Introduction
Worldwide, gastric cancer (GC) affects over a
million individuals and leads to ~783,000 deaths each year, being
the 5th most common type of cancer and the 3rd major cause of
cancer-related deaths, in both sexes (1). Despite the existence of different
patterns of acquisition (sporadic, familial or hereditary), the
most widely accepted mechanism of gastric carcinogenesis describes
the evolution from chronic atrophic gastritis into intestinal
metaplasia, dysplasia and finally, the occurrence of sporadic GC
(2,3). Other risk factors, such as
Helicobacter pylori, a high level of salt intake and genetic
polymorphisms in pro- and anti-inflammatory cytokine coding genes,
have been considered to have a significant impact, and the
interaction between these factors may be crucial for development of
cancer (4-6).
TP53 is the most commonly mutated gene in
numerous types of cancer; it encodes one of the most important
tumor suppressors proteins, p53, which impacts multiple pathways of
carcinogenesis, not only due to mutations, but also by the
de-regulation of p53 pathways (7,8). The
murine double minute 2 (MDM2) gene encodes for mdm2, an E3
ubiquitin ligase, that acts as the major p53 negative regulator
implicated in several types of cancer (9,10). Under
physiological conditions, mdm2 binds to the p53 transactivation
domain, leading to the inhibition of its transcriptional
activities, followed by the promotion of proteasomal degradation
and p53 export from the nucleus, inactivating its functions
(11). Some studies have shown that
MDM2 amplification occurs in GC, with an expected impact on
p53 pathways (12,13). Furthermore, similar to what has been
described for other types of cancer (14-19),
MDM2 polymorphisms that can lead to differential protein
activity may have an impact on GC susceptibility (20-23).
The aim of the present study was to summarize the
studies that analyzed MDM2 polymorphisms and their
associations with the risk of GC development by performing a
systematic review of published manuscripts.
Materials and methods
Literature search and study
selection
A systematic review of the literature was performed
using the Preferred Reporting Items for Systematic Reviews and
Meta-Analyses (PRISMA) guidelines (24). The literature search was performed
using PubMed and Scopus on 18th October 2019 (and revised on 31st
July 2020) using the following key words combination: ‘gastric
cancer AND polymorphism AND MDM2’. Different combinations of words
and MESH terms were tested and the selected query was the most
representative. The literature search was performed independently
by two of the authors without any restrictions on time, sample size
or population studied.
Studies were included if they met the following
inclusion criteria: i) Assessment of any MDM2 polymorphism and risk
of GC; ii) case-controlled design, and iii) genotype frequencies
for both cases and controls were provided. Studies were excluded if
i) they were published in any language other than English; ii)
duplicated data; iii) they used another study design rather than
case-control (reports of clinical cases, comments, series, reviews
and editorials); and iv) if insufficient data was provided or the
data was not available. Review studies were checked for their
references for other relevant studies. Articles which included ≥2
case-control tests or ≥2 single-nucleotide polymorphisms (SNPs)
were regarded as two or more different studies. Case-control
studies were the only selected type to be included as they provide
the necessary data for meta-analysis considering the association
with GC risk. The reference lists of the selected studies and prior
systematic reviews/meta-analysis was also reviewed and compared
with our list of included studies.
Data extraction
According to the PRISMA guidelines, each step was
performed independently by two investigators and discrepancies were
decided by a third investigator. Briefly, manuscripts were first
screened by analyzing titles and abstracts, based on the
inclusion/exclusion criteria. Full texts were then reviewed and
data extracted (first author, year of publication, original
country, the ethnicity of the population studied, genotyping
method, histological GC, numbers for cases and controls of all
genotypes). All studies were assessed for Hardy-Weinberg
equilibrium of genotypes distribution and a qualitative analysis
was performed based on the Newcastle-Ottawa Scale (NOS) and
considering important information for case-controlled studies
(Table SI) (25). All articles with a NOS scale score ≥8
were considered high-quality studies.
Statistical analysis
The collected data were analyzed using Review
Manager version 5.3 (The Nordic Cochrane Centre, The Cochrane
Collaboration) to assess the association between the different
genotypes and GC risk, by calculating the odds ratios (OR) and the
confidence intervals (CI) with P<0.05. Funnel and forest plots
were created to summarize the differences in the studies and their
significance, considering the relative weight of each study,
according to the random effects model.
