Introduction
Methotrexate (MTX), a folic acid antagonist,
exhibits anti-proliferative activity, and immune- regulatory and
anti-inflammatory properties (1-3).
The chemical structure of MTX is:
(2~)-2-[[4-[(2,4-diaminopteridin-6-yl) methyl methylamino]
benzoyl]amino] pentanedioic acid (4). MTX competitively inhibits the activity
of dihydrofolate (DHF) reductase enzyme (DHFR), which is a small
protein (~19 kDa, 186 amino acids) that catalyzes the reduction of
DHF into tertahydrofolate (THF) (1,5).
Moreover, THF is essential for the synthesis of purines and several
amino acids, as well as for DNA synthesis (5).
The clinical use of MTX as an antimetabolite in
cancer management is associated with dose-dependent toxic adverse
effects, such as alopecia, ulcerations, pulmonary toxicity,
abdominal discomfort, hepatotoxicity, myelosuppression and
nephrotoxicity (6,7). Moreover, the administration of MTX is
associated with neurotoxicity that is reported along with
neurological complications, delays in treatment and prolonged
hospitalization (8,9). MTX administration also causes genetic
alterations and DNA damage by enhancing the accumulation of
oxidative DNA lesions (10,11). Furthermore, MTX has been reported to
cause double-stranded breaks, which can result in chromosomal
relocations, and is extremely harmful to dividing cells (12,13).
Previous studies have revealed the significant role of oxidative
stress as a participating factor in neurotoxicity and
hepatotoxicity (14-16).
Hence, the excessive production of reactive oxygen species (ROS)
contributes to the incidence and advancement of MTX-induced
toxicity (17). The redox-state
altering properties of MTX have been suggested as an essential
immunosuppressive mechanism and found to induce apoptosis via ROS
production (1). The produced
reactive species react with different biological macromolecules,
thereby generating lipid peroxides that are capable of producing
additional ROS or converting them into reactive compounds that are
able to crosslinks within DNA and proteins, resulting in cellular
toxicity (14,18). Thus, it is hypothesized that cellular
oxidative damage with lipid peroxidation is a characteristic of
MTX-induced toxicity. Moreover, a decrease in tetrahydrobiopterin
levels (an important cofactor for nitric oxide synthesis that is
produced by the DHFR) potentiates MTX-induced ROS production
(1). Antioxidants function to
reverse the increase in oxidative stress induced by MTX. Currently,
to the best of our knowledge, there are no studies aiming to
antagonize the genotoxicity induced by MTX. Thus, identifying an
agent that can ameliorate the MTX-induced genotoxicity may
significantly improve the outcomes of patients who are administered
MTX.
Metformin, a biguanide anti-hyperglycemic drug, is
the first-line agent used in the management of type 2 diabetes
mellitus. It reduces hepatic glucose synthesis, decreases glucose
intestinal absorption and increases insulin sensitivity by
elevating peripheral glucose uptake and consumption (19,20).
Additionally, metformin is widely used in the treatment of
polycystic ovary syndrome and as an adjunct treatment for cancer
(19-22).
The molecular mechanisms responsible for the effects of metformin
may include, but are not limited to: The reduction of cellular
oxidative stress, the suppression of inflammation and the reduction
of levels of inflammatory biomarkers through 5' AMP-activated
protein kinase-dependent and independent pathways (23). Cheki et al (24) reported that metformin has potential
protective effects against cisplatin-induced genotoxicity in rat
bone marrow cells due to its antioxidant properties. Moreover,
another study demonstrated that metformin exerted protective
effects against acetaminophen-induced liver toxicity by reducing
overall hepatic oxidative stress (25). Thus, the present study aimed to
assess the potential ameliorative effects of metformin against
MTX-induced genotoxicity in cultured human lymphocytes.
Materials and methods
Participants
In the present study, peripheral venous blood was
collected from five male subjects (median age 30 years old). The
participants did not consume alcohol or drugs, did not smoke
cigarettes/or waterpipe tobacco and were not on any medications.
The blood samples were freshly collected from donors (pre-dietary
intake) on the same day of the experiments to diminish any possible
dietary effects. Written informed consent was obtained from all
participants who decided to participate in the present study after
providing them with an explanation of the aims and the objectives
of the present study, and the present study was approved by the
Institutional Review Board of Jordan University of Science and
Technology (approval no. 27/132/2020). Additionally, the present
study was performed in accordance with guidelines described in the
Declaration of Helsinki (26) for
research involving humans.
Human blood cell culture and drug
treatment
The blood samples were cultured within 1 h of
collection. Each sample of freshly drained blood (1 ml) was mixed
with 9 ml culture medium (PB-MAX™ Karyotyping Medium; Gibco; Thermo
Fisher Scientific, Inc.). PB-Max (Gibco; Thermo Fisher Scientific,
Inc.) is an optimized fully supplemented RPMI-1640 medium that
includes 15% FBS, L-glutamine and phytohaemagglutinin (a mitogen
that stimulates the division of blood lymphocytes) (10,27-29).
A stock solution of 2.2 mM MTX (≥98% purity,
Sigma-Aldrich; Merck KGaA) was prepared using sodium hydroxide as a
solvent. The final concentration of MTX in the culture medium was
0.5 µM, and it was added to the cell culture 24 h before
harvesting. Human cultured lymphocytes require ~24 h to complete
one cycle of cell division (30).
Therefore, the 24 h exposure window was selected to make sure that
cultured cells were exposed to drugs in all phases of the cell
cycle. The MTX concentration used was based on previously published
literature which showed that MTX-induced chromosomal damage
(5,10). Metformin (kindly provided by MS
Pharma-United Pharmaceutical Manufacturing Company®) was
added to the corresponding flasks. The final metformin
concentration in the culture medium was 12 µM and it was added at
the beginning of culture. The metformin concentration used in the
present study was based on previously published literature
(31). To assess the effects of MTX
and/or metformin on DNA, four different groups were used: Control,
metformin, MTX and metformin + MTX groups. A total of 2 h prior to
harvesting, 100 µl 10 µg/ml Colcemid (a spindle inhibitor; cat. no.
