Introduction
The characteristics of temporomandibular joint
dysfunction (TMJD) include musculoskeletal conditions and
craniofacial pain in the masticatory system that involve the joint,
masticatory muscles or muscle innervations. Various factors are
associated with the aetiology of TMJD, including growth and
developmental anomalies (1), trauma
(2), detrimental body posture
(3), parafunctional habits and
bruxism (4), and stress (5). The most common forms of TMJD are disc
internal derangement, which involves an abnormal anatomical
relationship between the articular disc and articulating surfaces,
and osteoarthritis (OA), which involves an abnormal anatomical
relationship between the articular disc and articulating surfaces
(6). Although the degenerative
changes in the temporomandibular joint (TMJ) have been reported to
be related to osteoclastogenesis (7), the molecular process underlying these
changes are yet to be elucidated.
Previous studies (8-11)
have reported increased concentrations of
monocyte–macrophage-derived cytokines in the synovial fluid of
patients with TMJD. The release of proteinases and stimulation of
the expression of degrading enzymes and inflammatory mediators may
be facilitated by various cytokines, including interleukin 1β
(IL-1β), interleukin-6 (IL-6) and tumour necrosis factor-α (TNF-α),
thereby leading to inflammation of the TMJ and degradation of bone
and cartilage (12). These results
highlight the potential role of cytokines in TMJD pathogenesis. Our
previous study showed that IL-6 expression in the synovial fluid
obtained from patients with TMJD was significantly correlated with
the two clinical parameters of TMJ locking and pain/jaw function
based on a visual analogue scale (VAS) (13). Additionally, the concentration of
elastin-digested peptides in the synovial fluid of patients with
TMJD was significantly associated with the TMJ locking duration,
the VAS and IL-6 expression (13).
In vitro, elastin-digested peptides act on human TMJ
synovial cells to promote IL-6 upregulation and MMP-12 (an
elastin-degrading enzyme) (13).
These findings suggest an inflammation model in the TMJ where
elastin is degraded by a harmful mechanical stimulus, and the
degradation products induce a pro-inflammatory cascade and increase
the expression of MMP-12.
Currently, there is limited information regarding
the involvement of elastin-degradation by MMP-12 in the process of
inflammatory responses and cartilage degradation by aggrecanases [a
disintegrin and metalloproteinase with thrombospondin motifs
(ADAMTS)-4 and ADAMTS-5] in vivo. The STR/Ort mice often
develop spontaneous OA of the medial tibial cartilage of the knee
joint Thus this mouse model is useful in studying the pathogenesis
of knee OA (14). The
histopathological lesions of knee OA in STR/Ort mice are
progressive and exhibit a high degree of similarity to that of
human knee OA. Moreover, 85% of STR/Ort mice demonstrate
histological OA lesions in the medial tibial cartilage by the time
they are 35 weeks old. Previous reports have shown that the STR/Ort
mice develop spontaneous OA-like lesions in the TMJ with age, and
thus, they may facilitate the study of the pathogenesis of TMJ OA
(15). The aim of the present study
was to investigate the role of MMP-12 and aggrecanases in STR/Ort
mice with TMJ OA.
Materials and methods
Assessment of the progression and
severity of OA
A total of 24 male STR/Ort mice (age, 10 weeks) and
8 sex-matched CBA control mice were used in the present study. The
average weight of the STR/Ort mice at the beginning of the
experiment (age, 10 weeks) was 24.1±1.6 g, whereas that of the CBA
control mice (age, 10 weeks) was 25.3±1.4 g. The mice TMJs were
removed by microsurgery and were fixed in 4% paraformaldehyde
solution for 24 h at room temperature. Decalcification of TMJs was
performed using 10% EDTA for 3 weeks at room temperature, and
subsequently stored in 70% ethanol at 4˚C until required for
further analysis. Samples were embedded in paraffin wax and
sectioned sagittally (5-µm thickness) through the entire joint.
Safranin-O (Sigma-Aldrich; Merck KGaA) staining was used at room
temperature to evaluate proteoglycan loss using a validated scoring
system as per the Osteoarthritis Research Society International
(OARSI) recommendations (16). The
joints were graded at 10-µm intervals through the joint in a
blinded manner by two experienced investigators, and were scored as
follows: 0, normal cartilage; 0.5, loss of Safranin-O without
structural changes; 1, small fibrillations without loss of
cartilage; 2, vertical clefts down to the layer immediately below
the superficial layer and some loss of surface lamina; 3, vertical
clefts/erosion to the calcified cartilage extending to <25% of
the articular surface; 4, vertical clefts/erosion to the calcified
cartilage extending to 25-50% of the articular surface; 5, vertical
clefts/erosion to the calcified cartilage extending to 50-75% of
the articular surface; and 6, vertical clefts/erosion to the
calcified cartilage extending to >75% of the articular surface.
Scores were added from all levels through the entire joint to
obtain the ‘summed score’, which reflected the severity of the
osteoarthritic lesion. OA predominantly affects the mandibular
condyle (15); therefore, the
average of the summed scores at both the sites was used for each
sample to obtain robust results.
All the animal experiments performed in the present
study were approved by the Ethics Committee of the Kanazawa
University Graduate School of Medical Science (approval no. 352-2).