Results
Characteristics of the included
studies
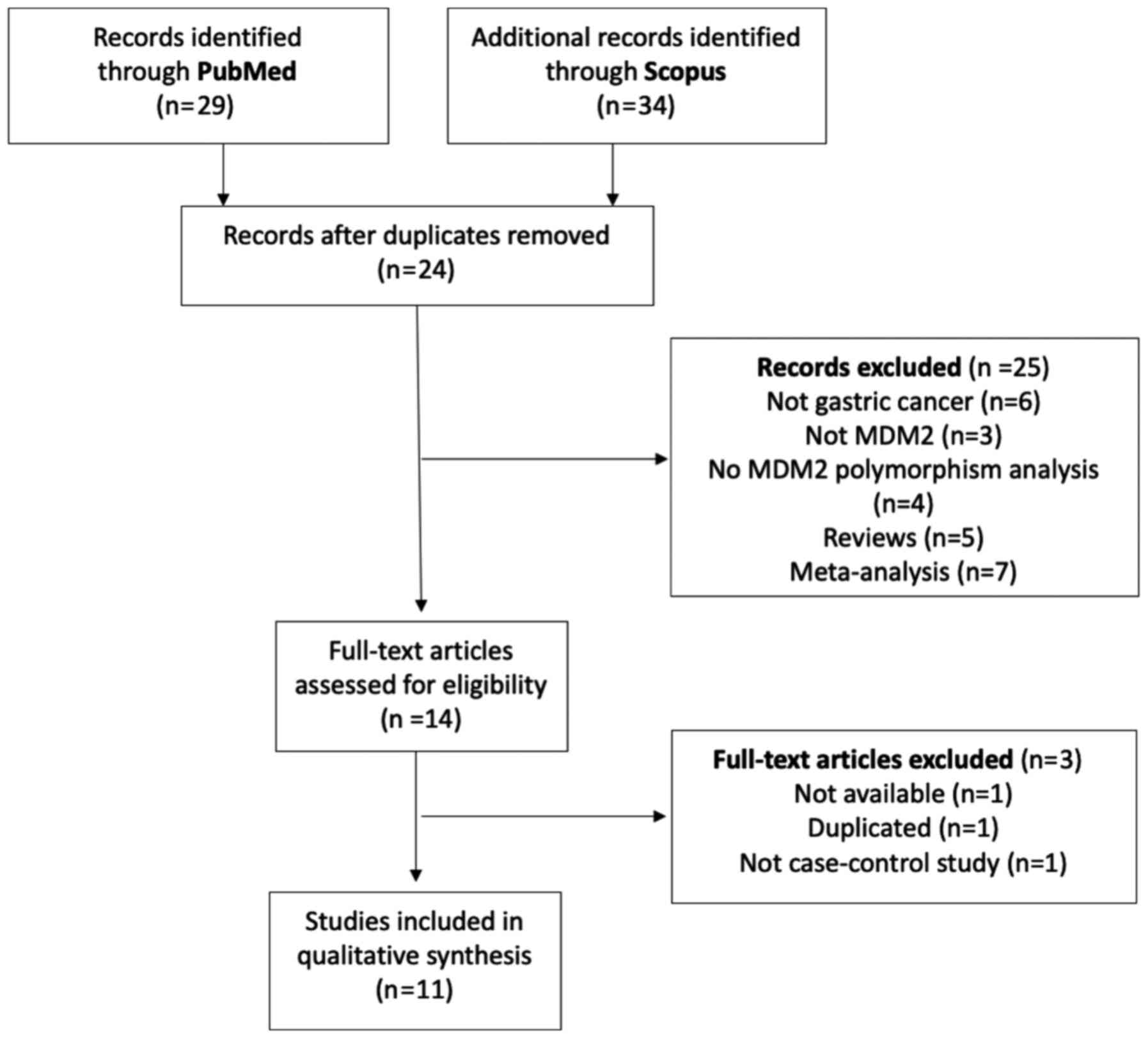
A flow diagram of study selection is presented in
Fig. 1. The literature search in
PubMed and Scopus provided a total of 63 manuscripts, of which 24
were duplicated between databases. All abstracts from the remaining
39 manuscripts were reviewed, and 25 were excluded for the
following reasons: Not GC (n=6), not MDM2 (n=3), no
MDM2 polymorphism analysis (n=4), reviews (n=5) and
meta-analysis (n=7). A total of 14 manuscripts were assessed for
full-review, of which three were excluded: One manuscript was not
available, one had a duplicated population of another published
study and one was not a case-controlled study. After the revision
process, a total of 11 articles were used for data analysis
(21,23,26-34).
Amongst the included studies, 10 studies were performed in Asian
populations and one in a Brazilian population.
Regarding the SNPs studied in association with GC, 1
study analyzed rs937283, 1 analyzed rs3730485 and 9 studies
analyzed the rs2279744 polymorphism. Both rs937283 and rs3730485
reports showed an association with GC, nevertheless there was only
one study of each in the literature, thus no further analysis was
performed. Regarding rs2279744, a total of 3,003 cases of GC and
3,676 controls were included in the present meta-analysis. The
genotyping information of the included studies is described in
Table I and the population
demographics of each study are presented in Table II. Amongst rs2279744, the genotype
distributions in the controls of 4 studies (23,28,29,31) were
not consistent with the HWE (P<0.050); and regarding the quality
analysis, one study was considered of ‘low quality’ (QA score
<8; Table I and SII).
 | Table IStudies included in the present
meta-analysis. |
Table I
Studies included in the present
meta-analysis.
| Studies assessing
each single nucleotide polymorphism | Genotyping
method | Cases genotyped, n
(%) | Controls genotyped,
n (%) | Hardy-Weinberg
Equilibrium P-value | Quality analysis
score | Refs. |
|---|
| rs937283 | | AA | AG | GG | AA | AG | GG | | | |
|
Chen et
al, 2018 | PCR-RFLP | 318 (69.1) | 123 (26.7) | 19 (4.1) | 600 (75.0) | 182 (22.8) | 18 (2.2) | 0.344 | 9 | (26) |
| rs3730485 | | InsIns | InsDel | DelDel | InsIns | InsDel | DelDel | | | |
|
Cavalcante
et al, 2017 | Multiplex PCR | 61 (50.8) | 46 (38.3) | 13 (10.8) | 274 (57.7) | 168 (35.4) | 33 (7.0) | 0.301 | 5 | (27) |
| rs2279744 | | TT | TG | GG | TT | TG | GG | | | |
|
Tas et
al, 2017 | PCR-RFLP | 4 (6.1) | 39 (60.0) | 22 (33.8) | 10 (14.9) | 45 (67.2) | 12 (17.9) | 0.005b | 8 | (28) |
|
Elingarami
et al, 2015 | qPCR | 28 (26.7) | 20 (19.0) | 57 (57.3) | 75 (63.6) | 36 (30.5) | 7 (5.9) | 0.348 | 8 | (21) |
|
Wu and
Zhang, 2015 | qPCR | 153 (23.8) | 288 (44.9) | 201 (31.3) | 255 (35.4) | 294 (40.8) | 171 (23.8) |
<0.001c | 8 | (31) |
|
Moradi et
al, 2013 | PCR-RFLP | 16 (7.7) | 156 (75.0) | 36 (17.3) | 60 (30.0) | 132 (66.0) | 8 (4.0) |
<0.001c | 8 | (29) |
|
Pan et
al, 2013 | PCR-RFLP | 173 (30.1) | 260 (45.3) | 141 (24.6) | 199 (34.7) | 296 (51.6) | 79 (13.8) | 0.060 | 9 | (30) |
|
Wang et
al, 2009 | PCR-RFLP | 74 (28.5) | 120 (46.1) | 66 (25.4) | 82 (31.5) | 141 (54.2) | 37 (14.2) | 0.057 | 11 | (32) |
|
Cho et
al, 2008 | PCR-RFLP | 64 (26.8) | 110 (46.0) | 65 (27.2) | 61 (20.4) | 152 (50.8) | 86 (28.8) | 0.680 | 6 | (33) |
|
Yang et
al, 2007 | PCR-RFLP | 107 (21.4) | 250 (50.0) | 143 (28.6) | 298 (29.8) | 498 (49.8) | 204 (20.4) | 0.877 | 11 | (34) |
|
Ohmiya et
al, 2006 | PCR-RFLP | 98 (23.9) | 188 (45.8) | 124 (30.2) | 99 (22.6) | 241 (55.0) | 98 (22.4) | 0.036a | 10 | (23) |
 | Table IIDemographic characteristics of the
cohorts used in the included studies. |
Table II
Demographic characteristics of the
cohorts used in the included studies.
