Arthritis is an umbrella term used to refer to
diseases that cause pain and inflammation of the joints, and it
characterized by painful inflammation and stiffness of the joints
(1). Osteoarthritis (OA) is one of
the most commonly diagnosed types of arthritis, and it is
considered a chronic, debilitating and prevalent joint disease,
accounting for ~23% of all cases of musculoskeletal disorders,
according to the Global Burden Disease study 2017(2). In brief, OA occurs due to the loss of
articular cartilage within the synovial joints with the natural
propensity to occur in elderly individuals (3,4). The
high count of years lived with disability of patients with OA makes
it one of the leading causes of disability, where patients with
advanced-stage OA tend to experience chronic pain and functional
impairment of the limbs, thus resulting in a poor quality of life
(1).
Generally, a healthy joint possesses a layer of
slippery tissue known as the articular cartilage, which is
comprised primarily of chondrocytes and extracellular matrix (ECM).
The ECM is predominantly made up of proteoglycan and type II
collagen fiber (5). With several
roles in the musculoskeletal system, the articular cartilage
lubricates the bones during angular movements and absorbs shock to
prevent the bones from impacting one another. This involves the
spreading of the load evenly across the joints during
weight-bearing activities (such as walking and weight-lifting), as
well as high-intensity activities (such as running and jumping).
Additionally, the articular cartilage also acts as a reservoir that
stores synovial fluid; a fluid that transports nutrients to the
joints (1).
Cartilage can be damaged through several factors,
such as injuries as well as autoimmune diseases including
rheumatoid arthritis (6). However,
it can also be damaged from wear-and-tear over time. Typically, the
occurrence of wear-and-tear are often counteracted by the repair
and renewal of articular cartilage. However, the regenerative
capacity is dependent on several aspects, including genetic
background, age, sex, body weight and the level of physical
activity an individual partakes in (7). When the cartilage damage outweighs the
regenerative capacity of the body, thinning of the articular
cartilage occurs followed by a progressive loss of articular
cartilage. Under such circumstances, the individual will thus be
diagnosed with OA (5).
OA is also referred to as degenerative arthritis due
to its tendency to develop as a person ages, as well as the fact
that it is characterized by the loss of the cartilage that leads to
constant friction followed by the eventual deformation of the bones
(1,8). In such events, the patient will
experience inflammation and subsequently, pain and stiffness to the
joints (7,8). OA can affect any joint in the body, but
often occurs in the knees, small joints of the fingers, lower back,
neck and hips (9). Though it was
typically accepted that it occurs as part of the aging process,
there are other causes of OA including congenital bone deformities,
joint overuse, traumatic injuries, obesity and genetic diseases,
such as Paget's disease and diabetes (7,10,11).
The primary process that underlies the development
of OA is the substantial degeneration of the structures within the
articular cartilage, thus causing severe pain and reduced mobility.
Unfortunately, the treatment options available for patients with OA
are palliative measures rather than curative. Symptom management,
with a focus on halting or slowing the progression of OA range from
physical and nonsurgical therapies to surgery, including: i)
Exercise programs for muscle strengthening and weight loss, and the
use of supporting devices such as braces; ii) pharmacological
interventions to alleviate pain; and iii) surgical interventions
(12,13). However, there are certain challenges
and limitations to all of these approaches as discussed in the
following subsections.
A basic attempt to treat OA involves the
introduction of an exercise program to strengthen the muscles
surrounding the affected joints, and to promote weight loss if
required. With regards to knee OA, where the knees act as the
pillar of support to the human body, a study demonstrated the
association between muscle weakness, particularly the quadriceps,
and the development of knee OA (14). In addition to weak muscle strength,
obesity which is suggested to be secondary to inactivity, is
well-established to favor the development of knee OA through
increased leverage, whereby the risk of knee OA is reported to
increase by 36% with every 2 units of body mass index (BMI) gained,
and the likelihood of developing knee OA by 4.2x in individuals
with a BMI >30 kg/m2 (15,16).
Hence, it was recommended that patients with OA increase the amount
of exercise they do, such as weight lifting and strength training
to increase muscle strength, as this has been proven to reduce pain
and improve physical function, as well as aid in weight-loss
(17-20).
However, the pain and physical restrictions that come with OA often
act as hurdles that keep OA patients from implementing and
sustaining such activities. In addition, maintaining a healthy
weight is another challenge faced by patients with OA, as it
requires (often substantial) changes to their lifestyle, long term
determination, and commitment to achieve noticeable results.
The general purpose of the braces is to provide
support, as well as align and immobilize the area of the affected
the joint (21). They prevent and
correct deformities, thereby improving function and assisting in
slowing the progression of the disease (22). There are several categories of braces
available for various purposes. The unloader knee brace is used
specifically for patients with OA to alleviate pain and improve
physical function. Other categories of braces include the
prophylactic knee brace, which is used to provide protection of the
healthy knees against injuries during athletic activities; the
patellofemoral knee brace which is used for anterior knee pain; and
the functional knee brace is used to improve stability of an
unstable knee in ligament injuries, such as a torn anterior
cruciate ligament (ACL) or post-ACL reconstruction (22). Studies have reported that the use of
braces has beneficial effects for patients with OA by reducing the
pain they experience, improving the physical function of the
affected joint, as well as delaying the need for surgical
interventions (23,24). However, despite the benefits of these
braces, it is only able to provide short-term pain relief, and are
inefficient for long-term management (25). Furthermore, the efficacy of the knee
braces varies between patients with OA; as indicated in certain
studies, the use of braces lacks symptomatic relief, may fit
poorly, and may cause discomfort when wearing the braces as well as
skin irritation (26,27).
The use of pharmacological treatments for OA is
often considered as a supplement in cases of severe OA, following
failure to relieve symptoms by non-pharmacological methods
(28). Some of the drugs used are
analgesics, such as acetaminophen and opioids, NSAIDs and COX-2
inhibitors (29). Although the
complementary usage of drugs and non-pharmacological regimen has
been shown to be most effective for pain management of OA, there
are safety concerns regarding the adverse effects of the drugs on
the human body, such as liver toxicity as well as renal,
cardiovascular and gastrointestinal side effects (29).
Surgery is considered as the final resort, when both
pharmacological and non-pharmacological regimens fail to relieve
the symptoms in patients with severe OA, particularly when their
joints have entered a state of severe damage, causing unbearable
pain, as well as deterioration of function in the affected patient.
The types of surgical treatments for OA include arthroscopic lavage
and debridement, cartilage repair, osteotomies and joint
arthroplasty (30). Arthroscopic
lavage and debridement are often performed as an initial surgical
option that entails the removal of fragments of the meniscus, loose
cartilage or osteophytes, and shaving of rough cartilage, and has
been shown to alleviate pain and improve physical function
(31). However, it has been
demonstrated in randomized controlled trials that arthroscopic
surgery was no more effective than a placebo surgery for treating
knee OA. In the early 2000's, two seminal studies by Kirkley et
al (32) and Moseley et
al (33), made an impact on the
use of arthroscopy for OA, in which the authors reported that
patients with OA who received arthroscopy lavage and debridement
did not show any improvements in the pain score and physical
functions compared with a placebo group who underwent a sham
surgery, a group that received optimized therapy, and a group that
underwent physical therapy. The publication of these two landmark
studies was followed by the update of the guidelines for the
treatment of OA by the United Kingdom's National Institute for
Health and Care Excellence, which no longer recommends arthroscopy
as a treatment for OA (1).
The final resort for a patient with OA who has
progressed to the most severe grade would be a total joint
replacement. However, the procedure for joint replacements may
cause unbearable pain and requires a long duration for
rehabilitation. The adverse outcomes observed in patients who
undergo total knee replacement surgery include myocardial
infarction, infections and pulmonary embolism (34).
The inconsistency of palliative treatments for OA
highlight the need for a more reliable and curative approach that
targets the root cause of OA; the degeneration of articular
cartilage. Hence, the notion of stem cell therapy has galvanized
intensive investigation into its potential use for treatment of OA,
due to its regenerative properties. Owing to their excellent
self-renewing capacity as well the ability to differentiate into
>200 cell types, the use of stem cells in cellular therapy has
ushered in an exciting new epoch for the fields of regenerative
medicine with grounds for optimism to address the present unmet
medical needs to treat a variety of degenerative diseases,
including OA (35). At present,
three types of stem cells are commonly studied with regard to stem
cell therapy: Embryonic stem cells (ESCs), induced pluripotent stem
cells (iPSCs), and adult stem cells, and these are discussed
below.
