Introduction
Esophageal achalasia, a typical esophageal
dyskinesia, is characterized by impaired relaxation of the lower
esophageal sphincter (LES), as well as abnormal peristaltic
movements of the esophageal body. Esophageal achalasia can impair
the ability to digest food and thus reduce a patient's quality of
life. Human herpes virus 1 encoded microRNAs have been identified
in biopsy samples of the LES muscle during peroral endoscopic
myotomy (POEM) for esophageal achalasia (1-3).
The disease was first reported ~300 years ago, but its underlying
cause remains unknown (4). Current
treatment strategies employ various methods, including endoscopic
balloon dilatation, botulinum toxin injection, laparoscopic Heller
myotomy, and surgical resection of the affected esophagus in
advanced and severe cases (5).
Recently, POEM has been established as a minimally invasive method
for treating achalasia (6). This
treatment is effective and safe, even in elderly patients, and
exhibits favorable short- and long-term prognoses (6,7). Sato
et al (8) performed peroral
endoscopic biopsies of the muscular layer termed POEM-b, with
histopathological and immunohistochemical analysis of POEM-b
samples showing signs of neurodegeneration rather than inflammatory
infiltration into the muscle layer (8). Based on the Chicago classification
criteria (9,10), high-resolution manometry revealed
that patients with type III achalasia tended to exhibit preserved
interstitial cells of Cajal, whereas patients with type I achalasia
were more likely to present with more severe fibrosis (8,11).
The proposed causative factors for achalasia are
diverse and multifactorial, with complex interactions between
autoimmune and inflammatory responses that can be initiated by
viral infections in genetically susceptible patients (2). One causative agent includes the herpes
simplex virus (HSV), a neurotrophic virus that predominates in
squamous epithelium, varicella-zoster virus, measles virus and
human papillomavirus (4,5,7,12). In addition, HSV type 1 (HSV 1) DNA
and RNA have been detected in the tissues of all patients with
achalasia, but not in the tissues of control subjects (13). Therefore, HSV 1 infection may be
significantly associated with the development and/or progression of
achalasia.
In our previous study, it was shown that the viral
agents hsv1-miR-H1 and hsv1-miR-H18, which are neurotropic
HSV-1-derived biomolecules, were overexpressed in the LES muscle of
Japanese achalasia cohorts (1).
Furthermore, ATG16L1 expression was lower, whereas interleukin
(IL)-1β expression was higher in the LES of patients with achalasia
compared with that of the control subjects. Therefore, ATG16L1 may
be targeted by hsv1-miR-H1, and the downregulation of ATG16L1 may
be related to the inflammatory response (14).
However, these data were collected from studies
conducted on LES tissues and not from the plasma of patients with
achalasia. Similarly, tissue cytokine analysis reports for patients
with achalasia are available. IL-17 is produced by 17 subsets of T
helper (Th) and is an essential mediator of autoinflammatory
diseases. The frequency of IL-17A-secreting cells in the intestinal
plexus of peripheral cells and esophageal tissue is reportedly
higher in patients with achalasia compared with the control
subjects (5,13). IL-4 is an anti-inflammatory cytokine
synthesized primarily by Th2 cells and it inhibits the synthesis of
IL-1β, tumor necrosis factor (TNF)-α, IL-6 and IL-17. Moreover,
patients with achalasia have a significantly higher proportion of
circulating and tissue IL-4+ cells compared with the
control subjects (5,13). IL-13 has exhibits similar functions
to that of IL-4, as well as regulating the type I collagen gene,
and it is therefore involved in fibrosis. Its expression pattern in
patients with achalasia is similar to that of IL-4 (5,13).
Furthermore, interferon (IFN)-γ released from Th1 cells is
essential for regulating immune responses. Patients with achalasia
have a significantly higher proportion of circulating and tissue
IFN-γ+/ CD4+ T cells compared with the
control subjects (5,13).
Cytokine storms are reportedly associated with a
variety of infectious and non-infectious diseases. COVID-19, which
is caused by SARS-CoV-2, is a potentially deadly disease that has
sparked a global pandemic. Blood cytokine and chemokine levels are
significantly higher in patients with COVID-19, including IL-1β,
IL-1ra, IL-7, IL-8, IL-9, IL-10, fibroblast growth factor-2 (FGF2),
colony-stimulating factor (CSF)3 [a granulocyte-macrophage
colony-stimulating factor (GM-CSF)], CSF2 (a GM-CSF), IFN-γ, CXCL10
(IP-10), chemokine (C-C motif) ligand 2 [CCL2 or monocyte
chemoattractant protein 1 (MCP1)], CCL3 (also known as MIP1α), CCL4
(also known as MIP1β), platelet-derived growth factor (PDGF)-BB,
TNF-α and vascular endothelial factor (VEGF) (15-17).
Some severe cases admitted to the intensive care unit exhibited
high levels of inflammatory cytokines, including IL-2, IL-7, IL-10,
CSF3, CXCL10, CCL2, CCL3 and TNF-α (15-17).
However, the concept of cytokine storms, as well as the biological
effects of cytokine overproduction, remain poorly defined.
As mentioned above, cytokines may be involved in the
inflammatory response. However, there are few reports on plasma
cytokine levels in patients with achalasia. In the present study,
the cytokine levels in the plasma of patients with achalasia before
and after POEM were measured, and compared with the values in
healthy control subjects.
Materials and methods
Ethical considerations
Written informed consent was obtained from all the
patients. The research protocol was approved by the Nagasaki
University Ethics Committee (approval no. 13012899) under the
ethical guidelines of the Declaration of Helsinki.
Peroral endoscopic muscular biopsy
sampling during POEM
The standard POEM procedure was performed as
previously described (6). Briefly,
the following steps were followed: Submucosal injection and
incision, submucosal tunneling, selective myotomy of the medial
circular muscle and subsequent closure of the mucosal entrance. All
patients who underwent POEM underwent endotracheal intubation with
general anesthesia and positive pressure ventilation, including
those who underwent surgery at Nagasaki University Hospital between
February 2013 and March 2014. Patients with any severe underlying
illnesses, such as cancer, patients who had undergone previous
surgical treatment and those who could not tolerate general
anesthesia were excluded. Patients were diagnosed with sporadic and
classical achalasia by routine analysis, including barium
follow-through and upper gastrointestinal endoscopy. Serum was
collected on the day of or the day before POEM, as well as ~90 days
after POEM (Table I). All samples,
including the control, were collected between February 2013 and
March 2016 at Nagasaki University Hospital. Plasma was stored at
-20˚C until required. Cytokine analysis was performed on plasma
collected from 10 healthy volunteers as controls (5 men and 5
women; age range, 37-54 years; median age, 44 years) and 12
patients with achalasia (4 men and 8 women, including 3 smokers;
age range, 28-85 years; median age, 57 years). According to
descriptive rules for achalasia of the esophagus (18), 4 patients presented with grade I, 7
patients had grade II and 1 patient had grade III achalasia.
