Introduction
Type 2 diabetes is a disease that occurs when blood
glucose levels are high, which is referred to as hyperglycemia. The
severity of hyperglycemia is determined by an imbalance between
glucose entering and leaving the circulation. Carbohydrates from
the diet are hydrolyzed by digestive enzymes. α-Glucosidase or
sucrase-isomaltase cleaves the α-glucoside bonds of disaccharides,
including sucrose, to monosaccharides. The absorption of dietary
monosaccharides by the intestine may be one of the risk factors
associated with diabetes mellitus (1). Glucose derived from starch or sucrose
is taken up by the epithelial cells through the brush border
membrane (BBM), predominantly by a sodium-dependent glucose
co-transporter (SGLT1) (2,3). A previous study has revealed that
glucose transporter 2 (GLUT2) is expressed at the BBM of
enterocytes during the digestive phase and may affect circulatory
glucose concentration (2).
Subsequently, glucose effluxes across the basolateral membrane
(BLM) to the blood circulation via GLUT2. Therefore, any factor
that influences the activity of SGLT1 and GLUT2 also alters the
absorption and metabolism of glucose.
Previous studies have reported that intestinal SGLT1
mRNA expression is increased in streptozotocin-induced diabetic
rats and mice (4,5). Additionally, Otsuka Long-Evans
Tokushima Fatty rats exhibiting increased intestinal SGLT1 mRNA
expression combined with impaired glucose tolerance ultimately
developed insulin resistance and hyperinsulinemia (6). Similarly, patients with type 2 diabetes
mellitus also exhibit elevated intestinal glucose uptake as a
result of increased SGLT1 mRNA and protein expression (7). Furthermore, GLUT2 protein expression is
increased at the BBM of enterocytes in streptozotocin-induced
diabetic rats (8) and in response to
elevated glucose levels in the intestinal lumen (9). Therefore, intestinal SGLT1 and GLUT2
have a significant impact on the progression of diabetes
mellitus.
Coffea arabica is one of the most widely
consumed beverages. In traditional Chinese medicine, the green
Coffea bean is classified as a blood circulation warmer,
liver qi (Chinese concept of energy) and menstrual regulation,
stimulant, heart orifice opening, detoxification and tonification
herb (a traditional Chinese medicine therapeutic concept that
nourishes and replenishes the qi and blood of weakened individuals)
(10). At present, the Coffea
species C. arabica (Arabica) and C. canephora
(Robusta) are economically important in the global coffee market.
The major constituents of Coffea include caffeine,
chlorogenic acid, diterpenes and trigonelline (11). Moderate intake of Coffea has
been linked to a reduced risk of chronic diseases, including type 2
diabetes, Parkinson's disease and liver disease (12-14).
As mentioned above, there are several studies on the antidiabetic
potential of roast Coffea. However, to the best of our
knowledge, the effects of Coffea arabica bean extract (CBE)
on glucose absorption and the related mechanisms remain unknown.
The aim of the present study was to examine the effects of CBE on
glucose absorption in human Caco-2 colorectal adenocarcinoma cells.
Additionally, the molecular mechanisms involved in glucose
transport in an in vitro model were determined.
Materials and methods
Chemicals
DMEM/F12, CelLytic MT mammalian tissue
lysis/extraction reagents, phloretin, chlorogenic acid, caffeine,
caffeic acid and ferulic acid were purchased from Sigma-Aldrich;
Merck KGaA. FBS was purchased from Gibco; Thermo Fisher Scientific,
Inc. Polyclonal rabbit anti-SGLT1 (1:250; cat. no. 07-1417), GLUT2
(1:250; cat. no. 07-1402-I), 5' AMP-activated protein kinase (AMPK;
1:250; cat. no. 07-1417) and phospho-AMPK (1:250; cat. no. 07-363)
were purchased from Merck KGaA. Monoclonal mouse alkaline
phosphatase (ALP; 1:500; cat. no. NB110-3638) antibodies were
obtained from Novus Biologicals, LLC. Monoclonal mouse anti-β-actin
(1:500; cat. no. ab8224) was purchased from Abcam.
2-(N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)-2-deoxyglucose
(2-NBDG) was obtained from Thermo Fisher Scientific, Inc. Phlorizin
was purchased from Tocris Bioscience. All other chemicals with high
purity were obtained from commercial sources.
CBE extract preparation, purification
and qualification
Coffea arabica beans were kindly provided by
Hillkoff, and a voucher specimen (no. NU003806) was deposited at
the herbarium of the Faculty of Biology, Naresuan University,
Phitsanulok, Thailand. Dried Coffea arabica beans were
weighed and blended thoroughly, followed by 100˚C water infusion
for 30 min. Subsequently, the liquid extract was filtered through
filter paper (Whatman plc; GE Healthcare Life Sciences) three
times. The filtrate was evaporated using a lyophilizer (Labconco).
The chemical constituents of CBE, caffeine, chlorogenic acid,
caffeic acid and ferulic acid (Sigma-Aldrich; Merck KGaA), were
used as references for validation and quantitation by
high-performance liquid chromatography (HPLC) with diode array
detection on an Agilent 1200 series chromatograph (LabX) at the
Science and Technology Service Centre, Faculty of Science, Chiang
Mai University (Chiang Mai, Thailand) according to the ISO/IEC
17025 method (International Organization for Standardization, 2005)
(15). The flow rate was 0.5 ml/min.
The mobile phase was a binary solvent system consisting of 2%
acetic acid dissolved in HPLC water (solvent A) and absolute
methanol (solvent B). The column used was an Eclipse XDB-C18
(4.6x150 mm; 5 µm) and the absorption wavelength selected was 280
nm.
Cell culture
The Caco-2 human colorectal adenocarcinoma cell line
was purchased from American Type Culture Collection. Cells in the
2nd-22nd passage were grown in DMEM/F12 containing 20 and 1%
penicillin-streptomycin in a humidified atmosphere with 5% CO2.
Caco-2 cells were seeded in 24- and 96-well plates at a density of
5x104 cells/well and were cultured at 37˚C for 18-21
days to create a differentiated Caco-2 monolayer. Differentiated
Caco-2 monolayer forms a continuous barrier between the apical and
basolateral compartments and express several morphological and
functional characteristics of the absorptive enterocytes as found
in the small intestine (16). The
medium was replaced every 3 days during culture.
Glucose transport in human intestinal
Caco-2 cells
Differentiated Caco-2 cells were washed three times
with PBS (pH 7.4) and incubated with 2 mM 2-NBDG in the presence or
absence of 0.1-1,000 µg/ml CBE, a molar ratio of four major active
compounds of chlorogenic acid (29.62 µg/ml), caffeine (5.11 µg/ml),
caffeic acid (3.16 µg/ml), ferulic acid (1.54 µg/ml), phlorizin
(0.5 mM) and phloretin (1 mM) for 4 h. The medium was removed and
the cells were washed three times with ice-cold PBS. The
fluorescence intensity was measured using a Synergy™ HT microplate
reader (BioTek Instruments, Inc.) at excitation and emission
wavelengths of 485 and 535 nm, respectively.
Determination of cell viability
The viability of Caco-2 cells after exposure to CBE
and other test compounds was determined using an MTT assay. Caco-2
cells were seeded at a density of 5x104 cells/well in
96-well plates and cultured for 18-21 days at 37˚C. On the day of
the experiment, culture medium with or without test compounds was
added and the cells were incubated for 4 h at 37˚C. After exposure,
the cells were washed with PBS, culture medium containing MTT
reagent was added and the cells were incubated for the next 4 h at
37˚C. At the end of the experiment, the MTT solution was aspirated
and the cells were washed once with ice-cold PBS. DMSO was added to
each well, and cells were incubated for a further 30 min at 37˚C.
The absorbance of dissolved formazan was measured at a wavelength
of 570 nm using an M965 AccuReader microplate reader (Metertech,
Inc.). The sample detected at a wavelength of 680 nm was also used
as a reference.
