Introduction
Stroke is a significant cause of disability and
mortality in the elderly (≥65 years) worldwide (1). Moreover, stroke incidence and
mortality rates remain high in young adults (18-50 years) in
developing and developed countries alike (2,3). In
Thailand, the incidence of stroke-associated mortality per 100,000
individuals has continued to increase, reaching 53.0% in 2019, up
from 47.1% in 2017 and 47.8% in 2018(4). Ischemic stroke (IS), involving the
occlusion of a major cerebral artery or its branches in the brain,
is the primary type of stroke globally (3). The development of stroke is affected
by the interaction between multiple risk factors, involving both
genetic and non-genetic factors (5,6).
Chronic inflammatory diseases and unhealthy lifestyle habits are
the most common risk factors associated with stroke (6). The inflammatory component has been
considered as one of the possible etiological factors in the early
stages of IS development, thereby promoting the formation of a
cerebral thrombus in association with the endothelium and
coagulation (7). After cerebral
ischemia, a robust inflammatory response is triggered by ischemic
neuronal cell death and the release of necrotic cell debris, which
induces the production of inflammatory cytokines and chemokines
from microglia and macrophage cells (8-10).
A dysregulated immune response, accompanied by an increased number
of inflammatory cells, high TNF-α production and the activation of
microglia cells has been shown to enhance neuroinflammation and
exacerbate ischemic brain injury in patients with IS and a mouse
model of cerebral ischemia (11).
Inflammatory cytokines and chemokines have been used as predictors
of stroke incidence and outcomes (12,13).
Thus, the genetic variation of inflammatory cytokine genes has
become a critical issue in research relating to the predictive risk
factors of IS occurrence (14,15),
as well as its severity and outcome (16), and this provides potential
therapeutic options for the treatment of inflammatory diseases
(17,18).
IL-6 and TNF-α are potent pro-inflammatory cytokines
that serve vital roles in the immune response during inflammation
(19). IL-6 levels have been
suggested as a potential biomarker that is associated with stroke
outcomes (20), short-term acute
ischemic stroke death (21) and
post-stroke dementia (22). It has
also been shown that the overexpression of TNF-α transcripts is an
independent risk factor for the incidence of IS (13). Furthermore, high TNF-α levels
post-stroke are associated with poor patient outcomes (23).
Single-nucleotide polymorphisms (SNPs) are the most
commonly studied genetic variations for IS susceptibility (24), and functional SNPs located in a
gene's promoter region regulate that gene's transcriptional
expression (25,26). A meta-analysis revealed that the
IL-6-174G/C polymorphism was significantly associated with
IS risk in Asian populations in both dominant and recessive
inheritance models (14). However,
other studies reported that the IL-6-174G/C polymorphism was
not associated with the risk of IS in North Indian (16,27)
and Chinese populations (28).
Moreover, no association of the IL-6-174G/C polymorphism was
observed with the expression levels of IL-6 in the plasma of
patients with IS and controls (29). With regard to TNF-α gene, it
was found that the TNF-α-308G/A polymorphism was associated
with a risk of IS development (15)
and hypertension (30) in an Asian
population. In addition, TNF-α-308 GA genotypes may be
protective factors for IS occurrence in East Asian populations
(31,32).
However, there remains considerable controversy
surrounding the two SNPs, IL-6-174G/C and
TNF-α-308G/A, which are located in the promoter region of
the gene (14-16,32).
These may be predictive risk factors of IS development and modify
the gene's transcriptional activities, thereby contributing to
changes in the gene's expression levels. Presumably, the
association between these two SNPs and IS risk may be attributed to
different ethnicities, environmental influences and experimental
design. To the best of our knowledge, there have been no reports on
the association of IL-6-174G/C and TNF-α-308G/A
polymorphisms with the risk of IS and the expression levels of IL-6
and TNF-α in Thailand. Therefore, the present study aimed to
investigate the association between IL-6-174G/C and
TNF-α-308G/A polymorphisms with IS susceptibility, their
interactions with clinical variables and the expression levels of
IL-6 and TNF-α in a southern Thai population.
Materials and methods
Sample size calculation
A sample size calculation was performed using the
freely available G*Power version 3.1.9.2 application power analysis
(33). To achieve a power of 95% at
an α of 0.05, the sample size was calculated based on the previous
reports (14,15). The estimated sample sizes were 367
and 420 subjects for the IL-6 and TNF-α genotypes,
respectively.