| | Cases | Controls | |
|---|
| Studies assessing
each single nucleotide polymorphism | Ethnicity | Age, years, n
(%) | Male, n (%) | Female, n (%) | Age, years, n
(%) | Male, n (%) | Female, n (%) | Refs. |
|---|
| rs937283 | | | | | | | | |
|
Chen et
al, 2018 | Chinese | ≤ 60, 252 (54.8%)
> 60, 208 (45.2%) | 323 (70.3) | 137 (29.7) | ≤ 60, 434 (54.3%)
> 60, 366 (45.7%) | 558 (69.7) | 242 (30.3) | (26) |
| rs3730485 | | | | | | | | |
|
Cavalcante
et al, 2017 | Brazilian |
57.02±1.29a | 66 (55.0) | 54 (45.0) |
55.59±0.91a | 165 (34.7) | 310 (65.3) | (27) |
| rs2279744 | | | | | | | | |
|
Tas et
al, 2017 | Turkish | 62.25
±10.95a | 53 (81.5) | 12 (18.5) |
62.34±10.54a | 53 (79.1) | 14 (20.9) | (28) |
|
Elingarami
et al, 2015 | Chinese | 64
(29-87)b | NA | NA | 63
(32-89)b | NA | NA | (21) |
|
Wu and
Zhang, 2015 | Chinese |
60.2±11.2a | 366 (57.0) | 276 (43.0) |
61.4±10.6a | 423 (58.7) | 297 (41.3) | (31) |
|
Moradi et
al, 2013 | Iranian |
67.8±8.84a | 120 (57.7) | 88 (42.3) |
64.2±5.14a | 124 (62.0) | 76 (38.0) | (29) |
|
Pan et
al, 2013 | Chinese |
58.56±12.09a | 399 (69.5) | 175 (30.5) |
58.29±11.88a | 399 (69.5) | 175 (30.5) | (30) |
|
Wang et
al, 2009 | Chinese |
58.6±12.4a | 176 (67.7) | 84 (32.3) |
58.3±11.6a | 176 (67.7) | 84 (32.3) | (32) |
|
Cho et
al, 2008 | Korean | 61
(22-85)c | 154 (64.4) | 85 (35.6) | 45d | 164 (54.8) | 135 (45.2) | (33) |
|
Yang et
al, 2007 | Chinese | ≤50, 14 (2.8%)
51-60, 62 (12.4%) 61-70, 165 (33.0%) >70, 259 (51.8%) | 430 (86.0) | 70 (14.0) | ≤50, 28 (2.8%)