Inhibition of disaccharidase activity
in Caco-2 cells
The dose of CBE used in this experiment was inferred
from the glucose transport experiment, which revealed that CBE at a
dose of 100 µg/ml reduced glucose transport to a similar extent to
that observed with CBE at a dose of 1,000 µg/ml. Therefore, CBE at
a dose of 100 µg/ml was selected for further experiments.
Inhibition of sucrase enzyme activity was determined as previously
described, with some modifications (17). Briefly, differentiated Caco-2 cells
were rinsed three times with Hank's balanced salt solution (HBSS)
(Sigma-Aldrich; Merck KGaA). The cells were subsequently incubated
with HBSS containing 28 mM sucrose, which is an α-glucosidase
substrate, in the presence or absence of 100 µg/ml CBE, a molar
ratio of four major active compounds of CBE at 100 µg/ml of
chlorogenic acid (29.62 µg/ml), caffeine (5.11 µg/ml), caffeic acid
(3.16 µg/ml), ferulic acid (1.54 µg/ml) and acarbose,
disaccharidase inhibitor (50 µM) at 37˚C for 40 min. After
incubation, the incubated solution was collected. The glucose
concentration was assayed using commercial enzymatic assay kits
(cat. no. BLT0002) obtained from Biotechnical, Co., Ltd. Cells were
lysed by three freeze-thaw cycles and protein concentration was
determined using a Bradford assay (Bio-Rad Laboratories, Inc.). The
data obtained are expressed as units per mg protein.
Reverse transcription-quantitative PCR
(RT-qPCR)
Previous studies have demonstrated that sterol
regulatory element-binding protein-1 (SREBP-1c) and hepatocyte
nuclear factor 1α (HNF1α) are involved in SGLT1 and GLUT2
expression and function. Therefore, the present study measured
SREBP-1c and HNF1α mRNA expression in Caco-2 cells after 4 h of
exposure to CBE and other test compounds. Total RNA was extracted
and purified from Caco-2 cells using TRIzol® reagent
(Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. First strand cDNA was synthesized using a SensiFAST™ cDNA
synthesis kit (Bioline; Meridian Bioscience) and qPCR was performed
using SYBR Real-Time PCR MasterMix (Bioline; Meridian Bioscience)
on a CFX Touch real-time PCR system (Bio-Rad Laboratories, Inc.).
PCR amplifications were performed in a 20-µl volume with the
following thermocycling conditions: A polymerase enzyme activation
step at 95˚C for 2 min; followed by 40 cycles consisting of 5 sec
of denaturation at 95˚C, 10 sec of annealing at 60˚C depending on
primers, and 10 sec of elongation at 72˚C. Forward and reverse
primers were purchased from Macrogen, Inc. and used at a final
concentration of 0.4 µM. The specific primer sets for human
SREBP-1c, HNF1α and GAPDH were as follows: Human (h)SREBP-1c
forward, 5'-ATACCACCAGCGTCTACC-3' and reverse,
5'-CACCAACAGCCCATTGAG-3'; hHNF1α forward,
5'-TACACCTGGTACGTCCGCAA-3' and reverse, 5'-CACTTGAAACGGTTCCTCCG-3';
and hGAPDH forward, 5'-AGCCTTCTCCATGGTGGTGAAGAC-3' and reverse,
5'-CGGAGTCAACGGATTTGGTCG-3'. The results were calculated using the
2-ΔΔCq method (18),
normalized to GAPDH mRNA levels and reported as the relative fold
change. qPCR amplification was performed in duplicate for each
synthesized cDNA set.
Subcellular fractionation and western
blot analysis
To measure protein expression of the glucose
transporters SGLT1, GLUT2, AMPK and phosphorylated AMPK (p-AMPK),
which is the SGLT1 and GLUT2 regulator (19,20),
subcellular fractions were prepared using differential
centrifugation. Caco-2 cells were lysed using lysis buffer
containing 1% complete protease inhibitor mixture according to the
manufacturer's protocol. Briefly, samples were disrupted using a
homogenizer and centrifuged at 5,000 x g for 10 min at 4˚C. The
supernatant was designated as whole-cell lysate. Half of the
supernatant was recentrifuged at 100,000 x g for 2 h at 4˚C. The
supernatant fraction from this step was designated as the cytosolic
fraction, while the pellet was re-suspended with the same buffer
and used as the membrane fraction. The protein concentration in
each sample was also determined using a Bradford assay (Bio-Rad
Laboratories, Inc.), and the samples were stored at -80˚C prior to
use. For western blot analysis, samples were resolved in 4X Laemmli
solution, electrophoresed by 10% SDS-PAGE and transferred onto PVDF
membranes (Merck KGaA). Non-specific binding was eliminated by
blocking with 5% (w/v) non-fat dry milk in 0.05% Tween-20 in
Tris-buffered saline (TBS-T) for 1 h at room temperature and
incubated overnight at 4˚C with polyclonal anti-rabbit SGLT1
(1:250; cat. no. 07-1417), polyclonal anti-rabbit GLUT2 (1:250;
cat. no. 07-1402-I), polyclonal anti-rabbit AMPK (1:250; cat. no.
07-1417), polyclonal anti-rabbit p-AMPK (1:250; cat. no. 07-363),
monoclonal anti-mouse ALP (BBM marker) (1:500; cat. no.
NB110-3638), monoclonal anti-mouse Na+K+ATPase (BLM marker) (1:500;
cat. no. NB300-146) or anti-mouse β-actin (1:500; cat. no. ab8224)
antibodies. The PVDF membrane was washed with TBS-T buffer and
incubated with horseradish peroxidase-conjugated ImmunoPure
secondary goat anti-rabbit or anti-mouse IgG (1:1,000 dilution;
cat. no. AP132P; Merck KGaA) for 1 h at room temperature. Proteins
were detected using Clarity Western ECL Substrate (Bio-Rad
Laboratories, Inc.) and semi-quantitatively analyzed using ImageJ
version 1.44p (National Institutes of Health).