Study participants
A total of 422 blood samples of Thai-Buddhist origin
were collected from Thungchon, Bansakha and Thaiburi sub-district
health promoting hospitals and the Thasala Hospital (Thasala,
Nakhon Si Thammarat, Thailand) between November 2019 and August
2020. Patients with IS were recruited based on the physician's
diagnosis, assessed using clinical symptoms, computed tomography
scans and/or magnetic resonance imaging. Patients were all
medically stable and received the same class of drug regimens. The
controls were individuals from the same demographic who attended a
checkup, but had no history of stroke. Clinical information,
including age, sex, blood pressure, BMI, biochemical parameters
(blood sugar and lipid profile), smoking habit and alcohol
consumption, were collected from reviews of medical records.
Subjects were excluded if they had a history of inflammatory
diseases, acute heart failure, myocardial infarction (MI),
hepatitis, cirrhosis, chronic renal failure and dialysis,
autoimmune diseases, immune-suppressive therapy or cancer. Amongst
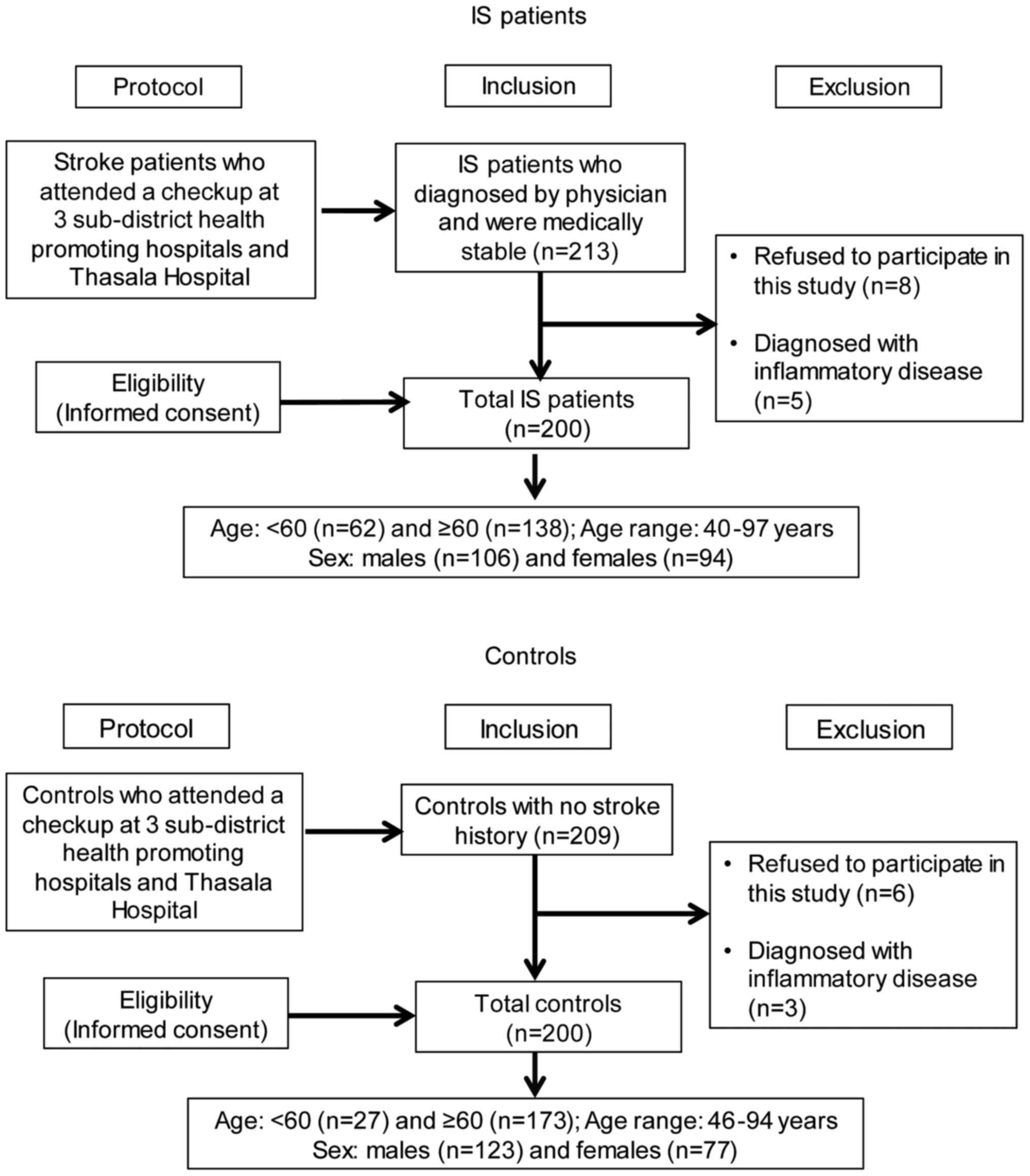
the 422 subjects, 22 subjects did not fulfill the inclusion
criteria (Fig. 1). Finally, a total
of 400 blood samples were obtained for genotyping analyses. Samples
were collected from 200 patients with IS (106 males and 94 females;
age range 40-97, median age 67 years) and 200 controls (123 males
and 77 females; age range 46-94, median age 66 years). The research
protocols were performed in accordance with the Declaration of
Helsinki guidelines (34) and
approved by the Human Research Ethics Committee of Walailak
University (approval no. WUEC-19-189-01). Informed consent was
acquired from each participant in this study.