The mice were housed in groups of four in individually vented cages
maintained at 21˚C±2˚C and a humidity range of 30-70% in a 12-h
light/dark cycle with ad libitum access to food and water.
The animals were monitored daily for any health and/or welfare
issues from the time of birth, including any possible defects or
significant changes in size during the first 2 weeks of life. The
mice were then sacrificed in a chamber by increasing the
CO2 concentration at a flow rate of 60%/min of volume.
Lack of respiration and faded eye colour were used to confirm
death.
Immunohistochemical analysis
The paraffin embedded sagittal sections of TMJs were
dewaxed and antigen retrieval were performed using Immunosaver
(FUJIFILM Wako Pure Chemical Corporation) for 45 min at 98˚C. The
sections were washed in PBS, and then rinsed in PBS containing 1%
BSA and 2% foetal calf serum. Immunohistochemical staining was
performed with antibodies against MMP-12 (cat. no. 22989-1-AP;
ProteinTech Group, Inc.), IL-6 (cat. no. ab6672; Abcam), ADAMTS-4
(cat. no. ab185722; Abcam) and ADAMTS-5 (cat. no. ab41037; Abcam).
Primary antibodies were diluted in PBS at a ratio of 1:1,000, and
the sections were then incubated with primary antibodies overnight
at 4˚C. Binding of these antibodies was detected using EnVision
Single Reagents (Dako; Agilent Technologies, Inc.). After washing,
the slides were incubated with 3,3'-diaminobenzidine
tetrahydrochloride (Sigma-Aldrich; Merck KGaA) and immediately
rewashed under tap water following colour development. Slides were
then counterstained with haematoxylin for 3 min at room temperature
(FUJIFILM Wako Pure Chemical Corporation), mounted with DPX
(FUJIFILM Wako Pure Chemical Corporation) and observed under a
light microscope at a magnification of x4-x100 (Olympus
Corporation). The specificity of staining was confirmed using a
Universal Negative Control for IS-Series Rabbit Primary Antibodies
(cat. no. IS60061; Dako; Agilent Technologies, Inc.) as a negative
control, according to the manufacturer's protocol.
Immunohistochemical staining was graded in a blinded manner by two
experienced investigators as follows: 0, no staining, 1; minor
staining, 2; marked staining; and 3, maximal staining, as described