 | Table IClinical characteristics of the 12
patients with achalasia. |
Table I
Clinical characteristics of the 12
patients with achalasia.
|
Characteristics | n |
|---|
| Age, years, median
(range) | 57 (28-85) |
| Sex, n (%) | |
|
Male | 4 (33.3) |
|
Female | 8 (66.7) |
| Smoking, n (%) | 3(25) |
| Grade, n (%) | |
|
1 | 4 (33.3) |
|
2 | 7 (58.3) |
|
3 | 1 (8.3) |
| Type, n (%) | |
|
Straight | 6(50) |
|
Sigmoid | 6(50) |
| Days after peroral
endoscopic; myotomy, median (range) | 97 (83-104) |
| Endoscopic balloon
dilatation, n (%) | 5 (41.7) |
| Body mass index,
median (range) | 20 (17-25) |
| Eckardt score,
median (range) | 9 (4-10) |
| Comorbidities, n
(%) | |
|
Diabetes | 1 (8.3) |
|
High blood
pressure | 3(25) |
|
Hyperuricemia | 1 (8.3) |
|
Down
syndrome | 1 (8.3) |
|
Psoriasis
vulgaris | 1 (8.3) |
|
Pneumonia | 1 (8.3) |
| White blood cell,
median (range) | 5,100
(3,600-11,300) |
| C-reactive protein,
mg/dl, mean (range) | 0.045
(0.01-6.67) |
Cytokine analysis
The Bio-Plex Pro™ Human Cytokine 27-plex assay kit
(cat. no. M500KCAF0Y) was purchased from Bio-Rad Laboratories, Inc.
The collected plasma samples were diluted 4-fold with the sample
diluent buffer. Antibody-bead conjugates were placed in the wells
of a 96 well microplate and incubated first with the diluted plasma
or the standard for 1 h at 20-25˚C with constant shaking, followed
by incubation with a biotin-labeled antibody for 30 min at 20-25˚C
and streptavidin-phycoerythrin conjugates for 10 min at 20-25˚C
(both after washing and shaking). The biotin-labeled antibody and
streptavidin-phycoerythrin conjugates were part of the assay kit.
After a further wash, the assay buffer was added to wells to
re-suspend the beads, and fluorescence was measured using an
automatic immunoassay analyzer (Bio-Plex® 200 System;
Bio-Rad Laboratories, Inc.). Finally, the cytokine concentration
was calculated from the standard curve.
Statistical analysis
Differences were analyzed using a Mann-Whitney U
test or a Dunn's test following a Kruskal-Wallis test. Statistical
analysis was performed using StatFlex version 7 (Artec Co., Ltd.).
A Shapiro-Wilk test using EZR version 1.54 (based on R version
4.1.0) was used to assess the distribution of the data. Data are
presented as box plots, with the minimum, 25th percentile, median,
75th percentile and maximum values forming the plots. P<0.05 was
considered to indicate a statistically significant difference.
Results
Patient characteristics
There was no significant differences in terms of age
between the patients and controls. However, a significant
difference in sex was observed in the expression of IL-7, IL-10,
IL-12, IL-17, FGF2, CSF3, CCL4 and VEGF; expression levels of these
cytokines were higher in females compared with males (Fig. S1A). Furthermore, serum IL-10 levels
in patients undergoing endoscopic balloon dilation before POEM were
higher than those in untreated patients (Fig. S1B). However, differences due to
other clinical patient characteristics were not observed.
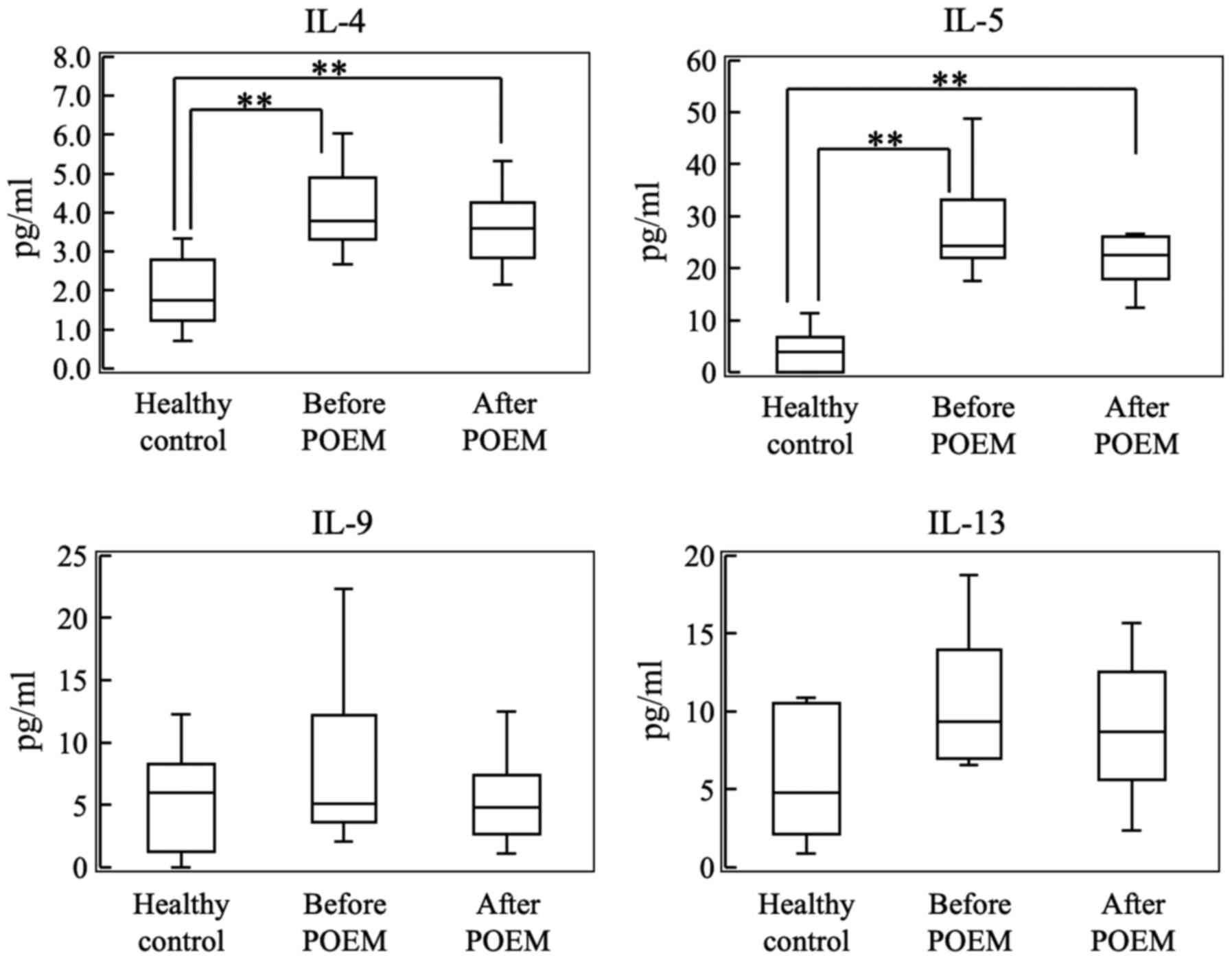
Cytokines are involved in the
differentiation and maintenance of Th17 cells
As shown in Fig. 1,
plasma levels of IL-17, IL-1β and CCL2 were significantly higher in
patients with achalasia compared with the control subjects.