Statistical analysis
Data are presented as the mean ± the standard error
of the mean. Statistical analysis was performed using SPSS version
23 (IBM Corp.). A one-way ANOVA followed by a post-hoc Dunnett's
test was used to compare differences between multiple groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Phenolic compounds in CBE
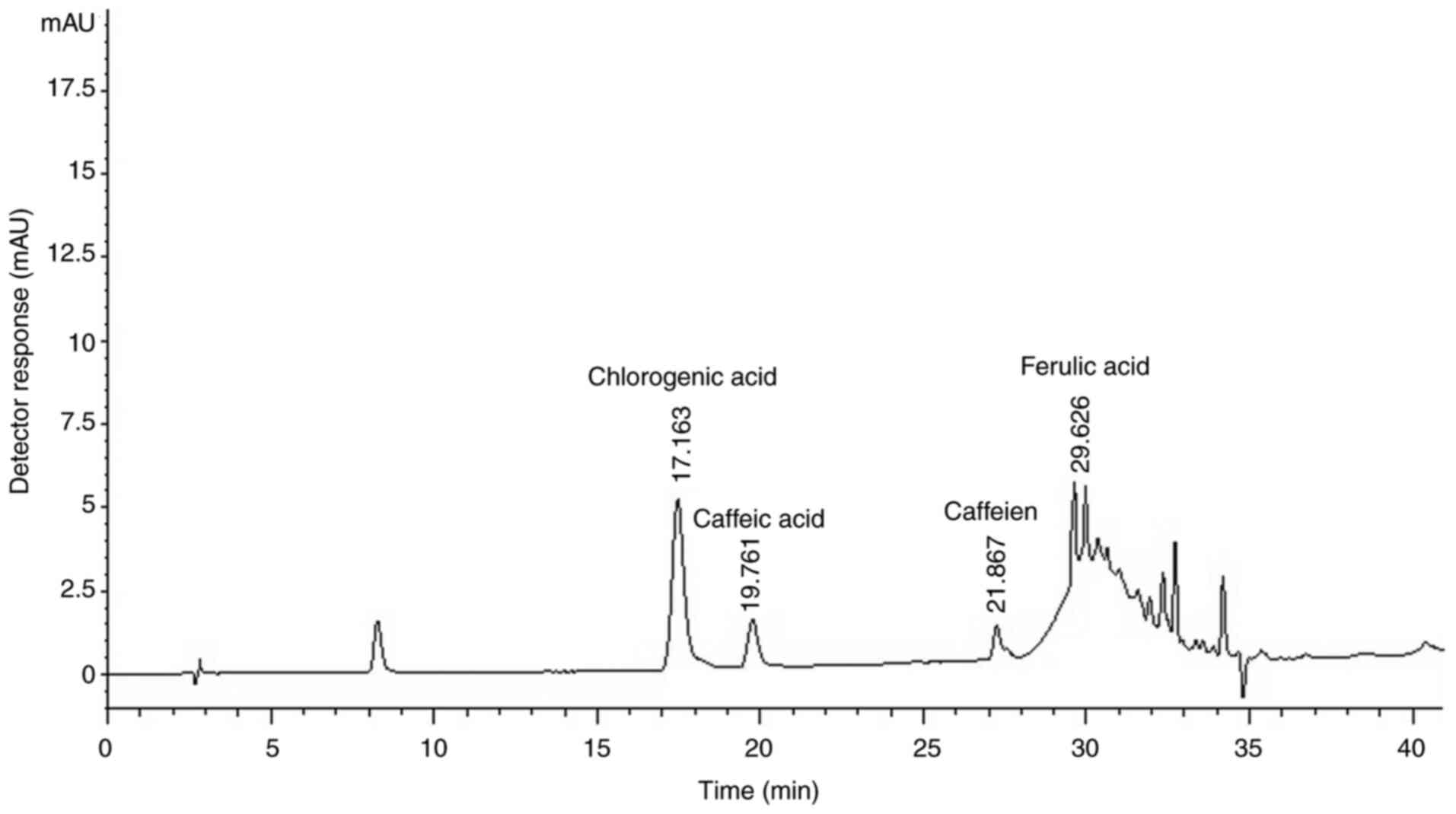
The HPLC chromatogram of CBE reliably detected
chlorogenic acid, caffeine, caffeic acid and ferulic acid with
retention times of 17.163, 19.761, 21.867 and 29.626 min,
respectively, compared with their respective references (Fig. 1). A relatively high content of
chlorogenic acid was obtained (296.2 mg/g) compared with other
phenolic compounds. In addition, caffeine, a major component in
Coffea arabica beans, was found at 51.1 mg/g of CBE, whereas
caffeic acid and ferulic acid were present at 31.6 and 15.4 mg/g of
CBE, respectively.
CBE inhibits glucose uptake in
intestinal Caco-2 cells
To further demonstrate the inhibitory effect of CBE
and its major constituents on glucose absorption, a glucose
transport experiment was performed. As shown in Fig. 2A, CBE at 10-1,000 µg/ml inhibited
glucose transport compared with the control similar to phlorizin
(an SGLT1 inhibitor) and phloretin (a GLUT2 inhibitor). These data
indicated that CBE may interfere with the transport function of
SGLT1 and GLUT2 resulting in decreased glucose uptake into Caco-2
cells. In addition, the major constituent, caffeic acid, also
reduced glucose uptake to a similar degree to 100 and 1,000 µg/ml
CBE, suggesting that inhibition of glucose uptake by CBE may be
partly affected by caffeic acid.
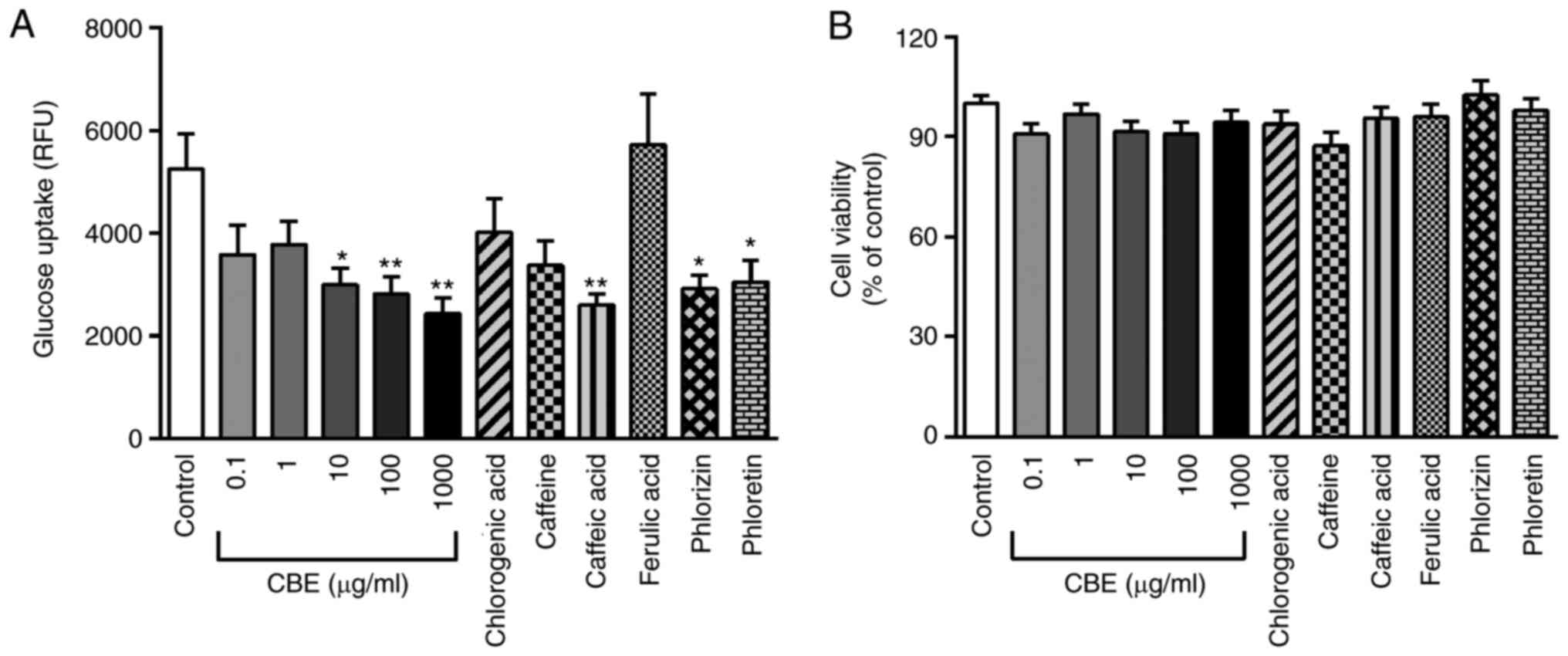 | Figure 2Effect of CBE and its constituents on
glucose transport. (A) Effect of treatment with 0.1-1,000 µg/ml CBE
and its constituents at a molar ratio concentration, including
chlorogenic acid (29.62 µg/ml), caffeine (5.11 µg/ml), caffeic acid
(3.16 µg/ml), ferulic acid (1.54 µg/ml), as well as the SGLT1
inhibitor phlorizin (0.5 mM) and GLUT2 inhibitor phloretin (1 mM)
for 4 h at 37˚C on glucose transport. (B) Viability of Caco-2 cells
after exposure to CBE, chlorogenic acid, caffeine, caffeic acid,
ferulic acid, phlorizin and phloretin. Values are presented as the
mean ± the standard error of the mean (n=6). *P<0.05,
**P<0.01 vs. control. CBE, Coffea arabica bean
extract; RFUY, relative fluorescence unit. |
Cell viability under exposure to test compounds was
also examined. CBE (0.1-1,000 µg/ml), caffeine, chlorogenic acid,
caffeic acid, ferulic acid, phlorizin and phloretin did not affect
Caco-2 cell viability compared with that of control cells (Fig. 2B). These data suggested that CBE and
its major components exerted an inhibitory effect on glucose
absorption without any cytotoxic effect.