51-60, 124 (12.4%) 61-70, 330 (33.0%) >70, 518 (51.8%) | 860 (86.0) | 140 (14.0) | (34) |
|
Ohmiya et
al, 2006 | Japanese |
62.8±11.8a | 302 (73.7) | 108 (26.3) |
53.4±10.1a | 326 (74.4) | 112 (25.6) | (23) |
Meta-analysis
Of the three SNPs described in the different
studies, only rs2279744 was the subject of more than one study with
genotyping data. Data analysis was performed considering the G
allele as the risk allele in different genetic models: i) the
dominant model (GG + TG vs. TT), and ii) the recessive model (GG
vs. TT + TG) (35). Meta-analysis
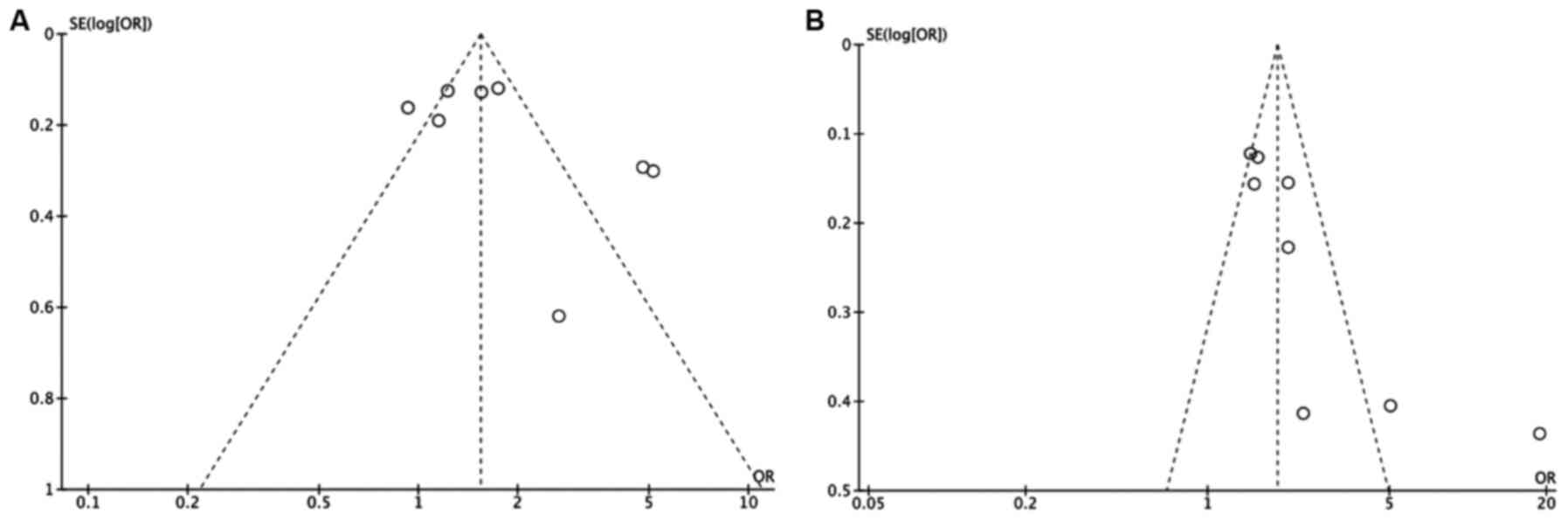
was performed only with studies with a QA score ≥8. In the funnel
plot analysis, three studies deviated from the expected outcomes
either in the dominant or recessive model analysis (P<0.05;
Fig. 2). The studies which showed
deviation from the expected outcomes, according to both genetic
models, were Elingarami et al (21) and Moradi et al (29), as well as also Ohmiya et al
(23) in the dominant model, which
may indicate publication bias. For this reason, they were
eliminated from the respective genetic model of the
meta-analysis.
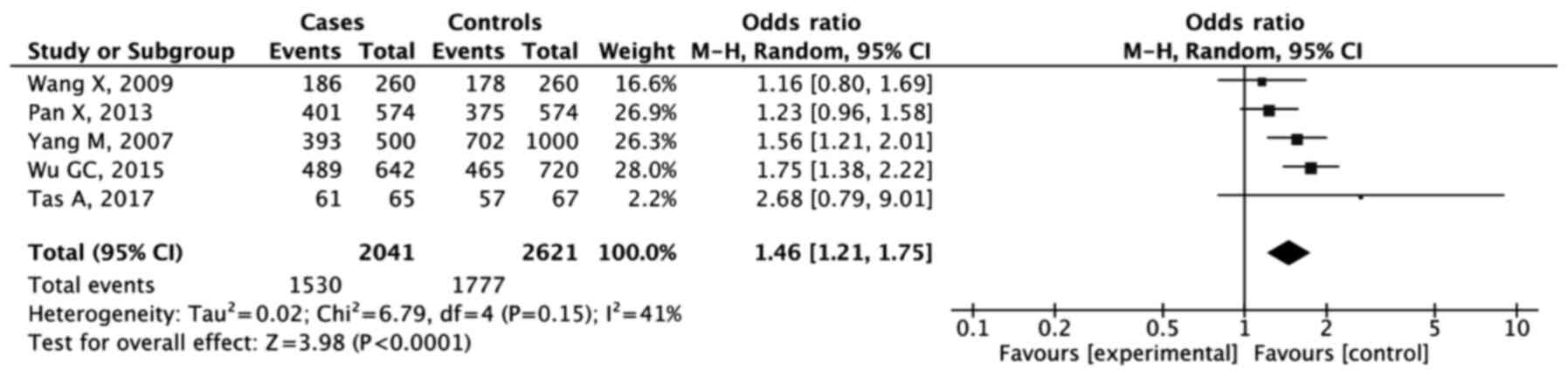
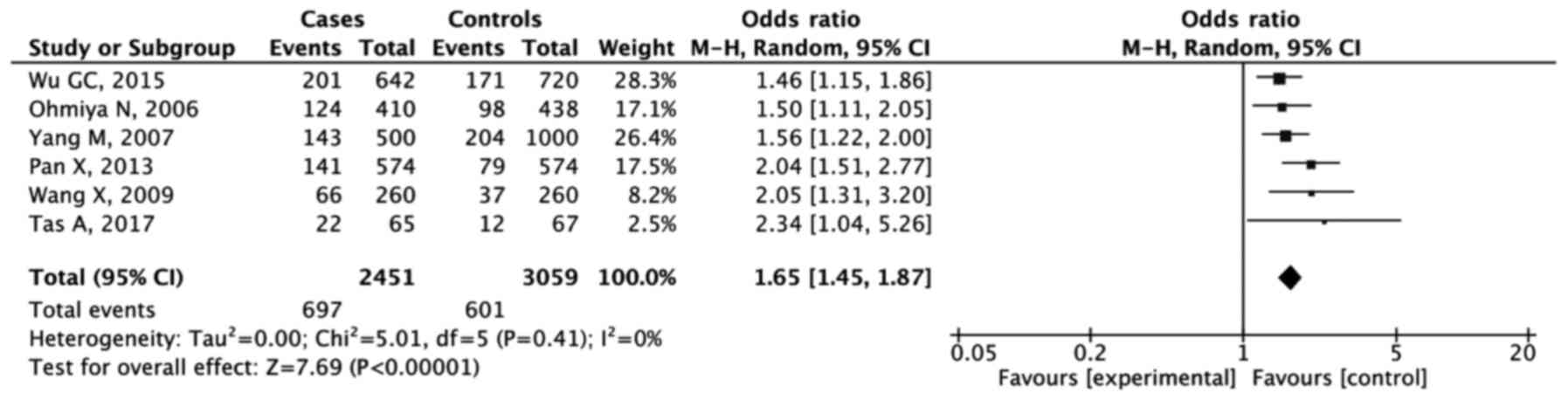
As shown in the forest plots (Figs. 3 and 4), the results of analysis of the dominant
model showed that GG + GT were significantly associated with
increased GC risk compared with the TT genotype (OR, 1.46; 95% CI,
1.21-1.75; P<0.001). The combined analysis for the recessive
model also showed an increased risk of GC development for the GG
genotype (OR, 1.65; 95% CI, 1.45-1.87; P<0.001).