previously (17).
Statistical analysis
The summed OARSI scores of joints are presented
using a box-whisker plot (median ± interquartile range). The
immunohistochemical scores are presented as the mean ± standard
error of the mean. There were eight samples in both the STR/Ort and
CBA groups. For comparisons between the samples, data were analysed
using Kruskal-Wallis one-way analysis of variance on ranks with a
post-hoc Dunnett's test for summed OARSI scores, or a Mann-Whitney
U test for immunohistochemical scores using SPSS version 23 (IBM
Corp.), as described previously (17,18).
P<0.05 was considered to indicate a statistically significant
difference.
Results
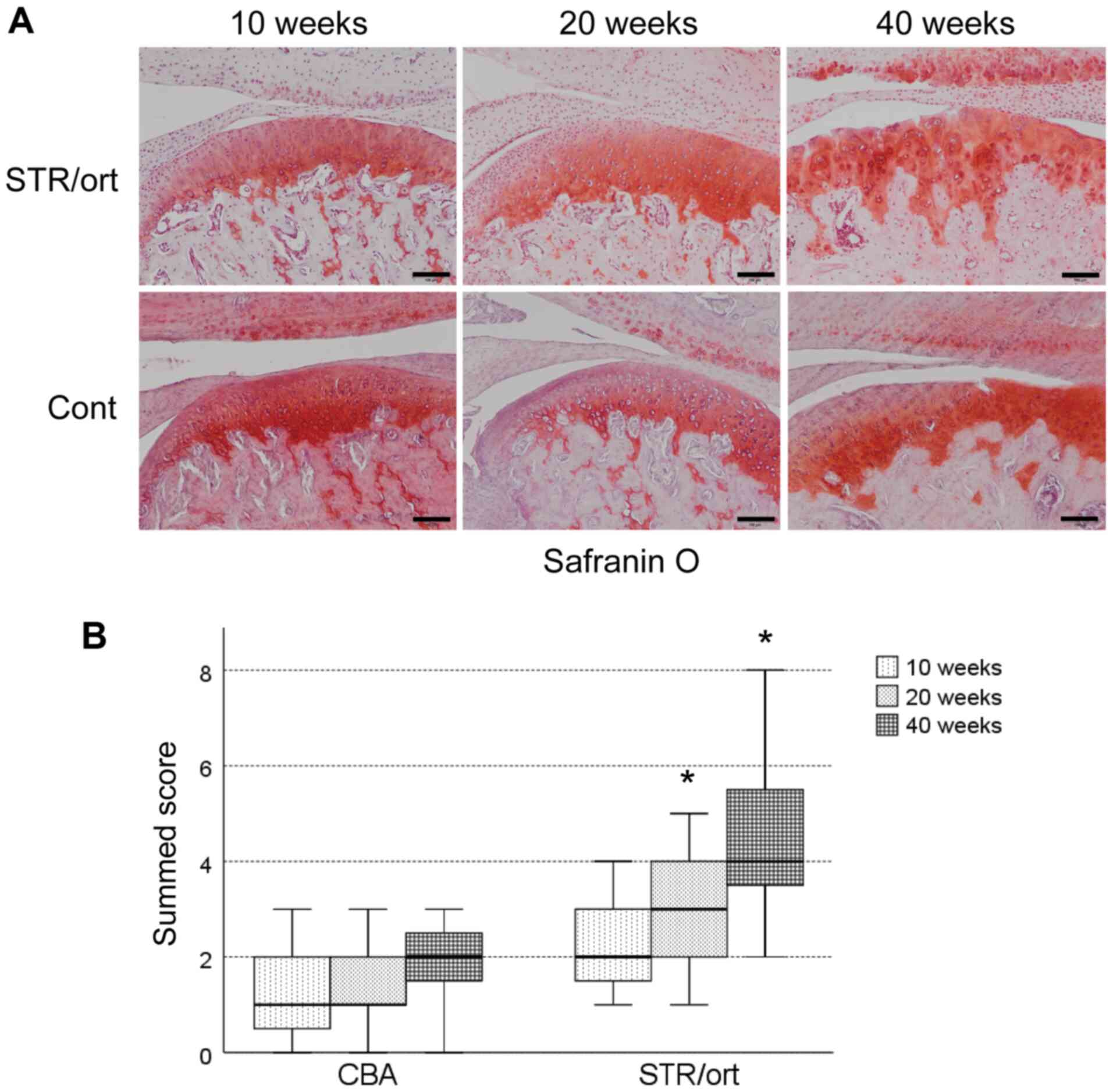
Cartilage degradation of the TMJs in
the STR/Ort mice
In order to examine the pathogenesis of spontaneous
OA, the TMJs of STR/Ort mice and control CBA mice were collected
and sectioned after 10, 20 or 40 weeks. Relative to the age-matched
control CBA mice, significant proteoglycan loss was observed in the
upper zone of the cartilage after 20 weeks of age, and this loss
was increased after 40 weeks (Fig.
1A). STR/Ort mice after 40 weeks exhibited vertical fissures in
the matrix into the mid zone, and the fissures were branched
(Fig. 1A). The data of the summed
scores showed that significant articular cartilage degeneration
started at 20 weeks of age in the STR/Ort mice and progressed
gradually until they were 40 weeks old; this was not observed in
the age-matched CBA mice (Fig.
1B).
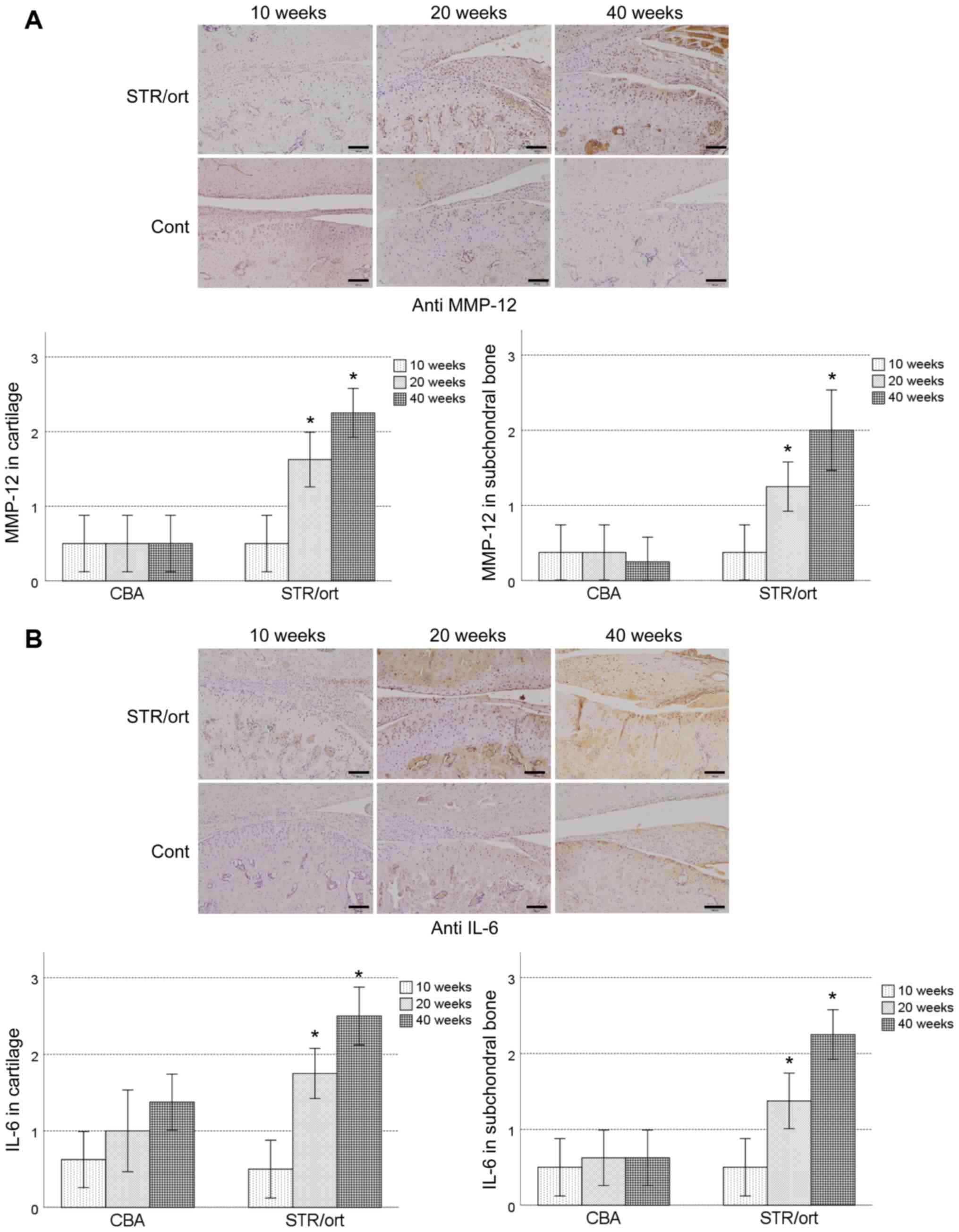
Expression of MMP-12, IL-6 and
aggrecanases
Immunostaining showed that MMP-12 was expressed in
the chondrocytes in the superficial zones after 20 weeks in the
STR/Ort mice, and this increased gradually until the mice were 40
weeks old (Fig. 2A). In contrast,
MMP-12 expression was detected in the chondrocytes in the CBA mice
at all time points (Fig. 2A). In the
subchondral bone, MMP-12 expression was observed in the
osteoblast-like and osteoclast-like cells, starting at 20 weeks of
age in the STR/Ort mice, and its levels increased with disease
severity until the mice were 40 weeks old (Fig. 2A). However, no MMP-12 expression was
