1. Introduction
Coronavirus disease 2019 (COVID-19) is a contagious
infectious disease caused by severe acute respiratory syndrome
coronavirus 2. Since being detected in Wuhan, Hubei Province,
China, on December 31, 2019, COVID-19 has rapidly spread to 219
countries worldwide, resulting in 126,890,643 individuals being
infected and 2,778,619 deaths (as of March 29th, 2021) (1). Among Southeast Asian countries,
Indonesia has experienced one of the highest increasing rates of
COVID-19 cases. Based on data obtained from the Indonesia Ministry
of Health on October 23, 2020, Indonesia had 386,432 COVID-19 cases
and 13,077 associated deaths (2).
Before the COVID-19 pandemic, there was a diverse
group of communicable diseases that received little consideration,
despite affecting 149 countries and infecting billions of
individuals, termed neglected tropical diseases (NTDs) (3). Based on the 10th Meeting of the
Strategic and Technical Advisory Group for NTD, the following 20
diseases are included in the group of NTDs: Chagas, dengue and
chikungunya, yaws, dracunculiasis, human African trypanosomiasis
(gambiense), leprosy, onchocerciasis, visceral leishmaniasis,
lymphatic filariasis, schistosomiasis, soil-transmitted
helminthiases (STHs), trachoma, buruli ulcer, echinococcosis,
foodborne trematodiases, taeniasis/neurocysticercosis, cutaneous
leishmaniasis, mycetoma, chromoblastomycosis and snakebite
envenoming (4).
The most common NTDs are found in low- and
middle-income countries, including Latin America, Africa and Asia.
NTDs are commonly found in tropical countries due to their humidity
and climate, which are suitable habitats for the development of
disease vectors (3). Individuals who
have limited access to clean water and who live in areas without
proper management of human waste are likely to be infected
(5), with women and children at the
highest risk for NTDs (5). During
the COVID-19 pandemic, efforts to eliminate NTDs have been
disturbed. In 2020, the World Health Organization (WHO) recommended
some advice regarding this emergency situation, primarily
suggesting the use of community-based engagement, active
surveillance (active case finding) and continuous health campaigns.
The elimination of NTDs must involve the effective collaboration
between health authorities and citizens (4). Therefore, the WHO encourages local
health authorities to strengthen NTD platforms, surveillance
mechanisms and health education (6).
As a tropical country, Indonesia faces a double
burden. For instance, the management of COVID-19, which currently
shows no significant progress, combined with the presence of NTDs
will remain a problem for public health (2). In October 2020, certain regions in
Indonesia entered the monsoon/rainy season, which potentially
increases the survival rate of some vectors of disease, such as
mosquitos, worms and bacteria, which carry causative agents of
disease (7). In addition, according
to the Indonesian Ministry of Health, five NTDs are still present
in Indonesia, including leprosy, filariasis, yaws, STHs and
schistosomiasis (8). The present
review will discuss the preparedness of Indonesia in facing NTDs
during the COVID-19 pandemic.
2. NTDs in Indonesia
Leprosy
Leprosy is one of the bacterial infectious diseases
caused by Mycobacterium (M.) leprae. The bacillus of
M. leprae was first detected by the Norwegian physician
Gerhard Armauer Hansen in the early 19th century, but the first
case was reported in the city of Rio de Janeiro, Brazil, in 1600
(9,10). M. leprae infects macrophages
and Schwan cells (11). Reproduction
of M. leprae occurs via binary fission and requires a
temperature between 27-30˚C for survival (12). Moreover, M. leprae is a
microorganism that has a predilection for the skin and nerves
(10). This bacillus can survive up
to 5 months in dry conditions, while it can survive up to 46 days
in wet soil (13). Furthermore,
M. leprae has a long incubation period, with an average time
of 4-5 years (14).
According to the WHO, leprosy can be classified for
therapeutic purposes based on the bacterial index, and is divided
into multibacillary and paucibacillary types. Patients are
classified as paucibacillary if they have a bacterial index of
<2+, whereas multibacillary cases have a bacterial index of ≥2+
(15). This disease primarily
affects the skin, eyes, peripheral nerves and mucosa of the upper
respiratory tract. Growths (nodules) can appear in the skin,
followed by other manifestations, such as painless ulcers on the
feet, loss of eyebrows, dry or thick skin and discoloured patches
of skin (16). These manifestations
in the skin can lead to stigma for individuals with leprosy. Thus,
health professionals should educate citizens on the transmission of
leprosy. For example, individuals cannot contract leprosy through
casual contact with others who have leprosy, such as through
hugging, shaking hands, sitting next to each other or dining
together. Leprosy is also not transmitted from the mother to the
new-born during pregnancy, and sexual contact is excluded as a
transmission route (16). Instead,
researchers consider that leprosy can be transmitted via persistent
sneezing or coughing. Thus, identifying the source of leprosy
transmission can be difficult (16).
A WHO report indicated that the number of leprosy
cases worldwide at the end of 2018 was 184,212, with a prevalence
rate of 0.2/10,000(17). The WHO
also launched a program titled ‘Global Leprosy Strategy 2016-2020:
Accelerating towards a Leprosy-Free World’ and mapped three core
pillars, which were as follows (17): i) First pillar, strengthening
government leadership through coordination and partnership; ii)
second pillar, stopping/treating leprosy and its complications;
iii) third pillar, stopping discrimination and stigma, as well as
supporting and promoting inclusion. Within the three pillars
established by the WHO, the outcome expected is having zero
disabilities among new patients (especially paediatric patients),
decreasing the grade 2 disability rate to <1 case per 1 million
individuals and having zero countries with legislation allowing bad
stigma of patients (17).
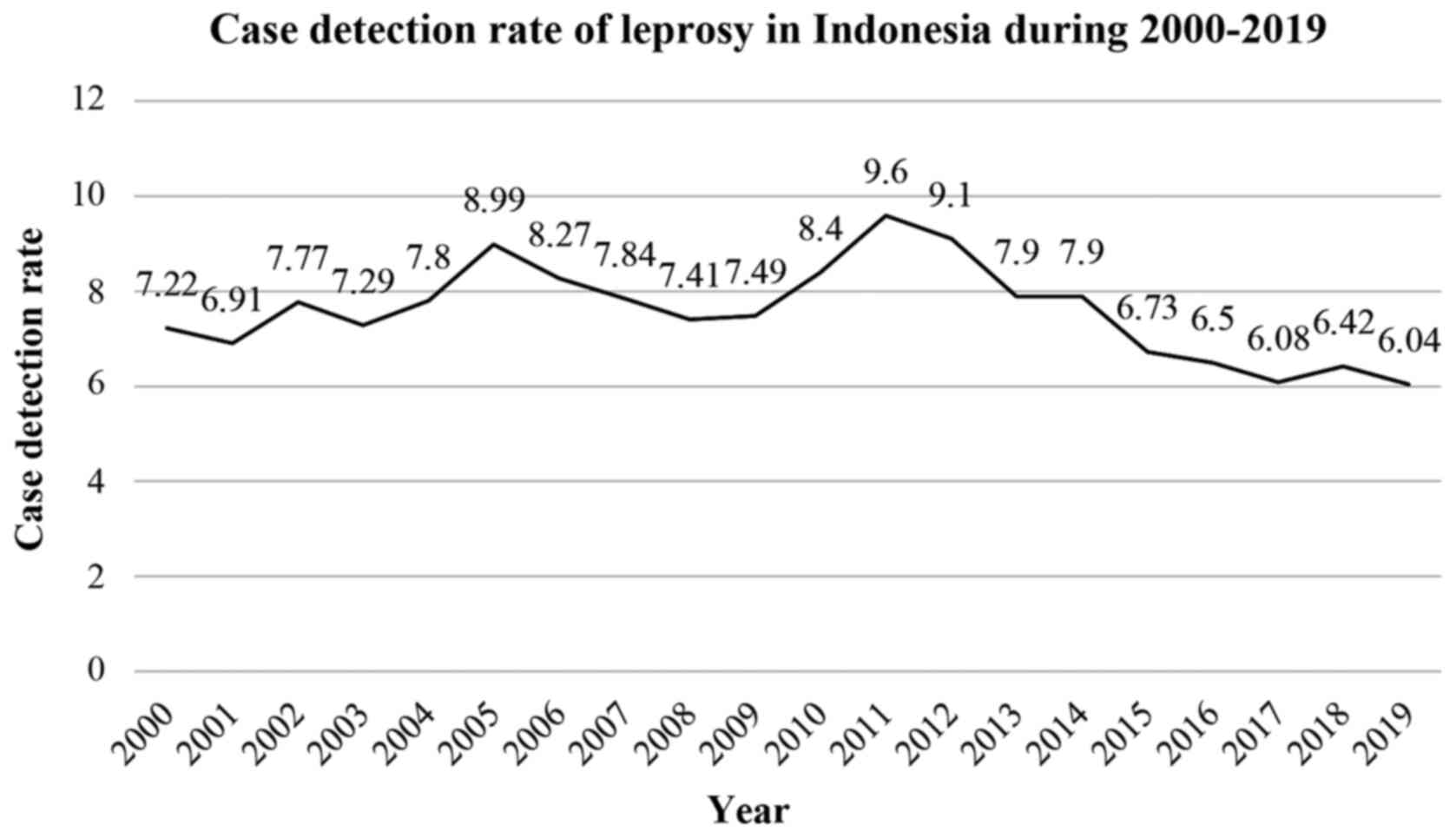
Indonesia is one of the top three countries that has
the highest number of cases of leprosy worldwide, after India and
Brazil. Data from 2017 indicated that 15,910 new cases of leprosy
were detected in Indonesia, with a case detection rate of 6.08%.