Hypertension was defined as a resting systolic blood
pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg, or a
requirement of antihypertensive drugs (35). Hyperlipidemia was defined as a total
cholesterol level of ≥240 mg/dl or a history of taking
antihyperlipidemic drugs (36).
Diabetes mellitus was defined as a fasting plasma glucose level
≥126 mg/dl or current use of glycemic control medication (37). Moreover, subjects that possessed ≥3
of the following five risk factors were diagnosed with metabolic
syndrome (38,39): BMI ≥27.0 kg/m2 in men and
≥25.0 kg/m2 in women; triglycerides of ≥150 mg/dl;
high-density lipoprotein cholesterol <40 mg/dl in men or <50
mg/dl in women; blood pressure ≥130/85 mmHg or patients currently
taking hypertensive medication; and fasting plasma glucose levels
of ≥100 mg/dl or patients currently taking insulin or hypoglycemic
medication. Smoking status referred to subjects that were current
smokers only. Subjects were considered alcohol drinkers if they
consumed ≥100 ml alcohol >3 times per week.
IL-6 and TNF-α genotyping
Ethylenediamine tetra-acetic acid blood samples of
3-5 ml from the patients with IS and controls were collected only
once at admission. Genomic DNA was extracted and purified using a
genomic DNA Mini kit (Geneaid Biotech, Ltd.). Then, DNA
concentration and purity were determined using a NanoDrop
spectrophotometer (NanoDrop OneC; Thermo Fisher
Scientific, Inc.) and stored at -20˚C until required for further
analysis. IL-6-174G/C (rs1800795) genotyping was performed
using a TaqMan™ SNP Genotyping assay, available with probe and
primers (assay ID: C_1839697_20; cat. no. 4351379; Thermo Fisher
Scientific, Inc.). Genotyping reactions were performed in a 10-µl
reaction volume and the amplification was conducted according to
the manufacturer's instructions as follows: Polymerase activation
at 95˚C for 10 min, followed by 50 cycles of denaturation at 95˚C
for 15 sec and annealing/extension at 60˚C for 1 min using a
StepOnePlus™ Real-Time PCR system (Thermo Fisher Scientific, Inc.).
Data were analyzed using StepOnePlus™ software (version 2.2.2;
Thermo Fisher Scientific, Inc.).
For TNF-α-308G/A (rs1800629) genotyping, the
primers were designed using the Primer3 and BLAST software
(ncbi.nlm.nih.gov/tools/primer-blast) from the National
Center for Biotechnology Information. The primer sequences were:
Forward, 5'-CTGGTCCCCAAAAGAAATGGAG-3' and reverse,
5'-CTGGGCCACTGACTGATTTGTG-3' (product size, 96 bp). The total
volume of the PCR reaction was 20 µl, which was composed of 1X HOT
FIREPol® EvaGreen® HRM mix (ROX) (cat. no.
08-33-00001; Solis BioDyne), 250 nM forward and reverse primers and
20 ng DNA samples. A quantitative-(q)PCR was set up in a 96-well
PCR plate and processed on a Quanstudio™ 5 Real-Time PCR system
(Thermo Fisher Scientific, Inc.) with the following thermocycling
conditions: Initial denaturation at 95˚C for 15 min, followed by 40
cycles of denaturation at 95˚C for 15 sec, annealing at 60˚C for 20
sec and elongation at 72˚C for 20 sec. The melt curve protocol was
set as follows: Denaturation at 95˚C for 15 sec, reannealing at
60˚C for 20 sec and melting from 60˚C to 95˚C with a ramp rate of
0.05˚C/sec. Data were analyzed via high resolution melting (HRM)
curve analysis (version 3.1; Thermo Fisher Scientific, Inc.). In
total, 184 DNA samples were randomly analyzed for the first round
of examination using HRM software. Among them, three different
patterns of different plots were observed, and the samples were
selected for DNA sequencing. The DNA sequencing results revealed
100% concordance with the results of the HRM analyses. The three
different genotypes obtained from the first round were submitted as
genotype controls and included in each plate. The qPCR and HRM
analyses were reanalyzed for the first round of 184 samples and
performed for all DNA samples.