detected in the subchondral bone of the CBA mice, regardless of age
(Fig. 2A).
IL-6 was expressed throughout the cartilage of both
the STR/Ort and CBA mice from 20 weeks of age, with expression in
the chondrocytes in the superficial zones. However, the expression
was significantly higher in the STR/Ort cartilage after 20 and 40
weeks relative to the age-matched CBA mice (Fig. 2B). In the subchondral bone, IL-6
expression was observed in the osteoblast-like and the
osteoclast-like cells, starting at 20 weeks of age in the STR/Ort
mice, and its levels increased with disease severity until the mice
were 40 weeks old (Fig. 2B). IL-6
expression in the subchondral bone in the STR/Ort cartilage was
significantly higher compared with the age-matched CBA mice,
excluding after 10 weeks (Fig. 2B).
However, no detectable IL-1β or TNF-α expression was observed in
the STR/Ort mice and CBA-strain mice, irrespective of age (data not
shown).
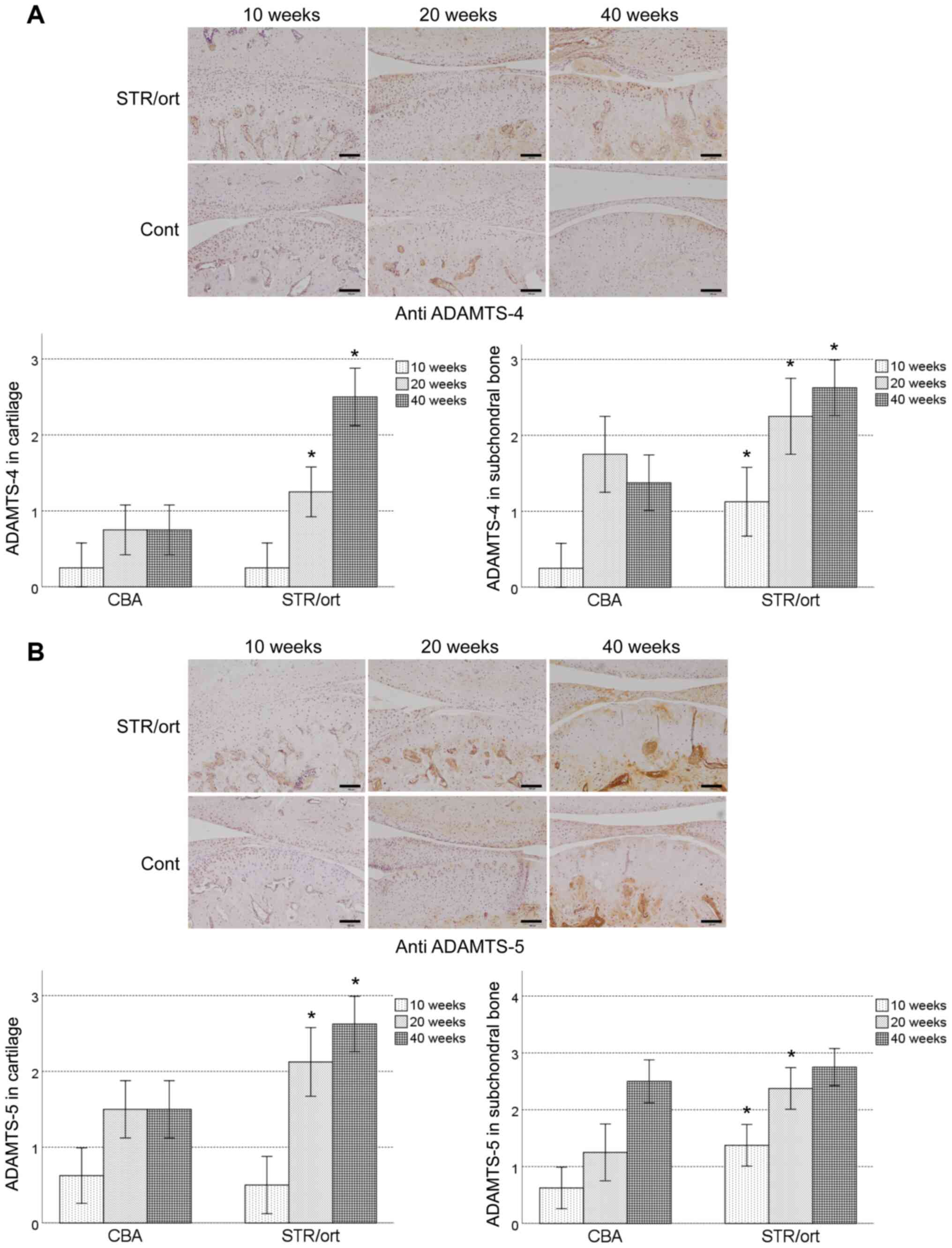
ADAMTS-4 and ADAMTS-5 were expressed throughout the
cartilage of both the STR/Ort and CBA mice from 20 weeks of age,
with expression in the chondrocytes in the superficial zones that
gradually increased till the mice were 40 weeks old (Fig. 3A and B). ADAMTS-5 was expressed in the middle
zone of the cartilage after 40 weeks of age in the STR/Ort mice.
ADAMTS-4 and ADAMTS-5 expression was significantly higher in the
STR/Ort cartilage compared with the age-matched CBA mice, excluding
after 10 weeks (Fig. 3A and B). In the subchondral bone, ADAMTS-4 and
ADAMTS-5 expression was observed in the osteoblast-like and
osteoclast-like cells, starting at 10 weeks of age in the STR/Ort
mice, and its levels increased with disease severity until the mice
were 40 weeks old (Fig. 3A and
B). ADAMTS-4 and ADAMTS-5 expression
levels were significantly higher in the STR/Ort subchondral bone
compared with the age-matched CBA mice (Fig. 3A and B).
Discussion
The primary aim of the present study was to
determine whether elastin-degradation by MMP-12 was involved in TMJ
inflammatory responses and cartilage degradation by aggrecanases
in vivo. STR/Ort mice were used as a model of TMJ OA. The
initial joint pathology caused by OA is difficult to characterise