The latest data revealed that the prevalence of leprosy in 2017 was
0.7 per 10,000 population (18). The
highest number of new cases was 3,373, as identified in East Java
between 2015-2017, followed by West Java with 1,813 new cases,
Central Java with 1,644 cases and South Sulawesi with 1,091 new
cases (8). Although the Indonesian
Ministry of Health has created a health campaign to eliminate
leprosy, the number of new cases in 2019 remained high. For
instance, current data revealed 16,186 new reported cases, with a
case detection rate of 6.04 (Fig. 1)
(8,18).
Hopes and challenges
Indonesia experienced numerous setbacks in leprosy
management before the COVID-19 pandemic, such as limited early
detection, lack of effective treatment, a less effective vaccine,
lack of understanding of the pathogenesis of peripheral nerve
damage, stigmatization among individuals with deformities and poor
management of chronic erythema (19). As one of the top three countries with
the highest number of leprosy cases, Indonesia must not forget
leprosy as one of its public health problems, even if there is a
current worldwide focus on the COVID-19 pandemic. Some aspects that
should be considered in managing leprosy are as follows: i)
Co-infection between COVID-19 and leprosy that has already been
reported, and this resulted in immunological consequences (20). However, in Indonesia, this
co-infection has not yet been reported; ii) there is increasing
stigmatization among patients with leprosy who are co-infected with
COVID-19; iii) there is limited access to health facilities due to
the prioritization of patients with COVID-19; and iv) poverty and
practicing a healthy lifestyle remain challenges.
Some recommendations for patients with leprosy
during the COVID-19 pandemic have been established by the Indian
Association of Dermatologists, Venereologists and Leprologists and
Special Interest Group, which are as follows (21): i) A health officer should be an
advocate for patients with leprosy, with the aim of implementing a
healthy and hygienic lifestyle (such as regular hand washing,
wearing a face mask and physical distancing) to prevent
co-infection of COVID-19 and to minimize the burden on the health
system; ii) individuals with leprosy should continue therapy and
practice all precautions; iii) all registered patients with leprosy
should be prescribed multidrug therapy; iv) some patients with
leprosy are at high risk of COVID-19, such as those taking
corticosteroids and those using immunosuppressants; and v)
neurological damage in patients with leprosy could lead to the
occurrence of lesions (especially in the eyes, feet and hands),
skin dryness and bone resorption that manifests in a tendency for
deformities (21).
3. Filariasis
Filariasis is a group of infectious diseases caused
by filarial worms. The nematode species identified as the source of
transmission includes Wuchereria (W.)
brancrofti, which causes the most infections (90%), followed
by Brugia (B.) malayi, which causes 10% of all
infections, and then B. timori I (data for the number of
infections not available) (22).
Moreover, mosquito vectors transmitting this parasite include
Culex, Anopheles, Aedes and Mansonia.
It has been reported that most cases of lymphatic filariasis in
Indonesia are caused by B. malayi (22). Current data from the WHO suggest that
893 million individuals from 49 countries in 2018 were infected by
filariasis and required chemotherapy to stop the transmission
(22). It has been shown that
filarial parasites can infect individuals through infective
mosquito bites (22). Furthermore,
children tend to be infected more often, and they experience hidden
damage to their lymphatic system (22).
In Indonesia, which is a tropical country with
various genera of mosquito, filariasis is a public health problem.
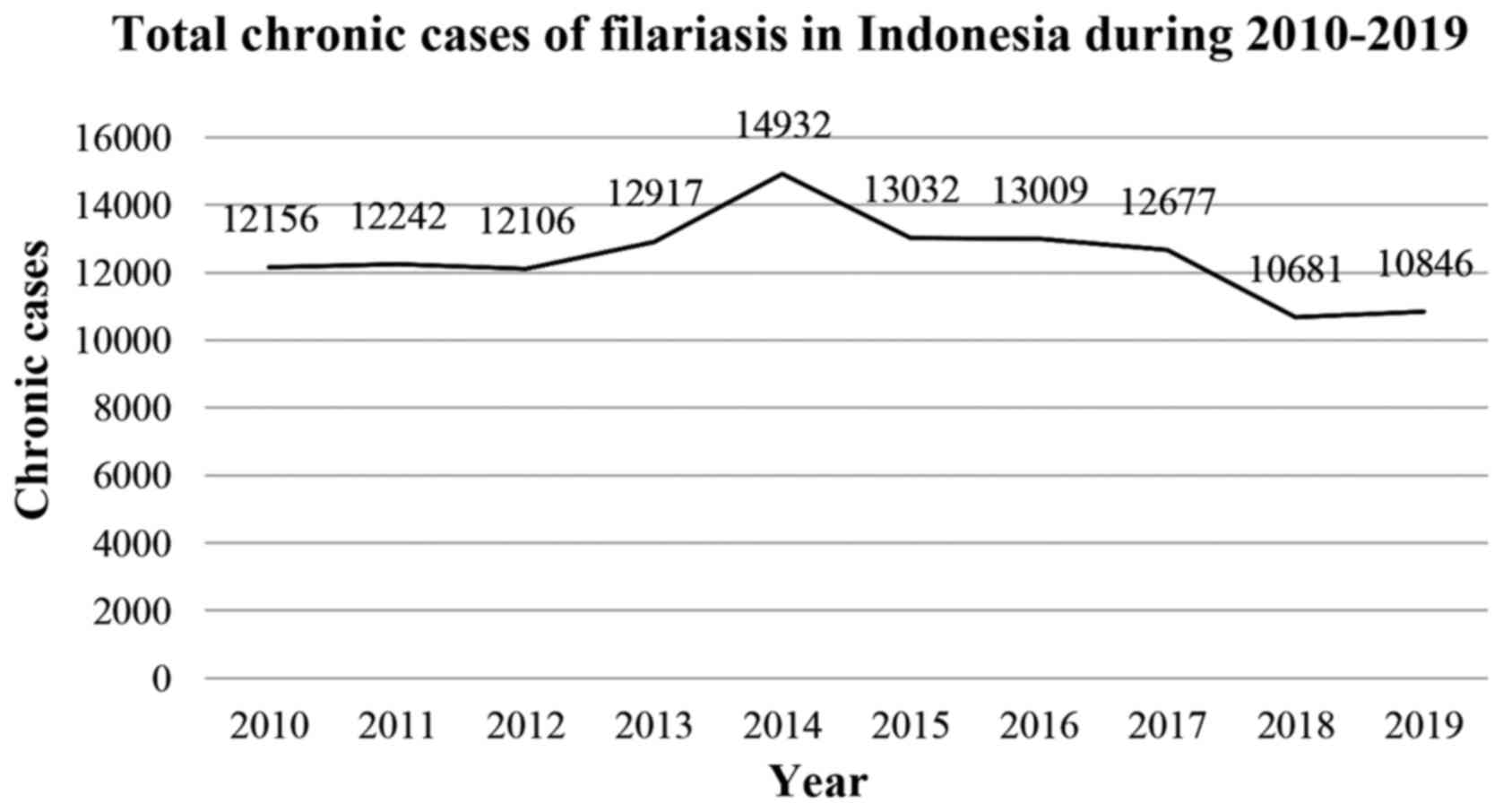
In 2019, there were 592 new cases of filariasis, with 10,681
chronic cases reported in the previous year (23). The province of Papua has the highest
number of chronic cases with 3,615 reported cases, followed by East
Nusa Tenggara with 1,542 cases, East Java with 781 cases, West
Papua with 622 cases and Aceh with 578 of cases (23). The number of filariasis cases in 2019
was 10,846 cases as shown in Fig.