ESCs are considered to be totipotent stem cells
derived from the fertilized zygote cell, wherein the embryo is
usually 4-5 days old (35,36). The use of embryonic stem cells has
generated ethical concerns, particularly with regard to how they
are obtained (37). Hence, it is
restricted for use in biomedical research only, and is to date,
illegal for use as a treatment of any diseases, as their remains a
notable bone of contention over the considerable ethical issues
that arise, given that an embryo must be aborted to obtain the ESCs
(37,38).
As one of the major revolutions in stem cell
research, the identification and interest in research on iPSCs was
spurred on by the ethical issues raised over the sourcing of ESCs
(39,40). iPSCs were first discovered in 2006 by
Takahashi and Yamanaka (39) who
successfully reprogrammed the terminally differentiated fibroblast
to an iPSC via introduction of four transcription factors, the so
called Yamanaka factors; Sox2, Oct3/4, Klf4 and c-Myc (39,41).
Similar to ESCs, iPSCs exhibit a high degree of pluripotency, with
the additional benefit of circumventing the ethical concerns
regarding the use of ESCs.
Despite its initial promise as a potential
substitute for ESCs however, the transition to iPSC research for
clinical applications highlighted several obstacles inherent to the
use of iPSCs for cellular therapy, which includes genomic
instability, immunogenicity, teratoma formation and clonal
variations amongst iPSCs derived from the same donor cells, thus
raising major concerns over the safety of their use clinically
(40,42,43).
Adult stem cells are usually found in differentiated
cells of specific tissues after birth, and are further categorized
into hematopoietic stem cells (HSCs) and mesenchymal stem cells
(MSCs) (44). The regeneration of
the damaged tissues using adult stem cells was greeted as a
breakthrough in relatively recent years, and has exhibited
encouraging outcomes when used for treatment of several chronic
degenerative conditions, such as degenerative disc disease,
Parkinson's disease and amyotrophic lateral sclerosis. Numerous
studies have demonstrated the safety and efficacy of adult stem
cells for the treatment of several diseases (45-47).
HSCs are the building blocks for the production of
blood cells, including the erythrocyte and leukocyte lineages, as
well as platelets (48,49). HSCs are found in the bone marrow and
the umbilical cord, and are now primarily used for the treatment of
the majority of disorders of blood cells, including primary immune
deficiencies, congenital cytopenia, and storage and metabolic
disorders (50,51). The transplantation of HSCs has been
shown to ameliorate bone lesions, a decline in cognitive and
central nervous function, as well as improving the survival of
children diagnosed with Hurler's syndrome (the most severe form of
mucopolysaccharidoses) (52).
Furthermore, the use of HSCs has been deemed to be curative in the
treatment of sickle cell diseases (53). There have been attempts to assess the
efficacy of HSCs for the treatment of OA, wherein a study by
Abdelmoaty et al (54) showed
that patients who received repeated injections of autologous
peripheral blood stem cells experienced improvements in physical
function as well as improved articular cartilage quality. At
present, the only FDA-approved stem cell products consist of
hematopoietic progenitor cells that are derived from umbilical cord
blood, solely for the treatment of blood disorders involving the
hematopoietic system (55).
MSCs are multipotent stem cells that are found
ubiquitously throughout the musculoskeletal system in the human
body, and can differentiate into various cell types including, but
not limited to, osteoblasts, chondrocytes, adipocytes, astrocytes
and cardiomyocytes (56-61).
They can be isolated from the HSCs based on their ability to adhere
readily to the plastic surfaces of tissue culture plates (56). MSCs can be derived from various
tissues in the body, including bone marrow, adipose tissue,
synovium, umbilical cord blood, dental pulp, amniotic fluid, dermis
and peripheral blood (62-67).
The characterization of MSCs are defined based on a guideline
proposed by the International Society for Cellular Therapy (ISCT);
the minimum criteria being that cells exhibit expression of
specific markers, including CD44, CD90 and CD105, but lack
expression of CD34, DC45 and CD133, as these are markers of HSCs
(68). The revelation of the
intrinsic nature of MSCs to regenerate and differentiate into
chondrocytes has increased interest in the investigation of MSCs,
and they show potential as an excellent alternative treatment
option for OA. One study that used bone marrow aspirates in
treating knee OA has successfully entered clinical trial phase 4,
and was registered in ClinicalTrials.gov (Identifier, NCT03289416). In this
clinical trial, undifferentiated cells found in the bone marrow
aspirate concentrate (BMA) were shown to promote healing of damaged
tissue, and aid in growth, repair and tissue regeneration. Whereas
the full benefits of BMA remain to be elucidated, studies have
shown that this treatment can relieve pain, and improve healing in
articular cartilage and bone grafts (69). Although MSCs can be derived from
numerous tissues in the body as mentioned previously, the two types
of MSCs that are widely studied for the treatment of OA are bone
marrow-derived MSCs (BMSCs) and adipose-derived stem cells (ASCs)
(70,71).
BMSCs are a population of fibroblast-like cells that
reside in the stroma of the bone marrow (66). These MSCs were initially isolated
from the bone marrow aspirate of the iliac crest, before the
subsequent emergence of MSCs derived from other tissues, such as
adipose tissue, umbilical cord and amniotic fluid (72,73).
Currently, BMSCs are regarded as the gold standard and remain the
most frequently investigated cell type, as they are hypothesized to
possess higher potential for chondrogenic differentiation (70). There are 58 registered clinical
trials in Clinicaltrials.gov (as of March
2021), on the use of BMSCs for treatment of osteoarthritis using
the key words ‘osteoarthritis’ and ‘bone marrow stem cells’.
However, only a handful of studies have been completed and have
published their results (74-81).
ASCs are stem cells isolated from adipose tissues.
They were first identified as an MSC in 2001(82), following which, they have been widely
studied for their potential therapeutic value in regenerative
medicine and tissue engineering (63,83-85).
Although ASCs exhibit similarities to the BMSCs, there are several
distinct characteristics between these 2 types of stem cells, such
as their differentiation potential and the complement of cell
surface markers. BMSCs express CD106 (a marker that is involved in
MSCs-mediated immunosuppression and the binding of hematopoietic
progenitor cells) which was found to be absent on ASCs (86,87) and
the ASCs express CD49d (α4 integrin that is involved in
facilitating leukocyte migration) which was not detected on BMSCs
(86). Unlike BMSCs, ASCs can be
obtained in high yields from adipose tissues, which can be found
abundantly in the body (84). It has
been estimated that MSCs account for 0.001-0.004% of the bone
marrow aspirate cells, whereas ASCs account for ~2% of the
lipoaspirate cells (84). The
isolation of ASCs can be performed via liposuction aspirates or
from subcutaneous adipose tissue fragments, and is less invasive
compared to BMSCs (63). As of March
2021, 52 registered clinical studies were found on Clinicaltrials.gov using the key words
‘osteoarthritis’ and ‘adipose stem cells’.
MSCs are favored over the other types of stem cells,
as they exhibit numerous advantages for therapeutic purposes, such
as their relative abundance, ease of isolation, their multilineage
differential potential, lower risk of malignant transformation,
immunomodulatory properties and the lack of ethical issues.
Previous reports have suggested that MSCs originate
from the perivascular niche, thus making it possible to isolate
them from various tissues in the body such as bone marrow, adipose
tissue, peripheral blood, the placenta and the umbilical cord
(72,73). However, the bone marrow and
subcutaneous adipose tissues remain the preferential sources of
obtaining MSCs, due to their relative abundance in the human body,
particularly in the subcutaneous adipose tissue (88).
MSCs can be differentiated into various cell
lineages. Over the years, in addition to the production of
osteocytes, chondrocytes and adipocytes from MSCs, studies have
also successfully induced MSCs to differentiate into
oligodendrocytes (89-91),
insulin-producing cells (71,92,93)
and cardiomyocytes (94),
highlighting their potential for the treatment of various
degenerative diseases, including diabetes mellitus (95), cardiovascular diseases (96,97) and
bone diseases (98).
MSCs are endogenously programmed to exhibit limited
proliferation capacity in cultures, after which cells enter a state
of senescence, preventing their ability to divide; this is termed
the ‘Hayflick limit’ (99).
Senescence is defined as a stress response that results in the
arrest of cell proliferation, thus preventing the propagation of
damaged cells and lowering the risk of malignant transformation in
the body (100).
Another characteristic of MSCs that contributes to
the advantage of using MSCs is that they have been shown to exhibit
immunomodulatory properties, wherein they secrete anti-inflammatory
cytokines to suppress both the adaptive and innate immune
responses, thus permitting their use as universal donor cells
without the need for immunosuppressants (101-103).
This is due to the presence of unique surface markers that permit
the MSCs to remain undetected by the immune system, including the
lack of expression of major histocompatibility complex class II and
co-stimulatory cluster of differentiation (CD) molecules such as
CD40 ligand, CD40, CD80 and CD86 (104-107).