DNA sequencing
To validate the genotyping results of the
IL-6-174G/C and TNF-α-308G/A polymorphisms, a random
selection of 10% of samples for re-genotyping were subjected to DNA
sequencing (Apical Scientific Sdn. Bhd.). Before re-genotyping, PCR
amplification of the two SNPs was performed using 5X
FIREPol® MasterMix (cat. no. 04-12-00125; Solis
BioDyne). The IL-6-174G/C polymorphism was amplified using
the redesigned forward primer, 5'-GTAAAACTTCGTGCATGACTTC-3' and
reverse primer, 5'-AATCTTTGTTGGAGGGTGAG-3', to obtain a product
size of 255 bp. To identify the TNF-α-308 polymorphism, the
following redesigned primers were used: Forward,
5'-GCCCCTCCCAGTTCTAGTTC-3' and reverse,
5'-CATCAAGGACCCCTCACACTC-3', to obtain the extended PCR product
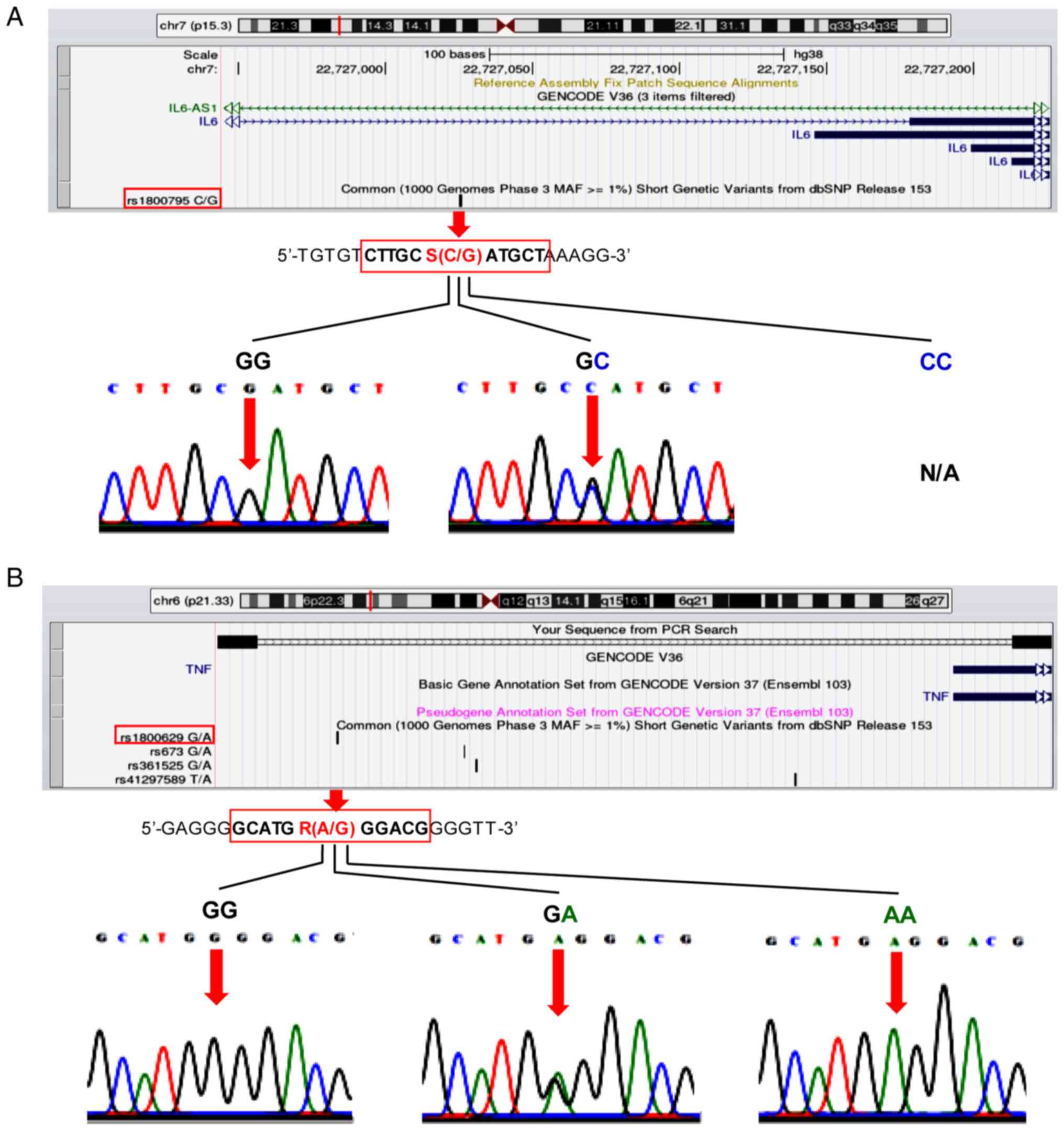
size of 225 bp for DNA sequencing. The UCSC In-Silico PCR online
informatics tool (genome.ucsc.edu/cgi-bin/hgPcr) was used to create the
UCSC Genome Browser on Human Dec. 2013 (GRCh38/hg38) Assembly in
order to visualize the position of the two polymorphisms in the
predicted PCR product [Fig. 2A
(IL-6, upper panel) and 2B (TNF-α, upper panel)].