in humans as OA is often diagnosed in the first instance at an
advanced stage. Animal models of OA may improve our understanding
of the early pathology of OA, including loss of proteoglycans and
fibrillations. Dreessen and Halata (19) showed age-related osteoarthritic
degeneration of the TMJ in male STR/1N mice, suggesting a systemic
basis of the disease. In the present study, significant articular
cartilage degeneration began at 20 weeks of age in the STR/Ort mice
and progressed gradually until 40 weeks relative to that in the
age-matched CBA mice. The progression that was observed until 40
weeks of age in the STR/Ort mice was consistent with the results of
a previous study (15). Conversely,
in this same previous study, significant articular cartilage
degeneration was not observed at 20 weeks of age in STR/Ort mice
compared with the age-matched CBA mice (15). The maximal OARSI scores in STR/Ort
mice at 20 and 40 weeks of age were 2 (highest OARSI score was 6).
These results suggested that TMJ osteoarthritis at 20 and 40 weeks
of age in STR/Ort mice showed initial or early joint pathology
caused by OA. Thus, the STR/Ort mouse model may be appropriate for
facilitating an understanding of the initial pathology of TMJ
OA.
Elastin fibres are major extracellular matrix
macromolecules that are critical for the maintenance of elasticity
and resilience of tissues, such as blood vessels, lungs and skin. A
previous study showed that the architecture of the elastin network
varies significantly with cartilage depth (20). Dense elastin fibres formed a
distinctive cobweb-like meshwork parallel to the cartilage surface
in the most superficial layer of the articular cartilage (20). Contrastingly, in the superficial
zone, the elastin fibres were found to be well organised in the
physiological orientation, parallel to the collagen fibres. In the
deep zone, no detectable elastin fibres were observed. In the
elastin-containing superficial cartilage, inflammation was
concomitant with elastolysis, leading to the generation of
elastin-digested peptides (21-23).
In these cases, a direct association between inflammation and
elastin-digested peptide levels has been previously established
(21-23).