This highlights the possibility for the use of allogeneic MSCs to
treat patients who do not meet the criteria for autologous stem
cell therapy.
Unlike ESCs, MSCs can be derived from various
tissues in the body and hence, the ethical concerns associated with
ESCs do not apply to MSCs. Although ESCs have received significant
interest due to their high-degree of pluripotency, the use of ESCs
in clinical applications remains controversial due to the safety
concerns over teratoma formation, as well as the ethical issues
with regard to sourcing (108,109).
Studies on MSC therapy for OA that have been
performed globally to evaluate their safety and efficacy, ranging
from proof-of-concept studies to randomized controlled clinical
trials, and have yielded positive results. Additionally, there are
no studies showing notable side effects of the use of BMSCs and
ASCs, and they have seen progressive improvements when used to
reduce pain, physical function, stabilization of cartilage defects
and even the thickening of articular cartilage in patients with OA.
Table I summarizes the randomized
controlled trials of BMSCs and ASCs as a treatment for OA performed
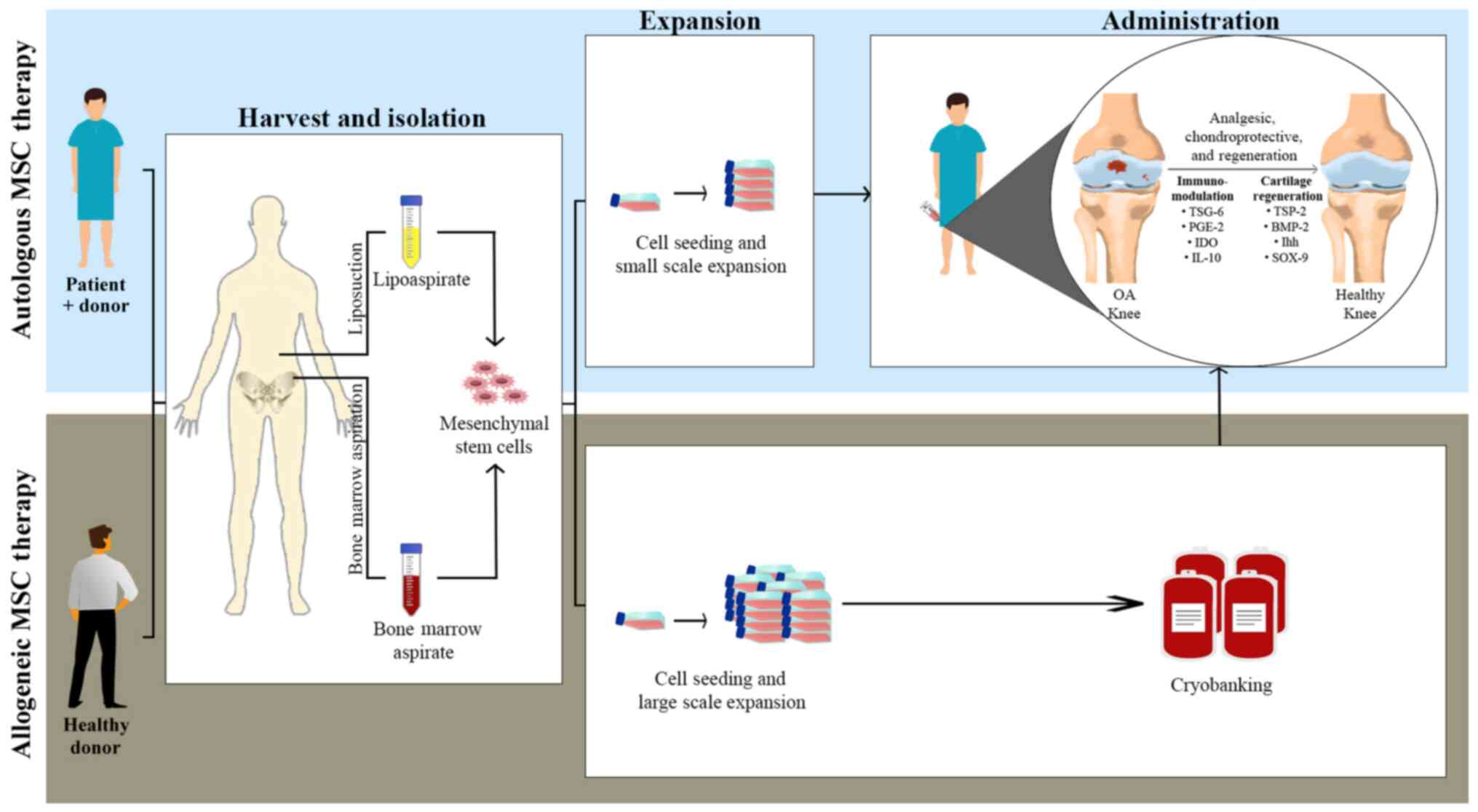
between 2010 and 2020. The general procedures used are as shown in
Fig. 1. Collectively, the studies
highlight their beneficial properties, exhibiting analgesic,
chondroprotective and anatomical regenerative properties.
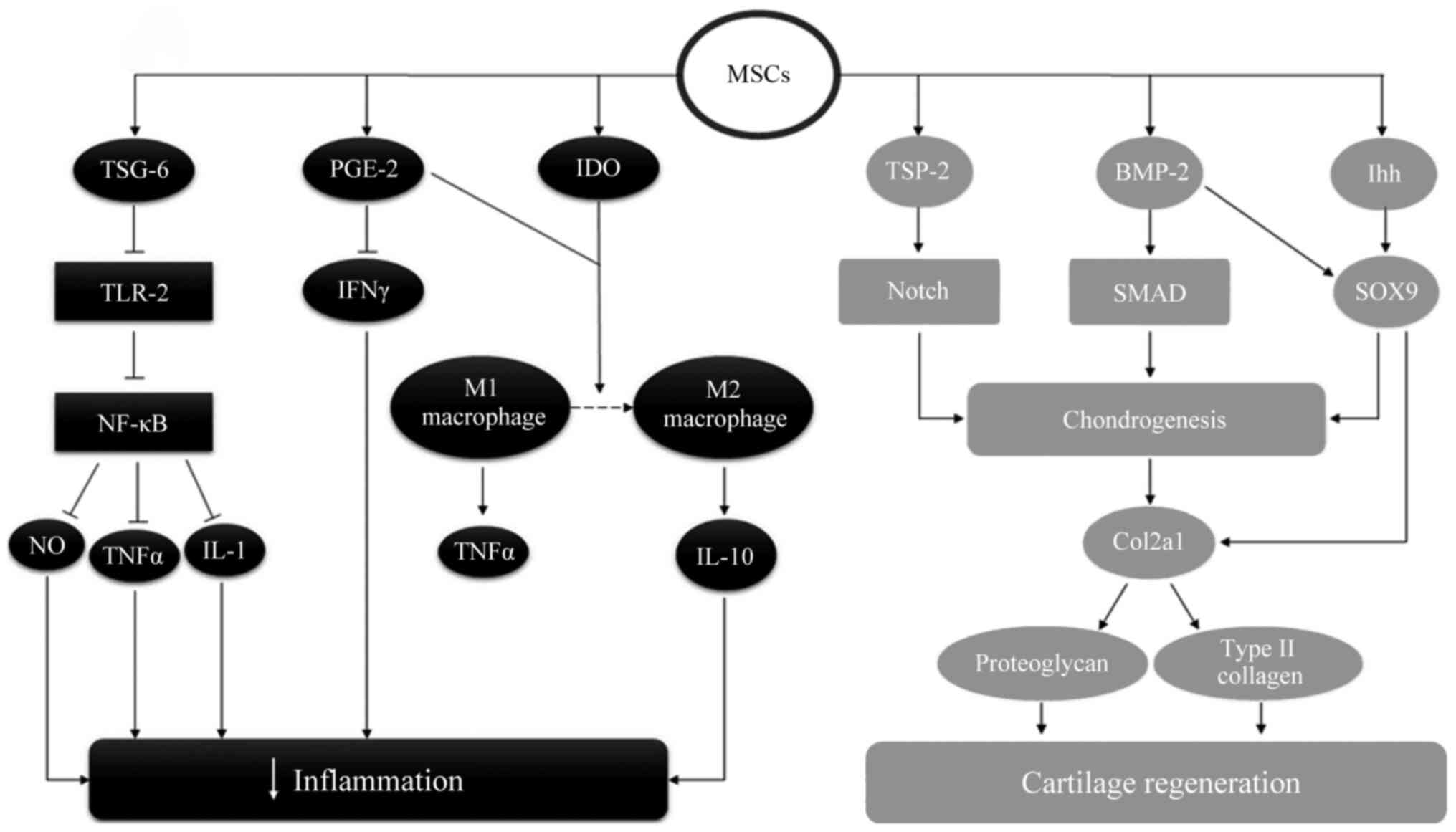
Although the specific molecular mechanisms by which
MSCs exert an analgesic effect remain to be elucidated, it is
hypothesized that they revolve around its immunomodulatory effects,
by inducing the synthesis of anti-inflammatory cytokines, such as
IL-10, and downregulating the production of pro-inflammatory
cytokines, such as IL-1, IL-6, TNFα and interferon (IFN)-γ
(110-114).