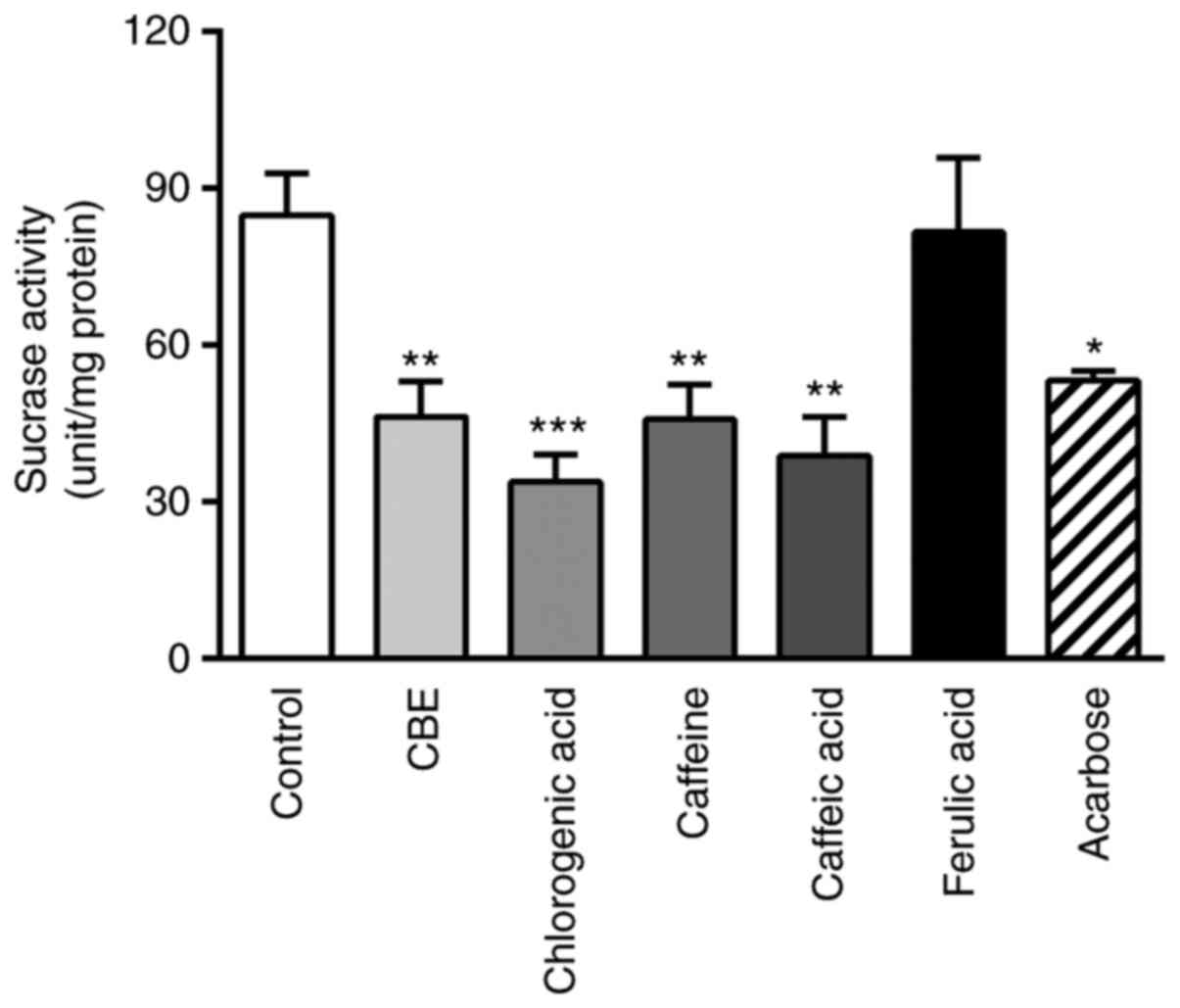
CBE attenuates sucrase activity in
intestinal Caco-2 cells
To determine the effect of CBE on sucrase activity,
the glucose concentration in the substrate solution was evaluated.
As shown in Fig. 3, co-incubation
with CBE at 100 µg/ml decreased the glucose concentration compared
with the control group. Furthermore, the constituents of CBE
(caffeine, chlorogenic acid and caffeic acid) were also reduced
compared with the control. The inhibitory effect was similar to
that of the positive control, acarbose, which is a sucrase enzyme
inhibitor. Therefore, CBE may not only reduce glucose transporter
expression but also decrease disaccharidase enzyme activity,
leading to decreased glucose transport across the human intestinal
cell membrane.
CBE downregulates SGLT1 and GLUT2
protein expression
To further determine whether the reduced rate of
glucose transport caused by CBE was due to decreased membrane
expression of SGLT1, cellular protein expression of SGLT1 was
determined using western blotting. As shown in Fig. 4A and B, SGLT1 membrane protein
expression was decreased by CBE and its constituent, caffeic acid,
compared with the control group. Similarly, phlorizin (an SGLT1
inhibitor) also decreased SGLT1 protein expression in Caco-2 cells,
whereas cells treated with caffeine, chlorogenic acid, ferulic acid
and phloretin did not exhibit any notable differences. Accordingly,
CBE-induced downregulation of SGLT1 membrane protein expression
resulted in a reduction of glucose uptake in human intestinal
epithelia.
