Introduction
The isolation of growth factors stimulating the
formation of bone from decalcified bone matrix has been shown in
several studies, characterising their activity in intra- and
extra-skeletal sites and their stimulatory/inhibitory effects on
cell populations involved in bone formation (1). Growth factors are also essential for
endochondral bone formation and they have been identified in
various components of the epiphyseal growth plate (2); however, quantitative studies on their
presence are lacking. In the present study, the data on the quality
and quantity of growth factors present in the epiphyseal growth
plate, and particularly in calcified cartilage (the site of initial
bone formation) was assessed for the first time. These data may
assist in identification of the growth factors most useful as a
potential treatment for damaged bones postnatally.
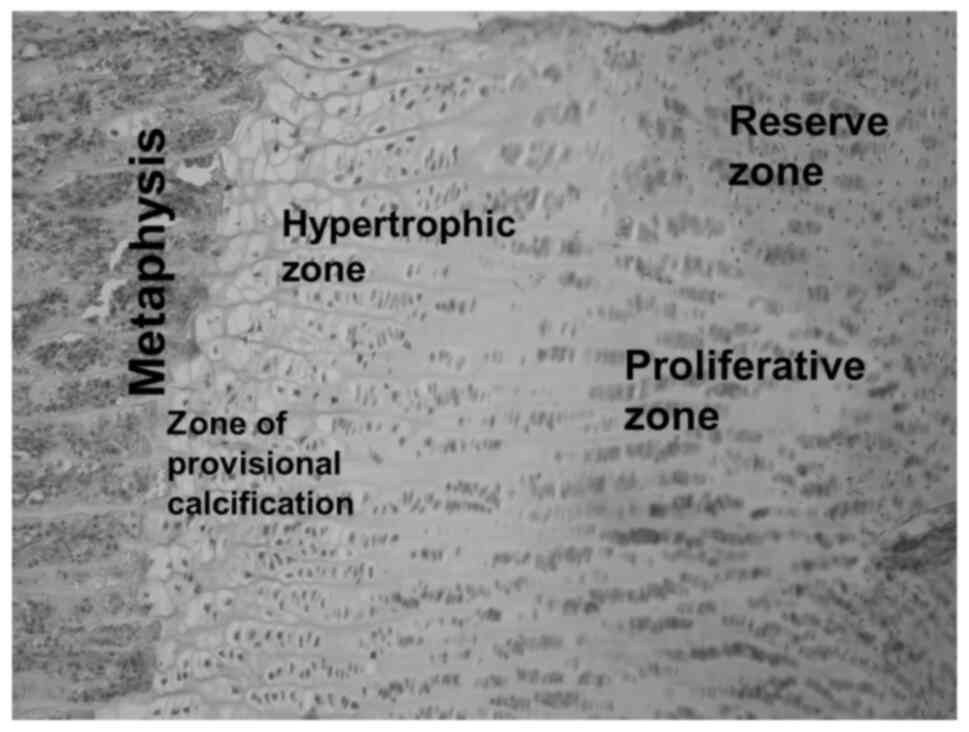
The epiphyseal growth plate, serving as the template
for bone formation, is composed of chondrocytes arranged in
reserve, proliferative and hypertrophic zones (3). Ossification of cartilage is preceded by
the deposition of a periosteal bone collar by osteoblasts
differentiating it from the surrounding mesenchymal cells (4). Chondrocytes in the proliferative and
hypertrophic zones form elongated columns separated from one
another by longitudinal septa of the cartilage matrix, whereas
chondrocytes within the columns are separated by transverse septa.
In the hypertrophic zone, chondrocytes become enlarged and deposit
calcium salts within longitudinal septa in the region adjacent to
the metaphysis, known as the zone of provisional calcification.
Approximately two-thirds of the longitudinal septa becomes
partially or completely calcified (5). Transverse septa remain non-calcified.
The metaphysis, in which osteoblasts deposit bone on the calcified
longitudinal septa, and which represents the initial site of bone
formation, is located just distal to the last intact transverse
septum at the base of each cell column (6,7).
Calcification of the epiphyseal growth plate begins
with the formation of matrix vesicles produced by chondrocytes in
the proliferative and hypertrophic zones (8,9). Matrix
vesicles contain enzymes that increase the local concentration of
orthophosphate and lead to the formation of apatite-like deposits
(7,10-12).
Matrix vesicles also carry bone morphogenetic proteins (BMPs)1-7
and vascular endothelial growth factor (VEGF), factors which serve
an important morphogenetic role in endochondral bone formation
(13). The presence of BMP1-7 was
also demonstrated immunocytochemically in chondrocytes of the
hypertrophic and calcifying zones during endochondral bone
formation (14). In extracts from
bovine articular cartilage, yet more growth factors were
identified, including cartilage-derived morphogenetic protein-1
(CDMP-1), also known as growth/differentiation factor-5 (GDF-5), as
well as BMP-14 and 2 (also referred to as CDMP-2 and GDF-6,
respectively) (15,16). Moreover, in rats, interstitial fluid
from the non-calcified portion of the articular-epiphyseal
cartilage complexes contained BMP-7, basic fibroblast growth factor
(bFGF) and numerous additional factors (17). Another factor, NELL-1 was found to
control the ossification of the cranial skeleton (18).
BMPs, excluding BMP-1, which is a metalloprotease,
are multi-functional growth factors that belong to the TGF-β
superfamily. Currently, >20 types of BMPs have been identified
(19). BMPs are involved in all
stages of epiphyseal cartilage formation, including mesenchyme
condensation, chondrocyte differentiation, hypertrophy, cartilage
matrix calcification and subsequent resorption by chondroclasts, as
well as the final bone deposition step (2,18-21).
GDF-5 and NELL-1 participate in bone repair and regeneration
(2,22). VEGF stimulates the formation of blood
vessels required for epiphyseal cartilage nutrition (23).
An improved understanding of the interaction of
various growth factors may emerge from studies on their signalling
pathways. For example, growth factors from the TGF-β superfamily
(BMPs/GDF-5) transduce signals by both the canonical SMAD-dependent
signalling pathway and the non-canonical SMAD-independent
signalling pathway. Both pathways converge at transcription
factors, for example, Runx2, to promote osteoblast differentiation
from mesenchymal precursor cells (24). NELL-1, which is not a member of the
TGF-β superfamily, also acts through the Runx2(18) and effectively induces the expansion
of a bone marrow subset of mesenchymal progenitor cells (24,25).
The presence of growth factors within matrix
vesicles (13) and in non-calcified
cartilage (14) suggests that they
may also be present within calcium deposits. Growth factors may be
released during calcified cartilage resorption and stimulate bone
formation. The quantitative profile of the growth factors that
accumulate in calcified matrix and participate in endochondral bone
formation remains to be fully elucidated. The primary obstacle is
the paucity of calcified cartilage in epiphyseal cartilage. Even in
large animals, obtaining a large amount of calcified cartilage from
the epiphyseal cartilages of long bones is technically difficult.
Based on the similarity in structure and function of epiphyseal
cartilage and costochondral junctions (26-28),
it was possible to harvest calcified cartilage from 24 ribs of one
animal. The choice of calves was dictated by the local preference
for calf meat assuring a regular supply of ribs and by the
availability of ELISA tests for numerous bovine growth factors.
Thus, costochondral junctions of calf ribs were collected, and
growth factors were determined both in non-calcified and calcified
matrices. The obtained data could be important both for an improved
understanding of the role of growth factors during the early stages
of bone formation and possibly, for assisting in the formulation of
treatment regimens useful in the treatment of bone
deficiencies.
Materials and methods
Preparation of calcified
cartilage
The cartilage was dissected from the ribs of
6-20-week-old calves of the Holstein Friesian dairy breed and
bought from a local butcher within 24 h of death. The animals were
killed by exsanguination after stunning in accordance with the
Polish norm (29). Costochondral
junction (an equivalent of the cartilage growth plate of long
bones) was cleared from the adhering tissues and hand broken at the
level of the metaphysis, the provisional zone of calcification.
Then, the calcified cartilage was scraped from the exposed surface
with a knife, lyophilised and pulverised in liquid nitrogen. On
average, 400 mg dried cartilage powder was obtained from one
animal. Ribs were collected from 32 animals (768 ribs). In order to
eliminate the possible individual differences between calves,
cartilage powder was pooled in batches containing harvest from
10-12 animals.