MSCs are able to secrete numerous soluble growth factors and
cytokines, including TNFα-stimulated gene/protein 6 (TSG-6),
prostaglandin E2 (PGE-2) and indoleamine 2,3-dioxygenase (IDO),
which contribute to their ability to mitigate pain (103,115-117).
It has been shown that the presence of MSCs results in the
production of TSG-6, leading to inhibition of the toll-like
receptors-2/nuclear factor κ-light-chain-enhancer of activated B
cells signaling pathway, followed by the subsequent downregulation
of inflammatory mediators, such as nitric oxide, TNFα and IL-1
(103,115). Upregulation of PGE-2 by MSCs leads
to inhibition of the IFN-γ, inducing the differentiation of M1-type
macrophages to M2-type macrophages (116). Similarly, the production of IDO by
MSCs also promotes the conversion of M1 macrophages to M2
macrophages (117).
The homing migratory ability of MSCs to sites of
injury and inflammation provides beneficial relief in both BMSC and
saline-treated knee in patients with bilateral OA (118-120).
The regenerative effect of MSCs on the cartilage has
been shown to involve the high expression of several genes
responsible for inducing chondrogenesis, and the subsequent
development of normal cartilage. These genes include the production
of thrombospondin-2, which promotes the Notch signaling pathway;
the production of bone morphogenetic protein 2, which induces the
SMAD signaling pathway; and the Indian hedgehog signaling pathway,
which promotes the expression of SOX9 followed by increased
expression of the Col2a1 gene, thus stimulating the production of
proteoglycans and type II collagen, all of which are involved in
cartilage regeneration (121-123).
The likely mechanism by which MSCs exert their anti-inflammatory
and cartilage regenerative effects is summarized in Fig. 2.
Several studies have highlighted the analgesic
effects of MSCs is addition to their cartilage regenerative
effects; however, follow-up is usually limited to 6-12 months
post-treatment. The long-term potential of MSC therapy may thus be
underrated, as the structural changes required for a prominent
effect take at least a year to occur (79,124).
In general, numerous reports have shown that as
little as a single injection of a 1x106 cell dose is
sufficient for initiation of their analgesic effects, although
eliciting chondrocyte regeneration response in cartilage requires a
much higher dose of at least 1x108 cells (81,125).
Hence, higher quality randomized, controlled clinical studies with
larger cohorts are required to strengthen the evidence and evaluate
the quality of its therapeutic outcomes. Additionally, established
protocols for consideration of the optimal dose, time of
intervention, method of delivery and safety precautions also
require extensive study.
Despite the promising aspects for the use of MSCs
clinically, this approach is considered relatively new and is
surrounded by challenges involved in its use for OA, and these are
discussed below.
The differentiation potential and proliferation
capacity of the MSCs are known to be affected by patients requiring
autologous MSC therapy. Certain metabolic conditions, such as
diabetes and obesity, can generate microenvironmental cues that
predispose the differentiation potential of MSCs towards adipocyte
differentiation rather than chondrocyte differentiation (126-128).
Besides, the proliferative capacity of MSCs decreased with age
(126).
One of the major challenges accompanying the use of
MSCs for treatment of OA is the unsustainable cellular and hyaline
cartilage phenotype of differentiated chondrocytes. Previous
studies have documented the possible involvement of these cells in
the development of heterotopic ossification, a process where bone
formation occurs in non-skeletal tissues (129-132).
These studies reported on the transient secretion of type II
collagen from the MSCs, followed by the up-regulated expression of
collagen type X, matrix metalloproteinase and alkaline phosphatase
activity, thus indicating a shift from the chondrogenic to a
hypertrophic phenotype that precedes osteogenesis, a phenomenon
that does not normally occur amongst the chondrocytes found in the
hyaline cartilage in the joints.
As mentioned earlier, the MSCs will enter a state of
senescence whereby they lose their ability to proliferate. This is
a double-edged characteristic, as it may lower the risk of
malignant tumors, but limit the therapeutic use of stem cells. It
was documented that the MSCs exhibited abnormalities in the
morphology of the cells, such as enlargement, reduced expression of
specific surface markers, and finally senescence as they reached
higher passage numbers (133,134).
The entry into a state of senescence in MSCs was shown to affect
the differential potential of the cells, the immunomodulation
capability and their migratory ability (133). Although cryopreservation of MSCs
can be used to address this issue, the process comes with its own
challenges; a decrease in viability, colony forming units and
integrin expression were observed after cryopreservation and
thawing of the cells (135).
MSC therapy requires highly skilled professionals,
as the culturing of MSCs must be performed with utmost care to
prevent contamination of the cells. Additionally, considerably more
research is required in order to establish a clear protocol for the
isolation, expansion, differentiation and pre-conditioning of MSCs,
and to determine the appropriate concentration of MSCs for use in
patients with OA.
While stem cell therapy may offer an attractive
option for treatment of currently uncurable diseases, the costs are
currently considerably high, owing to the need to cover the cost of
the individual harvesting, isolation, and expansion of cells in a
sterile facility. Additionally, the increasing popularity of stem
cell therapy has warranted the use widespread biobanking, and an
increased access to the various highly multipotent stem cells,
which could be at risk of exploitation. Hence, policies on
biobanking of stem cells must be regulated to address the possible
issues regarding its usage, control as well as patients'
consent.
The emergence of stem cell-based therapies has
brought about novel avenues to address the, as of yet, unmet
curative treatment for various degenerative disorders, including
OA. The multipotency of the MSCs, along with its self-renewal and
immunomodulatory properties, availability and ease of isolation
highlight the potential use of MSCs for cellular therapeutic
approaches in OA, and promising results have been demonstrated in
various pre-clinical and clinical trials. However, several
challenges are involved in this process, and this requires
standardized solutions before they can be recommended clinically.
Efforts on investigating and establishing protocols to increase the
stability of the chondrocyte-like phenotype of the MSCs is required
to raise the success rates of MSC-based treatment in patients with
OA, and to lower the cost. In addition, the appropriate
concentration of stem cells for specific treatments, and the
long-term follow-ups of patients with OA treated with MSCs should
be performed to investigate the long-term safety and efficacy of
MSC-based therapy.
Not applicable.
No funding was received.
Not applicable.
SJQL and NKW both wrote and revised the article.
Both authors read and approved the final manuscript. Data sharing
is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
National Clinical Guideline Centre (UK):
Osteoarthritis-Care and Μanagement in Αdults. London: National
Institute for Health and Care Excellence (UK), 2014.
|
|
2
|
James SL, Abate D, Hassen Abate K, Abay
SM, Abbafati C, Abbasi N, Abbastabar H, Abd-Allah F, Abdela J,
Abdelalim A, et al: Global, regional, and national incidence,
prevalence, and years lived with disability for 354 diseases and
injuries for 195 countries and territories, 1990-2017: A systematic
analysis for the Global Burden of Disease Study 2017. Lancet.
392:1789–1858. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Blagojevic M, Jinks C, Jeffery A and
Jordan KP: Risk factors for onset of osteoarthritis of the knee in
older adults: A systemic review and meta-analysis. Osteoarthritis
Cartilage. 18:24–33. 2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Loeser RF: Molecular mechanisms of
cartilage destruction: Mechanics, inflammatory mediators, and aging
collide. Arthritis Rheum. 54:1357–1360. 2006.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ng HY, Alvin Lee KX and Shen KX: Articular
cartilage: Structure, composition, injuries and repair. JSM Bone Jt
Dis. 1(1010)2017.
|
|
6
|
Ostrowska M, Maśliński W,
Prochorec-Sobieszek M, Nieciecki M and Sudoł-Szopińska I: Cartilage
and bone damage in rheumatoid arthritis. Reumatologia. 56:111–120.
2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Mahajan A, Verma S and Tandon V:
Osteoarthritis. J Assoc Physicians India. 53:634–641.
2005.PubMed/NCBI
|
|
8
|
Houard X, Goldring MB and Berenbaum F:
Homeostatic mechanisms in articular cartilage and role of
inflammation in osteoarthritis. Curr Rheumatol Rep.