Discussion
Dysregulated MDM2 expression has been shown
to associated with several types of cancer due to its p53
regulatory functions (9). Under
increased exposure to stress, p53 expression increases promoting
transcriptional activity that leads to cell cycle arrest, repair
and/or apoptosis (11). This
increased p53 expression leads to increased mdm2 protein expression
since the MDM2 P2 promoter is p53-dependent, establishing an
autoregulatory feedback loop (19).
Indeed, MDM2 is considered an oncogene because of its p53
inhibition function (19). Under
physiological conditions, mdm2 E3 ubiquitin ligase domain binds the
p53 transactivation domain, leading to the inhibition of the
transcriptional activity of p53, followed by an increase in
proteasomal degradation and ended by the export of p53 from the
cell nucleus, which is crucial for the repression of p53 suppressor
function (11). In addition to p53
regulation, MDM2 also serves an oncogenic function by
interfering with the functions of other proteins that participate
in several pathways, including DNA repair, apoptosis, motility and
invasion (19). Studies have shown
that MDM2 amplification has been detected in several types
of cancer, including GC (10,19). In
GC, MDM2 has been shown to be amplified (12) and overexpressed in GC tissues
(36,37), and this has also been associated with
a higher grade of tumor differentiation, deeper invasion and nodal
and distant metastasis (37).
Previous studies have shown an association between
MDM2 and GC (7,12). Considering the impact that some
genetic polymorphisms have on the risk of developing GC, a
systematic review was performed to clarify the associations between
MDM2 polymorphisms and GC. The NCBI SNP database contains a
total of 9,642 MDM2 polymorphisms; however, only a few have
been studied for their potential functional role in cancer. In the
present study, only three different MDM2 polymorphisms
(rs937283, rs3730485 and rs2279744) that have been studied in
association with GC development were found. The previous studies on
the rs2279744 and rs937283 polymorphisms suggested that they may
affect expression of MDM2, leading to higher degradation of
p53 and consequently to the loss of the primary tumor suppressor
pathways and therefore increase the risk of developing cancer.
The rs937283 polymorphism is characterized by an A
to G change in the nucleotide at position 2,164 of the MDM2
promoter region, and this seems to lead to an increase in mdm2
expression (26,38). This SNP was studied by Chen et
al (26), and they concluded
that it significantly increased the risk of developing GC in the
Chinese population. In their study, the G allele was associated
with an increased risk of developing GC either when G carriers vs.
AA (OR, 1.34; P=0.024), and despite not being statistically
significant, an association was observed for GG vs. A carriers (OR,
1.87; P=0.061). This SNP has been described to significantly
enhance the transcriptional activity of the MDM2 gene
increasing the mRNA and protein levels, and additionally, this
polymorphism has been studied in other types of cancer, such as
lung cancer (26), liver cancer
(39) and retinoblastoma (40), with similar effects reported.
The rs3730485 polymorphism, a 40 bp deletion in the
P1 promoter of MDM2, was studied by Cavalcante et al
(27) in a Brazilian population,
where it was shown to exert a protective effect against the
development of GC, and is associated with an homozygous insertion
of 40-bp (OR, 0.41; P=0.021). It has been suggested that the
insertion of this 40-bp insert may reduce the activity of MDM2 and
increase the availability of p53 in the cells reducing the chances
of developing cancer. This polymorphism has been differentially
associated with several types of cancer, including breast (41,42),
prostate (42), ovarian (43) and hepatocellular carcinoma (44). Furthermore, it has been suggested
that this SNP is in linkage disequilibrium with SNP 309, and
therefore its impact may also be dependent on the SNP 309 genotype
(18,43).
The rs2279744, also known as SNP 309, is the most
studied MDM2 polymorphism and it is characterized by a T to
G change in the nucleotide at position 309 of the P2 promoter of
MDM2. This genotype change seems to increase the affinity of
the SP1 transcription factor, thus increasing mdm2 expression, and
subsequently leading to increased inhibition of p53-dependent
pathways (45). This polymorphism
has been associated with several types of cancer, such as bladder
(46), endometrial (47), cervical (48), hepatocellular (44) and colorectal cancer (14,49),
amongst other types of cancer. The literature review identified a
total of 9 individual relevant case-controlled studies on the
MDM2 rs2279744 polymorphism and GC risk. Of note, all
studies were performed in Asian populations, most of them Chinese,
which is expected, taking into account that these populations have
the highest incidence rates of GC (50). Of the studies, 4 were not consistent
with HWE (23,28,29,31) and
the funnel plots revealed that some of these studies may have bias
that may be affecting their results. Nevertheless, the analysis
revealed a significant association between the SNP 309 G allele and
increased risk of GC development, particularly in the homozygous
model, in accordance with published studies (23,28,30-32,34).
Of the previously published meta-analyses regarding MDM2
SNPs and GC, all of them focused on SNP 309, 4 of which (with a
range of 5-11 included manuscripts) showed that the SNP 309 G
allele is associated with an increased risk of GC (51-54);
1 meta-analysis (which included 6 studies), showed the opposite
association, but only the recessive model was analyzed (55); and the remaining 2 meta-analyses were
not specific to GC; nevertheless they show an association between
SNP 309 and cancer, overall (45,56).