However, plasma levels of TNF-α, IL-6 and IL-8 were not
significantly increased in patients with achalasia when compared
with those of the control subjects. Furthermore, there were no
differences in cytokine levels in the plasma collected before and
after POEM.
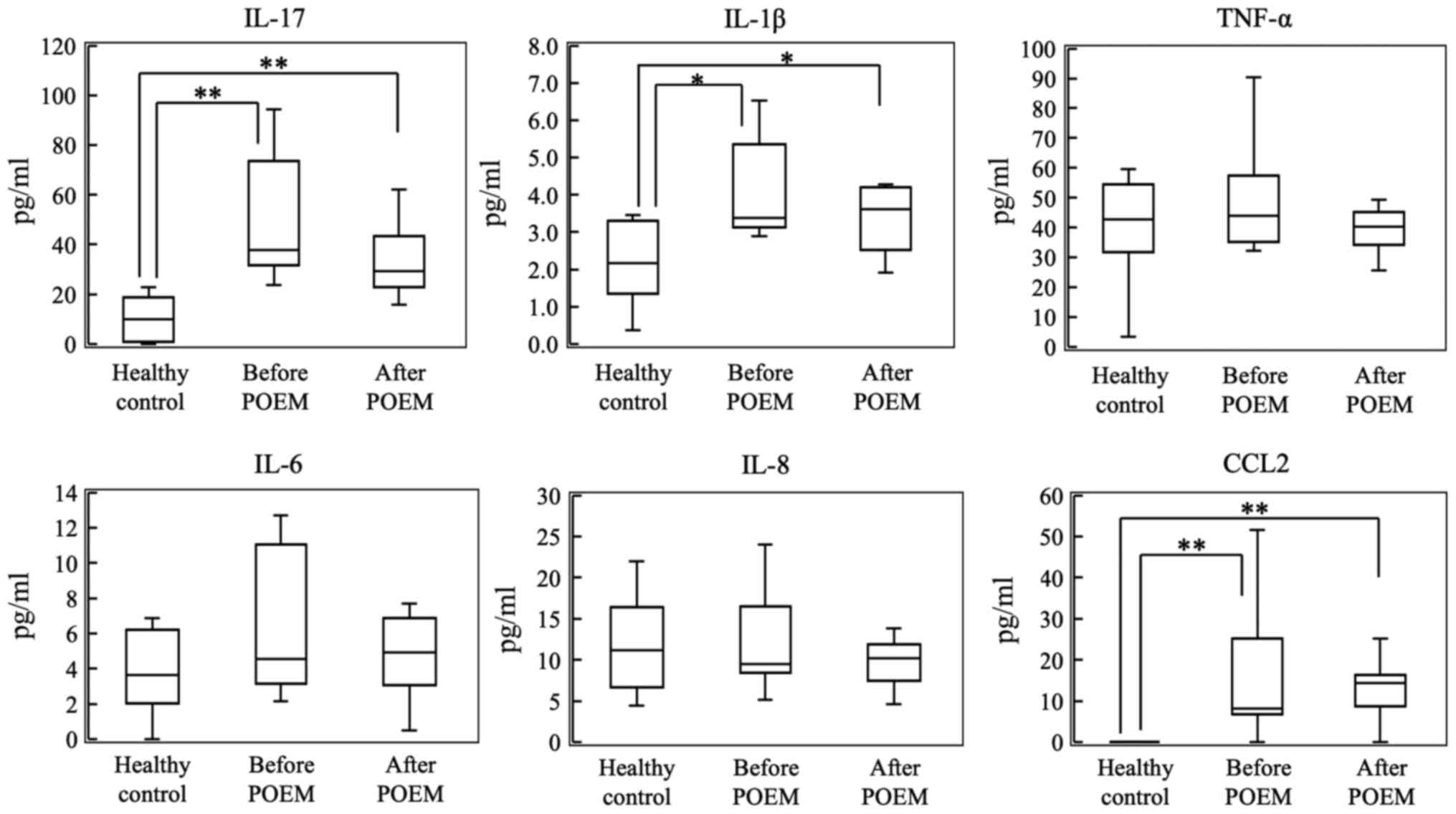 | Figure 1Plasma levels of IL-17, IL-1β, TNF-α,
IL-6, IL-8 and CCL2 in the control group, and before and after POEM
in the patients with achalasia. *P<0.05,
**P<0.01. POEM, peroral endoscopic myotomy; IL,
interleukin; TNF-α, tumor necrosis factor-α; CCL, chemokine (C-C
motif) ligand. |
Th2-derived cytokines
As shown in Fig. 2,
the plasma levels of IL-4 and IL-5 were significantly higher in the
plasma of achalasia patients compared with the control subjects.
However, plasma levels of IL-9 and IL-13 were not significantly
increased in patients with achalasia when compared with those in
the plasma of the control subjects. Furthermore, no differences in
cytokine levels were detected in the plasma collected before and
after POEM.
Cytokines are involved in the
differentiation and maintenance of Th1 cells
As shown in Fig. 3,
plasma levels of IL-1ra, IL-7, IL-12, IFN-γ and IL-2 were
significantly higher in patients with achalasia compared with the
control subjects. However, the plasma levels of CXCL10, IL-10 and
IL-15 did not significantly increase in patients with achalasia
when compared with those the control subjects. Furthermore, there
were no differences in cytokine levels in the plasma samples
collected before and after POEM.