2(23). In addition, the
prevalence of filariasis in Indonesia was <1% per 10,000
population in 2018(24).
A necessary effort to eliminate lymphatic filariasis
has been attempted via mass drug administration (MDA) or
large-scale treatment within at-risk communities once a year. A new
triple regimen (such as ivermectin, albendazole and
diethylcarbamazine) has been introduced by the WHO to treat
filariasis in endemic countries. These methods can kill the
microfilariae in the blood of the infected person so that
transmission by mosquitoes can be prevented (22). Since 1970, Indonesia has worked to
eliminate lymphatic filariasis, but the country still faces
challenges, such as program coordination regarding the geographical
area of Indonesia, lack of personnel, inadequate medication and the
lack of an education and awareness program (24).
In Indonesia, filariasis is the result of three
agents, namely, W. bancrofti, B. malayi and B.
timori. W. bancrofti can be found in both urban and
rural areas (25). Furthermore,
there are 20 species of mosquitoes that have been confirmed as
vectors of filariasis. In urban settings, bancroftian filariasis is
transmitted by Culex species, such as Culex
quinquefasciatus. However, in rural settings, bancroftian
filariasis is transmitted by Anopheles species, such as
Anopheles aconitus and Anopheles punctulatus complex
(25). A study in Pekalongan,
Central Java, reported the prevalence of W. bancrofti to be
4.4%, with a higher prevalence in adults compared with children
(26). MDA has been applied in
provinces in which filariasis bancrofti is endemic, and evaluations
of this intervention have shown varied results. For instance, the
evaluation of post-MDA in Jatimulya Bekasi indicated that
microfilaria of W. bancrofti in C. quinquefasciatus
was already non-existent, suggesting low transmission of lymphatic
filariasis (27). Another report
post-MDA in Papua reported the non-existence of W. bancrofti
in 358 dissected mosquitoes (28).
The COVID-19 pandemic has had a significant global
impact, with 126,890,643 confirmed cases and 2,778,619 deaths (as
of March 29th, 2021) (1). On April
1, 2020, the WHO issued a recommendation to postpone
community-based survey programs and case detection activities, as
well as mass treatment campaigns in tropical areas with NTDs until
further notice (29). Thus, the
provision of temporary MDA used in the lymphatic filariasis control
and elimination program was also stopped. However, the WHO plans to
analyse the impact of postponing MDA delivery on the 2030 goal by
considering other strategies to strengthen programs, and aims to
reduce the negative impact of the COVID-19 pandemic. Overall, with
these program delays, the WHO is considering accelerating progress
through biannual treatment or by increasing drug access to at least
65% of the at risk population (30).
Hopes and challenges
The 2020 targets proposed by the Global Program to
Eliminate Lymphatic Filariasis (GPELF) were not achieved due to the
impact of COVID-19. Therefore, the WHO will accelerate work to
achieve this target by 2030. Global estimates suggest a 74%
reduction in the number of infected individuals since the inception
of GPELF (30). The new target by
2030 is that 80% of endemic countries will have met the criteria
for eliminating public health problems, with the remaining 20% in
post-treatment surveillance, meaning that MDA is no longer
required. GPELF aims to reduce the prevalence of infection below
the target threshold and decrease the impact of morbidity in
individuals with lymphedema, hydrocele and chronic manifestations
of lymphatic filariasis. Essential treatments recommended for the
management of lymphedema and hydrocele should be available in 100%
of districts with individuals who have manifestations of lymphatic
filariasis (30). This goal is in
line with the universal health coverage goal of not excluding
anyone by 2030(31).
Studies evaluating the effectiveness of MDA in
Indonesia and the detection of microfilaria in mosquitoes are still
limited. In addition, health workers responsible for carrying out
the MDA campaign frequently face technical problems, such as
persuading the community in endemic areas to take antifilarial
drugs (32). Continuous consumption
of antifilarial drugs may cause uncomfortable conditions, and thus,
the elimination effort has been disturbed, although microfilaria
still exists. Another campaign involves the distribution of bed
nets to prevent mosquito bites. For this campaign, health workers
should collaborate with local citizens to increase their impact in
persuading the community to adopt this preventive measure (32).
4. Yaws
Yaws is a communicable disease caused by the
Treponema pallidum subspecies pertenue (33). This disease belongs to the same group
of bacteria that cause diseases such as venereal syphilis. Yaws is
a chronic deforming and debilitating infectious disease that
affects not only bone and skin but also cartilage. Most individuals
infected by yaws (75-80%) are children younger than 15 years, and
the incidence peak occurs in children between 6-10 years of age
(33). Moreover, the incubation
period ranges from 9-90 days, with an average of 21 days (33).
There are two stages of yaws infection. In the early
stage, the patient is infectious (33). During this stage, a papule develops
at the site of infection, which is full of the organisms. This
papule may persist for 3-6 months. Without adequate treatment, this
stage results in disseminated skin injuries over the body (33). The second stage is non-infectious and
typically appears 5 years after the onset of infection. The
diagnosis of yaws is based on these symptoms, and the disease is
confirmed via dark-field microscopy examination (33). Blood tests are not commonly used as
yaws is very similar to the bacterium that causes syphilis, and so
these diseases will show the same results (33).