In the present study, immunostaining showed that MMP-12 was
expressed in the chondrocytes in the superficial zones where
elastin is found physiologically. These data suggest that MMP-12
directly digested elastin in the superficial zone of the cartilage
and produced elastin-digested peptides. Our previous study
demonstrated that the concentration of elastin-digested peptides in
the synovial fluid of patients with TMJD was significantly
correlated with IL-6 expression, the duration of TMJ locking and
the VAS (13). IL-6 is generated at
the inflammation site and plays a major role in the acute phase
response (24). In the present
study, immunostaining showed that IL-6 was expressed in the
chondrocytes in the superficial zones where metalloelastase-12 is
expressed during the initial and early joint pathology caused by
OA. These data suggested that elastin peptides digested by MMP-12
induced IL-6 expression in the chondrocytes in the superficial zone
of the cartilage. However, whether the elastin-digested peptides
induced IL-6 expression directly in the chondrocytes, in
vitro or in vivo, remains unclear. In this study, it was
not possible to detect elastin-digested peptides in the joint
fluid, as, if they were present, their levels were below the limits
of detection. Further research using joint tissue-specific
elastin–overexpressing mice or joint tissue-specific elastin
knockout mice is warranted. In contrast, in the subchondral bone,
MMP-12 expression was observed in osteoblast- and osteoclast-like
cells in the 20-week-old STR/Ort mice and the frequency of
occurrence increased with disease severity until the mice were 40
weeks old. In a previous study, it was shown that MMP-12 was
present in the matrix adjacent to the osteoblast-like cells, bone
lining cells and osteoclasts in moderate and severe stages of OA
(25). In vitro and in
vivo animal studies revealed that MMP-12 cleaved bone matrix
proteins critical for osteoclast matrix interactions, and this was
induced only in response to critical situations (26). However, elastin fibres have not been
detected in the subchondral bone. A total of 90% of the organic
matrix of bone is made of type I collagen, which is not degradable
by MMP-12(27). From a quantitative
point of view, non-collagenous bone proteins, such as vitronectin,
osteonectin, osteopontin and bone sialoprotein, are minimally
present in the bone (26). It is
thus interesting that vitronectin and osteonectin are completely
degraded by MMP-12, but that osteopontin and bone sialoprotein are
cleaved in a very selective way (28). Therefore, the degradation fragments
of these minor bone proteins may potentially induce IL-6 expression
in osteoblasts and osteoclasts. Further research is necessary to
address this issue.
Following the onset of the inflammatory response,
IL-6 secreted in the TMJ may function as a chemoattractant to
recruit cell types that are crucial in tissue degradation (13). The stimulation of cartilage explants
with IL-6 potentiated proteoglycan (aggrecan) catabolism in the
articular cartilage has been previously reported (29). This catabolism was found to be
associated with aggrecanase activity. The aggrecanases are members
of the ADAMTS family of extracellular metzincin proteinases.
Amongst the various members of this family of proteins, ADAMTS-4
and ADAMTS-5 show the highest levels of aggrecanase activity
(30). Mice deficient in ADAMTS-5
were protected against aggrecan loss and cartilage degradation in
models of inflammatory arthritis (31) and OA (32). In this regard, IL-6 upregulation is
critical for the progression of arthritic diseases. IL-6
contributes to cartilage destruction in mouse models of
inflammatory arthritis (33,34); as per one report, aggrecan loss is
prevented in IL-6-deficient mice (34). IL-6 increases the mRNA expression
levels of ADAMTS-4 and ADAMTS-5 in bovine chondrocytes (35). Moreover, IL-6–null mice are protected
against cartilage damage both in the CIA model (36) and the antigen-induced arthritis model
(33). In the present study,
immunostaining revealed that ADAMTS-4 and ADAMTS-5 were expressed
in chondrocytes in the superficial zones where IL-6 was expressed
during the initial and early joint pathology caused by OA. These
data suggested that IL-6 induced ADAMTS-4 and ADAMTS-5 expression
in the chondrocytes, following cartilage degradation in the
superficial zone in vivo.
In conclusion, a model for the initiation of the
inflammatory and degradative process in the TMJ is proposed.
Harmful mechanical stimuli, particularly increased pressure, may
cause damage to the elastin fibres in the most elastin-rich
superficial layer of the articular cartilage. Elastin-digested
peptides are then generated as endogenous danger signals and induce
a pro-inflammatory cascade. This leads to upregulation of
pro-inflammatory mediators, such as IL-6 and MMP-12, triggering
further tissue damage that results in increased levels of
elastin-digested peptides. IL-6 induced ADAMTS-4 and ADAMTS-5
expression in the chondrocytes, following cartilage degradation.
Thus, a positive feedback loop is established and may result in
chronic inflammation and cartilage degradation of the TMJ in
vivo.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by Grants-in-Aid for
Scientific Research from the Ministry of Education, Science, Sports
and Culture, Japan (grant nos. 18K09764, 18K17166, 15H05042 and
17K11869).
Availability of data and material
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
HN was responsible for the conception and design of
the study. YYF, RJ KK, IK and KL performed the experiments. YYF and
HN wrote the manuscript. KO, GBG and SK assisted in the design of
the study. HN edited the manuscript. All authors have read and
approved the final manuscript. GBG and HN confirm the authenticity
of all the raw data.
Ethics approval and consent to
participate
All the studies using laboratory animals were
approved by the Institutional Animal Care and Use Committees of
Kanazawa University (approval no. AP183935). The protocols employed
in the present study adhered to all applicable institutional and
governmental guidelines for the humane care and use of laboratory
animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Power A and Carter L: Osteochondroma of
the mandibular condyle: an unusual case of dentofacial asymmetry.