477-30-5; Sigma-Aldrich; Merck KGaA) was added to arrest mitosis at
the metaphase stage. The procedure of human lymphocyte cell culture
preparation was performed according to previously published
protocols (10,29,32).
Chromosomal aberration (CA) assay
The cultured cells were transferred into screw
capped 15-ml tubes and centrifuged at 134 x g, 4˚C for 10 min. The
supernatant was then removed and the pellet was resuspended using
pre-warmed KCL solution (0.56% KCL) followed by incubation at 37˚C
for 30 min. Subsequently, the lymphocytes were centrifuged for 10
min at 134 x g, 4˚C to collect swollen lymphocytes. The supernatant
was then removed and the pellets were fixed by the addition of the
freshly prepared fixative, absolute methanol and glacial acetic
acid [3:1 (v/v)] in a drop-wise manner, followed by incubation in
the dark for 20 min at room temperature. The pellet was then washed
three times using the aforementioned fixative solution and
suspended in ~1 ml fixative solution. Then, the suspended solution
was dropped on pre-chilled slides to disperse the metaphases.
Finally, the slides were allowed to dry and were then dyed with 5%
Giemsa (Sigma-Aldrich; Merck KGaA) for 4 min at room temperature,
rinsed with distilled water and then air-dried for the CA test. CAs
were evaluated in 100 well-spread metaphases for each group/donor
(two repeats per donor were used) using a high-resolution light
microscope (Nikon Corporation) with immersion oil at x1,000
magnification. The CAs were classed as gaps or
breaks/exchanges.
Sister chromatid exchange (SCE)
assay
The blood cultures were treated with 25 µl
5-bromo-2-deoxyuridine (BrdU 0.01 g/ml; cat. no. 72218-68-9; Gibco;
Thermo Fisher Scientific, Inc.) prior to the incubation period. As
BrdU is highly susceptible to light, the procedures were performed
in the dark to prevent photolysis. The protocols of cell culture
preparation, cell harvesting/dropping and slide preparation were
comparable to those used in the CA assay. The prepared slides for
SCE were stained at room temperature for 22 min with 10 µg/ml
Hoechst 32285 dye, then rinsed with distilled water and mounted in
Mcllvaine buffer (0.18 g citric acid and 2 g sodium phosphate
dissolved in 100 ml distilled water; pH 8.0). Subsequently, the
prepared slides were exposed to two UV lamps (350 nm) at a distance
of 7 cm at 40˚C for 30 min. Subsequently, the slides were washed
carefully with distilled water and dried at room temperature. The
slides were then stained at room temperature for 4 min using 5%
Giemsa stain in Gurr buffer (pH 6.8; Gibco; Thermo Fisher
Scientific, Inc.), washed with distilled water and air-dried at
room temperature. For SCE scoring, ~50 well-spread second-division
metaphases (M2) per donor that contained 42-46 chromosomes were
scored using a high-resolution light microscope (Nikon Corporation)
with immersion oil at x1,000 magnification as described above. M2
chromosomes were distinguished based on the presence of two
differentially stained chromatids (one lightly and the other darkly
stained). Conversely, chromosomes in the M1 phase were
distinguished by having both chromatids darkly stained. Chromosomes
in the M3 phase were differentiated by exhibiting a combination of
differentially stained chromatids, whereas chromosomes in the M4
phase had both chromatids lightly stained (10). To perform the cell kinetics analysis,
the proliferative index (PI) was calculated via scoring 100
metaphases from each donor using the following formula: (1 x M1 + 2
x M2 + 3 x ≥M3)/100; where M1, M2 and M3 represent the number of
cells at the first, second and third metaphases, respectively
(29).
Statistical analysis
Data analysis was performed using GraphPad Prism
version 7 (GraphPad Software, Inc.). Multiple comparisons were
performed using a one-way ANOVA followed by a Tukey's post hoc
test. Data are presented as the mean ± standard error of the mean.
P<0.05 was considered to indicate a statistically significant
difference.
Results
The effect of metformin (12 µM) on MTX (0.5
µM)-induced genotoxicity in cultured human lymphocytes was examined
using CA assays. The frequency of CAs induced by the indicated
drugs is presented in Table I.
Treatment of the cultured cells with MTX significantly increased
the number of CAs. This included aberration exchanges
(P<0.0001), chromatid/chromosome breaks (P<0.0001) and gap
aberrations (P<0.0001). With respect to metformin, no
significant changes in the frequency of all examined CAs were
observed (P>0.05). However, co-treatment of the cultures with
MTX and metformin induced significantly fewer CAs compared with the
group treated with MTX alone (P<0.0001). The reduction in the
frequency of CAs ranged between 35% of aberrations exchanges to 67%
in gap aberrations. The trend in changes in the frequency of CAs
induced by MTX and metformin was observed in cells obtained from
different blood donors (Table
I).
 | Table IFrequency of chromosomal aberrations
per donor induced by the different treatmentsa. |
Table I
Frequency of chromosomal aberrations
per donor induced by the different treatmentsa.