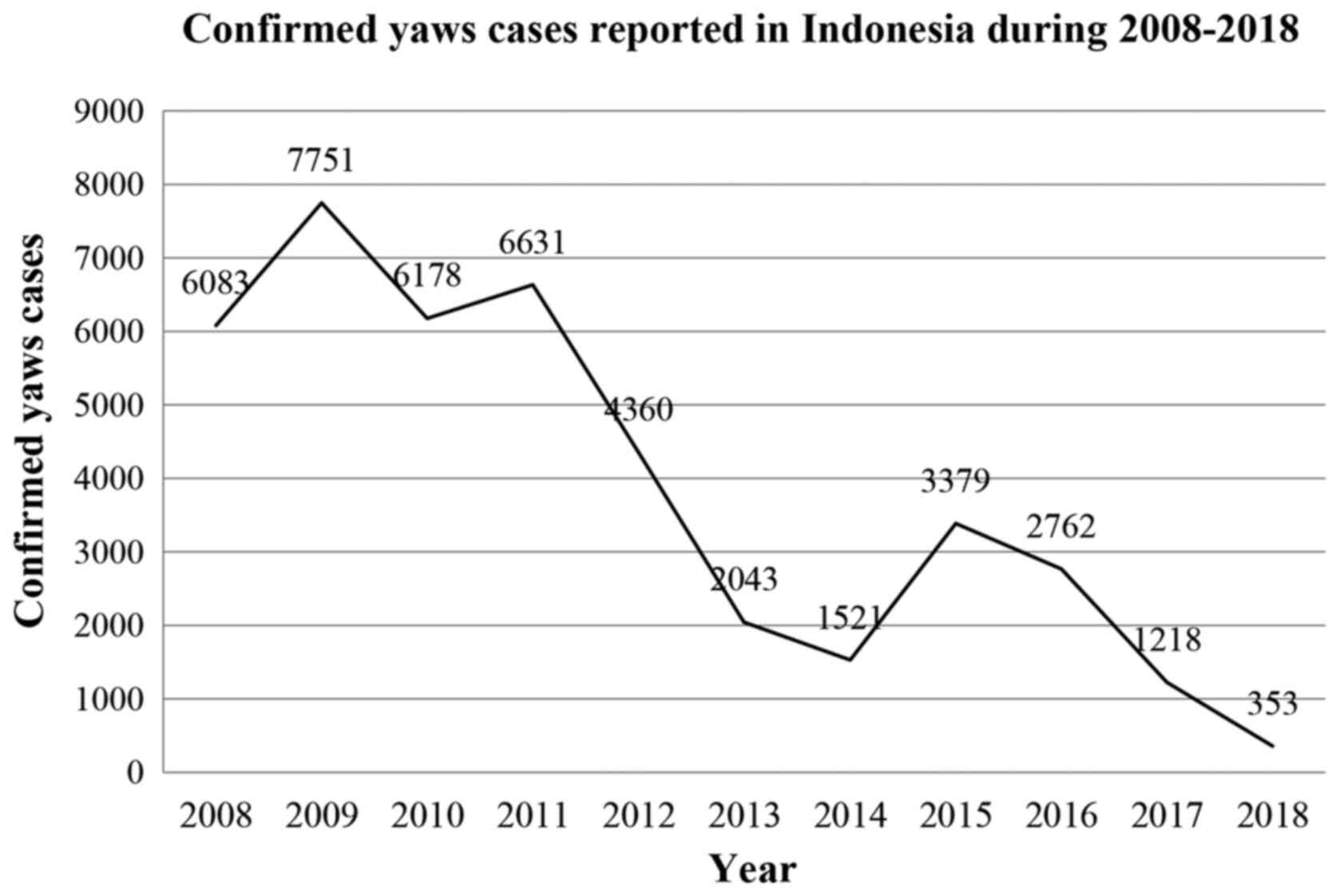
In Indonesia, yaws is present in 18 provinces and is
endemic in four islands, including Papua, Maluku, East Nusa
Tenggara, and Sulawesi (23). During
2016, eight provinces reported cases of yaws, five of which (Papua,
West Papua, Maluku, North Maluku and East Nusa Tenggara) were from
eastern Indonesia and included 99.7% of the total 2,762 cases.
There were 6,083 cases in 2008, which spiked to 7,751 cases in
2009. However, the number of cases decreased to 1,218 in 2017, and
gradually reduced to 353 cases in 2018 (Fig. 3) (23). In the last report in 2019, the
prevalence of yaws was 0.9 per 10,000 population (23).
There is currently no vaccine for yaws. Health
education and improved personal hygiene are the most recommended
critical tactics for prevention. Moreover, the most recommended
treatment for yaws is a single oral dose of the antibiotic
azithromycin (30 mg/kg, maximum 2 g) (33). In addition, benzathine penicillin
(single intramuscular dose) at 0.6 million units (for children
younger than 10 years) and 1.2 million units (individuals older
than 10 years) are used for patients with suspected clinical
treatment failure after azithromycin or for patients who cannot be
treated with azithromycin (33).
The WHO aimed to eradicate yaws in 2020 by using a
MDA of single-dose azithromycin for all individuals in the
community, regardless of infection status (34). This program comprised of the initial
mass treatment of endemic communities, with an evaluation conducted
every 6 months to actively identify and treat remaining cases
(active case finding) (23,35). Several approaches have been conducted
by the Indonesian Ministry of Health since 2013, such as active
screening, engaging communities, increasing the capacity of health
workers to diagnose and oversee treatment, and intersectoral
collaboration (19). However, the
obtained results of MDA in several districts remain low, as not all
districts have reported results, and serology surveys still observe
positive cases in several districts (36).
Hopes and challenges
There are hindrances to the eradication of yaws in
Indonesia. Prior to the COVID-19 pandemic, yaws management remained
an issue, due to restricted early detection by health workers,
topographical issues in endemic area, lack of surveillance and
reporting systems, and a lack of confirmatory laboratory testing
(19). Indonesia must consider that
yaws is an endemic disease in some districts. The COVID-19 outbreak
has affected the implementation of essential health services for
NTDs, including yaws. The areas of disruption are identified as
follows (37): i) Suspension of mass
interventions, active case finding and other community-based
activities; ii) delays in diagnosis; iii) discontinuance of routine
surveillance; and iv) discontinuance of population-based
surveys.
5. STHs
There is a high prevalence of STH infection across
South Asia and Southeast Asia, and this remains endemic in most
tropic and subtropic regions (38).
STHs contribute to the global burden of disease, with ~1.45 billion
individuals infected by STHs worldwide, and ~70% of these
infections are found in Asia (39).
In 2017, STH infection, mostly caused by Ascaris (A.)
lumbricoides, Trichuris trichiura, Ancylostoma
duodenale and Necator americanus, resulted in 1.9
million disability-adjusted life years (40). These parasitic helminths are
transmitted by eggs that are passed in the faeces of an infected
human, and these eggs can potentially contaminate areas with
inadequate sanitation. Individuals who live in contaminated
environments and have poor hygiene practices are at risk of
accidentally ingesting eggs through food, drinking water or unclean
hands (41).
Symptoms of STH infection depend on the phase of the
helminth life cycle and its intensity of infection. An infected
person with light and moderate intensity of infection may have
non-specific symptoms or be asymptomatic. However, patients who are
symptomatic may complain of a lack of appetite, abdominal pain or
discomfort and diarrhoea, which result in weight loss. Moreover,
heavy-intensity hookworm infection can lead to continuous blood
loss and a decline in erythrocytes and nutrients, and this
condition may result in severe anaemia. Severe anaemia can also
occur in patients with dysentery syndrome as a result of infection
with Trichuris trichiura (42). It has been shown that larva migration
of A. lumbricoides through pulmonary tissue may cause
eosinophilic pneumonia, also referred to as Loeffler syndrome. This
syndrome occurs 10-14 days after infection (42).
The diagnosis of STH infection by clinical symptoms
remains unspecific. The established diagnostic method for the
identification STH infection is microscopic identification, and it
is sufficient for detecting moderate to severe infection or
examination in endemic areas (43).
However, another highly sensitive method is required for the
evaluation of the efficacy, effectiveness and disease elimination
of drug interventions, such as serology and molecular technique,
yet these techniques may be costly and pose new problems in
resource-limited regions (43).
STH infection remains frequent in several rural
communities in Indonesia. A cross-sectional study conducted in
Sampang Regency in 2019 reported a high prevalence (71.4%) of STH
infection among school-aged children (SAC) (44). Another study in Central Sumba
District reported that the STH prevalence was very high, with 91%
of infection occurring in SAC (45).
The WHO has suggested an integrated strategy to
control the morbidity of STHs through preventive chemotherapy (PC),
the provision of clean water, access to adequate sanitation and
health and hygiene education (46).
The WHO recommend an annual or biannual administration of PC with
albendazole (400 mg) or mebendazole (500 mg) as a single dose for
the at-risk population, such as young children, preschool AC
(PSAC), SAC, non-pregnant adolescent girls, non-pregnant women of
reproductive age and pregnant women (46). As an effort to eliminate STH
infection as a public health problem in children, the WHO has
targeted ≥75% of PC coverage for PSAC and SAC (47). A previous study reported that PC
coverage for PSAC and SAC between 2010 and 2017 had consistently
increased. However, as of 2018, there were still 23 countries in
which PC coverage was below the target, one of which was Indonesia
(48). In addition, compared with
that in 2017, the global PC coverage in 2018 was decreased from 70
to 60% (49).