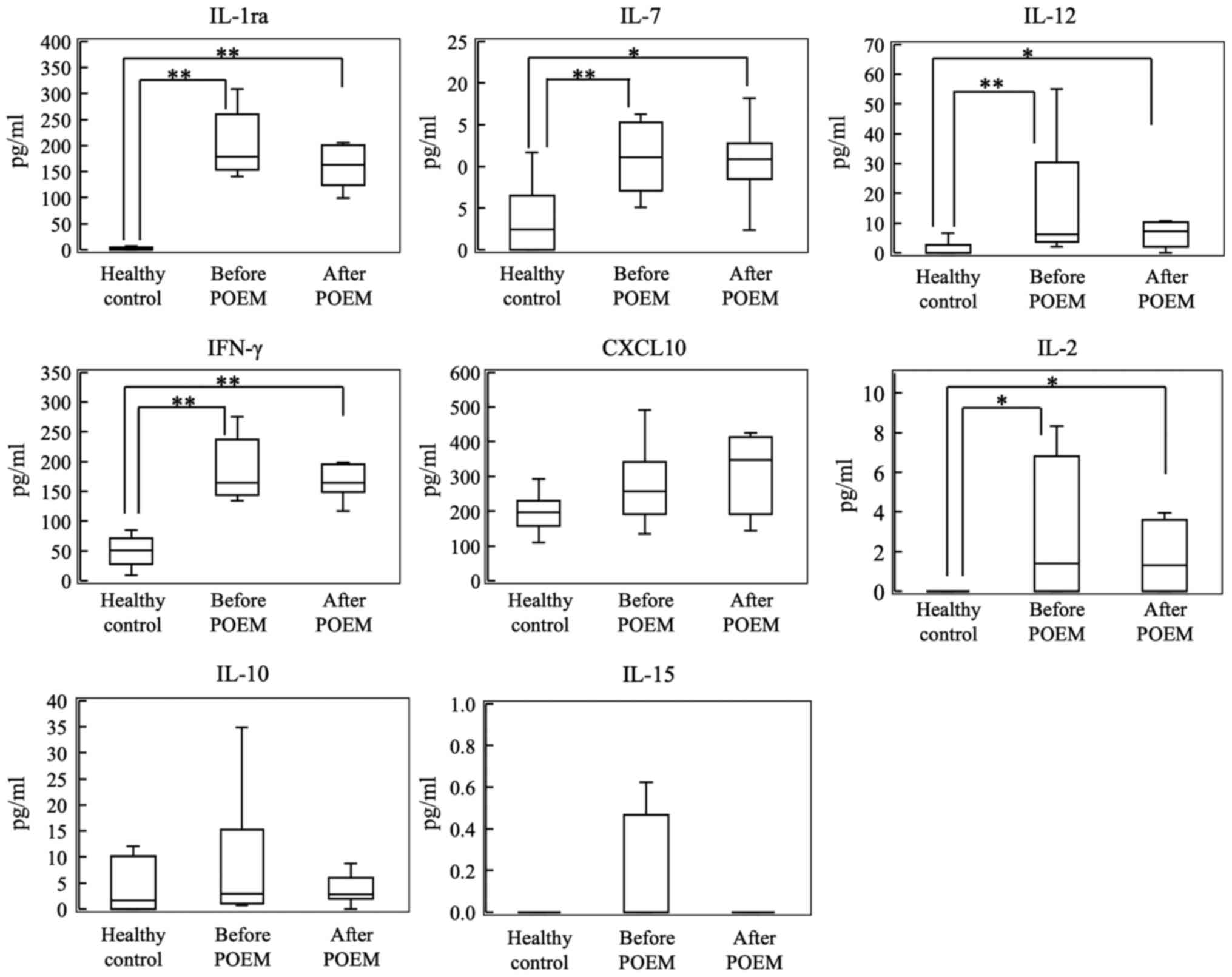 | Figure 3Plasma levels of IL-1ra, IL-7, IL-12,
IFN-γ, CXCL10, IL-2, IL-10 and IL-15 in the control group, and
before and after POEM in the patients with achalasia.
*P<0.05, **P<0.01. POEM, peroral
endoscopic myotomy; IL, interleukin; IFN-γ, interferon-γ. |
Growth factors and chemokines
As shown in Fig. 4,
plasma levels of FGF2, CSF2 and CSF3 levels were significantly
higher in patients with achalasia compared with the control
subjects. However, plasma levels of PDGF-BB, VEGF, CCL3, CCL4, CCL5
and CCL11 were not significantly increased in patients with
achalasia when compared with the control subjects. Furthermore, no
differences in cytokine levels were detected in the plasma samples
collected before and after POEM.
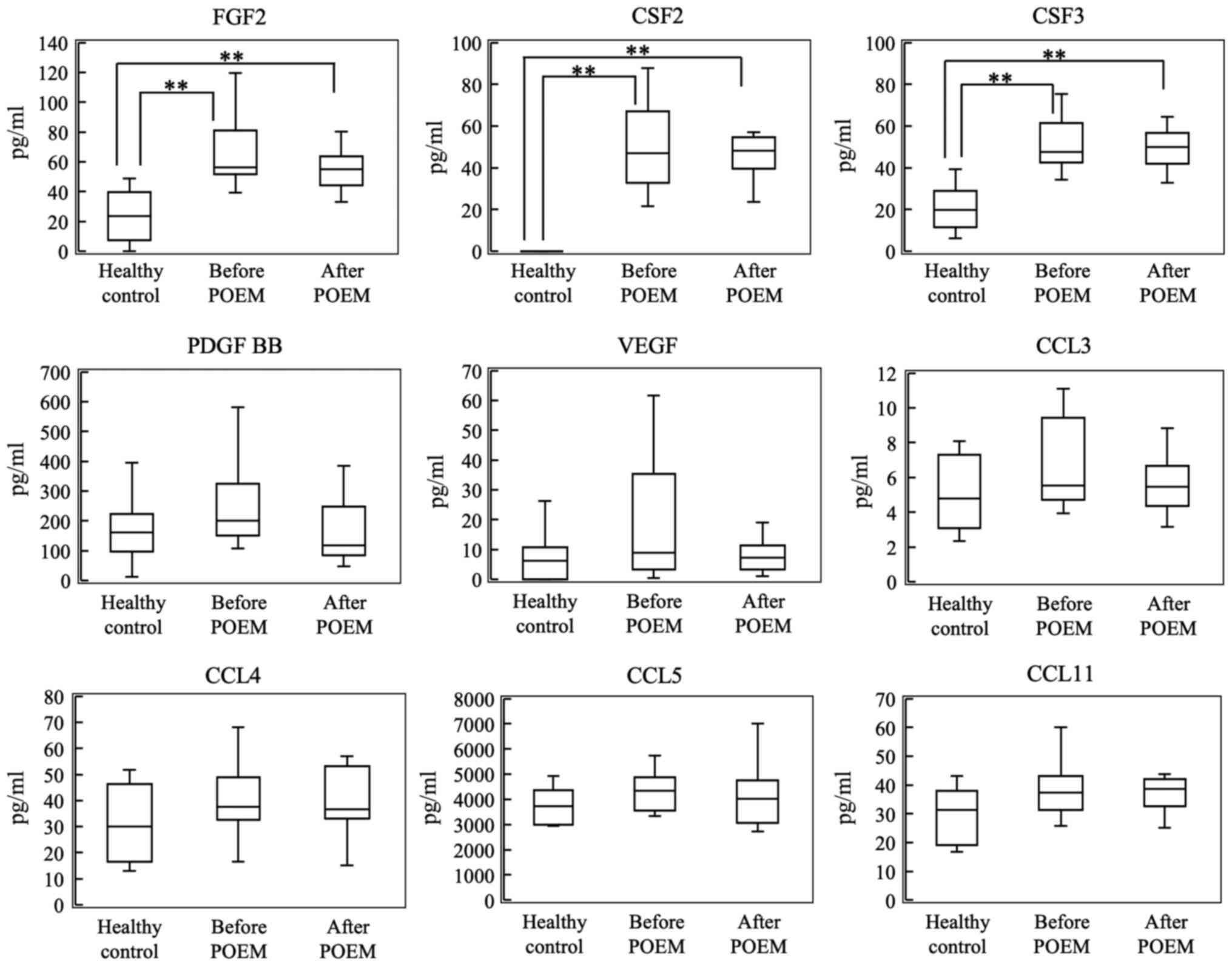 | Figure 4Plasma levels of FGF2, CSF2, CSF3,
PDGF BB, VEGF, CCL3, CCL4, CCL5 and CCL11 in the control group, and
before and after POEM in the patients with achalasia.