Dent Update. 42:369–370. 372:2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Nagori SA, Jose A, Bhutia O and
Roychoudhury A: Undiagnosed mandibular condylar fractures causing
temporomandibular joint ankylosis: A problem in northern India.
Natl Med J India. 27:251–255. 2014.PubMed/NCBI
|
|
3
|
Durham J, McDonald C, Hutchinson L and
Newton JL: Painful temporomandibular disorders are common in
patients with postural orthostatic tachycardia syndrome and impact
significantly upon quality of life. J Oral Facial Pain Headache.
29:152–157. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sierwald I, John MT, Schierz O, Hirsch C,
Sagheri D, Jost-Brinkmann PG and Reissmann DR: Association of
temporomandibular disorder pain with awake and sleep bruxism in
adults. J Orofac Orthop. 76:305–317. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Tosato JP, Caria PH, Gomes CA, Berzin F,
Politti F, Gonzalez TO and Biasotto-Gonzalez DA: Correlation of
stress and muscle activity of patients with different degrees of
temporomandibular disorder. J Phys Ther Sci. 27:1227–1231.
2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Fang PK, Ma XC, Ma DL and Fu KY:
Determination of interleukin-1 receptor antagonist, interleukin-10,
and transforming growth factor-beta1 in synovial fluid aspirates of
patients with temporomandibular disorders. J Oral Maxillofac Surg.
57:922–928; discussion 928-929. 1999.PubMed/NCBI View Article : Google Scholar
|
|
7
|
de Bont LG, Boering G, Liem RS, Eulderink
F and Westesson PL: Osteoarthritis and internal derangement of the
temporomandibular joint: A light microscopic study. J Oral
Maxillofac Surg. 44:634–643. 1986.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Shafer DM, Assael L, White LB and
Rossomando EF: Tumor necrosis factor-alpha as a biochemical marker
of pain and outcome in temporomandibular joints with internal
derangements. J Oral Maxillofac Surg. 52:786–791; discussion
791-792. 1994.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sandler NA, Buckley MJ, Cillo JE and Braun
TW: Correlation of inflammatory cytokines with arthroscopic
findings in patients with temporomandibular joint internal
derangements. J Oral Maxillofac Surg. 56:534–543; discussion
543-544. 1998.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sakamaki H, Ogura N, Kujiraoka H, Akiba M,
Abiko Y and Nagura H: Activities of plasminogen activator, plasmin
and kallikrein in synovial fluid from patients with
temporomandibular joint disorders. Int J Oral Maxillofac Surg.
30:323–328. 2001.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Takahashi T, Kondoh T, Fukuda M, Yamazaki
Y, Toyosaki T and Suzuki R: Proinflammatory cytokines detectable in
synovial fluids from patients with temporomandibular disorders.
Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 85:135–141.
1998.PubMed/NCBI View Article : Google Scholar
|
|
12
|
de Bont LG and Stegenga B: Pathology of
temporomandibular joint internal derangement and osteoarthrosis.
Int J Oral Maxillofac Surg. 22:71–74. 1993.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kobayashi K, Jokaji R, Miyazawa-Hira M,
Takatsuka S, Tanaka A, Ooi K, Nakamura H and Kawashiri S: Elastin
derived peptides are involved in the processes of human
temporomandibular disorder by inducing inflammatory responses in
synovial cells. Mol Med Rep. 16:3147–3154. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chambers MG, Cox L, Chong L, Suri N, Cover
P, Bayliss MT and Mason RM: Matrix metalloproteinases and
aggrecanases cleave aggrecan in different zones of normal cartilage
but colocalize in the development of osteoarthritic lesions in
STR/ort mice. Arthritis Rheum. 44:1455–1465. 2001.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kumagai K, Suzuki S, Kanri Y, Matsubara R,
Fujii K, Wake M, Suzuki R and Hamada Y: Spontaneously developed
osteoarthritis in the temporomandibular joint in STR/ort mice.
Biomed Rep. 3:453–456. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Glasson SS, Chambers MG, Van Den Berg WB
and Little CB: The OARSI histopathology initiative -
recommendations for histological assessments of osteoarthritis in
the mouse. Osteoarthritis Cartilage. 18 (Suppl 3):S17–S23.
2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Valverde-Franco G, Pelletier JP, Fahmi H,
Hum D, Matsuo K, Lussier B, Kapoor M and Martel-Pelletier J: In
vivo bone-specific EphB4 overexpression in mice protects both
subchondral bone and cartilage during osteoarthritis. Arthritis
Rheum. 64:3614–3625. 2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Nakamura H, Vo P, Kanakis I, Liu K and
Bou-Gharios G: Aggrecanase-selective tissue inhibitor of
metalloproteinase-3 (TIMP3) protects articular cartilage in a
surgical mouse model of osteoarthritis. Sci Rep.