Hopes and challenges
The STH infection preventive and control program
faces several challenges beyond 2020, as described by Freeman et
al (50): i) Inconsistency and
reduced comprehensiveness of monitoring the program's impact; ii)
expanding PC coverage for at-risk groups other than SAC; iii) the
possible emergence of drug resistance; iv) a weak diagnostic method
for assessing program requirements at the implementation stage; v)
low efficacy of available anthelmintic drugs and gaps in their
procurement; vi) lack of coordination with the water, sanitation
and hygiene sector; and vii) developing a new target for the
elimination of STHs post-2020
Since early 2020, the world has suffered from the
emergence of the COVID-19 pandemic, which has primarily affected
world health security, economics and healthcare providers, and has
exerted a potentially adverse effect on access to healthcare in
low- and middle-income countries (1. The COVID-19 pandemic
threatens the containment of NTDs, a situation which is a
significant health problem for tropical and subtropical countries,
where diseases of parasite infection, such as malaria and NTDs, are
prevalent, and co-infections are likely to occur. This condition
may result in a worse outcome, which is referred to as synergistic
endemics (51). Generally, helminth
infection regulates human IL-4 and IL-10 cytokines, which lead to
the differentiation of T helper cells 2 cells and downregulation of
the inflammation responses of IFN-γ, IL-6, IL-17 and TNF-α. This
mechanism allows us to block the helminths both locally and
systematically (52). However, the
cytokine storm observed in COVID-19 cases is suggested to prevent
this blockage. This scenario may occur in individuals who are
infected with parasites and live in a resource-limited area where
deworming is not conducted regularly (52).
In responding to the COVID-19 pandemic, the
Indonesian Ministry of Health has issued ‘Guidance for Toddler
Health Services during the COVID-19 Emergency Response’. The main
purpose of this policy is recommendations for cross-sector
coordination in disseminating efforts to prevent the transmission
of COVID-19, and implementing emergency conditions and routine
health services for toddlers during the spread of infection. Health
routine services for toddlers are carried out by applying the
triage method, the principles of infection prevention and control
and physical distancing (53). In
areas where the local government implements large-scale social
distancing, called Pembatasan Sosial Berskala Besar (PSBB; social
distancing in Indonesian), with positive cases of COVID-19, the
administration of PC for helminths infection is postponed (54). By contrast, in areas with no PSBB and
that have no COVID-19 cases, the administration of PC is conducted
by implementing health protocols for COVID-19 and maintaining
physical distancing (53).
The cessation of PC administration during COVID-19
is not an absolute choice. Several regions in Indonesia have
organized the administration of PC during this pandemic through the
Integrated Health Service Post (often termed Pos Pelayanan Terpadu
in Indonesian) and school-based community, as well as by complying
with health protocols such as using masks, sterilizing the
location, providing handwashing facilities, checking body
temperature and maintaining physical distancing (53). Prevention is key, and there is an
urgent requirement to develop parasitic and COVID-19 prevention
messages for SAC. For example, it is possible to develop an
entertaining education approach using a cartoon video that can be
applicable for television broadcasts or a poster that can be shared
on social media (50,55). However, all of these efforts require
significant support, not only from the local health office but also
from the government and other sectors.
6. Schistosomiasis
Schistosomiasis, or bilharzia, is a type of NTD
caused by a parasite referred to as the blood fluke (trematoda
worms) of the genus Schistosoma (S.). This disease is
typically found in tropical and subtropical zones, particularly in
communities with inadequate sanitation (56). The WHO has estimated that 240 million
individuals were infected and that >700 million individuals
lived in endemic zones in 2018(56).
There are two dominant types of Schistosomiasis, intestinal
and urogenital, and both are caused by the three most important
types of blood flukes: S. haematobium, S. mansoni and
S. japonicum (56).
Schistosomiasis in Indonesia is caused by S. japonicum,
which is also spread in several other Asian countries, such as
China, Japan, the Philippines, Vietnam, Laos, Thailand and Cambodia
(56).
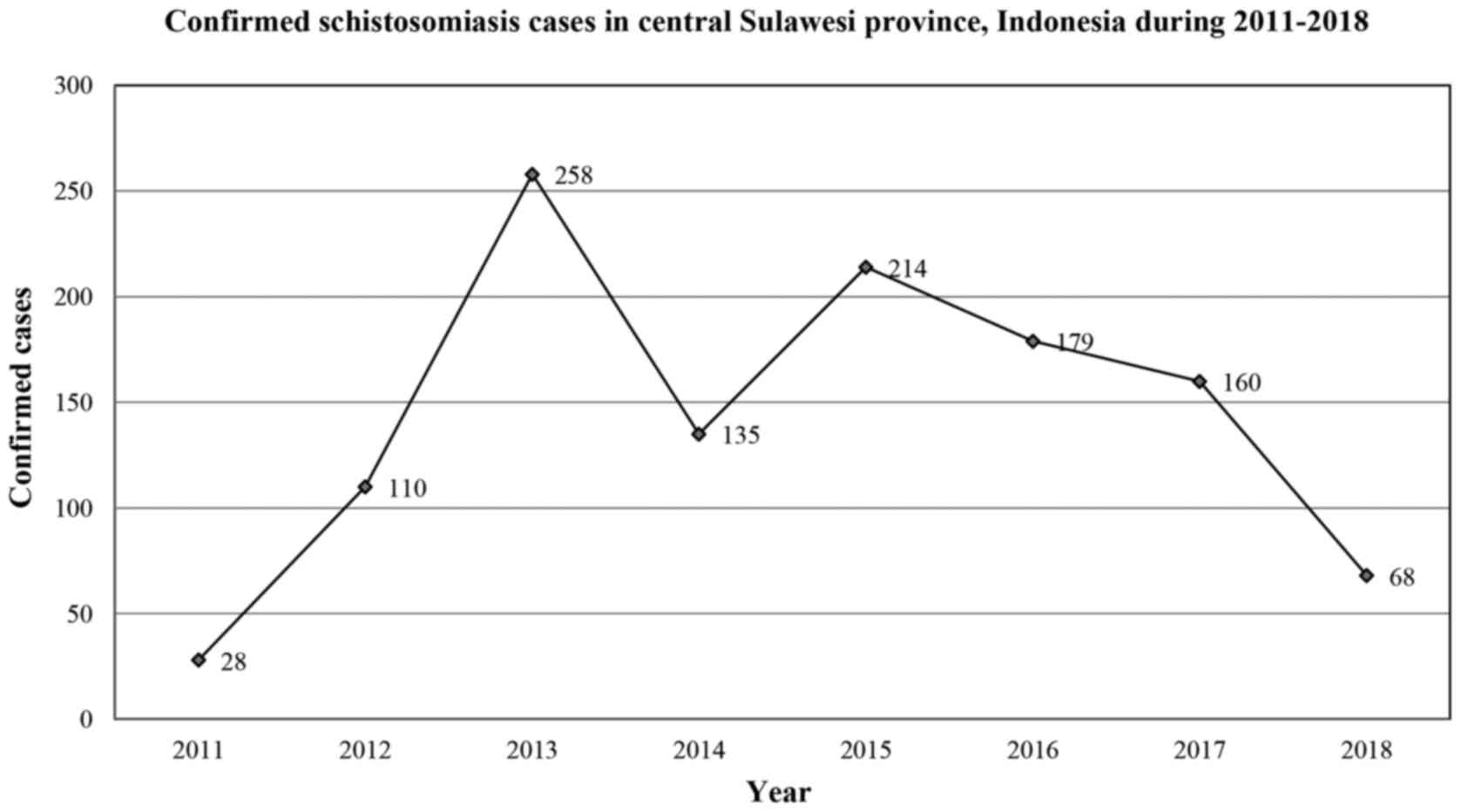
Schistosomiasis is endemic in two districts in
Indonesia, namely, Sigi (Lindu valley) and Poso (Napu and Bada
valley), both of which are located in the Central Sulawesi
Province. Data from 2011-2018 (Fig.
4) revealed a fluctuation in the prevalence of schistosomiasis
in Central Sulawesi. Moreover, data from 2011 indicated that 28 of
7,655 individuals (0.39%) had confirmed schistosomiasis, a rate
that increased to 258 of 14,180 (1.81%) individuals in 2013. The
latest data from 2018 suggest that the prevalence of
schistosomiasis has decreased to 68 of 20,342 (0.33%) examined
individuals (57).