Isolation of calcified cartilage
Cartilage powder (400 mg) was rehydrated in 40 ml
dH2O for 15 min at 4˚C and centrifuged at 500 x g for 5
min at 4˚C. The pellet was suspended in 10 ml dH2O and
further incubated for 5 min at 4˚C. After a second centrifugation
step (500 x g for 5 min at 4˚C), the pellet was re-suspended in 10
ml dH2O, placed atop a gradient consisting of 1.25 g and
1.0 g/ml of barium iodide in dH2O and spun down at 500 x
g for 10 min at 4˚C. The pellet contained large fragments of
calcified cartilage usually attached to some non-calcified
substance, whereas in the material from the gradient interface, the
latter predominated. Interface material was discarded, and the
pellets were rinsed in dH2O and lyophilised. The average
weight of a lyophilised pellet was 300 mg.
Isolation of growth factors
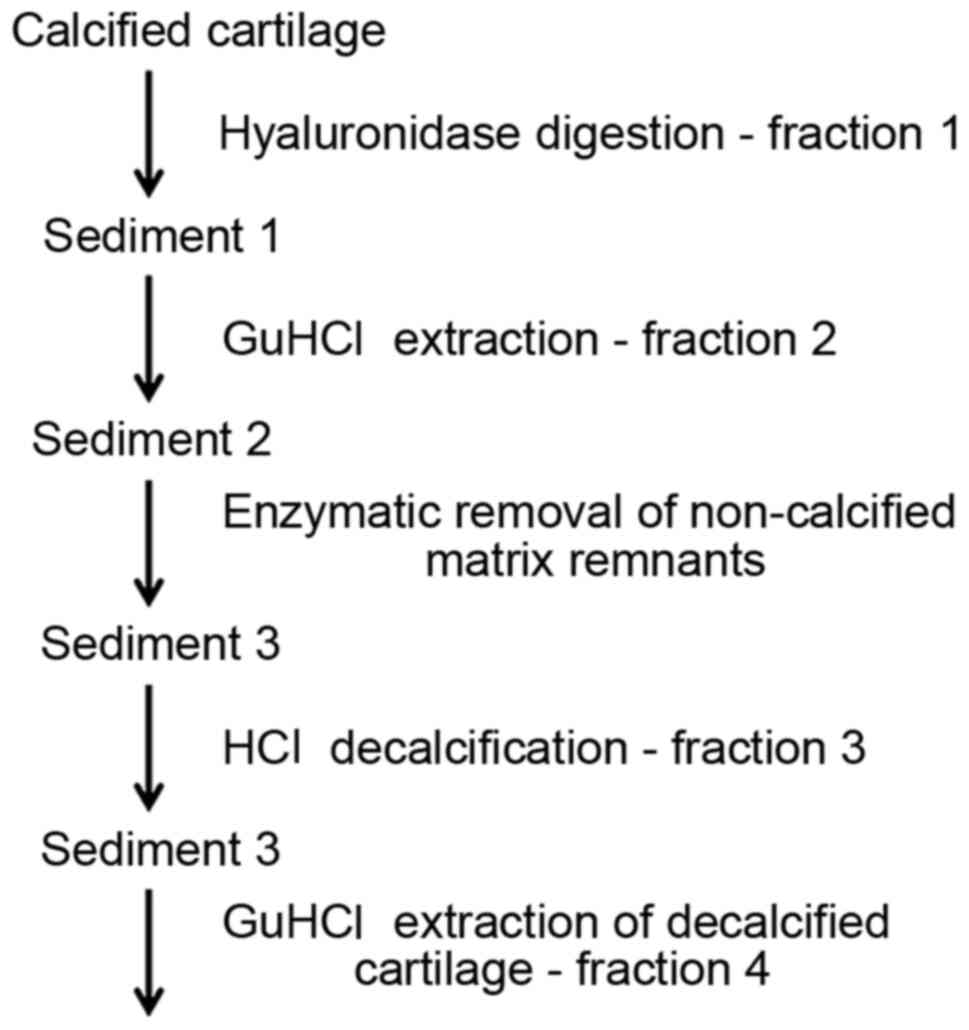
The process of the isolation of growth factors
involved: Hyaluronidase digestion; GuHCl extraction; enzymatic
removal of non-calcified matrix remnants; HCl decalcification;
GuHCl extraction of decalcified matrix (Fig. 1); and purification of growth factors
using HiTrap heparin affinity columns.
Hyaluronidase digestion. Pellets obtained
after gradient separation were digested in 10 ml 0.1% bovine testes
hyaluronidase (Sigma-Aldrich; Merck KGaA) in PBS (Sigma-Aldrich;
Merck KGaA) in order to obtain the fraction of growth factors
(fraction 1) present in the matrix adjacent to calcified cartilage
(30). The progress of digestion was
controlled by staining with 0.1% aqueous solution of toluidine blue
at room temperature for 10 min (Sigma-Aldrich; Merck KGaA).
Digestion lasting 4-5 h was found to be sufficient to remove all
traces of stainable material. Fraction 1 was separated from the
solid material (sediment 1) by centrifugation (500 x g for 10 min
at 4˚C), desalted in PD-10 columns (GE Healthcare Life Sciences)
and lyophilised. Next, lyophilised material was dissolved in 4 M
GuHCl in 50 mM TRIS (pH 8.0) with 0.15 M NaCl (all from
Sigma-Aldrich; Merck KGaA). GuHCl served as the binding buffer
during the separation of growth factors in heparin columns (GE
Healthcare Life Sciences).
GuHCl extraction after hyaluronidase
digestion. Sediment 1 was extracted with 4 M GuHCl (prepared as
above). Extraction was performed twice for 2 h at 4˚C each time.
Extracts were pooled (fraction 2) and applied to 4 M GuHCl heparin
columns.
Enzymatic removal of non-calcified matrix
remnants. In order to remove the remnants of the organic
material, the sediment (sediment 2) after extraction was digested
with 0.25% collagenase and 0.05% DNase solution (all from
Sigma-Aldrich; Merck KGaA) at 37˚C for 2 h, in 0.25% trypsin
(Sigma-Aldrich; Merck KGaA) at 37˚C for 1 h and again in
collagenase (under the same conditions). The enzymatic solutions
were discarded, and the calcified cartilage (sediment 3) was rinsed
twice with 4 M GuHCl for 15 min, followed by two changes of
dH2O.
HCl decalcification. Sediment 3 purified by
enzymatic digestion was decalcified in 4 ml 0.6 M HCl
(Sigma-Aldrich; Merck KGaA) for 30 min at room temperature. The
progress of decalcification was controlled by microscopic
observation. Then, the material was neutralised with 10 N NaOH
(Sigma-Aldrich; Merck KGaA), and the supernatant (fraction 3)
obtained by centrifugation (500 x g for 5 min at 4˚C), desalted in
PD-10 columns (GE Healthcare Life Sciences), lyophilised, dissolved
in 4 M GuHCl and applied to heparin columns.
GuHCl extraction of decalcified cartilage.
After HCl decalcification (sediment 4), the pellet was extracted
using GuHCl prepared as above, and the obtained fraction 4 was
applied to heparin columns.
Purification of growth factors by HiTrap heparin
affinity columns. Fractions were applied to HiTrap heparin
affinity columns (GE Healthcare Life Sciences) in the binding
buffer containing 20 mM Tris-HCl, 0.15 M NaCI, 4 M GuHCl (pH 8.0)
(all from Sigma-Aldrich; Merck KGaA). The elution buffer contained
20 mM Tris-HCl with 2 M NaCl (all from Sigma-Aldrich; Merck KGaA).
The eluate was desalted in PD-10 columns (GE Healthcare Life
Sciences), lyophilised, and used for growth factor determination by
ELISA and protein sequence analysis. All solutions used in this
work contained 10 µl 100x concentrated protease inhibitors (Thermo
Fisher Scientific, Inc.) per 1 ml.
ELISA
Growth factors were evaluated using immunoassay kits
for BMP-1 (cat. no. GR106213), BMP-3 (cat. no. GR106215), BMP-5
(cat. no. GR106217) (all from Genorise Scientific, Inc.), BMP-2
(cat. no. E11B0810), BMP-4 (cat. no. E11B0542), BMP-6 (cat. no.
E11B0395), BMP-7 (cat. no. E11B0390) and transforming growth
factor-β1 (TGF-β1; cat. no. E11T0009) (all from BlueGene), VEGF
(cat. no. MBS760448), mesencephalic astrocyte-derived neurotrophic
factor (MANF; cat. no. MBS288818), NEL-like protein-1 (NELL-1; cat.
no. MBS93668405), osteoclast-stimulating factor-1 (OSTF-1; cat. no.