The present study has some limitations. First,
regarding the genotyping methods, 1 study was performed using
quantitative PCR (31), whereas the
other five were performed using PCR-Restriction Fragment Length
Polymorphism (23,28,30,32,34). The
different methods of genotyping may impact the quality of the
genotyped data, since the specificity and sensitivity are variable
and therefore, some variation in the genotype distribution is
possible. Another source of limitations is the fact that the
majority of studies are from the Chinese population, and the
outcomes may not be applicable to individuals of other ethnicities.
Nevertheless, China has a higher incidence of GC, which may explain
the extra interest for studies of this nature, and may help to
predict the potential impact in other areas at high-risk of GC
(1). The low number of studies, some
of which had a smaller number of cases may also impact the quality
of the data analyzed. Indeed, qualitative analysis of studies
should be performed once there is more significant data. Another
issue is the importance of considering the role of mdm2 in the
molecular mechanisms underlying gastric carcinogenesis, and the
lack of information regarding its expression in precursor
lesions.
Numerous studies have shown the roles of genetic
polymorphisms of several genes in almost every aspect of cancer,
with a potential impact on the clinical outcomes. Several
MDM2 polymorphisms have been studied, and some of these
appear to affect protein expression, and therefore MDM2
variants may have an impact on the treatment response when treated
with MDM2 inhibitors, particularly in cases of cancer with a
low frequency of p53 mutations (57-59).
The present study revealed that three different MDM2 genetic
polymorphisms (rs937282, rs3730485 and rs2279744) have been studied
for their association with the development of GC. The present study
showed that these three MDM2 polymorphisms were associated
with GC development, particularly rs2279744 (SNP 309), which was
significantly associated with GC development. The fact that the
number of studies is low and the studied populations are primarily
Asian emphasizes the need for more studies in other populations to
corroborate the association of these polymorphisms with GC.
Supplementary Material
Scale for assessment of the quality of
the studies.
Quality assessment criteria scores for
each study.
Acknowledgements
Not applicable.
Funding
This study was supported by the Northern Portugal Regional
Operational Programme (NORTE 2020), under the Portugal 2020
Partnership Agreement, through the European Regional Development
Fund (grant no. NORTE-01-0145-FEDER-000027).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
MT obtained, analyzed and interpreted the data, and
drafted the article. AT acquired the data and drafted the article.
SC and CC contributed to the writing of the article and performed
the final review of the contents. RM conceived and designed the
study. HS conceived and designed the study, analyzed and
interpreted the data, and revised the manuscript. HS and MT confirm
the authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Röcken C: Molecular classification of
gastric cancer. Expert Rev Mol Diagn. 17:293–301. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Correa P: A human model of gastric
carcinogenesis. Cancer Res. 48:3554–3560. 1988.PubMed/NCBI
|
|
4
|
Amieva M and Peek RM Jr: Pathobiology of
Helicobacter pylori-Induced Gastric Cancer.
Gastroenterology. 150:64–78. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Cavatorta O, Scida S, Miraglia C, Barchi
A, Nouvenne A, Leandro G, Meschi T, De' Angelis GL and Di Mario F:
Epidemiology of gastric cancer and risk factors. Acta Biomed.
89:82–87. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Melchiades JL, Zabaglia LM, Sallas ML,
Orcini WA, Chen E, Smith MAC, Payão SLM and Rasmussen LT:
Polymorphisms and haplotypes of the interleukin 2 gene are
associated with an increased risk of gastric cancer. The possible
involvement of Helicobacter pylori. Cytokine. 96:203–207.
2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Busuttil RA, Zapparoli GV, Haupt S,
Fennell C, Wong SQ, Pang JM, Takeno EA, Mitchell C, Di Costanzo N,
Fox S, et al: Role of p53 in the progression of gastric cancer.
Oncotarget. 5:12016–12026. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Aubrey BJ, Strasser A and Kelly GL:
Tumor-suppressor functions of the TP53 pathway. Cold Spring Harb
Perspect Med. 6(a026062)2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Yu H, Huang YJ, Liu Z, Wang LE, Li G,
Sturgis EM, Johnson DG and Wei Q: Effects of MDM2 promoter
polymorphisms and p53 codon 72 polymorphism on risk and age at
onset of squamous cell carcinoma of the head and neck. Mol
Carcinog. 50:697–706. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
10
|
Baliou E, Nonni A, Keramopoulos D, Ragos
V, Tsiambas E, Patsouris E and Pavlakis K: Deregulation of p53-MDM2
auto-regulatory pathway in breast carcinoma. J BUON. 21:1099–1103.
2016.PubMed/NCBI
|
|
11
|
Bond GL, Hu W and Levine AJ: MDM2 is a
central node in the p53 pathway: 12 years and counting. Curr Cancer
Drug Targets. 5:3–8. 2005.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Günther T, Schneider-Stock R, Häckel C,
Kasper HU, Pross M, Hackelsberger A, Lippert H and Roessner A: Mdm2
gene amplification in gastric cancer correlation with expression of
Mdm2 protein and p53 alterations. Mod Pathol. 13:621–626.
2000.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ooi A, Oyama T, Nakamura R, Tajiri R,
Ikeda H, Fushida S, Nakamura H and Dobashi Y: Semi-comprehensive
analysis of gene amplification in gastric cancers using multiplex
ligation-dependent probe amplification and fluorescence in situ
hybridization. Mod Pathol. 28:861–871. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Qin X, Peng Q, Tang W, Lao X, Chen Z, Lai
H, Deng Y, Mo C, Sui J, Wu J, et al: An updated meta-analysis on
the association of MDM2 SNP309 polymorphism with colorectal cancer
risk. PLoS One. 8(e76031)2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Sousa H, Pando M, Breda E, Catarino R and
Medeiros R: Role of the MDM2 SNP309 polymorphism in the initiation
and early age of onset of nasopharyngeal carcinoma. Mol Carcinog.