15(375)2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Gignac MAM, Irvin E, Cullen K, Van Eerd D,
Beaton DE, Mahood Q, McLeod C and Backman CL: Men and women's
occupational activities and the risk of developing osteoarthritis
of the knee, hip, or hands: A systematic review and recommendations
for future research. Arthritis Care Res (Hoboken). 72:378–396.
2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Michael JW, Schlüter-Brust KU and Eysel P:
The epidemiology, etiology, diagnosis, and treatment of
osteoarthritis of the knee. Dtsch Arztebl Int. 107:152–162.
2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Helliwell P: Osteoarthritis and Paget's
disease. Br J Rheumatol. 34:1061–1063. 1995.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Mora JC, Przkora R and Cruz-Almeida Y:
Knee osteoarthritis: Pathophysiology and current treatment
modalities. J Pain Res. 11:2189–2196. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
de l'Escalopier N, Anract P and Biau D:
Surgical treatments for osteoarthritis. Ann Phys Rehabil Med.
59:227–233. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Øiestad BE, Juhl CB, Eitzen I and Thorlund
JB: Knee extensor muscle weakness is a risk factor for development
of knee osteoarthritis. A systematic review and meta-analysis.
Osteoarthritis Cartilage. 23:171–177. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
March LM and Bagga H: Epidemiology of
osteoarthritis in Australia. Med J Aust. 180:S6–S10.
2004.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Messier SP: Obesity and osteoarthritis:
Disease genesis and nonpharmacologic weight management. Rheum Dis
Clin North Am. 34:713–729. 2008.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Vincent KR and Vincent HK: Resistance
exercise for knee osteoarthritis. PM R. 4 (Suppl 5):S45–S52.
2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Rogers MW and Wilder FV: The effects of
strength training among persons with hand osteoarthritis: A
two-year follow-up study. J Hand Ther. 20:244–249; quiz 250.
2007.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Latham N and Liu CJ: Strength training in
older adults: The benefits for osteoarthritis. Clin Geriatr Med.
26:445–459. 2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Aguiar GC, Rocha SG, da Silva Rezende GA,
do Nascimento MR and Scalzo PL: Effects of resistance training in
individuals with knee osteoarthritis. Fisioter Mov. 29:589–596.
2016.
|
|
21
|
Thoumie P, Marty M, Avouac B, Pallez A,
Vaumousse A, Pipet LPT, Monroche A, Graveleau N, Bonnin A, Amor CB
and Coudeyre E: Effect of unloading brace treatment on pain and
function in patients with symptomatic knee osteoarthritis: The
ROTOR randomized clinical trial. Sci Rep. 8(10519)2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Chew KT, Lew HL, Date E and Fredericson M:
Current evidence and clinical applications of therapeutic knee
braces. Am J Phys Med Rehabil. 86:678–686. 2007.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lee PY, Winfield TG, Harris SR, Storey E
and Chandratreya A: Unloading knee brace is a cost-effective method
to bridge and delay surgery in unicompartmental knee arthritis. BMJ
Open Sport Exerc Med. 2(e000195)2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ostrander RV, Leddon CE, Hackel JG,
O'Grady CP and Roth CA: Efficacy of unloader bracing in reducing
symptoms of knee osteoarthritis. Am J Orthop (Belle Mead NJ).
45:306–311. 2016.PubMed/NCBI
|
|
25
|
Wilson B, Rankin H and Barnes CL:
Long-term results of an unloader brace in patients with
unicompartmental knee osteoarthritis. Orthopedics. 34:e334–e337.
2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Yu SP, Williams M, Eyles JP, Chen JS,
Makovey J and Hunter DJ: Effectiveness of knee bracing in
osteoarthritis: Pragmatic trial in a multidisciplinary clinic. Int
J Rheum Dis. 19:279–286. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Squyer E, Stamper DL, Hamilton DT, Sabin
JA and Leopold SS: Unloader knee braces for osteoarthritis: Do
patients actually wear them? Clin Orthop Relat Res. 471:1982–1991.
2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Nejati P, Farzinmehr A and Moradi-Lakeh M:
The effect of exercise therapy on knee osteoarthritis: A randomized
clinical trial. Med J Islam Repub Iran. 29(186)2015.PubMed/NCBI
|
|
29
|
Reid MC, Eccleston C and Pillemer K:
Management of chronic pain in older adults. BMJ.
350(h532)2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Rönn K, Reischl N, Gautier E and Jacobi M:
Current surgical treatment of knee osteoarthritis. Arthritis.
2011(454873)2011.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wang X, Wanyan P, Wang JM, Tian JH, Hu L,
Shen XP and Yang KH: A randomized, controlled trial to assess the
efficacy of arthroscopic debridement in combination with oral
medication versus oral medication in patients with gouty knee
arthritis. Indian J Surg. 77 (Suppl 2):S628–S634. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kirkley A, Birmingham TB, Litchfield RB,
Giffin JR, Willits KR, Wong CJ, Feagan BG, Donner A, Griffin SH,
D'Ascanio LM, et al: A randomized trial of arthroscopic surgery for
osteoarthritis of the knee. N Engl J Med. 359:1097–1107.
2008.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Moseley JB, O'Malley K, Petersen NJ, Menke
TJ, Brody BA, Kuykendall DH, Hollingsworth JC, Ashton CM and Wray
NP: A controlled trial of arthroscopic surgery for osteoarthritis
of the knee. N Engl J Med. 347:81–88. 2002.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Choi HG, Kwon BC, Kim JI and Lee JK: Total
knee arthroplasty reduces the risk of mortality in osteoarthritis
patients up to 12 years: A Korean national cohort longitudinal
follow-up study. J Orthop Surg (Hong Kong).
28(2309499020902589)2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Rajabzadeh N, Fathi E and Farahzadi R:
Stem cell-based regenerative medicine. Stem Cell Investig.
6(19)2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Landry DW and Zucker HA: Embryonic death
and the creation of human embryonic stem cells. J Clin Invest.
114:1184–1186. 2004.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Ede V and Obeagu EI: Ethical issues in
human embryonic stem cell research: A christian perspective. Int J
Med Sci Dent Res. 1:8–14. 2019.
|
|
38
|
Lo B and Parham L: Ethical issues in stem
cell research. Endocr Rev. 30:204–213. 2009.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Takahashi K and Yamanaka S: Induction of
pluripotent stem cells from mouse embryonic and adult fibroblast
cultures by defined factors. Cell. 126:663–676. 2006.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Shi Y, Inoue H, Wu JC and Yamanaka S:
Induced pluripotent stem cell technology: A decade of progress. Nat
Rev Drug Discov. 16:115–130. 2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Takahashi K, Tanabe K, Ohnuki M, Narita M,
Ichisaka T, Tomoda K and Yamanaka S: Induction of pluripotent stem
cells from adult human fibroblasts by defined factors. Cell.
131:861–872. 2007.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Gutierrez-Aranda I, Ramos-Mejia V, Bueno
C, Munoz-Lopez M, Real PJ, Mácia A, Sanchez L, Ligero G,
Garcia-Parez JL and Menendez P: Human induced pluripotent stem
cells develop teratoma more efficiently and faster than human
embryonic stem cells regardless the site of injection. Stem Cells.
28:1568–1570. 2010.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Zhang M, Wang L, An K, Cai J, Li G, Yang
C, Liu H, Du F, Han X, Zhang Z, et al: Lower genomic stability of
induced pluripotent stem cells reflects increased non-homologous
end joining. Cancer Commun (Lond). 38(49)2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Christensen R and Serakinci N: Adult Stem
Cells. In: Encyclopedia of Cancer. Springer, Heidelberg, pp1-5,
2015.
|
|
45
|
Centeno C, Markle J, Dodson E, Stemper I,
Williams CJ, Hyzy M, Ichim T and Freeman M: Treatment of lumbar
degenerative disc disease-associated radicular pain with
culture-expanded autologous mesenchymal stem cells: A pilot study
on safety and efficacy. J Transl Med. 15(197)2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Berry JD, Cudkowicz ME, Windebank AJ,
Staff NP, Owegi M, Nicholson K, McKenna-Yasek D, Levy YS, Abramov
N, Kaspi H, et al: NurOwn, phase 2, randomized, clinical trial in
patients with ALS: Safety, clinical, and biomarker results.
Neurology. 93:e2294–e2305. 2019.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Riecke J, Johns KM, Cai C, Vahidy FS,
Parsha K, Furr-Stimming E, Schiess M and Savitz SI: A meta-analysis
of mesenchymal stem cells in animal models of Parkinson's disease.
Stem Cells Dev. 24:2082–2090. 2015.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Ng AP and Alexander WS: Haematopoietic
stem cells: Past, present and future. Cell Death Discov.