|
Donor/treatment | Frequency of
chromatid/chromosome exchanges | Frequency of
chromatid/chromosome breaks | Frequency of
chromatid/chromosome gaps |
|---|
| Donor 1 |
|
Control | 0 | 0.03 | 0.12 |
|
Metformin | 0.01 | 0.03 | 0.06 |
|
Methotrexate | 0.06 | 0.16 | 0.25 |
|
Methotrexate
+ Metformin | 0.05 | 0.06 | 0.09 |
| Donor 2 |
|
Control | 0.01 | 0.04 | 0.1 |
|
Metformin | 0 | 0.02 | 0.05 |
|
Methotrexate | 0.08 | 0.17 | 0.28 |
|
Methotrexate
+ Metformin | 0.04 | 0.07 | 0.08 |
| Donor 3 |
|
Control | 0 | 0.05 | 0.13 |
|
Metformin | 0.01 | 0.02 | 0.08 |
|
Methotrexate | 0.07 | 0.16 | 0.25 |
|
Methotrexate
+ Metformin | 0.05 | 0.09 | 0.07 |
| Donor 4 |
|
Control | 0 | 0.03 | 0.1 |
|
Metformin | 0 | 0.04 | 0.06 |
|
Methotrexate | 0.07 | 0.19 | 0.26 |
|
Methotrexate
+ Metformin | 0.04 | 0.06 | 0.09 |
| Donor 5 |
|
Control | 0.01 | 0.04 | 0.12 |
|
Metformin | 0.01 | 0.04 | 0.05 |
|
Methotrexate | 0.06 | 0.22 | 0.27 |
|
Methotrexate
+ Metformin | 0.04 | 0.08 | 0.1 |
| Total mean |
|
Control | 0.004 | 0.038 | 0.114 |
|
Metformin | 0.006c,d | 0.030c,d | 0.060b-d |
|
Methotrexate | 0.068b,d | 0.180b,d | 0.262b |
|
Methotrexate
+ Metformin | 0.044a,b | 0.072a | 0.086a,b |
| P-value | P<0.0001 | P<0.0001 | P<0.0001 |
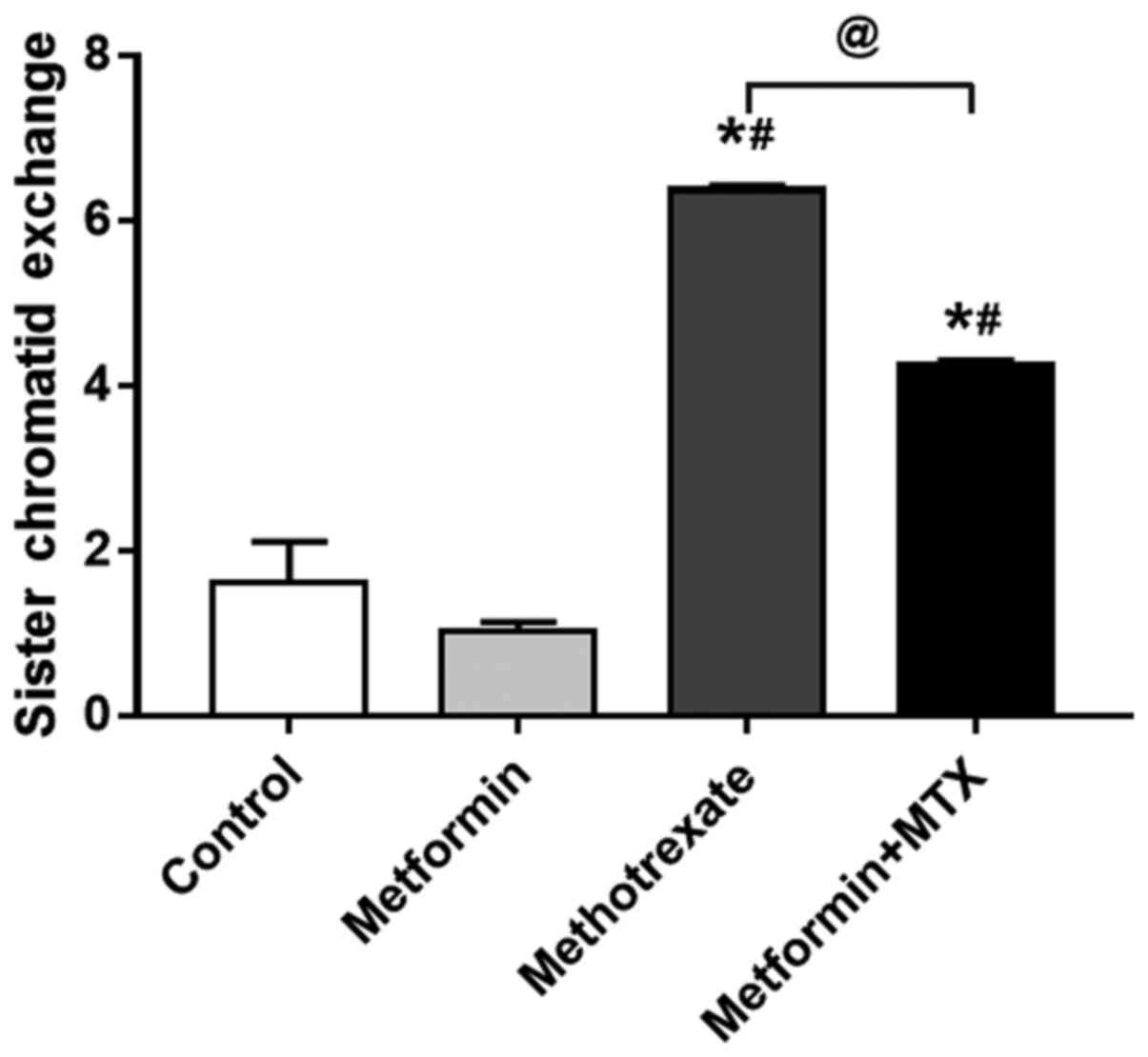
The SCE frequencies were increased in the
MTX-treated human lymphocytes compared with other treatment groups
(P<0.0001; Fig. 1). When the
cells were treated with metformin, lymphocytes were protected
against the genotoxic effects of MTX (P<0.0001). The results of
the present study revealed that MTX (0.5 µM) led to a statistically
significant increase in the frequency of SCEs in normal healthy
lymphocytes (P<0.0001). However, pre-treatment of the cells with
metformin (12 µM) induced a significant decrease in the SCE
frequency; it should be noted that the SCE frequency did not return
to normal levels. Furthermore, treatment of the cells with
metformin alone did not affect the frequency of SCE compared when
compared with the control group (P=0.4278).