**P<0.01. POEM, peroral endoscopic myotomy; IL,
interleukin; FGF2, fibroblast growth factor 2; CSF, colony
stimulating factor; PDGF, platelet-derived growth factor; VEGF,
vascular endothelial growth factor; CCL, chemokine (C-C motif)
ligand. |
Discussion
The hypothesis developed in the present study
regarding the mechanism of cytokine storm induction is shown in
Fig. 5. IL-1β induced in LES
stimulates the immune and inflammatory systems. IL-1β activates
macrophages, polymorphonuclear leukocytes (PMNs) and T cells. These
cells activate viral clearance and induce inflammation. Activated T
cells, B cells and natural killer (NK) cells produce and release
cytokines, which re-activate macrophages, PMNs and T cells.
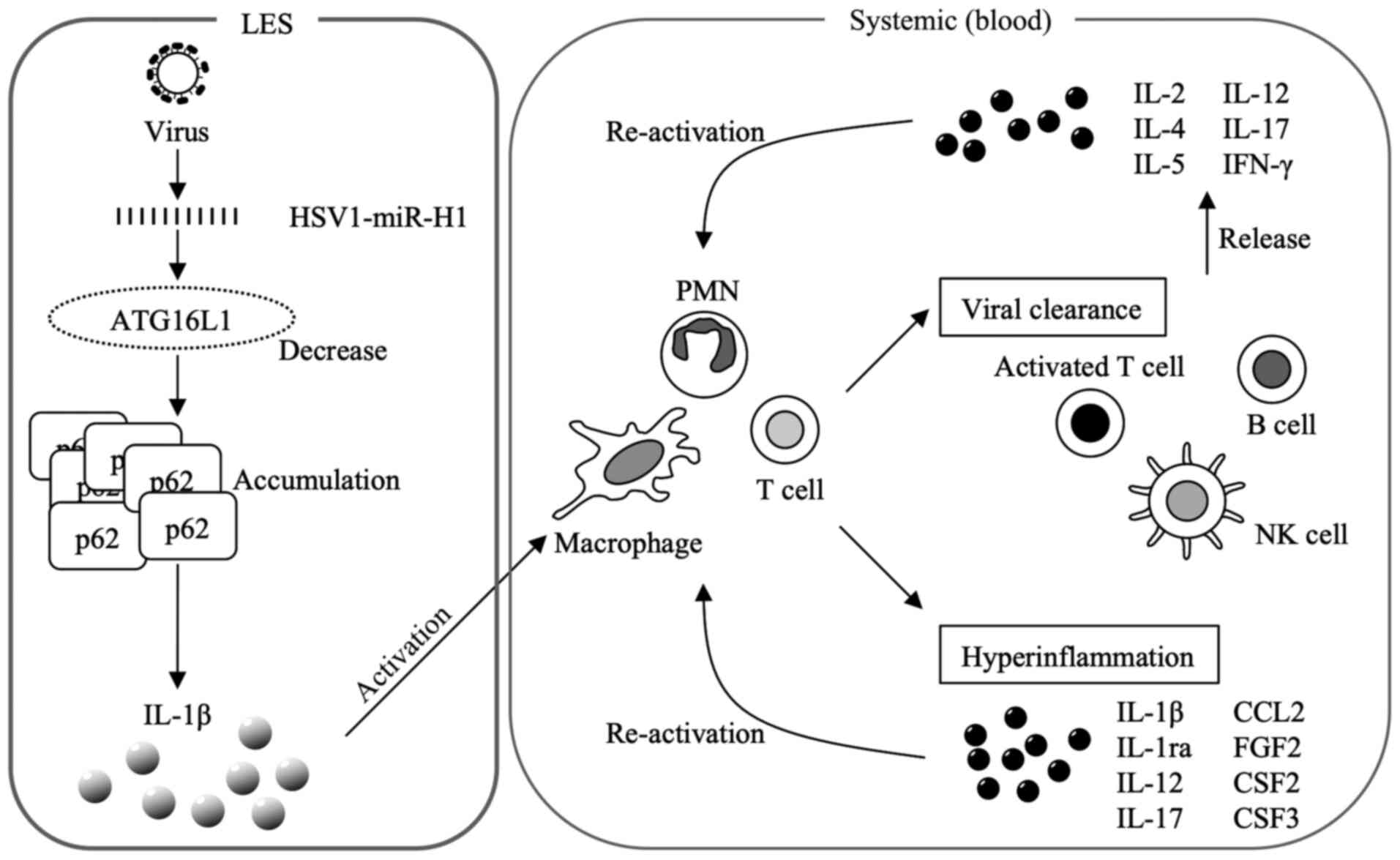 | Figure 5Mechanism of induction underlying the
cytokine storm. IL-1β activates macrophages, PMNs and T cells.
Macrophages, PMNs and T cells activate viral clearance and induce
hyperinflammation. The activated T, B and NK cells produce
cytokines. These released cytokines re-activate macrophages, PMNs
and T cells. IL, interleukin; PMN, polymorphonuclear leukocytes;
NK, natural killer; IFN-γ, interferon-γ; miR, microRNA; HSV herpes
simplex virus; CCL, chemokine (C-C motif) ligand; FGF2, fibroblast
growth factor 2; CSF, colony stimulating factor. |
The expression of IL-7, IL-10, IL-12, IL-17, FGF2,
CSF3, CCL4 and VEGF is higher in females than in males. Estrogen, a
female hormone, inhibits the production of Th1 pro-inflammatory
cytokines, such as IL-12, TNF-α and IFN-γ, and stimulates the
production of Th2 anti-inflammatory cytokines, such as IL-10, IL-4
and TGF-β (19). However, these
previous results differ from the results in the present. Thus,
there is a possibility of the influence of achalasia on cytokine
levels.