After initial activation at 95˚C for 5 min, PCR cycling conditions
of the two polymorphisms (95˚C for 15 sec, 54˚C for 30 sec and 72˚C
for 30 sec) were carried out for 30 cycles on a GeneAmp®
PCR System 9700 thermal cycler (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The presence of bands of the expected size was
confirmed via agarose gel electrophoresis. PCR products were
visualized on 1.5% agarose gel stained with SYBR™ Safe DNA Gel
Stain (cat. no. S33102; Thermo Fisher Scientific, Inc.). The DNA
sequencing results highlighted that they were reproducible with no
discrepancies.
ELISA for cytokine levels
A total of 268 samples from patients with IS and
controls based on 1:1 matching of sex, age ±4 years and BMI ±2.5
were selected to measure IL-6 and TNF-α levels. Amongst these, the
volume of plasma in 48 samples was not sufficient to perform the
test. The remaining 220 samples [110 patients with IS (63 males and
47 females), age range 49-88 years, median age 67 years and 110
controls (63 males and 47 females), age range 47-88 years, median
age 66 years] were subjected to ELISA. Centrifugation of blood
samples was performed at 1,200 x g for 5 min at room temperature.
Subsequently, plasma was aliquoted and stored at -80˚C for the
assessment of cytokine levels. The levels of TNF-α and IL-6 were
determined using commercially available ELISA MAX™ Deluxe Set Human
IL-6 (cat. no. 430504) and TNF-α (cat. no. 430204) kits, which were
conducted in accordance with the manufacturer's instructions
(BioLegend, Inc.).
Statistical analysis
SPSS version 25 was used to analyze the data (IBM,
Corp.). The normality of data was assessed using a
Kolmogorov-Smirnov test. The baseline characteristics of the
patients with IS and controls were examined using a χ2
test for categorical data and a Mann-Whitney U test for continuous
data. Categorical variables are reported as proportions, whereas
continuous variables are reported as the median [interquartile
range (IQR)]. A binomial test was used to analyze the difference
between the genotype and allele frequencies in patients with IS and
controls. The association of the genetic polymorphisms with the
risk of IS was determined using multivariate logistic regression.
The Enter method, which is the default in SPSS Statistics, was used
to enter all independent variables into the equation in one step
(40). The Hardy-Weinberg
equilibrium (HWE) was evaluated using a χ2 test. To
investigate the interaction between the IS genetic polymorphism and
clinical factors, multivariate logistic regression analysis was
performed. The difference in plasma levels between patients with IS
and controls were compared using a Mann-Whitney U test. The
difference in plasma levels of cytokines among genotypes in
patients with IS and controls was compared using a Mann-Whitney U
tests for two groups and Kruskal-Wallis tests followed by a Dunn's
posthoc test for three groups. P<0.05 was considered to indicate
a statistically significant difference.
Results
Characteristics of patients with IS
and controls
The baseline characteristics and clinical factors of
patients with IS and controls are presented in Table I. There were no significant
differences between the age or sex of patients between the IS and
controls (P>0.05). Hypertension (patients with IS, 77.0% vs.
controls, 39.0%), hyperlipidemia (patients with IS, 53.0% vs.
controls, 25.5%), smoking (patients with IS, 28.5% vs. controls,
19.5%) and alcohol consumption (patients with IS, 22.0% vs.
controls, 13.0%) were significantly more frequent in patients with
IS than in the controls. IS patients had considerably lower total
and low-density lipoprotein cholesterol levels than the controls
(P<0.001). Lower cholesterol levels in patients with IS may be
linked to the use of anti-lipidemic medications.
 | Table IClinical parameters of patients with
IS and controls. |
Table I
Clinical parameters of patients with
IS and controls.