10(9288)2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Dreessen D and Halata Z: Age-related
osteo-arthrotic degeneration of the temporomandibular joint in the
mouse. Acta Anat (Basel). 139:91–96. 1990.PubMed/NCBI View Article : Google Scholar
|
|
20
|
He B, Wu JP, Chen HH, Kirk TB and Xu J:
Elastin fibers display a versatile microfibril network in articular
cartilage depending on the mechanical microenvironments. J Orthop
Res. 31:1345–1353. 2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Duca L, Lambert E, Debret R, Rothhut B,
Blanchevoye C, Delacoux F, Hornebeck W, Martiny L and Debelle L:
Elastin peptides activate extracellular signal-regulated kinase 1/2
via a Ras-independent mechanism requiring both p110gamma/Raf-1 and
protein kinase A/B-Raf signaling in human skin fibroblasts. Mol
Pharmacol. 67:1315–1324. 2005.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Houghton AM, Quintero PA, Perkins DL,
Kobayashi DK, Kelley DG, Marconcini LA, Mecham RP, Senior RM and
Shapiro SD: Elastin fragments drive disease progression in a murine
model of emphysema. J Clin Invest. 116:753–759. 2006.PubMed/NCBI View
Article : Google Scholar
|
|
23
|
Robinet A, Fahem A, Cauchard JH, Huet E,
Vincent L, Lorimier S, Antonicelli F, Soria C, Crepin M, Hornebeck
W, et al: Elastin-derived peptides enhance angiogenesis by
promoting endothelial cell migration and tubulogenesis through
upregulation of MT1-MMP. J Cell Sci. 118:343–356. 2005.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Gabay C: Interleukin-6 and chronic
inflammation. Arthritis Res Ther. 8 (Suppl 2)(S3)2006.PubMed/NCBI View
Article : Google Scholar
|
|
25
|
Kaspiris A, Khaldi L, Chronopoulos E,
Vasiliadis E, Grivas TB, Kouvaras I, Dagkas S and Papadimitriou E:
Macrophage-specific metalloelastase (MMP-12) immunoexpression in
the osteochondral unit in osteoarthritis correlates with BMI and
disease severity. Pathophysiology. 22:143–151. 2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hou P, Troen T, Ovejero MC, Kirkegaard T,
Andersen TL, Byrjalsen I, Ferreras M, Sato T, Shapiro SD, Foged NT,
et al: Matrix metalloproteinase-12 (MMP-12) in osteoclasts: New
lesson on the involvement of MMPs in bone resorption. Bone.
34:37–47. 2004.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Chandler S, Cossins J, Lury J and Wells G:
Macrophage metalloelastase degrades matrix and myelin proteins and
processes a tumour necrosis factor-alpha fusion protein. Biochem
Biophys Res Commun. 228:421–429. 1996.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Fu JY, Lyga A, Shi H, Blue ML, Dixon B and
Chen D: Cloning, expression, purification, and characterization of
rat MMP-12. Protein Expr Purif. 21:268–274. 2001.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Flannery CR, Little CB, Hughes CE, Curtis
CL, Caterson B and Jones SA: IL-6 and its soluble receptor augment
aggrecanase-mediated proteoglycan catabolism in articular
cartilage. Matrix Biol. 19:549–553. 2000.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Tortorella MD and Malfait AM: Will the
real aggrecanase(s) step up: Evaluating the criteria that define
aggrecanase activity in osteoarthritis. Curr Pharm Biotechnol.
9:16–23. 2008.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Stanton H, Rogerson FM, East CJ, Golub SB,
Lawlor KE, Meeker CT, Little CB, Last K, Farmer PJ, Campbell IK, et
al: ADAMTS5 is the major aggrecanase in mouse cartilage in vivo and
in vitro. Nature. 434:648–652. 2005.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Glasson SS, Askew R, Sheppard B, Carito B,
Blanchet T, Ma HL, Flannery CR, Peluso D, Kanki K, Yang Z, et al:
Deletion of active ADAMTS5 prevents cartilage degradation in a
murine model of osteoarthritis. Nature. 434:644–648.
2005.PubMed/NCBI View Article : Google Scholar : Erratum in: Nature
446, 102, 2007.
|
|
33
|
Ohshima S, Saeki Y, Mima T, Sasai M,
Nishioka K, Nomura S, Kopf M, Katada Y, Tanaka T, Suemura M, et al:
Interleukin 6 plays a key role in the development of
antigen-induced arthritis. Proc Natl Acad Sci USA. 95:8222–8226.
1998.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Fosang AJ, Last K, Stanton H, Golub SB,
Little CB, Brown L and Jackson DC: Neoepitope antibodies against
MMP-cleaved and aggrecanase-cleaved aggrecan. Methods Mol Biol.
622:312–347. 2010.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Legendre F, Bogdanowicz P, Boumediene K
and Pujol JP: Role of interleukin 6 (IL-6)/IL-6R-induced signal
tranducers and activators of transcription and mitogen-activated
protein kinase/extracellular. J Rheumatol. 32:1307–1316.
2005.PubMed/NCBI
|
|
36
|
Sasai M, Saeki Y, Ohshima S, Nishioka K,
Mima T, Tanaka T, Katada Y, Yoshizaki K, Suemura M and Kishimoto T:
Delayed onset and reduced severity of collagen-induced arthritis in
interleukin-6-deficient mice. Arthritis Rheum. 42:1635–1643.
1999.PubMed/NCBI View Article : Google Scholar
|