3(17002)2017.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Yamamoto R, Wilkinson AC and Nakauchi H:
Changing concepts in hematopoietic stem cells. Science.
362:895–896. 2018.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Domen J, Wagers A and Weissman IL: Bone
marrow (Hematopoietic) stem cells. Stem Cell Information: The
National Institutes of Health resource for stem cell research:
13-34, 2006.
|
|
51
|
Morgan RA, Gray D, Lomova A and Kohn DB:
Hematopoietic stem cell gene therapy: Progress and lessons learned.
Cell Stem Cell. 21:574–590. 2017.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Taylor M, Khan S, Stapleton M, Wang J,
Chen J, Wynn R, Yabe H, Chinen Y, Boelens JJ, Mason RW, et al:
Hematopoietic stem cell transplantation for mucopolysaccharidoses:
Past, present, and future. Biol Blood Marrow Transplant.
25:e226–e246. 2019.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Walters MC: Update of hematopoietic cell
transplantation for sickle cell disease. Curr Opin Hematol.
22:227–233. 2015.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Abdelmoaty N, Alattar E, Ahmed K, Ibrahim
Y and Darwish B: Regenerative power of autologous peripheral blood
stem cell injection in knee osteoarthritis by a non-invasive
approach-MRI Study. Ann Rheum Dis. 73(1066)2014.
|
|
55
|
U.S Food and Drug Administration: Approved
Cellular and Gene Therapy Products, 2021.
|
|
56
|
Prockop DJ: Marrow stromal cells as stem
cells for nonhematopoietic tissues. Science. 276:71–74.
1997.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Makino S, Fukuda K, Miyoshi S, Konishi F,
Kodama H, Pan J, Sano M, Takahashi T, Hori S, Abe H, et al:
Cardiomyocytes can be generated from marrow stromal cells in vitro.
J Clin Invest. 103:697–705. 1999.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Wakitani S, Saito T and Caplan AI:
Myogenic cells derived from rat bone marrow mesenchymal stem cells
exposed to 5-azacytidine. Muscle Nerve. 18:1417–1426.
1995.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Nuttall ME, Patton AJ, Olivera DL, Nadeau
DP and Gowen M: Human trabecular bone cells are able to express
both osteoblastic and adipocytic phenotype: Implications for
osteopenic disorders. J Bone Miner Res. 13:371–382. 1998.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Pittenger MF, Mackay AM, Beck SC, Jaiswal
RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S and
Marshak DR: Multilineage potential of adult human mesenchymal stem
cells. Science. 284:143–147. 1999.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Bianco P, Robey PG and Simmons PJ:
Mesenchymal stem cells: Revisiting history, concepts, and assays.
Cell Stem Cell. 2:313–319. 2008.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Roufosse CA, Direkze NC, Otto WR and
Wright NA: Circulating mesenchymal stem cells. Int J Biochem Cell
Biol. 36:585–597. 2004.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Lindroos B, Suuronen R and Miettinen S:
The potential of adipose stem cells in regenerative medicine. Stem
Cell Rev Rep. 7:269–291. 2011.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Erices A, Conget P and Minguell JJ:
Mesenchymal progenitor cells in human umbilical cord blood. Br J
Haematol. 109:235–242. 2000.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Gronthos S, Mankani M, Brahim J, Robey PG
and Shi S: Postnatal human dental pulp stem cells (DPSCs) in vitro
and in vivo. Proc Natl Acad Sci USA. 97:13625–13630.
2000.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Haniffa MA, Wang XN, Holtick U, Rae M,
Isaacs JD, Dickinson AM, Hilkens CM and Collin MP: Adult human
fibroblasts are potent immunoregulatory cells and functionally
equivalent to mesenchymal stem cells. J Immunol. 179:1595–1604.
2007.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Sessarego N, Parodi A, Podesta M,
Benvenuto F, Mogni M, Raviolo V, Lituania M, Kunkl A, Ferlazzo G,
Bricarelli FD, et al: Multipotent mesenchymal stromal cells from
amniotic fluid: Solid perspectives for clinical application.
Haematologica. 93:339–346. 2008.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Dominici M, Le Blanc K, Mueller I,
Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A,
Prockop DJ and Horwitz E: Minimal criteria for defining multipotent
mesenchymal stromal cells. The International society for cellular
therapy position statement. Cytotherapy. 8:315–317. 2006.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Gianakos AL, Sun L, Patel JN, Adams DM and
Liporace FA: Clinical application of concentrated bone marrow
aspirate in orthopaedics: A systematic review. World J Orthop.
8:491–506. 2017.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Mohamed-Ahmed S, Fristad I, Lie SA,
Suliman S, Mustafa K, Vindenes H and Idris SB: Adipose-derived and
bone marrow mesenchymal stem cells: A donor-matched comparison.
Stem Cell Res Ther. 9(168)2018.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Gabr MM, Zakaria MM, Refaie AF,
Abdel-Rahman EA, Reda AM, Ali SS, Khater SM, Ashamallah SA, Ismail
AM, Ismail HEA, et al: From human mesenchymal stem cells to
insulin-producing cells: Comparison between bone marrow- and
adipose tissue-derived cells. Biomed Res Int.
2017(3854232)2017.PubMed/NCBI View Article : Google Scholar
|
|
72
|
da Silva Meirelles L, Chagastelles PC and
Nardi NB: Mesenchymal stem cells reside in virtually all post-natal
organs and tissues. J Cell Sci. 119:2204–2213. 2006.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Crisan M, Yap S, Casteilla L, Chen CW,
Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, et al: A
perivascular origin for mesenchymal stem cells in multiple human
organs. Cell Stem Cell. 3:301–313. 2008.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Orozco L, Munar A, Soler R, Alberca M,
Soler F, Huguet M, Sentís J, Sánchez A and García-Sancho J:
Treatment of knee osteoarthritis with autologous mesenchymal stem
cells: A pilot study. Transplantation. 95:1535–1541.
2013.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Lamo-Espinosa JM, Mora G, Blanco JF,
Granero-Moltó F, Nuñez-Córdoba JM, Sánchez-Echenique C, Bondía JM,
Aquerreta JD, Andreu EJ, Ornilla E, et al: Intra-articular
injection of two different doses of autologous bone marrow
mesenchymal stem cells versus hyaluronic acid in the treatment of
knee osteoarthritis: Multicenter randomized controlled clinical
trial (phase I/II). J Transl Med. 14(246)2016.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Garay-Mendoza D, Villarreal-Martínez L,
Garza-Bedolla A, Pérez-Garza DM, Acosta-Olivo C, Vilchez-Cavazos F,
Diaz-Hutchinson C, Gómez-Almaguer D, Jaime-Pérez JC and
Mancías-Guerra C: The effect of intra-articular injection of
autologous bone marrow stem cells on pain and knee function in
patients with osteoarthritis. Int J Rheum Dis. 21:140–147.
2018.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Vega A, Martín-Ferrero MA, Del Canto F,
Alberca M, García V, Munar A, Orozco L, Soler R, Fuertes JJ, Huguet
M, et al: Treatment of knee osteoarthritis with allogeneic bone
marrow mesenchymal stem cells: A randomized controlled trial.
Transplantation. 99:1681–1690. 2015.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Shadmanfar S, Labibzadeh N, Emadedin M,
Jaroughi N, Azimian V, Mardpour S, Kakroodi FA, Bolurieh T,
Hosseini SE, Chehrazi M, et al: Intra-articular knee implantation
of autologous bone marrow-derived mesenchymal stromal cells in
rheumatoid arthritis patients with knee involvement: Results of a
randomized, triple-blind, placebo-controlled phase 1/2 clinical
trial. Cytotherapy. 20:499–506. 2018.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Al-Najar M, Khalil H, Al-Ajlouni J,
Al-Antary E, Hamdan M, Rahmeh R, Alhattab D, Samara O, Yasin M,
Abdullah AA, et al: Intra-articular injection of expanded
autologous bone marrow mesenchymal cells in moderate and severe
knee osteoarthritis is safe: A phase I/II study. J Orthop Surg Res.
12(190)2017.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Anz AW, Hubbard R, Rendos NK, Everts PA,
Andrews JR and Hackel JG: Bone marrow aspirate concentrate is
equivalent to platelet-rich plasma for the treatment of knee
osteoarthritis at 1 year: A prospective, randomized trial. Orthop J
Sports Med. 2(2325967119900958)2020.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Chahal J, Gómez-Aristizábal A,
Shestopaloff K, Bhatt S, Chaboureau A, Fazio A, Chisholm J, Weston
A, Chiovitti J, Keating A, et al: Bone marrow mesenchymal stromal
cell treatment in patients with osteoarthritis results in overall
improvement in pain and symptoms and reduces synovial inflammation.