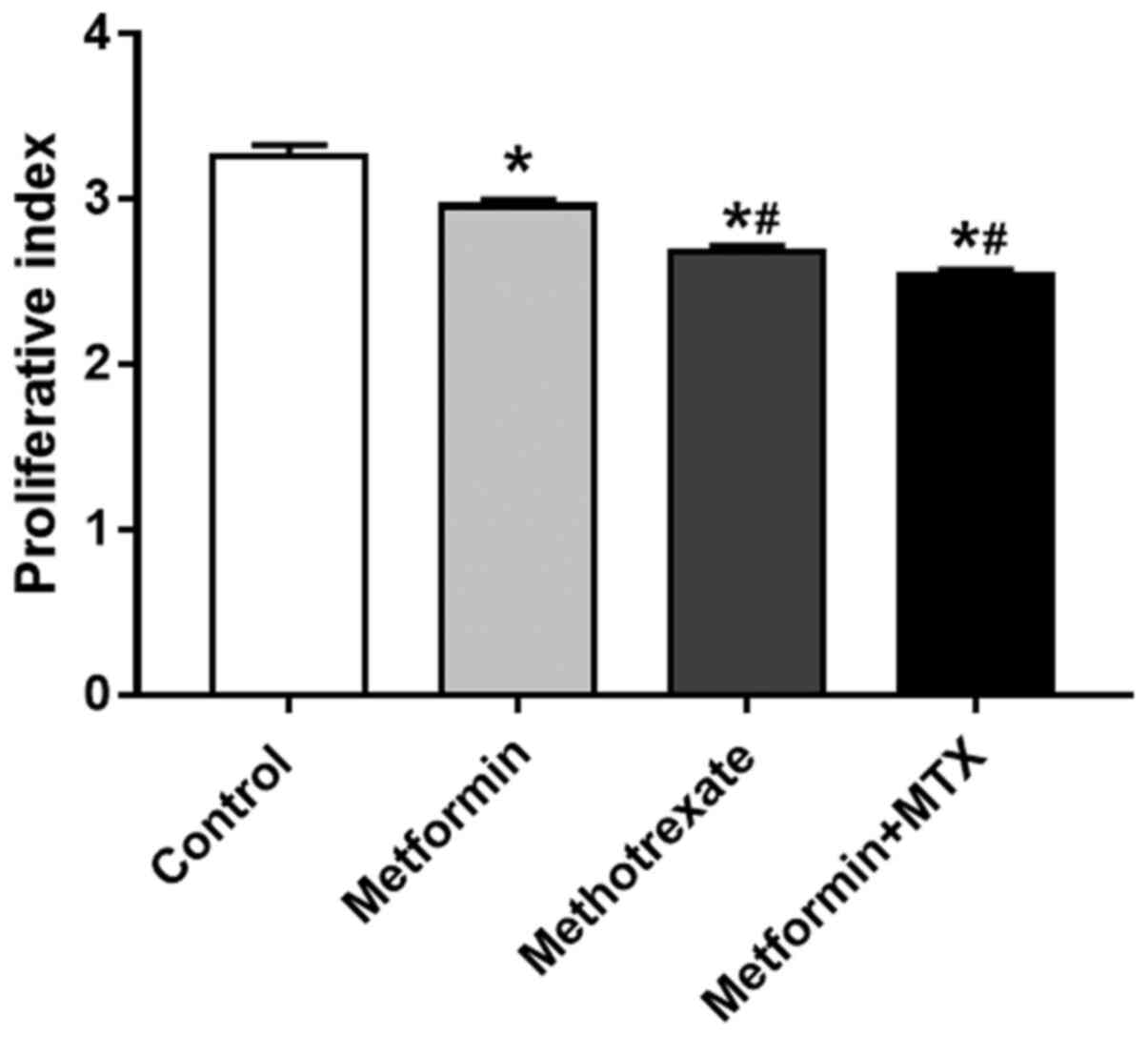
The effects of MTX and metformin on the PI are shown
in Fig. 2. MTX and metformin, when
used alone and when used in combination, induced a significant
decrease in the PI compared with the control group (P<0.0001;
Fig. 2). In addition, a significant
difference in the PI was observed between the group treated with
metformin alone and the MTX + metformin-treated group
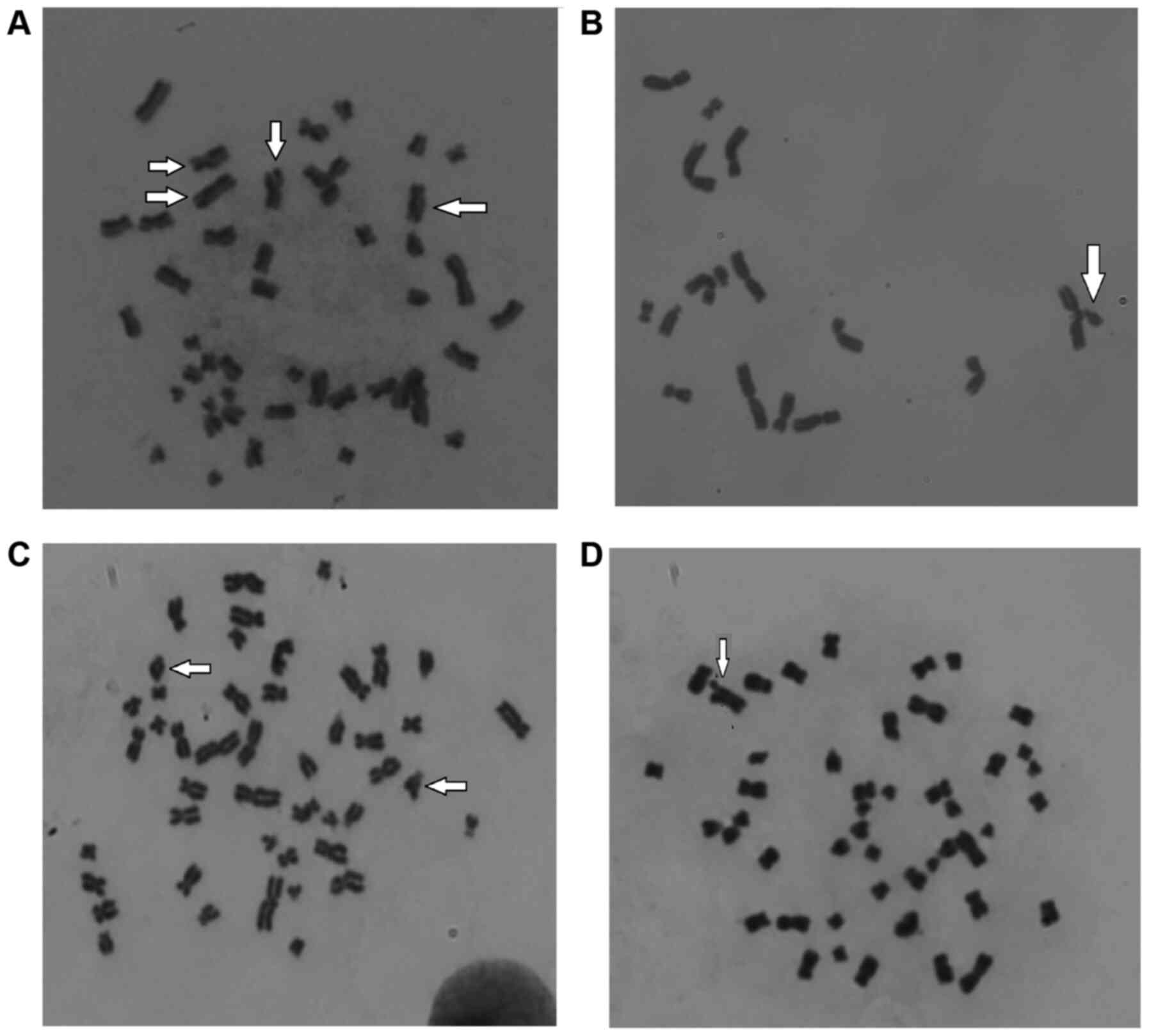
(P<0.0001). Fig. 3 provides
representative images and examples of the chromosomal damage (ring
and gap aberrations, SCEs and chromosomal exchange) observed in the
present study.
Discussion
Previous studies have shown that treatment with
several of the most effective anticancer agents causes direct
cellular toxicity (1,16,28).
Furthermore, treatment with various anticancer agents has been
demonstrated to exert carcinogenic, teratogenic and mutagenic
effects in experimental environments. For example, MTX
administration has been shown to enhance the accumulation of
oxidative DNA injuries, which, in turn, have been shown to induce
DNA damage (11,33). Hence, protecting normal cells from
conditions that may cause malignancy is an essential means to
inhibit long-term impairments or damage due to the administration
of chemotherapeutic agents.
Metformin remains the first-line medication used for
the treatment of patients with type 2 diabetes mellitus. Several
studies have demonstrated that metformin exhibits antioxidant,
anti-apoptotic and anti-inflammatory properties (34-37).
The aim of the present study was to investigate the potential
ameliorative effects of metformin on the genotoxicity induced by
MTX in human cultured lymphocytes. The cytogenetic effects were
examined by performing CA and SCE assays in vitro. Both CAs
and SCEs are commonly utilized as determining factors of DNA damage
and genotoxicity, and both assays are widely used for examining the
genotoxicity of therapeutic drugs and environmental agents
(38-41).
The primary results of the present study indicated
the ability of metformin to decrease the genotoxicity induced by
MTX, as shown by the reduction in the frequencies of CAs and SCEs
in the cells pre-treated with metformin. The results obtained in
the present study revealed that the SCE and CA frequencies in the
MTX treated cells were significantly higher compared with those in
the control group, showing the genotoxic effects of MTX on normal
healthy cells. These findings are in agreement with those of a
previous study, where the genotoxicity of MTX was also shown