IL-17 is an essential mediator of inflammatory
autoimmune diseases. It promotes neutrophil recruitment and innate
immune cell activation, enhances B cell function and induces
inflammatory cytokines (IL-1β and TNF-α). Under physiological and
pathological conditions, IL-17 stimulates T cells and increases
autoantibody production and inflammatory cytokines (TNF-α, IL-1β,
IL-6, IL-8, IL-17 and IL- 22). Chemokines (CCL2, CCL7, CCL20, CXCL1
and CXCL5) induce neutrophil recruitment through chemokine
regulation, activate innate immune cells, and enhance B cell
function (20). Plasma levels of
IL-17, IL-1β and CCL2 were significantly higher in patients with
achalasia compared with the control subjects. These data were
consistent with the higher tissue expression levels of IL-1β in
patients with achalasia compared with the control subjects
(21). These findings suggest that
IL-17 induced the expression of Il-1β and CCL2, which is
characteristic of the cytokine storm induced by COVID-19, in-turn
increasing the expression of IL-1β, IL-8, CCL2 and TNF-α (15-17).
IL-4, which is synthesized primarily by Th2 cells,
is an anti-inflammatory cytokine. It inhibits the synthesis of
IL-17, IL-1β, TNF-α and IL-6, and regulates B cell proliferation
and differentiation. Furthermore, it is a potent inhibitor of
apoptosis. IL-4 is a pro-fibrogenic cytokine (22,23) that
may also contribute to the fibrous outcome observed in the LES
muscle of patients with achalasia.
In the present study, it was shown that IL-4 levels
were higher in patients with achalasia compared with the control
subjects. Interestingly, the levels of two conflicting cytokines
were elevated, exhibiting a crucial feature of a cytokine storm
(17,24,25).
TNF-α, IL-6 and IL-8 levels were not significantly increased when
compared with control plasma samples, which could be attributed to
upregulation of IL-4. However, further research is required to
confirm this relationship. Moreover, the IL-4-expressing
CD4+ Th2 subset is characterized by the production of
IL-4, IL-5, IL-9 and IL-13, and serves a role in type 2 immunity to
combat infectious diseases. These cells suppress autoimmune
diseases mediated by Th1 cells (26). IL-5 levels were higher in patients
with achalasia compared with the control subjects; the levels of
IL-13, which exhibit an IL-4 like function, were also higher.
However, IL-9 levels did not increase, dissimilar to that observed
in the cytokine storm induced by COVID-19 (15-17).
These data suggest the need to further investigate the cytokine
storm in patients with achalasia.
The anti-inflammatory cytokine, IL-1ra, inhibits
IL-1β, regulating various IL-1-related immune responses and
inflammatory responses, particularly during the acute phase of
infection and inflammation (27-29).
IL-7 is essential for B and T cell development and maintenance of
naïve and memory T cells (30-32).
IL-12 acts on T and NK cells, presents a broad array of biological
functions and is essential for the differentiation of both Th1 and
Th2 cells (33-36).
Another critical cytokine, IFN-γ, released by Th1 cells, is also
essential for regulating the Th2 response; it is implicated in
regulating the immune response (35,37,38).
Specifically, this cytokine recruits leukocytes to infected tissues
to potentiate beneficial inflammation and stimulate macrophages to
engulf and kill bacteria (39-41).
Moreover, IFN-γ induces the production of several chemokines, such
as CXCR3, CXCL9, CXCL10 and CXCL11(20). IL-2 is a secreted cytokine produced
by activated CD4+ and CD8+ lymphocytes, and
is crucial for T cell and B cell lymphocyte proliferation (42-44).
IL-1ra, IL-7, IL-12, IFN-γ and IL-2 levels were significantly
higher in the plasma of patients with achalasia compared with the
control subjects. These data were consistent with the higher tissue
expression levels of IL-2 detected in patients with achalasia
compared with the control subjects (21). Additionally, CXCL10, IL-10 and IL-15
levels were higher in patients with achalasia compared with the
control subjects. These data are characteristic of the cytokine
storm induced by COVID-19, in which, increased levels of IL-1ra,
IL-7, IFN-γ, CXCL10, IL-2 and IL-10 are observed (15-17).
However, it is presumed that the significantly higher serum IL-10
levels in patients who underwent endoscopic balloon treatment are
identical to those of IL-10, which serves an essential role in skin
wound healing (45).
The plasma levels of FGF2, CSF2 and CSF3 were
significantly higher in patients with achalasia compared with the
control subjects. Additionally, PDGF-BB, VEGF, CCL3, CCL4, CCL5 and
CCL11 levels were higher in patients with achalasia compared with
the control subjects. As above, these data are characteristic of
the cytokine storm induced by COVID-19, in which the levels of
FGF2, CSF2, CSF3, PDGF-BB, VEGF, CCL3 and CCL4 levels are increased
(15-17).
IL-22 belongs to the IL-10 superfamily, and is
involved in the innate immune response to intestinal epithelial and
respiratory cell pathogens, tissue repair/regeneration processes
and antibody production. Th22 and Th17 cells synthesize IL-22.
Transforming growth factor (TGF)-β1 regulates cell growth,
proliferation, differentiation, inflammation, collagen synthesis
and apoptosis. The number of IL-22+ and
TGF-β1+ cells observed in the myenteric plexus of
esophageal biopsies are significantly higher in patients with
achalasia than in control subjects (5,13).
Moreover, serum levels of IL-22, IL-17A and IFN-γ were higher in 27
patients with achalasia compared with those in healthy volunteers
(46). In the present study, IL-22
and TGF-β levels were not evaluated, as these cytokines could not
be detected using the ELISA kit employed. Therefore, IL-22 and
TGF-β levels in patients with achalasia remain to be
investigated.
Taking plasma cytokine measurements before and after
POEM is a novel approach not previously used in studies on
achalasia, to the best of our knowledge. However, no significant
differences in cytokine production before and after POEM were
observed in the present study. Indeed, it is expected that POEM,
which treats only part of the muscle, does not affect plasma
cytokine levels. As POEM is a symptomatic treatment to improve food
passage, cyclooxygenase-2 inhibitors are required for preventative
treatment and suppression of inflammation.