50:73–79. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
16
|
Varmazyar S, Marashi SM, Shoja Z,
Tornesello ML, Buonaguro FM, Shahmahmoodi S, Safaie-Naraghi Z and
Jalilvand S: MDM2 gene polymorphisms and risk of classic Kaposi's
sarcoma among Iranian patients. Med Microbiol Immunol (Berl).
206:157–163. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Grochola LF, Zeron-Medina J, Mériaux S and
Bond GL: Single-nucleotide polymorphisms in the p53 signaling
pathway. Cold Spring Harb Perspect Biol. 2(a001032)2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Gansmo LB, Vatten L, Romundstad P, Hveem
K, Ryan BM, Harris CC, Knappskog S and Lønning PE: Associations
between the MDM2 promoter P1 polymorphism del1518 (rs3730485) and
incidence of cancer of the breast, lung, colon and prostate.
Oncotarget. 7:28637–28646. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Nag S, Qin J, Srivenugopal KS, Wang M and
Zhang R: The MDM2-p53 pathway revisited. J Biomed Res. 27:254–271.
2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Mendoza M, Mandani G and Momand J: The
MDM2 gene family. Biomol Concepts. 5:9–19. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Elingarami S, Liu H, Kalinjuma AV, Hu W,
Li S and He N: Polymorphisms in NEIL-2, APE-1, CYP2E1 and MDM2
genes are independent predictors of gastric cancer risk in a
Northern Jiangsu population (China). J Nanosci Nanotechnol.
15:4815–4828. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Moradi MT, Salehi Z, Aminian K and
Yazdanbod A: Effects of p53 codon 72 and MDM2 SNP309 polymorphisms
on gastric cancer risk among the Iranian population. Asian Pac J
Cancer Prev. 15:7413–7417. 2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ohmiya N, Taguchi A, Mabuchi N, Itoh A,
Hirooka Y, Niwa Y and Goto H: MDM2 promoter polymorphism is
associated with both an increased susceptibility to gastric
carcinoma and poor prognosis. J Clin Oncol. 24:4434–4440.
2006.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Page MJ, Moher D, Bossuyt PM, Boutron I,
Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: PRISMA 2020 explanation and elaboration: Updated
guidance and exemplars for reporting systematic reviews. BMJ.
372(n160)2021.PubMed/NCBI View
Article : Google Scholar
|
|
25
|
Wells GA, Shea B, O'Connell D, Peterson J,
Welch V, Losos M and Tugwell P: The Newcastle-Ottawa Scale (NOS)
for Assessing the Quality of Nonrandomised Studies in
Meta-Analyses. Ottawa Health Research Institute, Ottawa, Ontario,
1999.
|
|
26
|
Chen B, Wang J, Chen Y, Gu X and Feng X:
The MDM2 rs937283 A > G variant significantly increases the risk
of lung and gastric cancer in Chinese population. Int J Clin Oncol.
23:867–876. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Cavalcante GC, Amador MA, Ribeiro Dos
Santos AM, Carvalho DC, Andrade RB, Pereira EE, Fernandes MR, Costa
DF, Santos NP, Assumpção PP, et al: Analysis of 12 variants in the
development of gastric and colorectal cancers. World J
Gastroenterol. 23:8533–8543. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Tas A, Atabey M, Caglayan G, Bostanci ME,
Sahin Bolukbasi S, Topcu O and Silig Y: Investigation of the
association between the MDM2 T309G polymorphism and gastric cancer.
Biomed Rep. 7:469–473. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Moradi MT, Salehi Z, Asl SF, Aminian K and
Hashtchin AR: Helicobacter pylori infection and MDM2 SNP309
association with gastric cancer susceptibility. Genet Test Mol
Biomarkers. 17:794–798. 2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Pan X, Li Y, Feng J, Wang X, Hao B, Shi R
and Zhang G: A functional polymorphism T309G in MDM2 gene promoter,
intensified by Helicobacter pylori lipopolysaccharide, is
associated with both an increased susceptibility and poor prognosis
of gastric carcinoma in Chinese patients. BMC Cancer.
13(126)2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wu GC and Zhang ZT: Genetic association of
single nucleotide polymorphisms in P53 pathway with gastric cancer
risk in a Chinese Han population. Med Oncol. 32(401)2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wang X, Yang J, Ho B, Yang Y, Huang Z,
Zhang Z and Zhang G: Interaction of Helicobacter pylori with
genetic variants in the MDM2 promoter, is associated with gastric
cancer susceptibility in Chinese patients. Helicobacter.
14:114–119. 2009.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Cho YG, Choi BJ, Song JH, Kim CJ, Cao Z,
Nam SW, Lee JY and Park WS: No association of MDM2 T309G
polymorphism with susceptibility to Korean gastric cancer patients.
Neoplasma. 55:256–260. 2008.PubMed/NCBI
|
|
34
|
Yang M, Guo Y, Zhang X, Miao X, Tan W, Sun
T, Zhao D, Yu D, Liu J and Lin D: Interaction of P53 Arg72Pro and
MDM2 T309G polymorphisms and their associations with risk of
gastric cardia cancer. Carcinogenesis. 28:1996–2001.
2007.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Horita N and Kaneko T: Genetic model
selection for a case-control study and a meta-analysis. Meta Gene.
5:1–8. 2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kasper HU, Schneider-Stock R, Mellin W,
Günther T and Roessner A: P53-protein accumulation and MDM2-protein
overexpression in gastric carcinomas. No apparent correlation with
survival. Pathol Res Pract. 195:815–820. 1999.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Aboushousha T, Helal N, Hammam O, Ibrahim
M, Khaled S, Mostafa A and Anas A: Overview of MDM2 and B-RAF
Expression in Gastric Lesions. Open Access Maced J Med Sci.