MBS741961), (all from MyBioSource, Inc.), bFGF (cat. no. orb403256)
(Biorbyt), connective tissue growth factor (CTGF; cat. no.
RD-CTGF-Ra; RedDot), GDF-5 (cat. no. EK1504-BV-CAP; Boster
Biological Technology) and insulin-like growth factor-1 (IGF-1;
cat. no. MG100; Novateinbio) according to the manufacturers'
protocols. Proteins present in calcified cartilage from calf
costochondral junctions were sequenced.
Protein sequence analysis
Analysis and data processing was performed on a
commercial basis at the Mass Spectrometry Laboratory of the
Institute of Biochemistry and Biophysics of the Polish Academy of
Sciences, Warsaw, Poland according to the following procedure.
Trypsin digestion. Gel slices were subjected
to the standard ‘in-gel digestion’ procedure during which proteins
were reduced with 100 mM 1,4-dithiothreitol (Sigma-Aldrich; Merck
KGaA) for 30 min at 56˚C, alkylated with iodoacetamide (45 min in a
dark room at room temperature) and digested overnight with trypsin
(sequencing Grade Modified Trypsin; cat. no. V5111; Promega
Corporation). The resulting peptides were eluted from gels using
0.1% trifluoroacetic acid (TFA) and 2% acetonitrile (ACN) (both
from Sigma-Aldrich; Merck KGaA).
Mass spectrometry
The material for sequencing was served as 300 mg
pellets of enzymatically purified (hyaluronidase, collagenase,
DNase and trypsin) calcified cartilage from the costochondral
junction of calf ribs. A total of four samples were sequenced: A
sample of proteins released during decalcification in 0.6 N HCl,
and three samples of decalcified material. Peptide mixtures were
separated by liquid chromatography prior to molecular mass
measurements on the Orbitrap Velos mass Spectrometer (Thermo
Electron Corp.). A peptide mixture was applied to RP-18 precolumn
(nanoACQUITY Symmetry® C18; cat. no. 186003514; Waters
UK) using water containing 0.1% TFA as a mobile phase and then
transferred to a nano-HPLC RP-18 column (nanoACQUITY BEH C18; cat.
no. 186003545; Waters UK) with an ACN gradient (0-60% ACN in 70
min) in the presence of 0.05% formic acid with a flow rate of 150
nl/min. The column outlet was directly coupled to the ion source of
the spectrometer working in the regime of data-dependent MS to
MS/MS switch. A blank run ensuring lack of cross-contamination from
previous samples preceded each analysis. The mass spectrometry
proteomics data have been deposited to the ProteomeXchange
Consortium via the PRIDE (31)
partner repository with the dataset identifiers are PXD021781 and
10.6019/PXD021781.
Data processing
The acquired raw data were processed using Mascot
Distiller followed by database searches within the Mascot program
(Matrix Science, 8-processor on-site licence) against the National
Centre for Biotechnology Information (version 20100203). Search
parameters for precursors and product ions mass tolerance were 40
ppm and 0.8 Da respectively, with allowance made for one missed
trypsin cleavage and the following fixed modifications: Cysteine
carbamidomethylation and allowed variable modification - oxidation
(M). Peptides with Mascot Score exceeding the threshold value
corresponding to <5% false positive rate, calculated by the
Mascot procedure, were considered to be positively identified.
Histological procedures
Fragments of ribs were fixed in phosphate-buffered
formalin, pH 7.2 or in 70% ethanol at room temperature for 24 h,
decalcified in edetic acid (Sigma-Aldrich; Merck KGaA) at 37˚C for
6 days, and dehydrated and embedded in paraffin. Sections, 8 µM
thick, were stained with haematoxylin-eosin for 6 and 2 min
respectively, at room temperature.
Results
The concentration of growth factors assessed in the
calcified cartilage and detected in the material released during
all steps of purification is shown in Table I. Pooled material was used in all
determinations. BMP-7, GDF-5 and NELL-1 accounted for the majority
of growth factor content present compared with the remaining growth
factors detected (VEGF, BMP-2, BMP-3, BMP-4 and bFGF). TGF-β1 was
not released by hyaluronidase, but was present in small amounts in
the remaining groups. BMP-1, BMP-5, BMP-6, IGF-1, MANF, CTGF and
OSTF-1 were not detected by assays with detection ranges of
31-2,000 pg/ml for BMP-1; 125-8,000 pg/ml for BMP-5; 50-1,000 pg/ml
for BMP-6; 21.5-2,000 pg/ml for IGF-1; 6.25-400 pg/ml for CTGF;
50-1,000 pg/ml for OSTF-1; and 0.156-10 ng/ml for MANF. Thus, from
the 16 growth factors assessed in the calcified and non-calcified
material, bound to calcified cartilage matrix, 9 factors were
determined to be present by ELISA.
 | Table IConcentration of growth factors in
different fractions of calcified cartilage from calf costochondral
junctionsa. |
Table I
Concentration of growth factors in
different fractions of calcified cartilage from calf costochondral
junctionsa.
| Fractions | BMP-2 | BMP-3 | BMP-4 | BMP-7 | VEGF | bFGF | TGF-β1 | NELL-1 | GDF-5 |
|---|
| Fraction 1,
Hyaluronidase digestion | 38 | 6 | 40 | 236 | 70 | 12 | 0 | 587 | 226 |
| | 52 | 9 | 32 | 266 | 56 | 21.5 | 0 | 572 | 347 |
| Fraction 2, GuHCl
extraction after | 18 | 8 | 30 | 133 | 111 | 16 | 14 | 612 | 239 |
| hyaluronidase
digestion | 37 | 5 | 68 | 148 | 51 | 20 | 32 | 587 | 285 |
| Fraction 3, HCl
decalcification | 38 | 4 | 15 | 253 | 78 | 15 | 0 | 550 | 161 |
| after GuHCl
extraction | 10 | 5 | 90 | 276 | 37 | 15 | 23 | 387 | 207 |
| Fraction 4, GuHCl
extraction | 18 | 8 | 54 | 533 | 32 | 12.5 | 16 | 650 | 240 |
| after HCl
decalcification | 19 | 3 | 87 | 520 | 15.7 | 20.5 | 23 | 436 | 140 |
Protein sequential analysis showed the presence of
proteins characteristic of cartilage, such as collagen type II,
cartilage oligomeric matrix protein, aggrecan core protein,
hyaluronan, proteoglycan link protein 1, biglycan, chondroadherin
(data are available via ProteomeXchange: ID, PXD021781). Sequenced
material also contained >200 identifiable nuclear and
cytoplasmic components. In decalcified cartilage, certain proteins,
such as heterogeneous nuclear ribonucleoproteins, ADP-ribosylation
factor 4, α-enolase, alkaline phosphatase, phosphoglycerate mutase
1 and Annexin A6 were detected in all three samples. Others, for
example protein disulphide-isomerase A3, histone H2B type 1-K and
peptidyl-prolyl cis-trans isomerase B were found in only two
samples, and protein disulphide-isomerase A4, glutaminyl-peptide
cyclotransferase and protein/nucleic acid deglycase DJ-1 was only
present in one sample. Approximately 50 proteins were also released
from calcified cartilage during decalcification, but the profile
did not differ from the previous group notably. Blood proteins in
decalcified material were represented in all samples by serum
albumin, α-2-HS-glycoprotein, prothrombin, serotransferrin,
haemoglobin foetal subunit β, complement component C9, haemoglobin
subunit α, complement C3 and antithrombin-III; however, others,
such as apolipoprotein D or vitamin D-binding protein, were
identified in one sample only. Amongst the proteins released during
decalcification, certain proteins were absent in the former group,
for example angiogenin or leukocyte cell-derived chemotaxin-2.