(10). Moreover, it has been shown
that MTX substantially increases the frequency of CAs in cultured
human lymphocytes (42). Similarly,
Atteritano et al (43)
demonstrated a marked increase in the SCE frequency in the MTX
group in patients with rheumatoid arthritis. Another study by Said
Salem et al (44) reported a
significant increase in the number of micronucleated polychromatic
erythrocytes in mouse bone marrow cells treated with MTX compared
with the corresponding controls.
A suggested mechanism underlying the increase in CA
and SCE frequencies in cultured lymphocytes treated with MTX may
involve the generation of ROS induced by MTX, which potentiate
cellular damage (25). Several
anticancer agents, including MTX, induce cellular genotoxicity via
DNA oxidation, ROS production and reducing the total antioxidant
capacity (17,44). Several studies have demonstrated that
metformin exerts antioxidant activities by reducing the
malondialdehyde serum concentrations and increasing the activities
of superoxide dismutase, catalase and glutathione reductase
(45-47).
The results of the present study revealed that the administration
of metformin decreased the chromosomal damage induced by MTX, as
evidenced by a prominent decrease in the CA and SCE frequencies.
Collectively, it was suggested that metformin can attenuate the
genotoxic effects on normal cells induced by MTX by attenuating
oxidative stress through reducing ROS generation. This finding is
in agreement with the results reported in an animal study by Ashoka
and Mustak (48), which revealed the
protective effect of rutin, a potent antioxidant flavonoid
composite, in preventing MTX-induced genotoxicity. However, further
human in vivo studies are required to confirm these
results.
The results of the present study demonstrated that
metformin reduced the incidence of spontaneous SCE, break and gap
frequencies compared with the control. Hence, metformin appears to
decrease the spontaneous levels of SCEs and gap aberrations,
possibly via the reduction of the basal oxidative stress level.
However, the detailed mechanisms responsible for this suppressive
effect requires further investigation.
The protective effects of metformin on DNA damaging
agents was documented in several studies. For example, using HepG2
cells, metformin has been shown to protect against DNA damage
induced by formaldehyde (49). In a
study that was performed using cells derived from elderly subjects,
metformin has been shown to protect against pro-oxidant
stimulus-induced DNA damage (50).