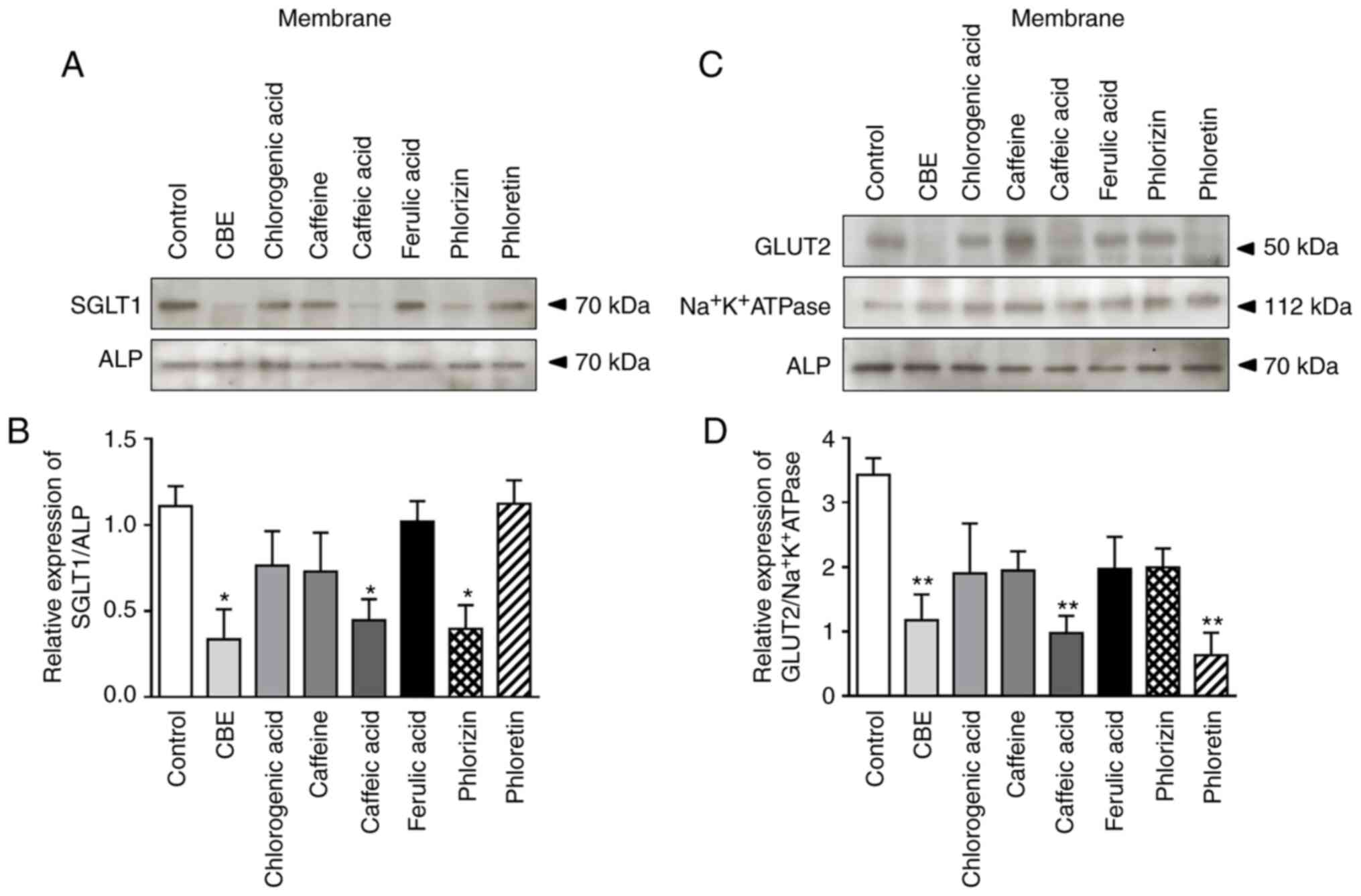 | Figure 4Effect of treatment with CBE (100
µg/ml), chlorogenic acid (29.62 µg/ml), caffeine (5.11 µg/ml),
caffeic acid (3.16 µg/ml), ferulic acid (1.54 µg/ml), phlorizin
(0.5 mM) and phloretin (1 mM), for 4 h at 37˚C, on glucose
transporters membrane protein expression. (A) Representative blot
of SGLT1 membrane protein expression, (B) semi-quantification of
relative SGLT1/ALP protein expression in each fraction, (C)
representative blot of GLUT2 membrane protein expression and (D)
semi-quantification of relative GLUT2/Na+K+ATPase protein
expression in each fraction. Values are presented as the mean ± the
standard error of the mean (n=3). *P<0.05,
**P<0.01 vs. control. CBE, Coffea arabica bean
extract; SGLT1, sodium glucose co-transporter 1; GLUT2 glucose
transporter 2; ALP, alkaline phosphatase. |
As mentioned above, GLUT2 is also associated with
intestinal glucose absorption in both the BBM and BLM. Therefore,
the present study further examined the effect of CBE on GLUT2
expression using western blotting. The results showed that GLUT2
membrane protein expression was reduced by CBE, caffeic acid and
phloretin (a GLUT2 inhibitor) (Fig.
4C and D). However, caffeine-, chlorogenic acid-, ferulic acid-
and phlorizin-treated cells did not exhibit any notable differences
(Fig. 4B). Therefore, the results
suggested that CBE downregulated glucose transport across the
jejunal epithelial cell membrane via GLUT2 inhibition.
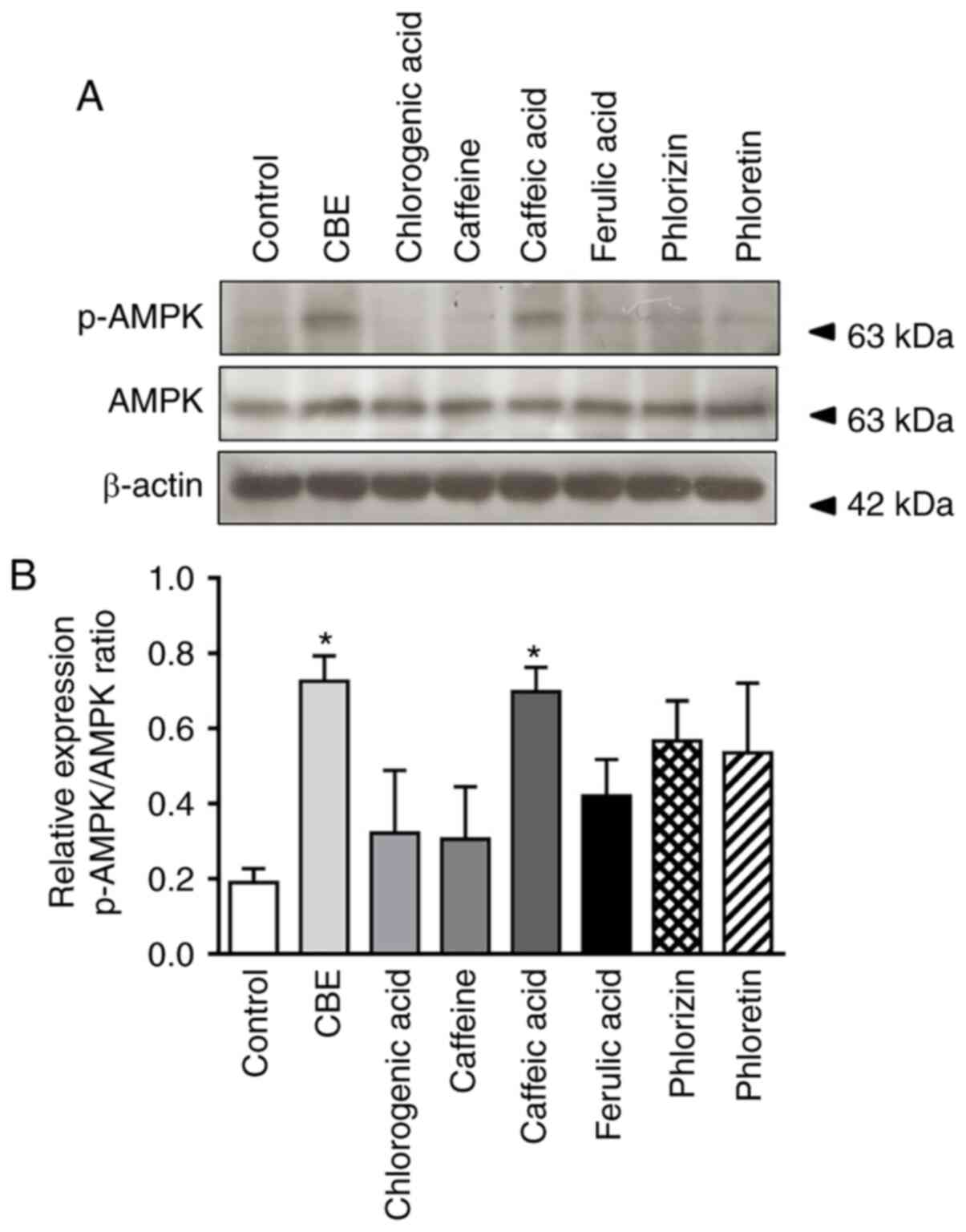
CBE inhibits SGLT1 expression via
activation of AMPK
To further elucidate the mechanism by which CBE
reduced glucose uptake, the effect of CBE on intestinal AMPK
activation and expression was further investigated. As shown in
Fig. 5, phosphorylation of AMPK, a
rate-limiting enzyme decelerating gluconeogenesis, was increased in
the CBE-treated group compared with the control group, similar to
the caffeic acid-treated group. Neither CBE constituents nor
glucose transporter inhibitors activated p-AMPK. Therefore, CBE and
caffeic acid exerted a suppressive effect on glucose absorption,
primarily via inhibition of SGLT1 expression at the translational
level, resulting in modulation of AMPK activity.