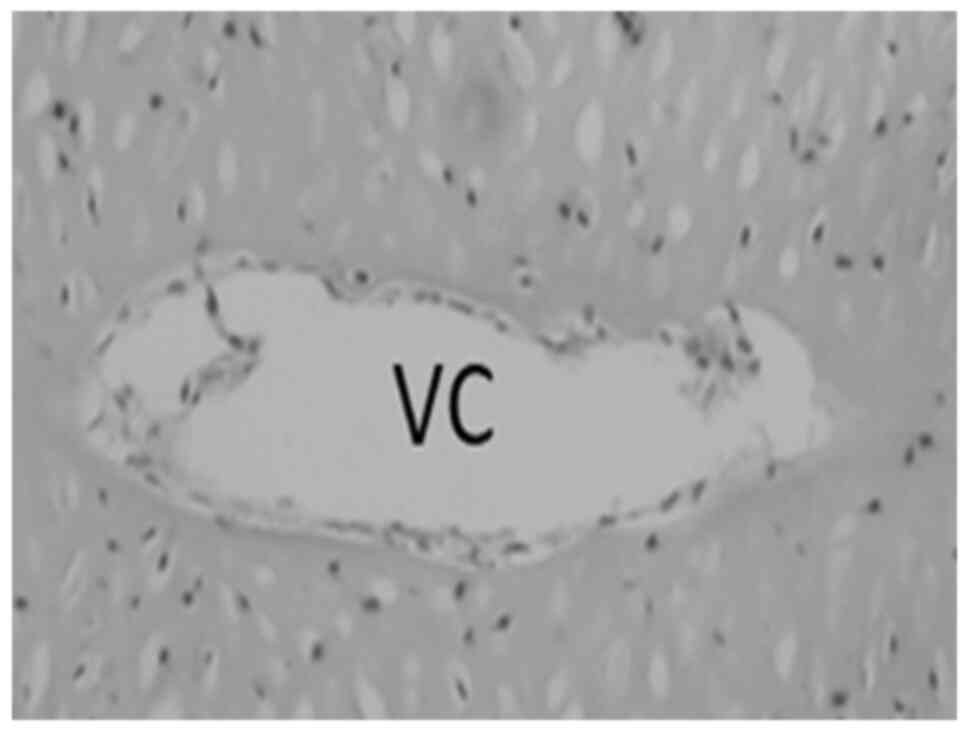
Their presence is not surprising, since core areas in large pieces
of permanent cartilage are supplied via vascular canals, to ensure
an adequate metabolic supply for the regions deep inside; reviewed
by Gabner et al (32). Such
canals were also present in the rib cartilage (Fig. 2). Among these proteins, several
growth factors were detected, three of which had a Mascot score
between 966 and 246; these were MANF, CTGF and OSTF-1. None
however, were detected by ELISA (Table
II).
 | Table IIGrowth factors found by sequence
analysis of proteins released during decalcification in 0.6 N HCl,
and in the decalcified material. |
Table II
Growth factors found by sequence
analysis of proteins released during decalcification in 0.6 N HCl,
and in the decalcified material.
| Factors | Mascot score
(number of matching peptides) |
|---|
| Mesencephalic
astrocyte-derived neurotropic | 966(7) |
| Connective tissue
growth factor | 739(8) |
| Osteoclast
stimulating factor | 243(4) |
| Hepatoma-derived
growth factor | 122(3) |
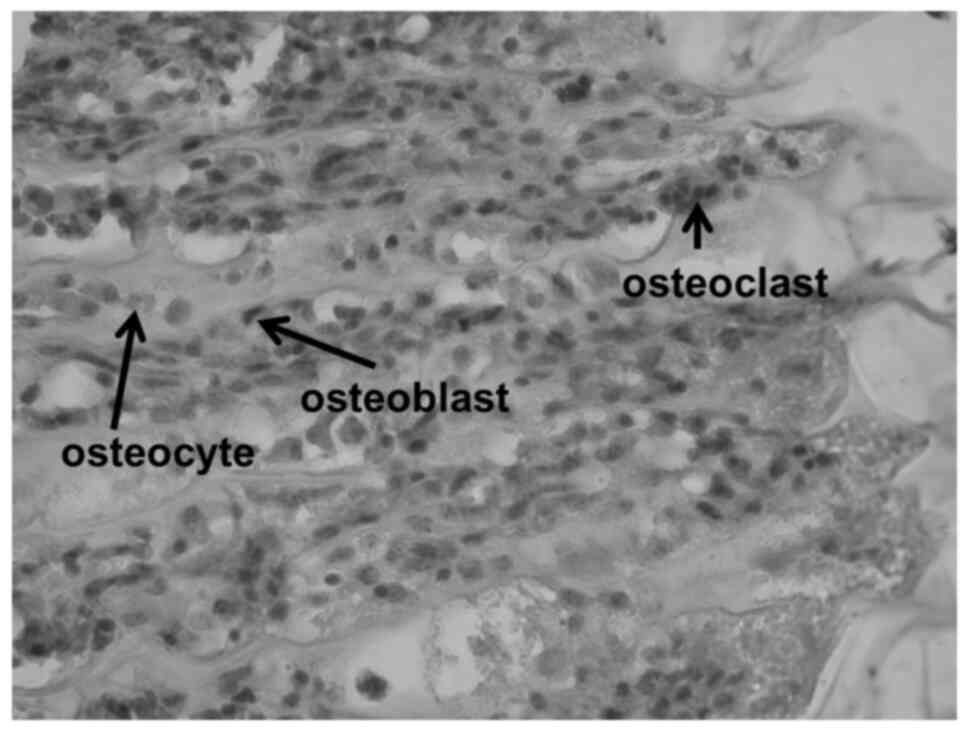
The structures and cell types forming the
costochondral junction are shown in the haematoxylin-eosin stained
histological sections (Figs. 3 and
4), prepared as an illustration for
the processes elaborated in the following discussion.
Discussion
Since the cartilage matrix within calcium deposits
contains growth factors important for bone cell differentiation and
activity, it is intriguing to see how they are made accessible to
osteoprogenitor cells. Resorption of non-calcified and calcified
cartilage occurs through two different mechanisms (5,33,34).
Non-calcified septa are invaded by capillary sprouts proceeding
from the metaphysis at the bottom of the growth plate. Endothelial
cells are accompanied by perivascular cells and pericytes, and by
mononuclear cells expressing cathepsin B, termed septoclasts
(5,33,35,36).
Calcified septa are resorbed by multinuclear cells called
osteoclasts or chondroclasts (33,37,38). The
osteoclasts extend their cell processes into the cartilage matrix
and entrap the calcified cartilage. They are followed by
tube-forming endothelial cells migrating from the capillary
sprouts, giving rise to the new capillaries (33,37,39,40).
Stromal cells follow the vascular elements, differentiate into
osteoblasts and deposit osteoid on the calcified longitudinal septa
(3,7,39).
Resorbing osteoclasts have different functionally separated zones.
Within the peripheral fusion zone, osteoclasts secrete protons and
proteolytic enzymes, dissolving the calcified matrix (41). In the uptake zone, osteoclasts
collect the degraded bone material (42,43).
Subsequently, this is transcytosed and discharged through the
functional secretory domain localised at the opposite pole of the
cell (44-47).
According to previously published data (13-17)
and the results of the present study, it is evident that
chondrocytes forming the epiphyseal growth plate produce growth
factors and deposit them within the matrix, which subsequently
undergoes mineralisation. These growth factors are accessible only
to osteoclasts, which release them presumably at a rate optimal for
the stimulation of various osteoprogenitor cells.
Schenk et al (5) reported that ~two-thirds of the
longitudinal septa in the epiphyseal growth plate is partially or
completely calcified, whereas the remainder is essentially
non-calcified. This close contact of calcified and non-calcified
matrices within longitudinal septa may explain why it was not
possible to eliminate non-calcified cartilage during pulverisation
in liquid nitrogen and gradient centrifugation.
Growth factors present in the non-calcified matrix
were released by hyaluronidase digestion followed by extraction
with GuHCl. In vivo, the factors were liberated by
septoclasts accompanying the capillary sprouts. According to Lee
et al (35), septoclasts
possess a cell apex with a ruffled border extending into the
transverse septum with signs of partially digested extracellular
matrix. Thus, it seems possible that septoclasts are able to
transport growth factors liberated from the non-calcified matrix by
a means that protects them from digestion, and is similar to that
in osteoclasts.