Finally, using human A549 cells, metformin conferred protection
against UVC-induced DNA damage (51). Thus, using different models,
metformin seems to be a potent option for use with agents that
induce cellular DNA damage.
In the present study, to assess the cytotoxicity of
MTX and metformin, cell kinetic analysis was performed, which
involves the determination of the PI. Following treatment of the
cultured cells with MTX for 24 h, it was found that MTX was
cytotoxic to healthy normal human lymphocytes, as evidenced by the
significant reduction in the calculated PI. However, metformin was
not capable of attenuating the cytotoxic effects of MTX, and a
slight further decrease in the PI was observed with the use of
metformin. The results of the present study are consistent with
those of previous research, where it has been demonstrated that MTX
reduced the PI of cultured human lymphocytes (10) and other cell types, such as neurons
and cancer cells. However, in a study that was conducted on rats,
treatment of animals with metformin ameliorated the reduction in
the number of proliferating cells, and the survival of the cells
and immature neurons induced by MTX in the brain (52). Thus, the combined effects of
metformin and MTX could differ according to model and or tissues
used. The mechanisms by which MTX induced reduction in cellular
proliferation include the induction of the intracellular ROS,
interference with pyrimidine metabolism, activation of cellular
apoptosis, reduction of methyltransferase activity and the
reduction in cellular utilization of folates (53,54).
Therefore, the observed weak impact of metformin on MTX-induced
inhibition of cellular proliferation could be due to the multiple
mechanisms utilized by this drug to mediate its effects.
Collectively, both metformin and MTX appeared to modulate changes
in the PI in cultured human lymphocytes; pre-treatment of the cells
with metformin did not lead to any marked alterations in the
MTX-mediated reduction in PI. The PI was evaluated to examine the
influence of metformin on the cytotoxicity of MTX, and was not
predicted to imitate the genotoxicity findings.
The present study has some limitations, including
the subjectivity in the genotoxicity parameter scoring (CAs, SCEs
and PI), and the lack of in vivo experiments. Hence, further
in vitro and in vivo studies are required to confirm
the findings presented herein, and to extrapolate a small in
vitro study to a large comprehensive analysis using larger
sample sizes. Moreover, MTX genotoxicity was detected at 24 h
following treatment with a single dose. Thus, further studies
investigating the dose-response effects with longer treatment
durations are required. This could provide a basis for future
experimental study to improve our understanding of the molecular
mechanism underlying the actions of MTX. Finally, in the present
study, oxidative stress biomarkers and how they are modulated by
the treatment were not investigated. Such experiments are
recommended in future investigations.
In conclusion, the present study demonstrated that
MTX was genotoxic to normal human cultured cells, as illustrated by
the results of the CA and SCE assays. Conversely, metformin exerted
an ameliorative effect against MTX-induced chromosomal injury, as
it significantly reduced the CA and SCE frequencies. As the present
study was conducted using an in vitro lymphocyte cell
culture model, the results are not generalizable to other cell
types, and thus additional studies are required.
Acknowledgements
We would like to thank the Deanship of Research at
Jordan University of Science and Technology for financial support
to conduct the present study.
Funding
The present study was funded the Deanship of Research at Jordan
University of Science and Technology (grant no. 2020/252 to
AR).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
AMR, MA and KHA conceived the study. AMR, KHA, OFK
and MA designed the study. AMR, KHA and OFK performed the
experiments. AMR and OFK analyzed the data. AMR, OFK and MA wrote
the manuscript. AMR, KHA, OFK and MA edited the manuscript. OFK and
AMR confirm the authenticity of all the raw data. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board of Jordan University of Science and Technology
(approval no. 27/132/2020). Written informed consent was obtained
from all study participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bedoui Y, Guillot X, Sélambarom J, Guiraud
P, Giry C, Jaffar-Bandjee MC, Ralandison S and Gasque P:
Methotrexate an old drug with new tricks. Int J Mol Sci.
20(20)2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Tian H and Cronstein BN: Understanding the
mechanisms of action of methotrexate: Implications for the
treatment of rheumatoid arthritis. Bull NYU Hosp Jt Dis.
65:168–173. 2007.PubMed/NCBI
|
|
3
|
Nedelcu RI, Balaban M, Turcu G, Brinzea A,
Ion DA, Antohe M, Hodorogea A, Calinescu A, Badarau AI, Popp CG, et
al: Efficacy of methotrexate as anti-inflammatory and
anti-proliferative drug in dermatology: Three case reports. Exp
Ther Med. 18:905–910. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Cai B, Liao A, Lee KK, Ban JS, Yang HS, Im
YJ and Chun C: Design, synthesis of methotrexate-diosgenin
conjugates and biological evaluation of their effect on
methotrexate transport-resistant cells. Steroids. 116:45–51.
2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Vitale L, Serpieri V, Lauriola M, Piovesan
A, Antonaros F, Cicchini E, Locatelli C, Cocchi G, Strippoli P and
Caracausi M: Human trisomy 21 fibroblasts rescue methotrexate toxic
effect after treatment with 5-methyl-tetrahydrofolate and
5-formyl-tetrahydrofolate. J Cell Physiol Jan 22, 2019 (Epub ahead
of print). doi: 10.1002/jcp.28140.
|
|
6
|
Campbell JM, Bateman E, Stephenson MD,
Bowen JM, Keefe DM and Peters MD: Methotrexate-induced toxicity
pharmacogenetics: An umbrella review of systematic reviews and
meta-analyses. Cancer Chemother Pharmacol. 78:27–39.
2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Howard SC, McCormick J, Pui CH, Buddington
RK and Harvey RD: Preventing and Managing Toxicities of High-Dose
Methotrexate. Oncologist. 21:1471–1482. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Deneux V, Leboucq N, Saumet L, Haouy S,
Akbaraly T and Sirvent N: Acute methotrexate-related neurotoxicity
and pseudo-stroke syndrome. Arch Pediatr. 24:1244–1248.