Water plays a significant role in the transmission
and spread of schistosomiasis. Human-to-environment transmission
typically occurs when infected individuals contaminate fresh water
with their faeces that contain parasite eggs, and the larval stages
of the parasite can infiltrate through the skin during contact with
contaminated water (58). Cercariae
are the short-lived and free-swimming larval phases of the
parasites, which are shed by snails that act as intermediate hosts
for the parasites. These larvae require 5-7 weeks to explicitly
develop into adult schistosomiasis, and the adult worms can live in
the human veins for up to three years (58). The female Schistosoma can produce
hundreds to thousands of prepared eggs each day. A small number of
eggs can cause immune reactions and damage the inner organs humans,
while the other eggs are released from the body (58).
The symptoms of schistosomiasis are the result of
immune responses to the worm's eggs. Most individuals have no
symptoms when they are first infected, but can develop a rash or
itchy skin within days after becoming infected. Furthermore,
several systemic symptoms may develop within 1-2 months of
infection, including fever, chills, muscle aches and cough. Without
prompt treatment, these symptoms can be chronic and persist for
several years in the human body (59).
Signs and symptoms of intestinal schistosomiasis
include stomach torment, haematochezia and diarrhoea. In severe
cases, enlargement of the liver and spleen may occur. Enlargement
of the liver is often connected to liquid aggregation in the
peritoneal cavity and hypertension of the stomach vein (60). On the other hand, schistosomiasis
also persists as urogenital schistosomiasis, with associated
urinary tract infection symptoms such as blood in the urine,
urinary tract fibrosis (bladder and ureter) or kidney failure in
most severe cases. Urogenital schistosomiasis occurs in women and
typically presents as genital injuries, vaginal bleeding, pain
during sexual activity and nodules in the vulva (60).
Diagnostic methods for schistosomiasis usually
consist of parasite detection from the stool and urine using the
Kato-Katz technique and urine microscopy, the detection of
antibodies in the serum, detection of the antigen and detection of
DNA. Kato-Katz is the most universally applied test due to it is
inexpensiveness, easy use and its easily improved diagnostic
sensitivity (61). Moreover,
serological and immunological testing may be useful in revealing
exposure to infection in non-endemic or low-transmission
communities areas (61).
Hopes and challenges
Schistosomiasis rarely causes mortality, but it can
lead to severe health and economic problems. For example,
schistosomiasis in children can cause anaemia, stunting and
cognitive impairment (56). The WHO
recommends an integrated strategy for the control of
schistosomiasis through a massive administration of treatment for
at-risk population groups, improved water supply and sanitation,
education in hygiene and controlling the snail population (56). Management of schistosomiasis by mass
treatment is typically chosen if the community prevalence is
>1%, while selective treatment is selected if the prevalence is
<1%. In Indonesia (8), the
control method of schistosomiasis consists of praziquantel dosage
regimens (60 mg/kg body weight). In 2011, a study in Indonesia
reported that praziquantel was the recommended treatment against
all stage of schistosomiasis. However, studies on the use of
different medications are still required, as the evaluation time is
fairly long (57).
The Indonesian Ministry of Health has published a
health campaign to eradicate schistosomiasis titled the
‘Schistosomiasis Roadmap Eradication 2018-2025’ (62). Collaboration and synergy across
sectors are important, such as public health, government and
community. Prior to the COVID-19 pandemic, the management of
schistosomiasis was challenging, and the outbreak of COVID-19 may
be exacerbating NTDs, as it has diverted both financial and human
resources. Potential strategies should be proposed to maintain the
synergy between the control of both the COVID-19 pandemic and NTDs
(62).
7. A view of NTDs and COVID-19 in the
Indonesian context
The first confirmed cases of COVID-19 in Indonesia
were detected in March 2020. Subsequently, the number of COVID-19
cases increased continuously, with 1,476,452 cases by March 29th,
2021, bringing the positivity rate to 13.5%. Moreover, to date,
there have been 39,983 mortalities caused by COVID-19 in Indonesia
(as of March 29th, 2021) (63). The
current strategies of the Indonesian government to reduce the
transmission of COVID-19 consist of the application of health
protocols, including washing hands regularly using soap and water,
and asking citizens to stay at home and minimize outdoor
activities, as well as practice physical distancing, wear masks,
stay updated regarding COVID-19 and postpone unnecessary travel
activity. Moreover, large-scale social restrictions have been
applied sporadically in regions with high numbers of cases, based
on Government Regulation no. 21/2020(54). The cumulative number of tests
performed since from the date of the first identified case to
February 3, 2021 is 6,280,182, with an average per day of 46,893
tests. The failure to reduce the transmission of COVID-19 led to
the highest number of active cases, namely, 175,326 cases, on
February 3, 2021. The latest update on February 3, 2021 shows the
rate of bed occupancy in isolation wards was also at its worst,
with the highest bed occupancy rate reported in Jakarta of 80%,
followed by East Kalimantan at 78.9% and Central Sulawesi at 70.2%
(63). A high demand for intensive
unit was also reported in Yogyakarta (83.8%), followed by Jakarta
(82.3%) and Gorontalo (77.8%) (63).
The number of COVID-19 cases in Indonesia seems likely to be
underreported due to the lack of testing. The wide gap between the
number of individuals tested and suspected cases indicates that
testing capacity in Indonesia remains limited.
As aforementioned, there are five NTDs in Indonesia
that require the initiation of a special elimination program. Due
to the pandemic, Indonesia needs to implement a strategic effort
for the effective management of NTDs. However, no guidelines for
the management of NTDs in Indonesia during the COVID-19 pandemic
have been released, nor has the number of cases of NTD during the
COVID-19 pandemic been reported. Future health policy makers should
take this into consideration, as data accessibility as a baseline
is important for formulating novel strategies. A retrospective
study in Jakarta reported a considerable increase in mortality
caused by COVID-19, as shown by burial records during 2020, with up
to a 61% increase in deaths compared with the previous year (2019)
(64). However, the mortality rate
was lower compared with that in developed countries due to the
demographics in Indonesia, which is dominated by a younger age
group (65). Overall, there is no
doubt that active case surveillance for the detection of new cases
of NTD will be disrupted during the pandemic, such as community
surveys for filariasis and STHs.
To minimize mortalities caused by a late response,
Indonesia should strengthen its efforts at NTD control.
Collaboration is key for successful NTD control during the COVID-19
era. The government and citizens should collaborate on active case
detection, and could use the technological sophistication that is
directly integrated in data centres. If internet networking is
limited in endemic areas, an alternative could be the use of a
short message service. Mobile health strategies that support NTD
programs have been applied in other countries as tools that can be
used to detect new cases or for patient follow-up (66).
8. Conclusion
Indonesia still faces some challenges for the
control of NTDs, especially during the COVID-19 pandemic, including
the inconsistent follow-up of multidrug therapy for patients with
leprosy resulting from an insecurity of patients regarding visiting
health facilities, as well as limited reports on the evaluation of
MDA for lymphatic filariasis, delayed diagnosis and active case
findings for yaws, a lack of coordination in the elimination of
STHs and lack evaluation of drugs used for schistosomiasis. This
review suggests to the Indonesian government to strengthen their
effort for NTD control through alternative methods, such as
collaborating with key citizens in the detection of new cases and
introducing mobile health as a method for detecting health problems
or for following up patient progress. Testing, tracing and
treatment to reduce the transmission of COVID-19 must be improved,
so that the gap between suspected cases and confirmed cases of
COVID-19 can be decreased. If the transmission of COVID-19 can be
decreased, then case detection and NTD control efforts can be
conducted effectively.