In the present study, the quantity of growth factors
in fractions obtained during each step of their isolation from
calcified cartilage of costochondral junctions was determined. The
present study was limited to only two determinations of each
factor; however, as can be seen is Table
I, the results were consistent in all steps of the isolation
procedure (hyaluronidase digestion and GuHCl extractions). The
quantities of the particular growth factors and general information
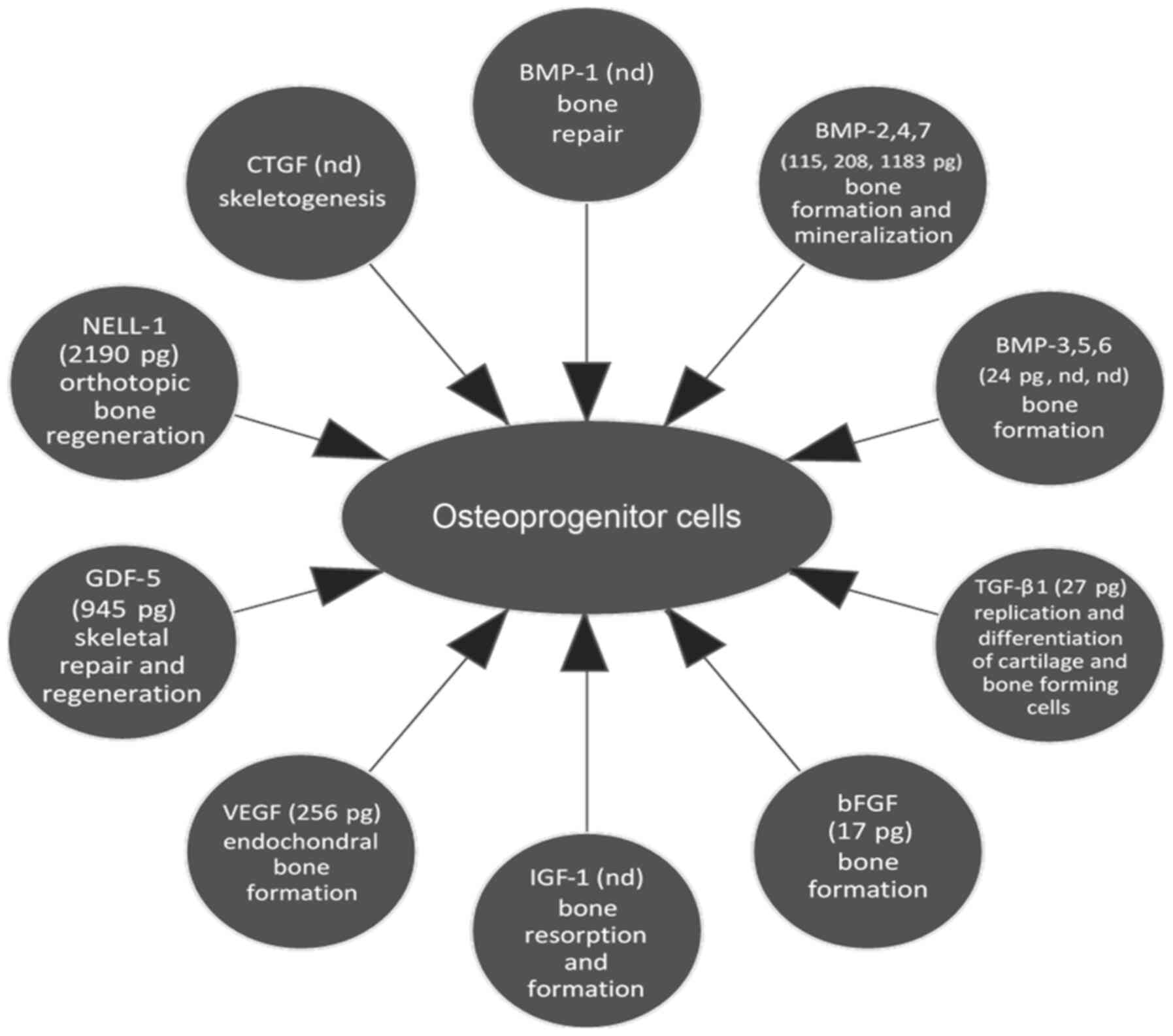
regarding their function is summarized in Table III. Factors affecting
osteoprogenitor cells are also presented in Fig. 5.
 | Table IIIGrowth factors that were present or
absent, based on ELISA, in the rib costochondral junctions, and
their role in endochondral ossification. |
Table III
Growth factors that were present or
absent, based on ELISA, in the rib costochondral junctions, and
their role in endochondral ossification.
| Growth factors | Functions | Concentration in
calf costochondral junctions, pg/300 mg dry cartilagea | Refs. |
|---|
| BMP-1 | Metalloprotease,
cleaves procollagen, participates in bone repair. | Not detected | (17) |
| BMP-2 | Induces
endochondral bone formation | 115 | (47,19,48) |
| BMP-4 | following in
vivo implantation; | 208 | |
| BMP-7 | participates in
bone mineralization. Differences in dose and time needed for
reactions. Facilitates both chondrogenic and osteogenic
differentiation of mesenchymal stem cells. | 1,182.50 | |
| BMP-3 | Induces
endochondral bone formation following in vivo implantation,
a negative modulator of bone formation, and an antagonist of BMP-2
signaling. | 23.5 | (19,48,50) |
| BMP-5 | Induces
endochondral bone formation following in vivo implantation,
and participates in bone mineralization. | Not detected | (19,47) |
| BMP-6 | Induces
endochondral bone formation following in vivo implantation,
and participates in bone mineralization. | Not detected | (19,47) |
| TGF-β1 | Regulates
replication and differentiation of cartilage and bone forming
cells. | 27 | (60) |
| bFGF | Stimulates bone
formation. | 16.75 | (76,77) |
| IGF-1 | Functions in
coupling bone resorption and formation. | Not detected | (75) |
| VEGF | Stimulates
angiogenesis and endochondral bone formation. | 255.5 | (22) |
| GDF-5 | Closely related to
BMP-5,6,7. Involved in skeletal repair and regeneration, inhibits
bone formation stimulated by BMP-2 and induces angiogenesis. | 945.5 | (17,53,54) |
| NELL-1 | Specific to the
osteochondral lineage, promotes orthotopic bone regeneration. | 2,190.50 | (16) |
| CTGF | Involved in
skeletogenesis. | Not detected | (78) |
| OSTF-1 | Stimulates
formation and activity of osteoclasts. | Not detected | (80) |
| MANF | Trophic factor for
dopamine neurons. Involved in chondrocyte endoplasmic reticulum
homeostasis. | Not detected | (79) |
When planning the experiments, it was expected that
the protein sequence analysis would assist in identifying proper
ELISA tests and limit the amount of material needed for growth
factors analysis.
Only three growth factors influenced osteoprogenitor
cells: OSTF-1, CTGF and MANF. Thus, for further analysis, growth
factors, which on the basis of published data were involved in
cartilage and bone formation were chosen. These were BMP-1-7
(14,19) GDF-5 (15,48),
NELL-1(18), TGF-β1(24), bFGF (49), VEGF (13) and IGF-1(49).
It seems reasonable to assume that growth factors
found in the non-calcified and calcified cartilage matrices (BMP-2,
BMP-3, BMP-4, BMP-7, GDF-5, NELL-1, TGF-β1, bFGF and VEGF) directed
the development of long bones by stimulating differentiation of
mesenchymal cells into osteoblasts, the formation of osteoclasts,
the formation of blood vessels and the deposition of osteoid on
calcified longitudinal septa. Several questions remain: What are
the specific roles of the factors? How is their action coordinated?
And are they released simultaneously or consecutively? Considerable
information on the action of particular factors alone, in pairs or
in triplets, has already been accumulated in previous studies;
however, elucidation of the role of the 9 factors at play (or more,
as some other factors not identified in the present study may be
present) is challenging.
In extracts from non-calcified and calcified
cartilage, the quantity of BMP-7 and GDF-5 (BMP-14) was ~10x higher
than that of BMP-2, and 5x higher than that of BMP-4; thus, the two
former factors may serve a dominant role in osteoprogenitor
stimulation. NELL-1, the third factor present in high
concentrations, is highly specific to the osteochondral lineage and
can promote orthotopic bone regeneration (18,25,50). The
small quantity of BMP-2 in relation to BMP-7 was unexpected, as in
the bone matrix, they appear to be present in comparable amounts.
It has been demonstrated that there is ~2 µg protein with bone
inductive activity (presumably primarily BMP-2) in 1 kg bovine bone
(22), and 1 µg BMP-7 in 1 kg bone
(51). Thus, in the present study,
the content of BMP-7 in non-calcified and calcified cartilage is
comparable or even higher than in bovine bone, whereas that of
BMP-2 is much lower. BMP-2 not only stimulates bone formation, but
significantly enhances osteoclastogenesis (52), the effect of which would probably not
be beneficial during the early stages of bone deposition. BMP-4,
which is less efficient than BMP-2 in promoting bone formation
(1,22,53), was
detected in the material at slightly higher concentrations than
BMP-2. It has previously been shown that the combined use of BMP-2,
BMP-5 and BMP-6 had an additive effect on matrix mineralisation
(52), but the latter two factors
were not detected in our samples, possibly due to the insufficient
sensitivity of the ELISA kits used. The quantity of BMP-3 detected
was negligible. BMP-3 is an inhibitor of osteogenesis in
vitro and of bone formation in vivo, and may antagonise
BMP-2 signalling (54).