CBE modulates GLUT2 expression through
HNF1α gene suppression
The present study further investigated the
mechanisms by which CBE inhibited glucose uptake. SREBP-1c and
HNF1α serve a critical role in glucose transporter regulation.
Therefore, the expression levels of these transcription factors in
Caco-2 cells were determined using qPCR. CBE at 100 µg/ml
suppressed HNF1α mRNA expression, similar to caffeic acid and
phloretin, a GLUT2 inhibitor (Fig.
6). However, there was no effect on SREBP-1c expression.
Therefore, these data suggested that CBE and its major component,
caffeic acid, had an inhibitory effect on GLUT2 transport function
and expression via HNF1α expression.
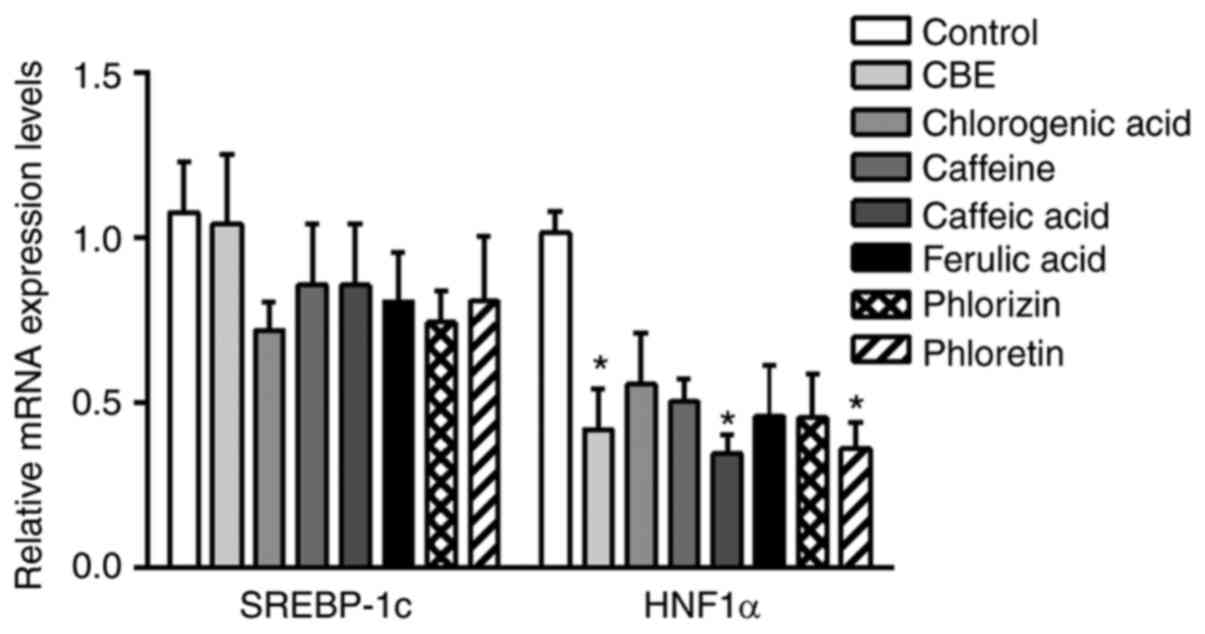 | Figure 6mRNA expression levels of glucose
transporter regulator genes, including SREBP-1c and HNF1α, in
Caco-2 cells treated with CBE (100 µg/ml) and its constituents at a
molar ratio concentration, including chlorogenic acid (29.62
µg/ml), caffeine (5.11 µg/ml), caffeic acid (3.16 µg/ml), ferulic
acid (1.54 µg/ml), phlorizin (0.5 mM) or phloretin (1 mM), for 4 h
at 37˚C. Values are presented as the mean ± the standard error of
the mean (n=5). *P<0.05 vs. control. CBE, Coffea
arabica bean extract; SREBP-1c, sterol regulatory element
binding protein-1c; HNF1α, hepatocyte nuclear factor 1α. |
Discussion
It has been previously shown that the diabetic
status enhances intestinal glucose transport across the BBM and BLM
by increasing SGLT1 and GLUT2 function and expression at both the
transcriptional and translational level (4,21-23).
In addition, the amount of SGLT1 protein is upregulated four-fold
in the gut of patients with type 2 diabetes mellitus, resulting in
a three-fold elevation of monosaccharide absorption compared with
healthy individuals (24).
Therefore, the identification of novel strategies to modulate these
key regulators in glucose absorption may be useful for preventing
further development of diabetes mellitus. A reduction of cellular
glucose transport indicates decreased cell surface glucose
transporter expression or interference in glucose transporter
trafficking. The results of the present study demonstrated that CBE
and one of its constituents, caffeic acid, inhibited SGLT1 and
GLUT2 membrane expression, resulting in reduced glucose absorption.
Similarly, a reduction of glucose uptake into enterocytes has been
reported in SGLT1-deficient mice (25). Furthermore, several natural
compounds, including flavonoids, anthocyanin-rich berry extract and
polyphenol-rich herbal extract, also inhibit glucose absorption via
downregulation of SGLT1 and GLUT2 expression (26-28).
AMPK serves a role in the regulation of cellular
glucose uptake, glycolysis, fatty acid oxidation and enzymes
required for ATP production (29-32).
Previous studies have demonstrated that AMPK regulates cellular
glucose uptake through the increased translocation of GLUT2 to the
BBM (20,33), whereas another study suggested that
AMPK decreases cellular glucose uptake by reducing the expression
levels of SGLT1 in cell membranes (20). Consistent with the effect of CBE on
AMPK activation in the present study, caffeic acid, one of the
major components found in CBE, also stimulates AMPK in Caco-2
cells, resulting in downregulation of SGLT1. Furthermore, CBE and
caffeic acid suppress GLUT2 protein expression, resulting in
reduced glucose uptake, suggesting that CBE and caffeic acid may
regulate GLUT2 protein expression. Although the direct effect of
CBE on AMPK activation was not investigated in the present study, a
previous study demonstrated that caffeic acid directly activates
AMPK in C-4I cells, whereas chlorogenic acid does not cause AMPK
stimulation (34,35). Therefore, CBE, primarily caffeic
acid, inhibits intestinal glucose absorption, partly via activation
of AMPK-mediated downregulation of SGLT1/GLUT2 expression.
A previous study showed that HNF1α is an important
transcription factor for endogenous SGLT1 expression in cultured
enterocytes (36). Additionally,
HNF1α is essential for the expression of GLUT2 transporter
(37,38). Consistently, the present study
demonstrated that CBE, caffeic acid and the GLUT2 inhibitor
phloretin downregulated HNF1α mRNA expression, resulting in
reduction of glucose transporter expression and function.
Similarly, SGLT1 expression is reduced following HNF1α- or
hepatocyte nuclear factor-1β-knockdown (36). Furthermore, GLUT2 expression is
decreased in HNF1α-/- mice (39) and INS1 cells expressing a dominant
negative inhibitor of HNF1α (38).
Therefore, downregulating intestinal SGLT1 and GLUT2 expression via
AMPK protein activation and HNF1α gene suppression by CBE
contributes to reduced transepithelial transport of glucose in the
intestinal epithelium.
CBE and its constituents (caffeine, chlorogenic acid
and caffeic acid) strongly inhibit sucrase activity in Caco-2
cells, similar to acarbose (a sucrase enzyme inhibitor). Similarly,
the mulberry leaf polyphenols arabinoxylan monosaccharide and
arabinoxylan oligosaccharide markedly decrease intestinal
α-glucosidase enzyme activity, making glucose unavailable for
absorption (40,41). Therefore, CBE downregulated glucose
transporter expression, and as a result, reduced glucose uptake
into intestinal absorptive cells. Furthermore, CBE interfered with
disaccharidase enzyme activity. Due to this distinctive mechanism
of glucose inhibition, CBE may be a novel glucose-lowering
supplement, broadening the available therapeutic options for
diabetes mellitus. However, it should be noted that the effect of
CBE on intestinal glucose absorption and its mechanisms require
further investigation in animal models and clinical trials.