| Variable | IS patients,
n=200 | Controls,
n=200 | P-value |
|---|
| Age,
yearsd | 67 (57.2-76.0) | 66 (62.0-73.0) | 0.810 |
| Sex, n (%) | | | |
|
Male | 106 (53.0) | 123 (61.5) | 0.086 |
|
Female | 94 (47.0) | 77 (38.5) | |
| Body mass index,
kg/m2d | 23 (20.2-24.7) | 24 (20.7-26.8) | 0.016a |
| Blood
pressured | | | |
|
Systolic
blood pressure, mmHg | 146
(132.0-169.8) | 142
(130.0-155.0) | 0.012a |
|
Diastolic
blood pressure, mmHg | 81 (73.0-95.0) | 79 (72.0-88.0) | 0.003b |
| Clinical factors, n
(%) | | | |
|
Hypertension | 154 (77.0) | 78 (39.0) |
<0.001c |
|
Hyperlipidemia | 106 (53.0) | 51 (25.5) |
<0.001c |
|
Diabetes
mellitus | 72 (36.0) | 86 (43.0) | 0.152 |
|
Metabolic
syndrome | 79 (39.5) | 74 (37.0) | 0.607 |
|
Smoking | 57 (28.5) | 39 (19.5) | 0.035a |
|
Alcohol
drinking | 44 (22.0) | 26 (13.0) | 0.018a |
| Blood
testsd | | | |
|
Fasting
blood glucose, mg/dl | 96
(88.0-109.0) | 97
(89.3-110.0) | 0.321 |
|
Total
cholesterol, mg/dl | 170
(141.0-203.0) | 203
(172.3-230.8) |
<0.001c |
|
Triglyceride,
mg/dl | 130
(94.2-176.0) | 126
(94.3-168.8) | 0.801 |
|
High-density
lipoprotein cholesterol, mg/dl | 49 (40.0-59.0) | 48 (41.0-58.8) | 0.945 |
|
Low-density
lipoprotein cholesterol, mg/dl | 103
(79.0-136.0) | 126
(101.0-149.8) |
<0.001c |
Associations of IL-6 and TNF-α
polymorphisms with the risk of IS development
The genotype distribution of IL-6-174G/C and
TNF-α-308G/A between patients with IS and controls was in
HWE (IL-6-174G/C, patients with IS, P=0.72 vs. controls,
P=0.66; TNF-α-308G/A, patients with IS, P=0.27 vs. controls,
P=0.48). It was identified that the IL-6-174 GG genotype and
G allele were the most prevalent genotype in both patients with IS
and controls, whilst the GC genotype and C allele were less common,
and the CC genotype was not observed in the research samples
(Table II). As illustrated in the
lower panel of Fig. 2A, the
representative electropherograms of the IL-6-174G/C
sequencing data revealed only two distinct genotypes (GG and GC).
There was no significant difference in the distribution of the
IL-6-174G/C genotype as well as G and C alleles between
patients with IS and controls. To determine whether the
IL-6-174G/C polymorphism was associated with IS risk,
multivariate logistic regression analysis was performed. The
results indicated that there was no association between
IL-6-174G/C polymorphism and the risk of IS occurrence in
dominant and recessive inheritance, after adjustment for sex
(Table III).
 | Table IIGenotype and allele frequencies of
IL-6 and TNF-α gene polymorphisms in the 200 patients
with IS and the 200 control individuals, and their associations
with the risk of IS susceptibility. |
Table II
Genotype and allele frequencies of
IL-6 and TNF-α gene polymorphisms in the 200 patients
with IS and the 200 control individuals, and their associations
with the risk of IS susceptibility.
| A,
IL-6-174G/C |
|---|
| Polymorphism | IS patients, n
(%) | Controls, n
(%) |
P-valueb | OR (95% CI) |
P-valuec |
|---|
| Genotype | | | | | |
|
GG | 190 (95.0) | 188 (94.0) | 0.959 | 1.000 | |
|
GC | 10 (5.0) | 12 (6.0) | 0.832 | 0.825
(0.348-1.955) | 0.661 |
|
CC | 0 (0.0) | 0 (0.0) | | | |
| Allele | | | | | |
|
G | 390 (97.5) | 388 (97.0) | 0.971 | 1.000 | |
|
C | 10 (2.5) | 12 (3.0) | 0.832 | 0.829
(0.354-1.941) | 0.666 |
|
MAF (%) | 2.5 | 3.0 | | | |
| B,
TNF-α-308G/A |
| Genotype | | | | | |
|
GG | 166 (83.0) | 181 (92.1) | 0.452 | 1.000 | |
|
GA | 31 (15.5) | 19 (7.9) | 0.119 | 1.747
(0.951-3.211) | 0.072 |
|
AA | 3 (1.5) | 0 (0.0) | 0.250 | | |
|
GA+AA | 34 (17.0) | 19 (7.9) | 0.053 | 1.951
(1.071-3.554) | 0.029a |
| Allele | | | | | |
|
G | 363 (90.7) | 381 (95.2) | 0.533 | 1.000 | |
|
A | 37 (9.3) | 19 (4.8) | 0.022a | 2.044
(1.154-3.620) | 0.014a |
|
MAF (%) | 9.3 | 4.8 | | | |
 | Table IIIAssociations of IL-6 and
TNF-α gene polymorphisms with the risk of IS development
according to different modes of inheritance. |
Table III
Associations of IL-6 and
TNF-α gene polymorphisms with the risk of IS development
according to different modes of inheritance.