GDF-5 (also referred to as CDMP-1/BMP-14) is closely
related to BMP-5, BMP-6 and BMP-7. It is present within the
cartilaginous cores of the developing long bones (15,16)
corresponding to calcified cartilage in the present study. The
expression of GDF-5 is required for proper skeletal patterning and
joint development in vertebrate limbs (55,56). Of
interest, GDF-5 was found to inhibit the BMP-2-induced increase in
alkaline phosphatase expression in the promyoblast C2C12 cell line,
and to inhibit bone formation stimulated by BMP-2 in vivo
after simultaneous implantation of both factors into rat muscle.
Thus, GDF-5 can act as an antagonist of BMP-2, which may have
important implications in processes where both factors act
simultaneously (57). In addition,
GDF-5 induced angiogenesis in both chick chorioallantoic membrane
and rabbit cornea, whereas BMP-2 did not (58). The results of the present study
suggest that the interference of BMP-2 with GDF-5 during
endochondral ossification is prevented or limited by the low levels
of the former. Nevertheless, whereas BMP-7, GDF-5 and NELL-1
accounted for the majority of the growth factors present, the
contribution of BMP-2 and BMP-4 to the deposition of bone in
endochondral ossification should not be neglected. BMP-5, BMP-6 and
BMP-7 show extensive sequence similarity to BMP-2, and the additive
or synergistic contribution of these molecules to osteogenic
activity has already been suggested (59). The lack of a detectable amount of
BMP-6 is surprising, since amongst BMP-2, BMP-4, BMP-6 and BMP-7,
BMP-6 is the most consistent and potent regulator of osteoblast
differentiation (60).
TGF-β1 regulates the replication and differentiation
of mesenchymal precursor cells, chondrocytes, osteoblasts and
osteoclasts, chondrocyte hypertrophy, growth plate maturation and
mineralisation (24,61-65).
Latent TGF-β1 stored in the bone matrix is released and activated
by osteoclasts (66). In the present
study, TGF-β1 was detected only in low quantities in GuHCl extracts
of non-calcified and calcified cartilage. Thus, in view of the
numerous TGF-β1 properties, it is difficult to ascribe to it a
specific function at the earliest stages of bone development.
VEGF is expressed by hypertrophic chondrocytes in
the epiphyseal growth plate. Members of the VEGF family are
essential coordinators of chondrocyte death, chondroclast function,
extracellular matrix remodelling, angiogenesis and endochondral
ossification (23,67-69).
BMPs, bFGF, TGF-β1, IGF-1 and vitamin D3 induce the expression of
VEGF released from osteoblasts (70). Osteoblast-derived VEGF is critical
for maintaining bone homeostasis by stimulating the differentiation
of mesenchymal stem cells to osteoblasts and repressing their
differentiation to adipocytes. VEGF also stimulates the
differentiation of monocytes to osteoclasts via a paracrine
mechanism (71-73).
VEGF may synergistically enhance BMP-induced bone formation
(74). VEGF and BMP-2 applied
together induced the differentiation of mesenchymal cells to
chondrocytes and osteoblasts, and these differentiated cells
produced VEGF, creating a favourable environment for
vascularisation in bony tissues (75,76).
Dilling et al (76) found the
immediate expansion of blood vessels in response to BMP-2,
identified brown fat cells as the source of VEGF, and related it to
the role of BMPs in the vascularisation of early embryos (77). In the present study, VEGF was
detected both in non-calcified and calcified cartilage, and could
stimulate angiogenesis as well as co-operate with other growth
factors in the differentiation of osteoblasts and osteoclasts.
bFGF inhibits the anabolic activity of IGF-1 and
BMP-7 in adult human articular chondrocytes (49). A low dose (0.1 mg/kg per day) of bFGF
stimulates endosteal and endochondral bone formation and reduced
periosteal bone formation in growing rats (78). A bFGF-loaded acellular dermal matrix
membrane resulted in similar bone regeneration as that observed
with BMP-2 through more efficient recruitment of mesenchymal stem
cells. Moreover, bone marrow mesenchymal stem cells pre-treated
with bFGF exhibited increased proliferation and osteogenic
differentiation potential compared with BMP-2 pre-treatment
(79).
CTGF (80), MANF
(81) and OSTF-1(48), although found by sequential analysis,
were not detected by ELISA. A short summary of their role in
cartilage physiology is presented in Table III.
BMP-2 and BMP-7 were used in clinics for the repair
of bone defects and fractures (82,83) and
for spine fusion (84); however,
adverse effects of BMP-2 were observed in procedures aimed at
cervical spine fusions soon afterwards (84). Moreover, BMP-2 is generally
upregulated in several types of tumours and is associated with
tumour cell proliferation, invasion, and at times a poor clinical
prognosis. The basic biological importance of BMPs in different
types of cancer raises potential concerns regarding their clinical
use (85).
In calf calcified cartilage, BMP-7, GDF-5 and NELL-1
were present in high concentrations and seem to be principal actors
in initial bone formation. The remaining factors could be
characters of the second plan. This poses the question of whether a
similar profile of factors serve a similar role in humans. This is
a challenging topic to study, due to the lack of suitable material
for study; however, sufficient amounts of calcified cartilage could
be obtained from other large animals for comparative evaluation. An
improved understanding of the function of growth factors during the
early stages of bone formation may possibly assist in identifying
effective regimens for bone regeneration.
In summary, in endochondral ossification, the
septoclasts invading non-calcified matrices release factors
required for the formation of osteoclasts. It may be the case, for
example, that BMP-2 not only stimulates bone formation, but
significantly enhances osteoclastogenesis, and that VEGF stimulates
differentiation of monocytes to osteoclasts (71-73).
Osteoclasts, once present, release growth factors stored in
calcified matrix, which are required for initial bone
deposition.
Acknowledgements
Not applicable.
Funding
Funding: This research was supported by the National Science
Centre, Poland (grant no. 2016/21/B/NZ1/00289).
Availability of data and materials
The datasets used and/or analysed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
SM designed the study. AI and AH performed the
experiments. AI, SM and AH analysed the data. AI, SM and AH wrote
the manuscript. All authors have read and approved the final
manuscript. AI, SM and AH confirmed the authenticity of all the raw
data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wozney JM: Overview of bone morphogenetic
proteins. Spine. 27 (Suppl 1):S2–S8. 2002.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Tsumaki N and Yoshikawa H: The role of
bone morphogenetic proteins in endochondral bone formation.
Cytokine Growth Factor Rev. 16:279–285. 2005.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Brighton CT: Structure and function of the
growth plate. Clin Orthop Relat Res. 136:22–32. 1978.PubMed/NCBI
|
|
4
|
Brochhausen C, Lehmann M, Halstenberg S,
Meurer A, Klaus G and Kirkpatrick CJ: Signalling molecules and
growth factors for tissue engineering of cartilage-what can we
learn from the growth plate? J Tissue Eng Regen Med. 3:416–429.
2009.PubMed/NCBI View
Article : Google Scholar
|
|
5
|
Schenk RK, Spiro D and Wiener J: Cartilage
resorption in the tibial epiphyseal plate of growing rats. J Cell
Biol. 34:275–291. 1967.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Mackie EJ, Tatarczuch L and Mirams M: The
skeleton: a multi-functional complex organ: the growth plate
chondrocyte and endochondral ossification. J Endocrinol.
211:109–121. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Arsenault AL and Hunziker EB: Electron
microscopic analysis of mineral deposits in the calcifying
epiphyseal growth plate. Calcif Tissue Int. 42:119–126.
1988.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Bonucci E: Fine structure of early
cartilage calcification. J Ultrastruct Res. 20:33–50.
1967.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Anderson HC: Vesicles associated with
calcification in the matrix of epiphyseal cartilage. J Cell Biol.
41:59–72. 1969.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ali SY, Sajdera SW and Anderson HC:
Isolation and characterization of calcifying matrix vesicles from
epiphyseal cartilage. Proc Natl Acad Sci USA. 67:1513–1520.
1970.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wuthier RE and Lipscomb GF: Matrix
vesicles: Structure, composition, formation and function in
calcification. Front Biosci. 16:2812–2902. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
12
|
Cui L, Houston DA, Farquharson C and
MacRae VE: Characterisation of matrix vesicles in skeletal and soft
tissue mineralisation. Bone. 87:147–158. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Nahar NN, Missana LR, Garimella R, Tague
SE and Anderson HC: Matrix vesicles are carriers of bone
morphogenetic proteins (BMPs), vascular endothelial growth factor
(VEGF), and noncollagenous matrix proteins. J Bone Miner Metab.