Stem Cells Transl Med. 8:746–757. 2019.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Zuk PA, Zhu M, Mizuno H, Huang J, Futrell
JW, Katz AJ, Benhaim P, Lorenz HP and Hedrick MH: Multilineage
cells from human adipose tissue: Implications for cell-based
therapies 2. Tissue Eng. 7:211–228. 2001.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Zuk PA, Zhu M, Ashjian P, De Ugarte DA,
Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P and Hedrick
MH: Human adipose tissue is a source of multipotent stem cells.
Raff M (ed). Mol Biol Cell. 13:4279–4295. 2002.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Coughlin RP, Oldweiler A, Mickelson DT and
Moorman CT III: Adipose-derived stem cell transplant technique for
degenerative joint disease. Arthrosc Tech. 6:e1761–e1766.
2017.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Freitag J, Bates D, Wickham J, Shah K,
Huguenin L, Tenen A, Paterson K and Boyd R: Adipose-derived
mesenchymal stem cell therapy in the treatment of knee
osteoarthritis: A randomized controlled trial. Regen Med.
14:213–230. 2019.PubMed/NCBI View Article : Google Scholar
|
|
86
|
De Ugarte DA, Alfonso Z, Zuk PA, Elbarbary
A, Zhu M, Ashjian P, Benhaim P, Hedrick MH and Fraser JK:
Differential expression of stem cell mobilization-associated
molecules on multi-lineage cells from adipose tissue and bone
marrow. Immunol Lett. 89:267–270. 2003.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Yang ZX, Han ZB, Ji YR, Wang YW, Liang L,
Chi Y, Yang SG, Li LN, Luo WF, Li JP, et al: CD106 identifies a
subpopulation of mesenchymal stem cells with unique
immunomodulatory properties. PLoS One. 8(e59354)2013.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Krampera M, Marconi S, Pasini A, Galiè M,
Rigotti G, Mosna F, Tinelli M, Lovato L, Anghileri E, Andreini A,
et al: Induction of neural-like differentiation in human
mesenchymal stem cells derived from bone marrow, fat, spleen and
thymus. Bone. 40:382–390. 2007.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Kennea NL, Waddington SN, Chan J,
O'Donoghue K, Yeung D, Taylor DL, Al-Allaf FA, Pirianov G, Themis
M, Edwards AD, et al: Differentiation of human fetal mesenchymal
stem cells into cells with an oligodendrocyte phenotype. Cell
Cycle. 8:1069–1079. 2009.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Shah S, Yin PT, Uehara TM, Chueng ST, Yang
L and Lee KB: Guiding stem cell differentiation into
oligodendrocytes using graphene-nanofiber hybrid scaffolds. Adv
Mater. 26:3673–3680. 2014.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Steffenhagen C, Dechant FX, Oberbauer E,
Furtner T, Weidner N, Küry P, Aigner L and Rivera FJ: Mesenchymal
stem cells prime proliferating adult neural progenitors toward an
oligodendrocyte fate. Stem Cells Dev. 21:1838–1851. 2012.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Allahverdi A, Abroun S, Jafarian A,
Soleimani M, Taghikhani M and Eskandari F: Differentiation of human
mesenchymal stem cells into insulin producing cells by using a
lentiviral vector carrying PDX1. Cell J. 17:231–242.
2015.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Chen LB, Jiang XB and Yang L:
Differentiation of rat marrow mesenchymal stem cells into
pancreatic islet beta-cells. World J Gastroenterol. 10:3016–3020.
2004.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Singh A, Singh A and Sen D: Mesenchymal
stem cells in cardiac regeneration: A detailed progress report of
the last 6 years (2010-2015). Stem Cell Res Ther.
7(82)2016.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Solis MA, Moreno Velásquez I, Correa R and
Huang LLH: Stem cells as a potential therapy for diabetes mellitus:
A call-to-action in Latin America. Diabetol Metab Syndr.
11(20)2019.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Sun R, Li X, Liu M, Zeng Y, Chen S and
Zhang P: Advances in stem cell therapy for cardiovascular disease
(Review). Int J Mol Med. 38:23–29. 2016.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Terashvili M and Bosnjak ZJ: Stem cell
therapies in cardiovascular disease. J Cardiothorac Vasc Anesth.
33:209–222. 2019.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Paspaliaris V and Kolios G: Stem cells in
osteoporosis: From biology to new therapeutic approaches. Stem
Cells Int. 2019(1730978)2019.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Mercado-Sáenz S, Ruiz-Gómez MJ,
Morales-Moreno F and Martínez-Morillo M: Cellular aging: Theories
and technological influence. Brazilian Arch Biol Technol.
53:1319–1332. 2010.
|
|
100
|
Shay JW and Wright WE: Role of telomeres
and telomerase in cancer. Semin Cancer Biol. 21:349–353.
2011.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Keating A: Mesenchymal stromal cells: New
directions. Cell Stem Cell. 10:709–716. 2012.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Le Blanc K and Mougiakakos D: Multipotent
mesenchymal stromal cells and the innate immune system. Nat Rev
Immunol. 12:383–396. 2012.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Prockop DJ and Oh JY: Mesenchymal
stem/stromal cells (MSCs): Role as guardians of inflammation. Mol
Ther. 20:14–20. 2012.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Ryan JM, Barry FP, Murphy JM and Mahon BP:
Mesenchymal stem cells avoid allogeneic rejection. J Inflamm
(Lond). 2(8)2005.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Klyushnenkova E, Mosca JD, Zernetkina V,
Majumdar MK, Beggs KJ, Simonetti DW, Deans RJ and McIntosh KR: T
cell responses to allogeneic human mesenchymal stem cells:
Immunogenicity, tolerance, and suppression. J Biomed Sci. 12:47–57.
2005.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Di Nicola M, Carlo-Stella C, Magni M,
Milanesi M, Longoni PD, Matteucci P, Grisanti S and Gianni AM:
Human bone marrow stromal cells suppress T-lymphocyte proliferation
induced by cellular or nonspecific mitogenic stimuli. Blood.
99:3838–3843. 2002.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Tse WT, Pendleton JD, Beyer WM, Egalka MC
and Guinan EC: Suppression of allogeneic T-cell proliferation by
human marrow stromal cells: Implications in transplantation.
Transplantation. 75:389–397. 2003.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Lee H, Shamy GA, Elkabetz Y, Schofield CM,
Harrsion NL, Panagiotakos G, Socci ND, Tabar V and Studer L:
Directed differentiation and transplantation of human embryonic
stem cell-derived motoneurons. Stem Cells. 25:1931–1939.
2007.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Su W, Zhou M, Zheng Y, Fan Y, Wang L, Han
Z, Kong D, Zhao RC, Wu JC, Xiang R and Li Z: Bioluminescence
reporter gene imaging characterize human embryonic stem
cell-derived teratoma formation. J Cell Biochem. 112:840–848.
2011.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Aggarwal S and Pittenger MF: Human
mesenchymal stem cells modulate allogeneic immune cell responses.
Blood. 105:1816–1822. 2005.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Li H and Fu X: Mechanisms of action of
mesenchymal stem cells in cutaneous wound repair and regeneration.
Cell Tissue Res. 348:371–377. 2012.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Maxson S, Lopez EA, Yoo D,
Danilkovitch-Miagkova A and Leroux MA: Concise review: Role of
mesenchymal stem cells in wound repair. Stem Cells Transl Med.
1:142–149. 2012.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Williams CG, Kim TK, Taboas A, Malik A,
Manson P and Elisseeff J: In vitro chondrogenesis of bone
marrow-derived mesenchymal stem cells in a photopolymerizing
hydrogel. Tissue Eng. 9:679–688. 2003.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Zhang W, Ge W, Li C, You S, Liao L, Han Q,
Deng W and Zhao RC: Effects of mesenchymal stem cells on
differentiation, maturation, and function of human monocyte-derived
dendritic cells. Stem Cells Dev. 13:263–271. 2004.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Choi H, Lee RH, Bazhanov N, Oh JY and
Prockop DJ: Anti-inflammatory protein TSG-6 secreted by activated
MSCs attenuates zymosan-induced mouse peritonitis by decreasing
TLR2/NF-κB signaling in resident macrophages. Blood. 118:330–338.
2011.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Németh K, Leelahavanichkul A, Yuen PS,
Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller
BH, Brown JM, et al: Bone marrow stromal cells attenuate sepsis via
prostaglandin E(2)-dependent reprogramming of host macrophages to
increase their interleukin-10 production. Nat Med. 15:42–49.