6:1795–1802. 2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Jiao Y, Jiang Z, Wu Y, Chen X, Xiao X and
Yu H: A Functional Polymorphism (rs937283) in the MDM2 Promoter
Region is Associated with Poor Prognosis of Retinoblastoma in
Chinese Han Population. Sci Rep. 6(31240)2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Chen B, Wang J, Wang J, Zhang J, Gu X and
Feng X: The Study of MDM2 rs937283 Variant and Cancer
Susceptibility in a Central Chinese Population. Technol Cancer Res
Treat. 17(1533033818801550)2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Cao Q, Wang Y, Song X and Yang W:
Association between MDM2 rs2279744, MDM2 rs937283, and p21
rs1801270 polymorphisms and retinoblastoma susceptibility. Medicine
(Baltimore). 97(e13547)2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Gallegos-Arreola MP, Márquez-Rosales MG,
Sánchez-Corona J, Figuera LE, Zúñiga-González G, Puebla-Pérez AM,
Delgado-Saucedo JI and Montoya-Fuentes H: Association of the
Del1518 Promoter (rs3730485) Polymorphism in the MDM2 Gene with
Breast Cancer in a Mexican Population. Ann Clin Lab Sci.
47:291–297. 2017.PubMed/NCBI
|
|
42
|
Hashemi M, Amininia S, Ebrahimi M,
Simforoosh N, Basiri A, Ziaee SAM, Narouie B, Sotoudeh M,
Mollakouchekian MJ, Rezghi Maleki E, et al: Association between
polymorphisms in TP53 and MDM2 genes and susceptibility to prostate
cancer. Oncol Lett. 13:2483–2489. 2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Gansmo LB, Bjørnslett M, Halle MK,
Salvesen HB, Romundstad P, Hveem K, Vatten L, Dørum A, Lønning PE
and Knappskog S: MDM2 promoter polymorphism del1518 (rs3730485) and
its impact on endometrial and ovarian cancer risk. BMC Cancer.
17(97)2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Dong D, Gao X, Zhu Z, Yu Q, Bian S and Gao
Y: A 40-bp insertion/deletion polymorphism in the constitutive
promoter of MDM2 confers risk for hepatocellular carcinoma in a
Chinese population. Gene. 497:66–70. 2012.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Wan Y, Wu W, Yin Z, Guan P and Zhou B:
MDM2 SNP309, gene-gene interaction, and tumor susceptibility: An
updated meta-analysis. BMC Cancer. 11(208)2011.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Avirmed S, Wang BS, Selenge B, Sanjaajamts
A, Ganbat B, Erdenebileg U, Purevsuren M, Jigjidsuren S, Batmunkh M
and Lee YJ: Association between MDM2-SNP309 and p53R72P
polymorphisms and the risk of bladder cancer in the Mongolian
population. Mol Clin Oncol. 7:412–420. 2017.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Zou X, Zhang Y, Zhang L, Li J, Zhu C,
Cheng Q, Zhou J and Chen Y: Association between MDM2 SNP309 and
endometrial cancer risk: A PRISMA-compliant meta-analysis. Medicine
(Baltimore). 97(e13273)2018.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Knappskog S and Lønning PE: MDM2 SNP309
and risk of cervical cancer. Tumour Biol. 35:6185–6186.
2014.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Wang W, Du M, Gu D, Zhu L, Chu H, Tong N,
Zhang Z, Xu Z and Wang M: MDM2 SNP309 polymorphism is associated
with colorectal cancer risk. Sci Rep. 4(4851)2014.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Shen W, Hu P, Cao JQ, Liu XX and Shao JH:
MDM2 oncogene, E3 ubiquitin protein ligase T309G polymorphism and
risk of oesophageal or gastric cancer: Meta-analysis of 15 studies.
J Int Med Res. 42:1065–1076. 2014.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Chen W, Wu Q and Ren H: Meta-analysis of
associations between MDM2 SNP309 polymorphism and gastric cancer
risk. Biomed Rep. 2:105–111. 2014.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Song B, Duan ZY, Zhong YH, Lei N, Yang YQ
and Luo KY: Meta-analysis of the MDM2 T309G polymorphism and
gastric cancer risk. Asian Pac J Cancer Prev. 14:6649–6651.
2014.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Ma Y, Bian J and Cao H: MDM2 SNP309
rs2279744 polymorphism and gastric cancer risk: A meta-analysis.
PLoS One. 8(e56918)2013.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Tian X, Tian Y, Ma P, Sui CG, Meng FD, Li
Y, Fu LY, Jiang T, Wang Y, Ji FJ, et al: Association between MDM2
SNP309 T>G and risk of gastric cancer: A meta-analysis. Asian
Pac J Cancer Prev. 14:1925–1929. 2013.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Chen B, Cao L, Hu KW, Zhang JW, Meng XL
and Xiong MM: MDM2 SNP309 is an ethnicity-dependent risk factor for
digestive tract cancers. Tumour Biol. 35:3431–3438. 2014.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Ohnstad HO, Castro R, Sun J, Heintz KM,
Vassilev LT, Bjerkehagen B, Kresse SH, Meza-Zepeda LA and Myklebost
O: Correlation of TP53 and MDM2 genotypes with response to therapy
in sarcoma. Cancer. 119:1013–1022. 2013.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Tisato V, Voltan R, Gonelli A, Secchiero P
and Zauli G: MDM2/X inhibitors under clinical evaluation:
Perspectives for the management of hematological malignancies and
pediatric cancer. J Hematol Oncol. 10(133)2017.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Gupta A, Shah K, Oza MJ and Behl T:
Reactivation of p53 gene by MDM2 inhibitors: A novel therapy for
cancer treatment. Biomed Pharmacother. 109:484–492. 2019.PubMed/NCBI View Article : Google Scholar
|