26:514–519. 2008.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Anderson HC, Hodges PT, Aguilera XM,
Missana L and Moylan PE: Bone morphogenetic protein (BMP)
localization in developing human and rat growth plate, metaphysis,
epiphysis, and articular cartilage. J Histochem Cytochem.
48:1493–1502. 2000.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chang SC, Hoang B, Thomas JT, Vukicevic S,
Luyten FP, Ryba NJ, Kozak CA, Reddi AH and Moos M Jr:
Cartilage-derived morphogenetic proteins. New members of the
transforming growth factor-beta superfamily predominantly expressed
in long bones during human embryonic development. J Biol Chem.
269:28227–28234. 1994.PubMed/NCBI
|
|
16
|
Reddi AH: Cartilage morphogenetic
proteins: Role in joint development, homoeostasis, and
regeneration. Ann Rheum Dis. 62 (Suppl 2):ii73–ii78.
2003.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hyc A, Moskalewski S and Osiecka-Iwan A:
Influence of cartilage interstitial fluid on the mRNA levels of
matrix proteins, cytokines, metalloproteases and their inhibitors
in synovial membrane. Int J Mol Med. 38:937–942. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhang X, Zara J, Siu RK, Ting K and Soo C:
The role of NELL-1, a growth factor associated with
craniosynostosis, in promoting bone regeneration. J Dent Res.
89:865–878. 2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Sheikh Z, Javaid MA, Hamdan N and Hashmi
R: Bone regeneration using bone morphogenetic oroteins and various
biomaterial carriers. Materials (Basel). 8:1778–1816.
2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Cao X and Chen D: The BMP signaling and in
vivo bone formation. Gene. 357:1–8. 2005.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kishigami S and Mishina Y: BMP signaling
and early embryonic patterning. Cytokine Growth Factor Rev.
16:265–278. 2005.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wozney JM: The bone morphogenetic protein
family and osteogenesis. Mol Reprod Dev. 32:160–167.
1992.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Gerber HP, Vu TH, Ryan AM, Kowalski J,
Werb Z and Ferrara N: VEGF couples hypertrophic cartilage
remodeling, ossification and angiogenesis during endochondral bone
formation. Nat Med. 5:623–628. 1999.PubMed/NCBI View
Article : Google Scholar
|
|
24
|
Wu M, Chen G and Li YP: TGF-β and BMP
signaling in osteoblast, skeletal development, and bone formation,
homeostasis and disease. Bone Res. 4(16009)2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
James AW, Shen J, Tsuei R, Nguyen A,
Khadarian K, Meyers CA, Pan HC, Li W, Kwak JH, Asatrian G, et al:
NELL-1 induces Sca-1+ mesenchymal progenitor cell
expansion in models of bone maintenance and repair. JCI Insight.
2(2)2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Brighton CT, Sugioka Y and Hunt RM:
Cytoplasmic structures of epiphyseal plate chondrocytes.
Quantitative evaluation using electron micrographs of rat
costochondral junctions with special reference to the fate of
hypertrophic cells. J Bone Joint Surg Am. 55:771–784.
1973.PubMed/NCBI
|
|
27
|
Byard RW, Foster BK and Byers S:
Immunohistochemical characterisation of the costochondral junction
in SIDS. J Clin Pathol. 46:108–112. 1993.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Clark CC, Tolin BS and Brighton CT: The
effect of oxygen tension on proteoglycan synthesis and aggregation
in mammalian growth plate chondrocytes. J Orthop Res. 9:477–484.
1991.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Poland TPotRo: Dziennik Ustaw SCG. Law
Gazette SCG, pp1-7, 2003.
|
|
30
|
Brem G and Nowshari MA: Nuclear transfer
in cattle. Methods Mol Biol. 254:213–226. 2004.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Perez-Riverol Y, Csordas A, Bai J,
Bernal-Llinares M, Hewapathirana S, Kundu DJ, Inuganti A, Griss J,
Mayer G, Eisenacher M, et al: The PRIDE database and related tools
and resources in 2019: Improving support for quantification data.
Nucleic Acids Res. 47:D442–D450. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Gabner S, Häusler G and Böck P: Vascular
canals in permanent hyaline cartilage: Development, corrosion of
nonmineralized cartilage matrix, and removal of matrix degradation
products. Anat Rec (Hoboken). 300:1067–1082. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Schenk RK, Wiener J and Spiro D: Fine
structural aspects of vascular invasion of the tibial epiphyseal
plate of growing rats. Acta Anat (Basel). 69:1–17. 1968.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Deckers MM, Van Beek ER, Van Der Pluijm G,
Wetterwald A, Wee-Pals LV, Cecchini MG, Papapoulos SE and Löwik CW:
Dissociation of angiogenesis and osteoclastogenesis during
endochondral bone formation in neonatal mice. J Bone Miner Res.
17:998–1007. 2002.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Lee ER, Lamplugh L, Shepard NL and Mort
JS: The septoclast, a cathepsin B-rich cell involved in the
resorption of growth plate cartilage. J Histochem Cytochem.
43:525–536. 1995.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Gartland A, Mason-Savas A, Yang M, MacKay
CA, Birnbaum MJ and Odgren PR: Septoclast deficiency accompanies
postnatal growth plate chondrodysplasia in the toothless (tl)
osteopetrotic, colony-stimulating factor-1 (CSF-1)-deficient rat
and is partially responsive to CSF-1 injections. Am J Pathol.
175:2668–2675. 2009.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Anderson CE and Parker J: Invasion and
resorption in enchondral ossification. An electron microscopic
study. J Bone Joint Surg Am. 48:899–914. 1966.PubMed/NCBI
|
|
38
|
Włodarski KH, Brodzikowska A and Kuzaka B:
Are chondroclasts and osteoclasts identical? Folia Biol (Krakow).
62:143–147. 2014.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Lewinson D and Silbermann M: Chondroclasts
and endothelial cells collaborate in the process of cartilage
resorption. Anat Rec. 233:504–514. 1992.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Shibata S, Suzuki S and Yamashita Y: An
ultrastructural study of cartilage resorption at the site of
initial endochondral bone formation in the fetal mouse mandibular
condyle. J Anat. 191:65–76. 1997.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Baron R: Molecular mechanisms of bone
resorption by the osteoclast. Anat Rec. 224:317–324.
1989.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Gupta A, Edwards JC and Hruska KA:
Cellular distribution and regulation of NHE-1 isoform of the NA-H
exchanger in the avian osteoclast. Bone. 18:87–95. 1996.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Stenbeck G and Horton MA: Endocytic
trafficking in actively resorbing osteoclasts. J Cell Sci.
117:827–836. 2004.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Nesbitt SA and Horton MA: Trafficking of
matrix collagens through bone-resorbing osteoclasts. Science.
276:266–269. 1997.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Cappariello A, Maurizi A, Veeriah V and
Teti A: The great beauty of the osteoclast. Arch Biochem Biophys.
558:70–78. 2014.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Takito J, Inoue S and Nakamura M: The
sealing zone in osteoclasts: A self-organized structure on the
bone. Int J Mol Sci. 19(19)2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Salo J, Lehenkari P, Mulari M, Metsikkö K
and Väänänen HK: Removal of osteoclast bone resorption products by
transcytosis. Science. 276:270–273. 1997.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Reddy S, Devlin R, Menaa C, Nishimura R,
Choi SJ, Dallas M, Yoneda T and Roodman GD: Isolation and
characterization of a cDNA clone encoding a novel peptide (OSF)
that enhances osteoclast formation and bone resorption. J Cell
Physiol. 177:636–645. 1998.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Loeser RF, Chubinskaya S, Pacione C and Im
HJ: Basic fibroblast growth factor inhibits the anabolic activity
of insulin-like growth factor 1 and osteogenic protein 1 in adult
human articular chondrocytes. Arthritis Rheum. 52:3910–3917.
2005.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Tanjaya J, Zhang Y, Lee S, Shi J, Chen E,
Ang P, Zhang X, Tetradis S, Ting K, Wu B, et al: Efficacy of
intraperitoneal administration of PEGylated NELL-1 for bone
formation. Biores Open Access. 5:159–170. 2016.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Sampath TK, Maliakal JC, Hauschka PV,
Jones WK, Sasak H, Tucker RF, White KH, Coughlin JE, Tucker MM and
Pang RH: Recombinant human osteogenic protein-1 (hOP-1) induces new
bone formation in vivo with a specific activity comparable with
natural bovine osteogenic protein and stimulates osteoblast
proliferation and differentiation in vitro. J Biol Chem.