2017.PubMed/NCBI View Article : Google Scholar : (In French).
|
|
9
|
Watanabe K, Arakawa Y, Oguma E, Uehara T,
Yanagi M, Oyama C, Ikeda Y, Sasaki K, Isobe K, Mori M, et al:
Characteristics of methotrexate-induced stroke-like neurotoxicity.
Int J Hematol. 108:630–636. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Rababa'h AM, Hussein SA, Khabour OF and
Alzoubi KH: The protective effect of cilostazol in genotoxicity
induced by methotrexate in human cultured lymphocytes. Curr Mol
Pharmacol. 13:137–143. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Shahin AA, Ismail MM, Saleh AM, Moustafa
HA, Aboul-Ella AA and Gabr HM: Protective effect of folinic acid on
low-dose methotrexate genotoxicity. Z Rheumatol. 60:63–68.
2001.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Shen J, Deininger P, Hunt JD and Zhao H:
8-Hydroxy-2'-deoxyguanosine (8-OH-dG) as a potential survival
biomarker in patients with nonsmall-cell lung cancer. Cancer.
109:574–580. 2007.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Martin SA, McCarthy A, Barber LJ, Burgess
DJ, Parry S, Lord CJ and Ashworth A: Methotrexate induces oxidative
DNA damage and is selectively lethal to tumour cells with defects
in the DNA mismatch repair gene MSH2. EMBO Mol Med. 1:323–337.
2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Taylor OA, Hockenberry MJ, McCarthy K,
Gundy P, Montgomery D, Ross A, Scheurer ME and Moore IM: Evaluation
of biomarkers of oxidative stress and apoptosis in patients with
severe methotrexate neurotoxicity: A case series. J Pediatr Oncol
Nurs. 32:320–325. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Akbulut S, Elbe H, Eris C, Dogan Z, Toprak
G, Otan E, Erdemli E and Turkoz Y: Cytoprotective effects of
amifostine, ascorbic acid and N-acetylcysteine against
methotrexate-induced hepatotoxicity in rats. World J Gastroenterol.
20:10158–10165. 2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Cetinkaya A, Bulbuloglu E, Kurutas EB and
Kantarceken B: N-acetylcysteine ameliorates methotrexate-induced
oxidative liver damage in rats. Med Sci Monit. 12:BR274–BR278.
2006.PubMed/NCBI
|
|
17
|
Abo-Haded HM, Elkablawy MA, Al-Johani Z,
Al-Ahmadi O and El-Agamy DS: Hepatoprotective effect of sitagliptin
against methotrexate induced liver toxicity. PLoS One.
12(e0174295)2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Gaschler MM and Stockwell BR: Lipid
peroxidation in cell death. Biochem Biophys Res Commun.
482:419–425. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Dumitrescu R, Mehedintu C, Briceag I,
Purcărea VL and Hudita D: Metformin-clinical pharmacology in PCOs.
J Med Life. 8:187–192. 2015.PubMed/NCBI
|
|
20
|
Chen K, Li Y, Guo Z, Zeng Y, Zhang W and
Wang H: Metformin: Current clinical applications in nondiabetic
patients with cancer. Aging (Albany NY). 12:3993–4009.
2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Meyerhardt JA, Irwin ML, Jones LW, Zhang
S, Campbell N, Brown JC, Pollak M, Sorrentino A, Cartmel B,
Harrigan M, et al: Randomized phase II trial of exercise,
metformin, or both on metabolic biomarkers in colorectal and breast
cancer survivors. JNCI Cancer Spectr. 4(pkz096)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Rababa'h AM, Matani BR and Ababneh MA: The
ameliorative effects of marjoram in dehydroepiandrosterone induced
polycystic ovary syndrome in rats. Life Sci.
261(118353)2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zeng J, Zhu L, Liu J, Zhu T, Xie Z, Sun X
and Zhang H: Metformin protects against oxidative stress injury
induced by ischemia/reperfusion via regulation of the
lncRNA-H19/miR-148a-3p/Rock2 Axis. Oxid Med Cell Longev.
2019(8768327)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Cheki M, Ghasemi MS, Rezaei Rashnoudi A
and Erfani Majd N: Metformin attenuates cisplatin-induced
genotoxicity and apoptosis in rat bone marrow cells. Drug Chem
Toxicol: May 9, 2019 (Epub ahead of print). doi:
10.1080/01480545.2019.1609024.
|
|
25
|
Tripathi SS, Singh S, Garg G, Kumar R,
Verma AK, Singh AK, Bissoyi A and Rizvi SI: Metformin ameliorates
acetaminophen-induced sub-acute toxicity via antioxidant property.
Drug Chem Toxicol: Sep 2, 2019 (Epub ahead of print). doi:
10.1080/01480545.2019.1658769.
|
|
26
|
World Medical Association. World Medical
Association Declaration of Helsinki: Ethical principles for medical
research involving human subjects. JAMA. 310:2191–2194.
2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Alzoubi KH, Bayraktar E, Khabour O and
Al-Azzam SI: Vitamin B12 protects against DNA damage induced by
hydrochlorothiazide. Saudi Pharm J. 26:786–789. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Al-Eitan LN, Alzoubi KH, Al-Smadi LI and
Khabour OF: Vitamin E protects against cisplatin-induced
genotoxicity in human lymphocytes. Toxicol In Vitro.
62(104672)2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Rababa'h AM, Khabour OF, Alzoubi KH,
Al-Momani D and Ababneh M: Assessment of genotoxicity of
levosimendan in human cultured lymphocytes. Curr Mol Pharmacol.
12:160–165. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Fowler S, Roush R and Wise J: Concepts of
Biology. OpenStax College. Rice University, 2013.
|
|
31
|
Othman EM, Oli RG, Arias-Loza PA, Kreissl
MC and Stopper sH: Metformin protects kidney cells from
insulin-mediated genotoxicity in vitro and in male Zucker diabetic
fatty rats. Endocrinology. 157:548–559. 2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Khabour OF, Enaya FM, Alzoubi K and
Al-Azzam SI: Evaluation of DNA damage induced by norcantharidin in
human cultured lymphocytes. Drug Chem Toxicol. 39:303–306.