2009.PubMed/NCBI View Article : Google Scholar
|
|
117
|
François M, Romieu-Mourez R, Li M and
Galipeau J: Human MSC suppression correlates with cytokine
induction of indoleamine 2,3-dioxygenase and bystander M2
macrophage differentiation. Mol Ther. 20:187–195. 2012.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Shapiro SA, Kazmerchak SE, Heckman MG,
Zubair AC and O'Connor MI: A prospective, single-blind,
placebo-controlled trial of bone marrow aspirate concentrate for
knee osteoarthritis. Am J Sports Med. 45:82–90. 2017.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Kanaya A, Deie M, Adachi N, Nishimori M,
Yanada S and Ochi M: Intra-articular injection of mesenchymal
stromal cells in partially torn anterior cruciate ligaments in a
rat model. Arthroscopy. 23:610–617. 2007.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Eseonu OI and De Bari C: Homing of
mesenchymal stem cells: Mechanistic or stochastic? Implications for
targeted delivery in arthritis. Rheumatology (Oxford). 54:210–218.
2015.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Horie M, Choi H, Lee RH, Reger RL,
Ylostalo J, Muneta T, Sekiya I and Prockop DJ: Intra-articular
injection of human mesenchymal stem cells (MSCs) promote rat
meniscal regeneration by being activated to express Indian hedgehog
that enhances expression of type II collagen. Osteoarthritis
Cartilage. 20:1197–1207. 2012.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Jeong SY, Ha J, Lee M, Jin HJ, Kim DH,
Choi SJ, Oh W, Yang YS, Kim JS, Kim BG, et al: Autocrine action of
thrombospondin-2 determines the chondrogenic differentiation
potential and suppresses hypertrophic maturation of human umbilical
cord blood-derived mesenchymal stem cells. Stem Cells.
33:3291–3303. 2015.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Scarfì S: Use of bone morphogenetic
proteins in mesenchymal stem cell stimulation of cartilage and bone
repair. World J Stem Cells. 8:1–12. 2016.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Oliver KS, Bayes M, Crane D and Pathikonda
C: Clinical outcome of bone marrow concentrate in knee
osteoarthritis. J Prolotherapy. 7:937–946. 2015.
|
|
125
|
Jo CH, Lee YG, Shin WH, Kim H, Chai JW,
Jeong EC, Kim JE, Shim H, Shin JS, Shin IS, et al: Intra-articular
injection of mesenchymal stem cells for the treatment of
osteoarthritis of the knee: A proof-of-concept clinical trial. Stem
Cells. 32:1254–1266. 2014.PubMed/NCBI View Article : Google Scholar
|
|
126
|
Dexheimer V, Mueller S, Braatz F and
Richter W: Reduced reactivation from dormancy but maintained
lineage choice of human mesenchymal stem cells with donor age. PLoS
One. 6(e22980)2011.PubMed/NCBI View Article : Google Scholar
|
|
127
|
Ferone A and Messina G: Sera of overweight
patients alter adipogenesis and osteogenesis of bone marrow
mesenchymal stromal cells, a phenomenon that also persists in
weight loss individuals. J Stem Cell Res Ther. 6(1000347)2016.
|
|
128
|
Mansilla E, Díaz Aquino V, Zambn D, Marin
GH, Mártire K, Roque G, Ichim T, Riordan NH, Patel A, Sturla F, et
al: Could metabolic syndrome, lipodystrophy, and aging be
mesenchymal stem cell exhaustion syndromes? Stem Cells Int.
2011(943216)2011.PubMed/NCBI View Article : Google Scholar
|
|
129
|
Johnstone B, Hering TM, Caplan AI,
Goldberg VM and Yoo JU: In vitro chondrogenesis of bone
marrow-derived mesenchymal progenitor cells. Exp Cell Res.
238:265–272. 1998.PubMed/NCBI View Article : Google Scholar
|
|
130
|
Tatebe M, Nakamura R, Kagami H, Okada K
and Ueda M: Differentiation of transplanted mesenchymal stem cells
in a large osteochondral defect in rabbit. Cytotherapy. 7:520–530.
2005.PubMed/NCBI View Article : Google Scholar
|
|
131
|
Pelttari K, Winter A, Steck E, Goetzke K,
Hennig T, Ochs BG, Aigner T and Richter W: Premature induction of
hypertrophy during in vitro chondrogenesis of human mesenchymal
stem cells correlates with calcification and vascular invasion
after ectopic transplantation in SCID mice. Arthritis Rheum.
54:3254–3266. 2006.PubMed/NCBI View Article : Google Scholar
|
|
132
|
Mackay AM, Beck SC, Murphy JM, Barry FP,
Chichester CO and Pittenger MF: Chondrogenic differentiation of
cultured human mesenchymal stem cells from marrow. Tissue Eng.
4:415–428. 1998.PubMed/NCBI View Article : Google Scholar
|
|
133
|
Turinetto V, Vitale E and Giachino C:
Senescence in human mesenchymal stem cells: Functional changes and
implications in stem cell-based therapy. Int J Mol Sci.
17(1164)2016.PubMed/NCBI View Article : Google Scholar
|
|
134
|
Gu Y, Li T, Ding Y, Sun L, Tu T, Zhu W, Hu
J and Sun X: Changes in mesenchymal stem cells following long-term
culture in vitro. Mol Med Rep. 13:5207–5215. 2016.PubMed/NCBI View Article : Google Scholar
|
|
135
|
Irioda AC, Cassilha R, Zocche L, Francisco
JC, Cunha RC, Ferreira PE, Guarita-Souza LC, Ferreira RJ, Mogharbel
BF, Garikipati VN, et al: Human adipose-derived mesenchymal stem
cells cryopreservation and thawing decrease α4-Integrin expression.
Stem Cells Int. 2016(2562718)2016.PubMed/NCBI View Article : Google Scholar
|
|
136
|
Bastos R, Mathias M, Andrade R, Bastos R,
Balduino A, Schott V, Rodeo S and Espregueira-Mendes J:
Intra-articular injections of expanded mesenchymal stem cells with
and without addition of platelet-rich plasma are safe and effective
for knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc.
11:3342–3350. 2018.PubMed/NCBI View Article : Google Scholar
|
|
137
|
Bastos R, Mathias M, Andrade R, Amaral
RJFC, Schott V, Balduino A, Bastos R, Miguel Oliveira J, Reis RL,
Rodeo S and Espregueira-Mendes J: Intra-articular injection of
culture-expanded mesenchymal stem cells with or without addition of
platelet-rich plasma is effective in decreasing pain and symptoms
in knee osteoarthritis: A controlled, double-blind clinical trial.
Knee Surg Sports Traumatol Arthrosc. 28:1989–1999. 2020.PubMed/NCBI View Article : Google Scholar
|
|
138
|
Emadedin M, Labibzadeh N, Liastani MG,
Karimi A, Jaroughi N, Bolurieh T, Hosseini SE, Baharvand H and
Aghdami N: Intra-articular implantation of autologous bone
marrow-derived mesenchymal stromal cells to treat knee
osteoarthritis: A randomized, triple-blind, placebo-controlled
phase 1/2 clinical trial. Cytotherapy. 20:1238–1246.
2018.PubMed/NCBI View Article : Google Scholar
|
|
139
|
Hernigou P, Auregan JC, Dubory A,
Flouzat-Lachaniette CH, Chevallier N and Rouard H: Subchondral stem
cell therapy versus contralateral total knee arthroplasty for
osteoarthritis following secondary osteonecrosis of the knee. Int
Orthop. 42:2563–2571. 2018.PubMed/NCBI View Article : Google Scholar
|
|
140
|
Gupta PK, Chullikana A, Rengasamy M,
Shetty N, Pandey V, Agarwal V, Wagh SY, Vellotare PK, Damodaran D,
Viswanathan P, et al: Efficacy and safety of adult human bone
marrow-derived, cultured, pooled, allogeneic mesenchymal stromal
cells (Stempeucel®): Preclinical and clinical trial in
osteoarthritis of the knee joint. Arthritis Res Ther.
18(301)2016.PubMed/NCBI View Article : Google Scholar
|
|
141
|
Song Y, Du H, Dai C, Zhang L, Li S, Hunter
DJ, Lu L and Bao C: Human adipose-derived mesenchymal stem cells
for osteoarthritis: A pilot study with long-term follow-up and
repeated injections. Regen Med. 13:295–307. 2018.PubMed/NCBI View Article : Google Scholar
|
|
142
|
Lee WS, Kim HJ, Kim KI, Kim GB and Jin W:
Intra-Articular injection of autologous adipose tissue-derived
mesenchymal stem cells for the treatment of knee osteoarthritis: A
phase IIb, randomized, placebo-controlled clinical trial. Stem
Cells Transl Med. 8:504–511. 2019.PubMed/NCBI View Article : Google Scholar
|