, Murray KF, Rosenthal P, Abrams SH, Scheimann AO,
Sanyal AJ, Chalasani N, et al: Nonalcoholic Steatohepatitis
Clinical Research Network: Effect of vitamin E or metformin for
treatment of nonalcoholic fatty liver disease in children and
adolescents: The TONIC randomized controlled trial. JAMA.
305:1659–1668. 2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Labruna G, Pasanisi F, Nardelli C, Caso R,
Vitale DF, Contaldo F and Sacchetti L: High leptin/adiponectin
ratio and serum triglycerides are associated with an ‘at-risk’
phenotype in young severely obese patients. Obesity (Silver
Spring). 19:1492–1496. 2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Korourian S, Hakkak R, Ronis MJJ, Shelnutt
SR, Waldron J, Ingelman-Sundberg M and Badger TM: Diet and risk of
ethanol-induced hepatotoxicity: Carbohydrate-fat relationships in
rats. Toxicol Sci. 47:110–117. 1999.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zucker LM: Fat mobilization in vitro and
in vivo in the genetically obese Zucker rat ‘fatty’. J Lipid Res.
13:234–243. 1972.PubMed/NCBI
|
|
23
|
Zucker LM and Zucker TF: Fatty, a new
mutation in the rat. J Hered. 52:275–278. 1961.
|
|
24
|
Hakkak R, Gauss CH, Bell A and Korourian
S: Short-term soy protein isolate feeding prevents liver steatosis
and reduces serum ALT and AST levels in obese female zucker rats.
Biomedicines. 6(6)2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zheng J, Woo SL, Hu X, Botchlett R, Chen
L, Huo Y and Wu C: Metformin and metabolic diseases: A focus on
hepatic aspects. Front Med. 9:173–186. 2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lu CH, Hung YJ and Hsieh PS: Additional
effect of metformin and celecoxib against lipid dysregulation and
adipose tissue inflammation in high-fat fed rats with insulin
resistance and fatty liver. Eur J Pharmacol. 789:60–67.
2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Srinivasan S, Ambler GR, Baur LA, Garnett
SP, Tepsa M, Yap F, Ward GM and Cowell CT: Randomized, controlled
trial of metformin for obesity and insulin resistance in children
and adolescents: Improvement in body composition and fasting
insulin. J Clin Endocrinol Metab. 91:2074–2080. 2006.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Love-Osborne K, Sheeder J and Zeitler P:
Addition of metformin to a lifestyle modification program in
adolescents with insulin resistance. J Pediatr. 152:817–822.
2008.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Burgert TS, Duran EJ, Goldberg-Gell R,
Dziura J, Yeckel CW, Katz S, Tamborlane WV and Caprio S: Short-term
metabolic and cardiovascular effects of metformin in markedly obese
adolescents with normal glucose tolerance. Pediatr Diabetes.
9:567–576. 2008.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wilson DM, Abrams SH, Aye T, Lee PD,
Lenders C, Lustig RH, Osganian SV and Feldman HA: Glaser Pediatric
Research Network Obesity Study Group. Metformin extended release
treatment of adolescent obesity: A 48-week randomized,
double-blind, placebo-controlled trial with 48-week follow-up. Arch
Pediatr Adolesc Med. 164:116–123. 2010.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kay JP, Alemzadeh R, Langley G, D'Angelo
L, Smith P and Holshouser S: Beneficial effects of metformin in
normoglycemic morbidly obese adolescents. Metabolism. 50:1457–1461.
2001.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Atabek ME and Pirgon O: Use of metformin
in obese adolescents with hyperinsulinemia: A 6-month, randomized,
double-blind, placebo-controlled clinical trial. J Pediatr
Endocrinol Metab. 21:339–348. 2008.PubMed/NCBI View Article : Google Scholar
|
|
33
|
El-Lakkany NM, Seif El-Din SH, Sabra AA,
Hammam OA and Ebeid FA: Co-administration of metformin and
N-acetylcysteine with dietary control improves the biochemical and
histological manifestations in rats with non-alcoholic fatty liver.
Res Pharm Sci. 11:374–382. 2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Ghadge A, Harsulkar A, Karandikar M,
Pandit V and Kuvalekar A: Comparative anti-inflammatory and
lipid-normalizing effects of metformin and omega-3 fatty acids
through modulation of transcription factors in diabetic rats. Genes
Nutr. 11(10)2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Sui Y, Kong X, Fan R, Ye Y, Mai H, Zhuo S,
Lu W, Ruan P, Fang S and Yang T: Long-term treatment with metformin
in the prevention of fatty liver in Zucker diabetic fatty rats.
Diabetol Metab Syndr. 11(94)2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Chen D, Jia D, Wu X, Shi K, Ren C, Dou Y,
Guo M, Wang J, Ma M, Wu Z, et al: A novel metformin derivative
showed improvement of lipid metabolism in obese rats with type 2
diabetes. Clin Exp Pharmacol Physiol. 47:1382–1392. 2020.PubMed/NCBI View Article : Google Scholar
|