Acknowledgements
We would express our gratitude to the Indonesian
Ministry of Health for the providing the data regarding the number
of cases from annually released data, which is entitled the
Indonesia Health Profile.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analysed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
SF was responsible for conceptualization, manuscript
preparation and validation. SMDP was responsible for manuscript
preparation, data analysis and grammatical checks. ZS was
responsible for manuscript preparation and investigation of the
data. FKNH was responsible for manuscript preparation and data
collection. HRW was responsible for manuscript preparation and data
analysis. THS was responsible as the supervisor and corresponding
author, and was involved in manuscript preparation. FA was
responsible for manuscript revision and providing data analysis. SS
was responsible for supervision and manuscript preparation. All
authors read and approved the final manuscript. Data sharing is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
World Health Organization: WHO coronavirus
disease (COVID-19) dashboard. WHO Health Emergency Dashboard.
https://covid19.who.int/. Accessed March 29,
2021.
|
|
2
|
Indonesian Ministry of Health: COVID-19.
https://infeksiemerging.kemkes.go.id/. Accessed
October 23, 2020.
|
|
3
|
World Health Organization: Neglected
Tropical Diseases. Hidden Successes, Emerging Opportunities,
Geneva, 2009.
|
|
4
|
World Health Organization: Neglected
tropical diseases. https://www.who.int/neglected_diseases/en/. Accessed
October 23, 2020.
|
|
5
|
Centres for Disease Control and
Prevention: Neglected Tropical Diseases. https://www.cdc.gov/globalhealth/ntd/. Accessed
October 23, 2020.
|
|
6
|
World Health Organization and the United
Nations Children's Fund (UNICEF): Community-Based Health Care,
Including Outreach and Campaigns, in the Context of the COVID-19
Pandemic. Geneva, p39, 2020.
|
|
7
|
Indonesian Ministry of Health: Dengue
Situation in Indonesia 0n 2016. https://pusdatin.kemkes.go.id/article/view/16090700001/situasi-demam-berdarah-dengue2016.html.
Accessed October 23, 2020.
|
|
8
|
Indonesian Ministry of Health: Profile
Kesehatan Indonesia Tahun 2018. Ministry of Health Indonesia,
pp107-108, 2018.
|
|
9
|
Trautman JR: A brief history of Hansen's
disease. Bull NY Acad Med. 60:689–695. 1984.PubMed/NCBI
|
|
10
|
Bhat RM and Prakash C: Leprosy: An
overview of pathophysiology. Interdiscip Perspect Infect Dis.
2012(181089)2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Rambukkana A, Zanazzi G, Tapinos N and
Salzer JL: Contact-dependent demyelination by Mycobacterium
leprae in the absence of immune cells. Science. 296:927–931.
2002.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Shepard CC: Temperature optimum of
Mycobacterium leprae in mice. J Bacteriol. 90:1271–1275.
1965.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Desikan KV: Sreevatsa. Extended studies on
the viability of Mycobacterium leprae outside the human
body. Lepr Rev. 66:287–295. 1995.PubMed/NCBI
|
|
14
|
Suzuki K, Udono T, Fujisawa M, Tanigawa K,
Idani G and Ishii N: Infection during infancy and long incubation
period of leprosy suggested in a case of a chimpanzee used for
medical research. J Clin Microbiol. 48:3432–3434. 2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
World Health Organization: WHO Expert
Committee on Leprosy [Seventh Report] 874. Geneva, 1998.
|
|
16
|
Centres for Disease Control and
Prevention: How Do People Get Hansen's Disease? U.S Department of
Health and Human Services. https://www.cdc.gov/leprosy/transmission/index.html.
Accessed October 23, 2020.
|
|
17
|
World Health Organization: Global Leprosy
Strategy 2016-2020: Accelerating Towards a Leprosy-Free World.
https://www.who.int/lep/resources/9789290225096/en/.
Accessed October 23, 2020.
|
|
18
|
Indonesian Ministry of Health: Hapuskan
Stigma dan Diskriminasi terhadap Kusta. InfoDatin Pusat Data dan
Informasi Kementrian Kesehatan RI. pp1-11, 2018 (In
Indonesian).
|
|
19
|
Wibawa T and Satoto TB: Magnitude of
neglected tropical diseases in Indonesia at postmillennium
development goals era. J Trop Med. 2016(5716785)2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Saxena S, Khurana A, B S, Sardana K,
Agarwal A, Muddebihal A, Raina A and Paliwal P: Severe type 2
leprosy reaction with COVID-19 with a favourable outcome despite
continued use of corticosteroids and methotrexate and a hypothesis
on the possible immunological consequences. Int J Infect Dis.
103:549–551. 2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Rathod S, Suneetha S, Narang T, Bhardwaj
A, Gupta SK, Kamoji SG, Ashwini PK, Pradhan S, Rather SP, Patnaik
S, et al: Management of leprosy in the context of COVID-19
pandemic: recommendations by SIG leprosy (IADVL Academy). Indian
Dermatol Online J. 11:345–348. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
World Health Organization: Lymphatic
Filariasis. https://www.who.int/health-topics/lymphatic-filariasis#tab=tab_1.
Accessed October 23, 2020.
|
|
23
|
Indonesian Ministry of Health: Data dan
Informasi Profil Kesehatan Indonesia 2019 8. Profil Kesehatan
Indonesia. Indonesia Ministry of Health, Jakarta, pp1-213, 2020 (In
Indonesian).
|
|
24
|
Indonesian Ministry of Health: Situasi
Filariasis di Indonesia. Infodatin Pusat Data dan Informasi
Kementerian Kesehatan RI. pp1-12, 2019 (In Indonesian).
|
|
25
|
Hoedojo. Vectors of malaria and filariasis
in Indonesia. Bul Penelit Kesehat. 17:181–189. 1989.
|
|
26
|
Ginandjar P, Saraswati LD, Suparyanto D,
Sakundarno M and Supali T: The prevalence of lymphatic filariasis
in elementary School Children Living in endemic areas: A baseline
survey prior to Mass Drug Administration in Pekalongan
District-Indonesia. Iran J Public Health. 47:1484–1492.
2018.PubMed/NCBI
|
|
27
|
Astuti EP, Hendri J, Ipa M and Ruliansyah
A: Identification of Wuchereria bancrofti in Culex
quinquefasciatus Post-Mass Drug Administration (MDA) Lymphatic
Filariasis in Bekasi District. Indonesian Ministry of Health,
Indonesia, 2020.
|
|
28
|
Suweni K and Soeyoko SS: Filariasis
bancrofti epidemiology post mass drug administration in Waris
District Keerom Regency Province of Papua. Trop. Med J. 3:57–63.
2013.
|
|
29
|
World Health Organization: WHO issues
interim guidance for implementation of NTD programmes. https://www.who.int/neglected_diseases/news/LF-reporting-continued-progress-towards-elimination-as-php/en/.
Accessed November 12, 2020.
|
|
30
|
World Health Organization: Alternative
Mass Drug Administration Regimens to Eliminate Lymphatic
Filariasis. Geneva: World Health Organization; (2017). https://www.who.int/lymphatic_filariasis/resources/9789241550161/en/.
Accessed November 12, 2020.
|
|
31
|
Michael E, Singh BK, Mayala BK, Smith ME,
Hampton S and Nabrzyski J: Continental-scale, data-driven
predictive assessment of eliminating the vector-borne disease,
lymphatic filariasis, in sub-Saharan Africa by 2020. BMC Med.
15(176)2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Bhullar N and Maikere J: Challenges in
mass drug administration for treating lymphatic filariasis in
Papua, Indonesia. Parasit Vectors. 3(70)2010.PubMed/NCBI View Article : Google Scholar
|
|
33
|
World Health Organization: Yaws. Geneva:
World Health Organization; (2020). Yaws (Endemic treponematoses)
(who.int) Accessed November 12, 2020.
|
|
34
|
World Health Organization: Report of a
Global Meeting on Yaws Eradication Surveillance, Monitoring and
Evaluation. Geneva, 2018. Accessed November 12, 2020.
|
|
35
|
Stolk WA, Prada JM, Smith ME, Kontoroupis
P, de Vos AS, Touloupou P, Irvine MA, Brown P, Subramanian S, Kloek
M, et al: Are alternative strategies required to accelerate the
global elimination of lymphatic filariasis? Insights from
mathematical models. Clin Infect Dis. 66 (Suppl 4):S260–S266.