A limitation of the present study is that plasma
biomarker levels may not correctly reflect tissue inflammation in
the LES and esophageal mucosa. Furthermore, the sample size of
patients with achalasia and the number of cytokines that can be
detected with the ELISA kit employed may have limited the observed
associations. In particular, the plasma mRNA or protein levels of
ATG16L1 were not assessed as previously reported (14). However, the findings of this study
may encourage further investigation with larger cohorts and a
broader panel of biomarkers. Moreover, according to a previous
study, TGF-β1, TGF-β2, TGF-β3, IL-1ra, IL-17, IL-18, IFN-γ, MIG,
PDGF-BB, CXCL10 (also known as IP-10) and stem cell growth factor-B
levels are significantly higher in patients with achalasia compared
with control subjects (47). TGF-β2,
IL-1ra, IL-2ra, IL-18, MIG, IFN-γ, SDF-1a, CCL11 (also known as
eotaxin), PDGF-BB, CXCL10, CCL2 and TRAIL levels were significantly
higher in type III achalasia compared with type I/II achalasia
(47). It has been reported that
IL-6 levels are significantly higher in patients with achalasia
compared with patients with eosinophilic esophagitis (EoE), and
they do not differ amongst the three achalasia subtypes (48). Another limitation of the present
study is that no comparison was performed between achalasia
subtypes or between achalasia and other benign esophageal diseases
(especially EoE).
In conclusion, a cytokine storm can occur in
patients with achalasia; this knowledge may assist in improving our
understanding of this disease and in the development of a suitable
treatment.
Supplementary Material
Plasma levels of cytokine expression
before POEM. (A) Plasma levels of cytokine expression in females
and males. (B) Plasma levels of IL-10 expression in the controls,
and be patients had and had not received previous treatment.
*P<0.05. FGF2, fibroblast growth factor 2; CSF3,
colony stimulating factor 3; CCL, chemokine (C-C motif) ligand; IL,
interleukin; VEGF, vascular endothelial growth factor; POEM,
peroral endoscopic myotomy; +, had received previous treatment; -,
had not received previous treatment.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are included in the published article.
Authors' contributions
TK and AY substantially contributed to the
acquisition, analysis and interpretation of data, and drafted the
manuscript. KO, HM, NY and YI substantially contributed to the
acquisition, analysis, and interpretation of data. KN contributed
to the conception and design of the study. HI made substantial
contributions to the conception and design of the study, and to
drafting of the manuscript. All authors have read and approved the
final manuscript. TK and AY confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
The study protocol used in the present study
followed the ethical guidelines of the Declaration of Helsinki and
was approved by the Nagasaki University Ethics Committee (approval
no. 13012899). Written informed consent was obtained from all
patients for participation in the present study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ikebuchi Y, Kanda T, Ikeda H, Yoshida A,
Sakaguchi T, Urabe S, Minami H, Nakao K, Kuwamoto S, Inoue H, et
al: Identification of human herpes virus 1 encoded microRNAs in
biopsy samples of lower esophageal sphincter muscle during peroral
endoscopic myotomy for esophageal achalasia. Dig Endosc.
32:136–142. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kahrilas PJ and Boeckxstaens G: The
spectrum of achalasia: Lessons from studies of pathophysiology and
high-resolution manometry. Gastroenterology. 145:954–965.
2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Minami H, Isomoto H, Miuma S, Kobayashi Y,
Yamaguch N, Urabe S, Matsushima K, Akazawa Y, Ohnita K, Takeshima
F, et al: New endoscopic indicator of esophageal achalasia:
‘Pinstripe pattern’. PLoS One. 10(e0101833)2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ghoshal UC, Daschakraborty SB and Singh R:
Pathogenesis of achalasia cardia. World J Gastroenterol.
18:3050–3057. 2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Furuzawa-Carballeda J, Torres-Landa S,
Valdovinos MÁ, Coss-Adame E, Martín Del Campo LA and
Torres-Villalobos G: New insights into the pathophysiology of
achalasia and implications for future treatment. World J
Gastroenterol. 22:7892–7907. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Inoue H, Shiwaku H, Iwakiri K, Onimaru M,
Kobayashi Y, Minami H, Sato H, Kitano S, Iwakiri R, Omura N, et al:
Clinical practice guidelines for peroral endoscopic myotomy. Dig
Endosc. 30:563–579. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Isomoto H and Ikebuchi Y: Japanese
guidelines for peroral endoscopic myotomy: 1st edition. Dig Endosc.
31:27–29. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Sato H, Inoue H, Ikeda H, Sato C, Santi
EGR, Phalanusitthepha C, Aoyagi Y and Kudo SE: In vivo
histopathological assessment of the muscularis propria in achalasia
by using endocytoscopy (with video). Endosc Int Open. 2:E178–E182.
2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Bredenoord AJ, Fox M, Kahrilas PJ,
Pandolfino JE, Schwizer W and Smout AJ: International High
Resolution Manometry Working Group. Chicago classification criteria
of esophageal motility disorders defined in high resolution
esophageal pressure topography. Neurogastroenterol Motil. 24 (Suppl
1):57–65. 2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kahrilas PJ, Bredenoord AJ, Fox M, Gyawali
CP, Roman S, Smout AJ, Pandolfino JE, Bhatia S, Boeckxstaens G, Bor
S, et al: International High Resolution Manometry Working Group:
The Chicago Classification of esophageal motility disorders, v3.0.
Neurogastroenterol Motil. 27:160–174. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Nakajima N, Sato H, Takahashi K, Hasegawa
G, Mizuno K, Hashimoto S, Sato Y and Terai S: Muscle layer
histopathology and manometry pattern of primary esophageal motility
disorders including achalasia. Neurogastroenterol Motil. 29:1–8.
2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Pressman A and Behar J: Etiology and
pathogenesis of idiopathic achalasia. J Clin Gastroenterol.
51:195–202. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Furuzawa-Carballeda J, Aguilar-León D,
Gamboa-Domínguez A, Valdovinos MA, Nuñez-Álvarez C,
Martín-del-Campo LA, Enríquez AB, Coss-Adame E, Svarch AE,
Flores-Nájera A, et al: Achalasia - an autoimmune inflammatory
disease: A cross-sectional study. J Immunol Res.
2015(729217)2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kanda T, Yoshida A, Ikebuchi Y, Ikeda H,
Sakaguchi T, Urabe S, Minami H, Nakao K, Inoue H and Isomoto H:
Autophagy-related 16-like 1 is influenced by human herpes virus
1-encoded microRNAs in biopsy samples from the lower esophageal
sphincter muscle during per-oral endoscopic myotomy for esophageal
achalasia. Biomed Rep. 14(7)2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Nile SH, Nile A, Qiu J, Li L, Jia X and
Kai G: COVID-19: Pathogenesis, cytokine storm and therapeutic
potential of interferons. Cytokine Growth Factor Rev. 53:66–70.