| A,
IL-6-174G/C |
|---|
| Polymorphism | Adjusted OR (95%
CI) | P-value |
|---|
| C dominance, G wild
type | | |
|
GG | 1.000 | |
|
GC or
CC | 0.801
(0.337-1.907) | 0.617 |
| C recessive, G wild
type | | |
|
GG or
GC | 1.000 | |
|
CC | 1.162
(0.072-18.805) | 0.916 |
| B,
TNF-α-308G/A |
| Polymorphism | Adjusted OR (95%
CI) | P-value |
| A dominance, G wild
type | | |
|
GG | 1.000 | |
|
GA or
AA | 1.971
(1.080-3.599) | 0.027a |
| A recessive, G wild
type | | |
|
GG or
GA | 1.000 | |
|
AA | 4.138
(0.456-37.544) | 0.207 |
For the TNF-α-308G/A polymorphism, the GG
genotype was found most frequently, followed by the GA and AA
genotypes, in the patients with IS; however, the AA genotype was
not found in the controls (Table
II). The TNF-α-308G/A representative electropherograms
of the three genotypes (GG, GA and AA) were shown in Fig. 2B, lower panel. No significant
difference in the distribution of the genotypes between patients
with IS and controls was observed, whilst there was a significant
difference (P=0.022) in the frequency of the TNF-α-308 A
allele between patients with IS (9.3%) and controls (4.8%).
Multivariate logistic regression analysis revealed that the GA and
AA genotypes, and the A allele of TNF-α-308 increased the
risk of IS development (GA and AA genotypes vs. GG genotype,
OR=1.951; 95% CI=1.071-3.554; P=0.029; and A allele vs. G allele,
OR=2.044; 95% CI=1.154-3.620; P=0.014). When the mode of
inheritance was dominant, the GA or AA genotype had a 1.97-fold
higher risk of IS development compared with the GG genotype, after
adjusting for sex (adjusted OR=1.971; 95% CI=1.080-3.599; P=0.027),
as shown in Table III. Therefore,
the A allele appears to be a risk factor for IS development in a
dominant mode of inheritance. However, when the mode of inheritance
was recessive, the AA genotype was not an IS risk factor.
Interaction of IL-6 and TNF gene
polymorphisms with clinical variables in IS susceptibility
To further investigate the interaction between genes
and clinical factors, an interaction analysis of the
IL-6-174G/C and TNF-α-308G/A polymorphisms with
clinical factors was performed using multivariate logistic
regression (Tables IV and V, respectively). Carriers of the
IL-6-174 GG genotype who had hypertension, hyperlipidemia or
consumed alcohol exhibited an increased risk for IS compared with
those without these clinical features (hypertensive subjects,
adjusted OR=4.095, 95% CI=2.604-6.441, P<0.001; hyperlipidemic
subjects, adjusted OR=2.979, 95% CI=1.850-4.797, P<0.001; and
alcohol consumption, adjusted OR=1.968, 95% CI=1.050-3.688;
P=0.035; Table IV).
Non-hypertensive subjects who had the IL-6-174 GC genotype
were less likely to develop IS compared with those who had the GG
genotype (adjusted OR=0.087, 95% CI=0.009-0.797; P=0.031).
 | Table IVInteraction of IL-6-174G/C
polymorphism with clinical factors, as determined using
multivariate logistic regression analysis. |
Table IV
Interaction of IL-6-174G/C
polymorphism with clinical factors, as determined using
multivariate logistic regression analysis.