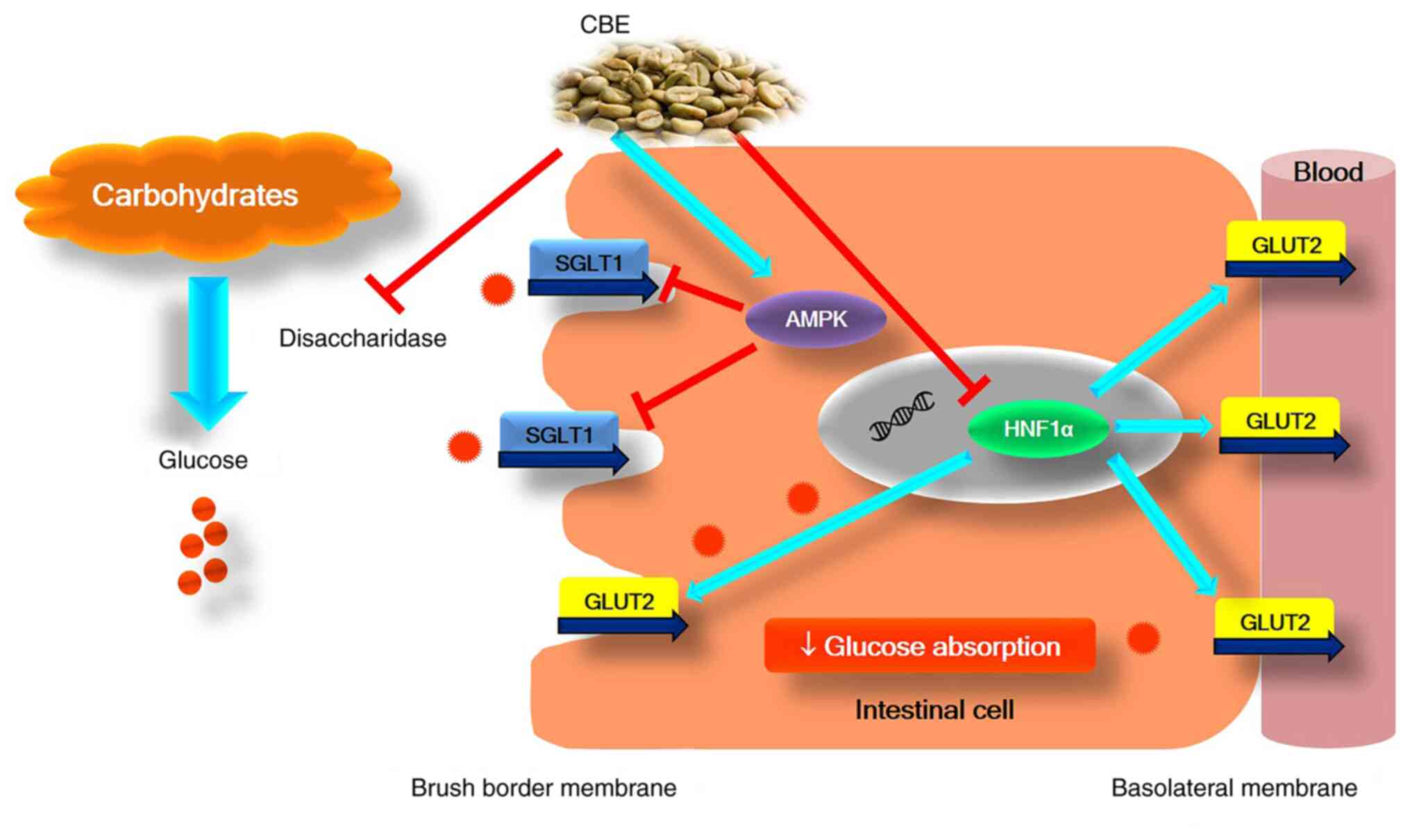
In conclusion, to the best of our knowledge, the
present study is the first to demonstrate the effective
antihyperglycemic action of CBE by reducing intestinal glucose
absorption via activation of AMPK, suppressing HNF1α gene
expression, downregulating SGLT1 and GLUT2 expression and function,
and interfering with sucrase enzyme activity (Fig. 7). Therefore, CBE may hold promise as
an intestinal glucose absorption inhibitor, and further in
vivo studies and clinical trials are required to elucidate
whether CBE has an overall antihyperglycemic effect in diabetes
mellitus.
Acknowledgements
The authors would like to thank Mr Jakkapong Inchai
(Department of Physiology, Faculty of Medicine, Chiang Mai
University) for his technical assistance.
Funding
The present study was supported by funding from the Unit of
Excellent in Research and Product Development of Coffee, University
of Phayao (grant nos. UoE62007 and UoE63004), the School of Medical
Science, University of Phayao, Phayao, Thailand (grant no. 631009)
and the National Higher Education Science Research and Innovation
Policy Council (grant no. FF64-RIB008).
Availability of data and materials
All data generated and/or analyzed during the
present study are included in the published article.
Authors' contributions
AO was involved in conceptualization, methodology,
validation, investigation, writing the original draft,
visualization, supervision and project administration. AD was
involved in designing the methodology, and reviewing and editing of
the manuscript. CS was involved in designing the methodology, and
in reviewing, editing and revising of the manuscript. All authors
have read and approved the final manuscript. AO, AD and CS confirm
the authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wright EM, Loo DD and Hirayama BA: Biology
of human sodium glucose transporters. Physiol Rev. 91:733–794.
2011.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wolffram S, Blöck M and Ader P:
Quercetin-3-glucoside is transported by the glucose carrier SGLT1
across the brush border membrane of rat small intestine. J Nutr.
132:630–635. 2002.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Yoshikawa T, Inoue R, Matsumoto M, Yajima
T, Ushida K and Iwanaga T: Comparative expression of hexose
transporters (SGLT1, GLUT1, GLUT2 and GLUT5) throughout the mouse
gastrointestinal tract. Histochem Cell Biol. 135:183–194.
2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Miyamoto K, Hase K, Taketani Y, Minami H,
Oka T, Nakabou Y and Hagihira H: Diabetes and glucose transporter
gene expression in rat small intestine. Biochem Biophys Res Commun.
181:1110–1117. 1991.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ogata H, Seino Y, Harada N, Iida A, Suzuki
K, Izumoto T, Ishikawa K, Uenishi E, Ozaki N, Hayashi Y, et al:
KATP channel as well as SGLT1 participates in GIP secretion in the
diabetic state. J Endocrinol. 222:191–200. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Fujita Y, Kojima H, Hidaka H, Fujimiya M,
Kashiwagi A and Kikkawa R: Increased intestinal glucose absorption
and postprandial hyperglycaemia at the early step of glucose
intolerance in Otsuka Long-Evans Tokushima Fatty rats.
Diabetologia. 41:1459–1466. 1998.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Dyer J, Wood IS, Palejwala A, Ellis A and
Shirazi-Beechey SP: Expression of monosaccharide transporters in
intestine of diabetic humans. Am J Physiol Gastrointest Liver
Physiol. 282:G241–G248. 2002.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Corpe CP, Basaleh MM, Affleck J, Gould G,
Jess TJ and Kellett GL: The regulation of GLUT5 and GLUT2 activity
in the adaptation of intestinal brush-border fructose transport in
diabetes. Pflugers Arch. 432:192–201. 1996.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kellett GL and Helliwell PA: The diffusive
component of intestinal glucose absorption is mediated by the
glucose-induced recruitment of GLUT2 to the brush-border membrane.