2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Directorate General of Disease Prevention
and Control. Indonesian Ministry of Health: Integrasi Sosialisasi
Program Pencegahan dan Pengendalian Penyakit Kusta dan Frambusia;
2019. kemkes.go.id. Accessed November 12, 2020.
|
|
37
|
Neglected tropical diseases: Impact of
COVID-19 and WHO's response - Maladies tropicales négligées. Impact
de la COVID-19 et réponse de l'OMS. Wkly Epidemiol Rec. 39:461–468.
2020.
|
|
38
|
Silver ZA, Kaliappan SP, Samuel P,
Venugopal S, Kang G, Sarkar R and Ajjampur SS: Geographical
distribution of soil transmitted helminths and the effects of
community type in South Asia and South East Asia - A systematic
review. PLoS Negl Trop Dis. 12(e0006153)2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Pullan RL, Smith JL, Jasrasaria R and
Brooker SJ: Global numbers of infection and disease burden of soil
transmitted helminth infections in 2010. Parasit Vectors.
7(37)2014.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Kyu HH, Abate D, Abate KH, Abay SM,
Abbafati C, Abbasi N, Abbastabar H, Abd-Allah F, Abdela J,
Abdelalim A, et al: GBD 2017 DALYs and HALE Collaborators: Global,
regional, and national disability-adjusted life-years (DALYs) for
359 diseases and injuries and healthy life expectancy (HALE) for
195 countries and territories, 1990-2017: A systematic analysis for
the Global Burden of Disease Study 2017. Lancet. 392:1859–1922.
2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
World Health Organization:
Soil-transmitted helminth infections. Geneva: World Health
Organization; (2020). Soil-transmitted helminths (who.int) Accessed November 12, 2020.
|
|
42
|
Jourdan PM, Lamberton PHL, Fenwick A and
Addiss DG: Soil-transmitted helminth infections. Lancet.
391:252–265. 2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Mbong Ngwese M, Prince Manouana G, Nguema
Mour PA, Ramharte M, Esen M and Adégnika AA, Esen M and Adégnika
AA: Diagnostic techniques of soil-transmitted helminths: Impact on
control measures. Trop Med Infect Dis. 5(5)2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Kurniati M, Budiono B and Sulistyawati SW:
Intestinal Protozoa infections in relation to nutritional status of
the students Mandangin Island elementary School 6 in Sampang
regency. JUXTA J Ilm Mhs Kedokt Univ Airlangga. 10(25)2019.
|
|
45
|
Mau F: Prevalence and intensity of
soil-Tansmitted helminth infections Among elementary School
Students in West Sumba and Central Sumba districts East Nusa
Tenggara, Indonesia. J Med Sci Clin Res 5, 2017.
|
|
46
|
World Health Organization: Preventive
Chemotherapy to Control Soil-Transmitted Helminth Infection in
At-Risk Population Groups. p75, 2017.
|
|
47
|
World Health Organization. Helminth
Control in School-Age Children Helminth Control in School Age
Children 90; (2011).
|
|
48
|
World Health Organization: 2030 Targets
for Soil-Transmitted Helminthiases Control Programmes. 2020.
|
|
49
|
Montresor A, Mupfasoni D, Mikhailov A,
Mwinzi P, Lucianez A, Jamsheed M, Gasimov E, Warusavithana S,
Yajima A, Bisoffi Z, et al: The global progress of soil-transmitted
helminthiases control in 2020 and World Health Organization targets
for 2030. PLoS Negl Trop Dis. 14(e0008505)2020.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Freeman MC, Akogun O, Belizario V Jr,
Brooker SJ, Gyorkos TW, Imtiaz R, Krolewiecki A, Lee S,
Matendechero SH, Pullan RL, et al: Challenges and opportunities for
control and elimination of soil-transmitted helminth infection
beyond 2020. PLoS Negl Trop Dis. 13(e0007201)2019.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Gutman JR, Lucchi NW, Cantey PT,
Steinhardt LC, Samuels AM, Kamb ML, Kapella BK, McElroy PD,
Udhayakumar V and Lindblade KA: Malaria and parasitic neglected
tropical diseases: Potential syndemics with COVID-19? Am J Trop Med
Hyg. 103:572–577. 2020.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Trasia RF: Covid-19 dan Koinfeksi Penyakit
Parasit. Med Hospitalia J Clin Med. 7:298–303. 2020.
|
|
53
|
Indonesian Ministry of Health: Panduan
Pelayanan Kesehatan Balita Pada Masa Tanggap Darurat COVID-19.
Indonesian Ministry of Health, pp1-30, 2020.
|
|
54
|
Cabinet Secretariat of The Republic of
Indonesia: Health Minister Signs Regulation of Guidelines to
Propose Large-scale Social Restrictions amid COVID-19 Pandemic.
https://setkab.go.id/en/health-minister-signs-regulation-on-guidelines-to-propose-large-scale-social-restrictions-amid-covid-19-pandemic/.
Accessed November 16, 2020.
|
|
55
|
Okereke M, Ukor NA, Adebisi YA, Ogunkola
IO, Favour Iyagbaye E, Adiela Owhor G and Lucero-Prisno DE III:
Impact of COVID-19 on access to healthcare in low- and
middle-income countries: Current evidence and future
recommendations. Int J Health Plann Manage. 36:13–17.
2021.PubMed/NCBI View Article : Google Scholar
|
|
56
|
World Health Organization:
Schistosomiasis. https://www.who.int/health-topics/schistosomiasis#tab=tab_1.
Accessed November 16, 2020.
|
|
57
|
Nurwidayati A, Frederika PP and Sudomo M:
Fluktuasi schistosomiasis di daerah Endemis provinsi Sulawesi
tengah tahun 2011-2018. Buletin Penelitian Kesehatan. 47:199–206.
2019.
|
|
58
|
Centres for Disease Control and
Prevention: Parasites-schistosomiasis. https://www.cdc.gov/parasites/schistosomiasis/disease.html.
Accessed November 16, 2020.
|
|
59
|
Colley DG, Bustinduy AL, Secor WE and King
CH: Human schistosomiasis. Lancet. 383:2253–2264. 2014.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Nelwan ML: Schistosomiasis: Life cycle,
diagnosis, and control. Curr Ther Res Clin Exp. 91:5–9.
2019.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Ajibola O, Gulumbe BH, Eze AA and
Obishakin E: Tools for detection of schistosomiasis in resource
limited settings. Med Sci (Basel). 6(39)2018.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Indonesia Ministry of Development Planning
and Agency: Bappenas. Siaran Pers Roadmap Eradikasi
Schistosomiasis. https://www.bappenas.go.id/files/5715/1617/7261/Siaran_Pers_-_Roadmap_Eradikasi_Schistosomiasis_20182025_Wujud_Komitmen_Pemerintah_Atasi_Penyakit_Demam_Keong.pdf;
(2018-25): Wujud Komitmen Pemerintah Atasi Penyakit Demam Keong.
Jakarta, Indonesia, 2018.
|
|
63
|
World Health Organization: Coronavirus
Disease 2019 (COVID-19) situation report, 48. World Health
Organization. 19:1–20. 2021.
|
|
64
|
Elyazar IR, Surendra H, Ekawati L,
Djaafara BA, Nurhasim A, Hidayana I, Widyastuti W, Oktavia D,
Adrian V, Salama N, et al: Excess mortality during the first ten
months of COVID-e9 Epidemic at Jakarta, Indonesia. medRxiv.
69:1–13. 2020.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Surendra H, Elyazar IR, Djaafara BA,
Ekawati LL, Saraswati K, Adrian V, Oktavia D, Salama N, Lina RN,
Andrianto A, et al: Clinical characteristics and mortality
associated with COVID-19 in Jakarta, Indonesia: a hospital-based
retrospective cohort study. medRxiv. 3768:1–21. 2020.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Martindale S, Mableson HE, Kebede B, Kiros
FH, Tamiru A, Mengistu B, Krueger A, Mackenzie CD and Kelly-Hope
LA: A comparison between paper-based and m-Health tools for
collating and reporting clinical cases of lymphatic filariasis and
podoconiosis in Ethiopia. mHealth. 4:49. 2018.PubMed/NCBI View Article : Google Scholar
|