2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Rothan HA and Byrareddy SN: The
epidemiology and pathogenesis of coronavirus disease (COVID-19)
outbreak. J Autoimmun. 109(102433)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu
Y, Zhang L, Fan G, Xu J, Gu X, et al: Clinical features of patients
infected with 2019 novel coronavirus in Wuhan, China. Lancet.
395:497–506. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Japan Esophageal Society. Descriptive
rules for achalasia of the esophagus, June 2012: 4th Edition.
Esophagus. 14:275–289. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Salem ML: Estrogen, a double-edged sword:
Modulation of TH1- and TH2-mediated inflammations by differential
regulation of TH1/TH2 cytokine production. Curr Drug Targets
Inflamm Allergy. 3:97–104. 2004.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Dardalhon V, Korn T, Kuchroo VK and
Anderson AC: Role of Th1 and Th17 cells in organ-specific
autoimmunity. J Autoimmun. 31:252–256. 2008.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Facco M, Brun P, Baesso I, Costantini M,
Rizzetto C, Berto A, Baldan N, Palù G, Semenzato G, Castagliuolo I,
et al: T cells in the myenteric plexus of achalasia patients show a
skewed TCR repertoire and react to HSV-1 antigens. Am J
Gastroenterol. 103:1598–1609. 2008.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Huaux F, Liu T, McGarry B, Ullenbruch M
and Phan SH: Dual roles of IL-4 in lung injury and fibrosis. J
Immunol. 170:2083–2092. 2003.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Nguyen JK, Austin E, Huang A, Mamalis A
and Jagdeo J: The IL-4/IL-13 axis in skin fibrosis and scarring:
Mechanistic concepts and therapeutic targets. Arch Dermatol Res.
312:81–92. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Fajgenbaum DC and June CH: Cytokine storm.
N Engl J Med. 383:2255–2273. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Yiu HH, Graham AL and Stengel RF: Dynamics
of a cytokine storm. PLoS One. 7(e45027)2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wynn TA: Type 2 cytokines: Mechanisms and
therapeutic strategies. Nat Rev Immunol. 15:271–282.
2015.PubMed/NCBI View
Article : Google Scholar
|
|
27
|
Dinarello CA: Overview of the IL-1 family
in innate inflammation and acquired immunity. Immunol Rev.
281:8–27. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhang J-M and An J: Cytokines,
inflammation and pain. Int Anesthesiol Clin. 69:482–489. 2009.
|
|
29
|
Martin P, Goldstein JD, Mermoud L,
Diaz-Barreiro A and Palmer G: IL-1 family antagonists in mouse and
human Skin inflammation. Front Immunol. 12(652846)2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Fry TJ and Mackall CL: The many faces of
IL-7: From lymphopoiesis to peripheral T cell maintenance. J
Immunol. 174:6571–6576. 2005.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Surh CD and Sprent J: Homeostasis of naive
and memory T cells. Immunity. 29:848–862. 2008.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Chetoui N, Boisvert M, Gendron S and
Aoudjit F: Interleukin-7 promotes the survival of human
CD4+ effector/memory T cells by up-regulating Bcl-2
proteins and activating the JAK/STAT signalling pathway.
Immunology. 130:418–426. 2010.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Vignali DA and Kuchroo VK: IL-12 family
cytokines: Immunological playmakers. Nat Immunol. 13:722–728.
2012.PubMed/NCBI View
Article : Google Scholar
|
|
34
|
Lu X: Impact of IL-12 in Cancer. Curr
Cancer Drug Targets. 17:682–697. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Zhang Y, Zhang Y, Gu W and Sun B: TH1/TH2
cell differentiation and molecular signals. Adv Exp Med Biol.
841:15–44. 2014.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Seder RA: The role of IL12 in the
regulation of Th1 and Th2 differentiation. Res Immunol.
146:473–476. 1995.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Kaiko GE, Horvat JC, Beagley KW and
Hansbro PM: Immunological decision-making: How does the immune
system decide to mount a helper T-cell response? Immunology.
123:326–338. 2008.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Schoenborn JR and Wilson CB: Regulation of
interferon-gamma during innate and adaptive immune responses. Adv
Immunol. 96:41–101. 2007.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Chaplin DD: Overview of the immune
response. J Allergy Clin Immunol. 125 (Suppl 2):S3–S23.
2010.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Schroder K, Hertzog PJ, Ravasi T and Hume
DA: Interferon-γ: An overview of signals, mechanisms and functions.
J Leukoc Biol. 75:163–189. 2004.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Bastos KR, Barboza R, Sardinha L, Russo M,
Alvarez JM and Lima MR: Role of endogenous IFN-γ in macrophage
programming induced by IL-12 and IL-18. J Interferon Cytokine Res.
27:399–410. 2007.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Bachmann MF and Oxenius A: Interleukin 2:
From immunostimulation to immunoregulation and back again. EMBO
Rep. 8:1142–1148. 2007.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Ross SH and Cantrell DA: Signaling and
function of interleukin-2 in T lymphocytes. Annu Rev Immunol.
36:411–433. 2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Lipsky PE, Hirohata S, Jelinek DF,
McAnally L and Splawski JB: Regulation of human B lymphocyte
responsiveness. Scand J Rheumatol Suppl. 76 (Suppl 76):229–235.
1988.PubMed/NCBI View Article : Google Scholar
|
|
45
|
King A, Balaji S, Le LD, Crombleholme TM
and Keswani SG: Regenerative wound healing: The role of
interleukin-10. Adv Wound Care (New Rochelle). 3:315–323.
2014.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Furuzawa-Carballeda J, Coss-Adame E,
Romero-Hernández F, Zúñiga J, Uribe-Uribe N, Aguilar-León D,
Valdovinos MA, Núñez-Álvarez CA, Hernández-Ramírez DF,
Olivares-Martínez E, et al: Esophagogastric junction outflow
obstruction: Characterization of a new entity? Clinical,
manometric, and neuroimmunological description. Neurogastroenterol
Motil. 32(e13867)2020.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Chen WF, Liu ZQ, Pu ZN, Xu JQ, Yao L, Wu
XY, Xu XY, Xu JX, Zhu Y, Wang Y, et al: Multiplex immunoassays
reveal increased serum cytokines and chemokines associated with the
subtypes of achalasia. Neurogastroenterol Motil.
32(e13832)2020.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Clayton S, Cauble E, Kumar A, Patil N,
Ledford D, Kolliputi N, Lopes-Virella MF, Castell D and Richter J:
Plasma levels of TNF-α, IL-6, IFN-γ, IL-12, IL-17, IL-22, and IL-23
in achalasia, eosinophilic esophagitis (EoE), and gastroesophageal
reflux disease (GERD). BMC Gastroenterol. 19(28)2019.PubMed/NCBI View Article : Google Scholar
|