Biochem J. 350:155–162. 2000.PubMed/NCBI
|
|
10
|
Dharmananda S: Medicine IfT: Coffee in
China and the Analysis of Coffee According to Traditional Chinese
Medicine. ITM, Portland, OR, 2003.
|
|
11
|
Nuhu AA: Bioactive micronutrients in
coffee: Recent analytical approaches for characterization and
quantification. ISRN Nutr. 2014(384230)2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Miran J: Guest Editor's Introduction:
Space, Mobility, and Translocal Connections across the Red Sea Area
since 1500. Northeast Afr Stud. 12:ix–xxvi. 2012.
|
|
13
|
Oliveira AL, Cabral FA, Eberlin MN and
Cordello HM: Sensory evaluation of black instant coffee beverage
with some volatile compounds present in aromatic oil from roasted
coffee. Food Sci Technol (Campinas). 29:76–80. 2009.
|
|
14
|
Salinardi TC, Rubin KH, Black RM and
St-Onge MP: Coffee mannooligosaccharides, consumed as part of a
free-living, weight-maintaining diet, increase the proportional
reduction in body volume in overweight men. J Nutr. 140:1943–1948.
2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
International Organization for
Standardization: ISO/IEC 17025: General requirements for the
competence of testing and calibration laboratories. International
Standard ISO/IEC 17025, pp1-39, 2005.
|
|
16
|
Verhoeckx K, Cotter P, López-Expósito I,
Kleiveland C, Lea T, Mackie A, Requena T, Swiatecka D and Wichers H
(eds): Caco-2 Cell Line. In: The Impact of Food Bioactives on
Health. Springer, Cham, 2015.
|
|
17
|
Pan GY, Huang ZJ, Wang GJ, Fawcett JP, Liu
XD, Zhao XC, Sun JG and Xie YY: The antihyperglycaemic activity of
berberine arises from a decrease of glucose absorption. Planta Med.
69:632–636. 2003.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Sopjani M, Bhavsar SK, Fraser S, Kemp BE,
Föller M and Lang F: Regulation of Na+-coupled glucose
carrier SGLT1 by AMP-activated protein kinase. Mol Membr Biol.
27:137–144. 2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Walker J, Jijon HB, Diaz H, Salehi P,
Churchill T and Madsen KL: 5-aminoimidazole-4-carboxamide riboside
(AICAR) enhances GLUT2-dependent jejunal glucose transport: A
possible role for AMPK. Biochem J. 385:485–491. 2005.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Debnam ES, Ebrahim HY and Swaine DJ:
Diabetes mellitus and sugar transport across the brush-border and
basolateral membranes of rat jejunal enterocytes. J Physiol.
424:13–25. 1990.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Fedorak RN, Cheeseman CI, Thomson AB and
Porter VM: Altered glucose carrier expression: Mechanism of
intestinal adaptation during streptozocin-induced diabetes in rats.
Am J Physiol. 261:G585–G591. 1991.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Miyamoto K, Takagi T, Fujii T, Matsubara
T, Hase K, Taketani Y, Oka T, Minami H and Nakabou Y: Role of
liver-type glucose transporter (GLUT2) in transport across the
basolateral membrane in rat jejunum. FEBS Lett. 314:466–470.
1992.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Williamson G: Possible effects of dietary
polyphenols on sugar absorption and digestion. Mol Nutr Food Res.
57:48–57. 2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Röder PV, Geillinger KE, Zietek TS,
Thorens B, Koepsell H and Daniel H: The role of SGLT1 and GLUT2 in
intestinal glucose transport and sensing. PLoS One.
9(e89977)2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Alzaid F, Cheung HM, Preedy VR and Sharp
PA: Regulation of glucose transporter expression in human
intestinal Caco-2 cells following exposure to an anthocyanin-rich
berry extract. PLoS One. 8(e78932)2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Farrell TL, Ellam SL, Forrelli T and
Williamson G: Attenuation of glucose transport across Caco-2 cell
monolayers by a polyphenol-rich herbal extract: Interactions with
SGLT1 and GLUT2 transporters. Biofactors. 39:448–456.
2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kwon O, Eck P, Chen S, Corpe CP, Lee JH,
Kruhlak M and Levine M: Inhibition of the intestinal glucose
transporter GLUT2 by flavonoids. FASEB J. 21:366–377.
2007.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Carling D: The role of the AMP-activated
protein kinase in the regulation of energy homeostasis. Novartis
Found Symp. 286:72–85, 162-163, 196-203. 2007.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Horie T, Ono K, Nagao K, Nishi H,
Kinoshita M, Kawamura T, Wada H, Shimatsu A, Kita T and Hasegawa K:
Oxidative stress induces GLUT4 translocation by activation of
PI3-K/Akt and dual AMPK kinase in cardiac myocytes. J Cell Physiol.
215:733–742. 2008.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Jensen TE, Rose AJ, Hellsten Y,
Wojtaszewski JF and Richter EA: Caffeine-induced Ca(2+) release
increases AMPK-dependent glucose uptake in rodent soleus muscle. Am
J Physiol Endocrinol Metab. 293:E286–E292. 2007.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Winder WW and Thomson DM: Cellular energy
sensing and signaling by AMP-activated protein kinase. Cell Biochem
Biophys. 47:332–347. 2007.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Gabler NK, Radcliffe JS, Spencer JD, Webel
DM and Spurlock ME: Feeding long-chain n-3 polyunsaturated fatty
acids during gestation increases intestinal glucose absorption
potentially via the acute activation of AMPK. J Nutr Biochem.
20:17–25. 2009.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Tsuda S, Egawa T, Ma X, Oshima R, Kurogi E
and Hayashi T: Coffee polyphenol caffeic acid but not chlorogenic
acid increases 5'AMP-activated protein kinase and
insulin-independent glucose transport in rat skeletal muscle. J
Nutr Biochem. 23:1403–1409. 2012.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Tyszka-Czochara M, Bukowska-Strakova K,
Kocemba-Pilarczyk KA and Majka M: Caffeic acid targets AMPK
signaling and regulates tricarboxylic acid cycle anaplerosis while
metformin downregulates HIF-1α-induced glycolytic enzymes in human
cervical squamous cell carcinoma lines. Nutrients.
10(841)2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Balakrishnan A, Stearns AT, Rhoads DB,
Ashley SW and Tavakkolizadeh A: Defining the transcriptional
regulation of the intestinal sodium-glucose cotransporter using
RNA-interference mediated gene silencing. Surgery. 144:168–173.
2008.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Ban N, Yamada Y, Someya Y, Miyawaki K,
Ihara Y, Hosokawa M, Toyokuni S, Tsuda K and Seino Y: Hepatocyte
nuclear factor-1alpha recruits the transcriptional co-activator
p300 on the GLUT2 gene promoter. Diabetes. 51:1409–1418.
2002.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Párrizas M, Maestro MA, Boj SF, Paniagua
A, Casamitjana R, Gomis R, Rivera F and Ferrer J: Hepatic nuclear
factor 1-alpha directs nucleosomal hyperacetylation to its
tissue-specific transcriptional targets. Mol Cell Biol.
21:3234–3243. 2001.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Shih DQ, Screenan S, Munoz KN, Philipson
L, Pontoglio M, Yaniv M, Polonsky KS and Stoffel M: Loss of
HNF-1alpha function in mice leads to abnormal expression of genes
involved in pancreatic islet development and metabolism. Diabetes.
50:2472–2480. 2001.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Li Q, Wang C, Liu F, Hu T, Shen W, Li E,
Liao S and Zou Y: Mulberry leaf polyphenols attenuated postprandial
glucose absorption via inhibition of disaccharidases activity and
glucose transport in Caco-2 cells. Food Funct. 11:1835–1844.
2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Malunga LN, Eck P and Beta T: Inhibition
of intestinal α-glucosidase and glucose absorption by feruloylated
arabinoxylan mono- and oligosaccharides from corn bran and wheat
aleurone. J Nutr Metab. 2016(1932532)2016.PubMed/NCBI View Article : Google Scholar
|