2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
El-Sheikh AA, Morsy MA, Abdalla AM,
Hamouda AH and Alhaider IA: Mechanisms of thymoquinone hepatorenal
protection in methotrexate-induced toxicity in rats. Mediators
Inflamm. 2015(859383)2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Luo X, Hu R, Zheng Y, Liu S and Zhou Z:
Metformin shows anti-inflammatory effects in murine macrophages
through Dicer/microribonucleic acid-34a-5p and microribonucleic
acid-125b-5p. J Diabetes Investig. 11:101–109. 2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Nna VU, Abu Bakar AB, Md Lazin M and
Mohamed M: Antioxidant, anti-inflammatory and synergistic
anti-hyperglycemic effects of Malaysian propolis and metformin in
streptozotocin-induced diabetic rats. Food Chem Toxicol.
120:305–320. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Yang X, Ding H, Qin Z, Zhang C, Qi S,
Zhang H, Yang T, He Z, Yang K, Du E, et al: Metformin prevents
renal stone formation through an antioxidant mechanism in vitro and
in vivo. Oxid Med Cell Longev. 2016(4156075)2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Kolivand S, Motevaseli E, Cheki M,
Mahmoudzadeh A, Shirazi A and Fait V: The anti-apoptotic mechanism
of metformin against apoptosis induced by ionizing radiation in
human peripheral blood Mononuclear Cells. Klin Onkol. 30:372–379.
2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Stults DM, Killen MW, Marco-Casanova P and
Pierce AJ: The Sister Chromatid Exchange (SCE) assay. Methods Mol
Biol. 2102:441–457. 2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Mosesso P and Cinelli S: In vitro
cytogenetic assays: Chromosomal aberrations and micronucleus tests.
Methods Mol Biol. 2031:79–104. 2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Mahmoodi M, Soleyman-Jahi S, Zendehdel K,
Mozdarani H, Azimi C, Farzanfar F, Safari Z, Mohagheghi MA,
Khaleghian M, Divsalar K, et al: Chromosomal aberrations, sister
chromatid exchanges, and micronuclei in lymphocytes of oncology
department personnel handling anti-neoplastic drugs. Drug Chem
Toxicol. 40:235–240. 2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Norppa H, Bonassi S, Hansteen IL, Hagmar
L, Strömberg U, Rössner P, Boffetta P, Lindholm C, Gundy S, Lazutka
J, et al: Chromosomal aberrations and SCEs as biomarkers of cancer
risk. Mutat Res. 600:37–45. 2006.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Mondello C, Giorgi R and Nuzzo F:
Chromosomal effects of methotrexate on cultured human lymphocytes.
Mutat Mutat Res Lett. 139:67–70. 1984.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Atteritano M, Mazzaferro S, Mantuano S,
Bagnato GL and Bagnato GF: Effects of infliximab on sister
chromatid exchanges and chromosomal aberration in patients with
rheumatoid arthritis. Cytotechnology. 68:313–318. 2016.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Said Salem NI, Noshy MM and Said AA:
Modulatory effect of curcumin against genotoxicity and oxidative
stress induced by cisplatin and methotrexate in male mice. Food
Chem Toxicol. 105:370–376. 2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Chukwunonso Obi B, Chinwuba Okoye T,
Okpashi VE, Nonye Igwe C and Olisah Alumanah E: Comparative study
of the antioxidant effects of metformin, glibenclamide, and
repaglinide in alloxan-induced diabetic rats. J Diabetes Res.
2016(1635361)2016.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Tang G, Yang H, Chen J, Shi M, Ge L, Ge X
and Zhu G: Metformin ameliorates sepsis-induced brain injury by
inhibiting apoptosis, oxidative stress and neuroinflammation via
the PI3K/Akt signaling pathway. Oncotarget. 8:97977–97989.
2017.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Cahova M, Palenickova E, Dankova H,
Sticova E, Burian M, Drahota Z, Cervinkova Z, Kucera O, Gladkova C,
Stopka P, et al: Metformin prevents ischemia reperfusion-induced
oxidative stress in the fatty liver by attenuation of reactive
oxygen species formation. Am J Physiol Gastrointest Liver Physiol.
309:G100–G111. 2015.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Ashoka CH and Mustak M: Antigenotoxic
effects of rutin against methotrexate genotoxicity in Swiss albino
mice. Curr Trends Biotechnol Pharm. 13:163–177. 2019.
|
|
49
|
Mai X, Zhou F, Lin P, Lin S, Gao J, Ma Y,
Fan R, Ting W, Huang C, Yin D, et al: Metformin scavenges
formaldehyde and attenuates formaldehyde-induced bovine serum
albumin crosslinking and cellular DNA damage. Environ Toxicol.
35:1170–1178. 2020.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Kanigür-Sultuybek G, Ozdas SB, Curgunlu A,
Tezcan V and Onaran I: Does metformin prevent short-term
oxidant-induced dna damage? In vitro study on lymphocytes from aged
subjects. J Basic Clin Physiol Pharmacol. 18:129–140.
2007.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Lee YS, Doonan BB, Wu JM and Hsieh TC:
Combined metformin and resveratrol confers protection against
UVC-induced DNA damage in A549 lung cancer cells via modulation of
cell cycle checkpoints and DNA repair. Oncol Rep. 35:3735–3741.
2016.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Sritawan N, Prajit R, Chaisawang P,
Sirichoat A, Pannangrong W, Wigmore P and Welbat JU: Metformin
alleviates memory and hippocampal neurogenesis decline induced by
methotrexate chemotherapy in a rat model. Biomed Pharmacother.
131(110651)2020.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Wessels JA, Huizinga TW and Guchelaar HJ:
Recent insights in the pharmacological actions of methotrexate in
the treatment of rheumatoid arthritis. Rheumatology (Oxford).
47:249–255. 2008.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Alqarni AM and Zeidler MP: How does
methotrexate work? Biochem Soc Trans. 48:559–567. 2020.PubMed/NCBI View Article : Google Scholar
|