267:20352–20362. 1992.PubMed/NCBI
|
|
52
|
Wutzl A, Rauner M, Seemann R, Millesi W,
Krepler P, Pietschmann P and Ewers R: Bone morphogenetic proteins
2, 5, and 6 in combination stimulate osteoblasts but not
osteoclasts in vitro. J Orthop Res. 28:1431–1439. 2010.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Gao X, Usas A, Lu A, Tang Y, Wang B, Chen
CW, Li H, Tebbets JC, Cummins JH and Huard J: BMP2 is superior to
BMP4 for promoting human muscle-derived stem cell-mediated bone
regeneration in a critical-sized calvarial defect model. Cell
Transplant. 22:2393–2408. 2013.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Bahamonde ME and Lyons KM: BMP3: To be or
not to be a BMP. J Bone Joint Surg Am. 83-A (Suppl 1):S56–S62.
2001.PubMed/NCBI
|
|
55
|
Storm EE, Huynh TV, Copeland NG, Jenkins
NA, Kingsley DM and Lee SJ: Limb alterations in brachypodism mice
due to mutations in a new member of the TGF beta-superfamily.
Nature. 368:639–643. 1994.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Polinkovsky A, Robin NH, Thomas JT, Irons
M, Lynn A, Goodman FR, Reardon W, Kant SG, Brunner HG, van der
Burgt I, et al: Mutations in CDMP1 cause autosomal dominant
brachydactyly type C. Nat Genet. 17:18–19. 1997.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Klammert U, Mueller TD, Hellmann TV,
Wuerzler KK, Kotzsch A, Schliermann A, Schmitz W, Kuebler AC,
Sebald W and Nickel J: GDF-5 can act as a context-dependent BMP-2
antagonist. BMC Biol. 13(77)2015.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Yamashita H, Shimizu A, Kato M, Nishitoh
H, Ichijo H, Hanyu A, Morita I, Kimura M, Makishima F and Miyazono
K: Growth/differentiation factor-5 induces angiogenesis in vivo.
Exp Cell Res. 235:218–226. 1997.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Celeste AJ, Iannazzi JA, Taylor RC, Hewick
RM, Rosen V, Wang EA and Wozney JM: Identification of transforming
growth factor beta family members present in bone-inductive protein
purified from bovine bone. Proc Natl Acad Sci USA. 87:9843–9847.
1990.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Friedman MS, Long MW and Hankenson KD:
Osteogenic differentiation of human mesenchymal stem cells is
regulated by bone morphogenetic protein-6. J Cell Biochem.
98:538–554. 2006.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Kingsley DM: The TGF-beta superfamily: New
members, new receptors, and new genetic tests of function in
different organisms. Genes Dev. 8:133–146. 1994.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Longobardi L, Li T, Myers TJ, O'Rear L,
Ozkan H, Li Y, Contaldo C and Spagnoli A: TGF-β type II
receptor/MCP-5 axis: At the crossroad between joint and growth
plate development. Dev Cell. 23:71–81. 2012.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Chen G, Deng C and Li YP: TGF-β and BMP
signaling in osteoblast differentiation and bone formation. Int J
Biol Sci. 8:272–288. 2012.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Derynck R and Akhurst RJ: Differentiation
plasticity regulated by TGF-beta family proteins in development and
disease. Nat Cell Biol. 9:1000–1004. 2007.PubMed/NCBI View
Article : Google Scholar
|
|
65
|
Centrella M, McCarthy TL and Canalis E:
Skeletal tissue and transforming growth factor beta. FASEB J.
2:3066–3073. 1988.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Dallas SL, Rosser JL, Mundy GR and
Bonewald LF: Proteolysis of latent transforming growth factor-beta
(TGF-beta)-binding protein-1 by osteoclasts. A cellular mechanism
for release of TGF-beta from bone matrix. J Biol Chem.
277:21352–21360. 2002.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Nagao M, Hamilton JL, Kc R, Berendsen AD,
Duan X, Cheong CW, Li X, Im HJ and Olsen BR: Vascular endothelial
growth factor in cartilage development and osteoarthritis. Sci Rep.
7(13027)2017.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Patil AS, Sable RB and Kothari RM:
Occurrence, biochemical profile of vascular endothelial growth
factor (VEGF) isoforms and their functions in endochondral
ossification. J Cell Physiol. 227:1298–1308. 2012.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Bluteau G, Julien M, Magne D,
Mallein-Gerin F, Weiss P, Daculsi G and Guicheux J: VEGF and VEGF
receptors are differentially expressed in chondrocytes. Bone.
40:568–576. 2007.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Zelzer E and Olsen BR: Multiple roles of
vascular endothelial growth factor (VEGF) in skeletal development,
growth, and repair. Curr Top Dev Biol. 65:169–187. 2005.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Hu K and Olsen BR: Osteoblast-derived VEGF
regulates osteoblast differentiation and bone formation during bone
repair. J Clin Invest. 126:509–526. 2016.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Liu Y and Olsen BR: Distinct VEGF
functions during bone development and homeostasis. Arch Immunol
Ther Exp (Warsz). 62:363–368. 2014.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Liu Y, Berendsen AD, Jia S, Lotinun S,
Baron R, Ferrara N and Olsen BR: Intracellular VEGF regulates the
balance between osteoblast and adipocyte differentiation. J Clin
Invest. 122:3101–3113. 2012.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Li B, Wang H, Qiu G, Su X and Wu Z:
Synergistic effects of vascular endothelial growth factor on bone
morphogenetic proteins induced bone formation in vivo: Influencing
factors and future research directions. BioMed Res Int.
2016(2869572)2016.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Kakudo N, Kusumoto K, Wang YB, Iguchi Y
and Ogawa Y: Immunolocalization of vascular endothelial growth
factor on intramuscular ectopic osteoinduction by bone
morphogenetic protein-2. Life Sci. 79:1847–1855. 2006.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Dilling CF, Wada AM, Lazard ZW, Salisbury
EA, Gannon FH, Vadakkan TJ, Gao L, Hirschi K, Dickinson ME, Davis
AR, et al: Vessel formation is induced prior to the appearance of
cartilage in BMP-2-mediated heterotopic ossification. J Bone Miner
Res. 25:1147–1156. 2010.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Hogan BL: Bone morphogenetic proteins in
development. Curr Opin Genet Dev. 6:432–438. 1996.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Nagai H, Tsukuda R and Mayahara H: Effects
of basic fibroblast growth factor (bFGF) on bone formation in
growing rats. Bone. 16:367–373. 1995.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Du M, Zhu T, Duan X, Ge S, Li N, Sun Q and
Yang P: Acellular dermal matrix loading with bFGF achieves similar
acceleration of bone regeneration to BMP-2 via differential effects
on recruitment, proliferation and sustained osteodifferentiation of
mesenchymal stem cells. Mater Sci Eng C. 70:62–70. 2017.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Arnott JA, Lambi AG, Mundy C, Hendesi H,
Pixley RA, Owen TA, Safadi FF and Popoff SN: The role of connective
tissue growth factor (CTGF/CCN2) in skeletogenesis. Crit Rev
Eukaryot Gene Expr. 21:43–69. 2011.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Bell PA, Dennis EP, Hartley CL, Jackson
RM, Porter A, Boot-Handford RP, Pirog KA and Briggs MD:
Mesencephalic astrocyte-derived neurotropic factor is an important
factor in chondrocyte ER homeostasis. Cell Stress Chaperones.
24:159–173. 2019.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Termaat MF, Den Boer FC, Bakker FC, Patka
P and Haarman HJ: Bone morphogenetic proteins. Development and
clinical efficacy in the treatment of fractures and bone defects. J
Bone Joint Surg Am. 87:1367–1378. 2005.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Vukicevic S, Oppermann H, Verbanac D,
Jankolija M, Popek I, Curak J, Brkljacic J, Pauk M, Erjavec I,
Francetic I, et al: The clinical use of bone morphogenetic proteins
revisited: A novel biocompatible carrier device OSTEOGROW for bone
healing. Int Orthop. 38:635–647. 2014.PubMed/NCBI View Article : Google Scholar
|
|
84
|
James AW, LaChaud G, Shen J, Asatrian G,
Nguyen V, Zhang X, Ting K and Soo C: A Review of the Clinical Side
Effects of Bone Morphogenetic Protein-2. Tissue Eng Part B Rev.
22:284–297. 2016.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Epstein NE: Basic science and spine
literature document bone morphogenetic protein increases cancer
risk. Surg Neurol Int. 5 (Suppl 15):S552–S560. 2014.PubMed/NCBI View Article : Google Scholar
|