| Polymorphism | Clinical
factor | IS patients,
n=200 | Controls,
n=200 | Adjusted OR (95%
CI) | P-value |
|---|
|
IL-6-174G/C | Hypertension | | | | |
|
GG | No | 45 | 113 | 1.000 | |
|
GC | No | 1 | 9 | 0.087
(0.009-0.797)c | 0.031a |
|
GG | Yes | 145 | 75 | 4.095
(2.604-6.441)c |
<0.001b |
|
GC | Yes | 9 | 3 | 2.372
(0.571-9.852)c | 0.235 |
|
IL-6-174G/C | Hyperlipidemia | | | | |
|
GG | No | 89 | 141 | 1.000 | |
|
GC | No | 5 | 8 | 0.791
(0.219-2.852)d | 0.720 |
|
GG | Yes | 101 | 47 | 2.979
(1.850-4.797)d |
<0.001b |
|
GC | Yes | 5 | 4 | 0.705
(0.164-3.030)d | 0.639 |
|
IL-6-174G/C | Smoking | | | | |
|
GG | No | 138 | 152 | 1.000 | |
|
GC | No | 5 | 9 | 0.449
(0.126-1.597)e | 0.216 |
|
GG | Yes | 52 | 36 | 1.608
(0.929-2.784)e | 0.090 |
|
GC | Yes | 5 | 3 | 1.763
(0.315-9.866)e | 0.519 |
|
IL-6-174G/C | Alcohol
drinking | | | | |
|
GG | No | 151 | 164 | 1.000 | |
|
GC | No | 5 | 10 | 0.488
(0.140-1.705)f | 0.261 |
|
GG | Yes | 39 | 24 | 1.968
(1.050-3.688)f | 0.035a |
|
GC | Yes | 5 | 2 | 1.967
(0.317-12.193)f | 0.468 |
 | Table VInteraction of the
TNF-α-308G/A polymorphism with clinical factors, as
determined using multivariate logistic regression analysis. |
Table V
Interaction of the
TNF-α-308G/A polymorphism with clinical factors, as
determined using multivariate logistic regression analysis.
| Polymorphism | Clinical
factor | IS patients,
n=200 | Controls,
n=200 | Adjusted OR (95%
CI) | P-value |
|---|
|
TNF-α-308G/A | Hypertension | | | | |
|
GG | No | 42 | 110 | 1.000 | |
|
GA+AA | No | 4 | 12 | 0.448
(0.133-1.513)c | 0.196 |
|
GG | Yes | 124 | 71 | 2.655
(1.721-4.094)c |
<0.001b |
|
GA+AA | Yes | 30 | 7 | 4.585
(1.892-11.110)c | 0.001b |
|
TNF-α-308G/A | Hyperlipidemia | | | | |
|
GG | No | 79 | 135 | 1.000 | |
|
GA+AA | No | 15 | 14 | 1.291
(0.546-3.054)d | 0.560 |
|
GG | Yes | 87 | 46 | 2.253
(1.401-3.624)d | 0.001b |
|
GA+AA | Yes | 19 | 5 | 3.016
(1.027-8.862)d | 0.045a |
|
TNF-α-308G/A | Smoking | | | | |
|
GG | No | 118 | 145 | 1.000 | |
|
GA+AA | No | 25 | 16 | 1.466
(0.686-3.134)e | 0.324 |
|
GG | Yes | 48 | 36 | 1.404
(0.800-2.462)e | 0.237 |
|
GA+AA | Yes | 9 | 3 | 4.010
(0.889-18.092)e | 0.071 |
|
TNF-α-308G/A | Alcohol
drinking | | | | |
|
GG | No | 126 | 156 | 1.000 | |
|
GA+AA | No | 30 | 18 | 1.650
(0.811-3.355)f | 0.167 |
|
GG | Yes | 40 | 25 | 1.934
(1.037-3.608)f | 0.038a |
|
GA+AA | Yes | 4 | 1 | 2.721
(0.288-25.693)f | 0.382 |
In the present study, hypertensive and
hyperlipidemic subjects carrying TNF-α-308 GA and AA
genotypes had the highest risk of IS prevalence compared with
non-hypertensive and non-hyperlipidemic subjects carrying the GG
genotype (hypertensive subjects, adjusted OR=4.585, 95%
CI=1.892-11.110, P=0.001; and hyperlipidemic subjects, adjusted
OR=3.016, 95% CI=1.027-8.862, P=0.045) (Table V). Carriers of the GG genotype who
had hypertension, hyperlipidemia and who consumed alcohol were
susceptible to a higher risk of IS compared with subjects with the
same genetic background but lacking these three clinical factors
(hypertensive subjects, adjusted OR=2.655, 95% CI=1.721-4.094,
P<0.001; hyperlipidemic subjects, adjusted OR=2.253, 95%
CI=1.401-3.624, P=0.001; and alcohol drinkers, adjusted OR=1.934,
95% CI=1.037-3.608; P=0.038). Moreover, there was no interaction
between the TNF-α-308 GA and AA genotype with smoking or
alcohol consumption.
Associations of IL-6-174G/C and
TNF-α-308G/A polymorphisms with plasma IL-6 and TNF-α levels
A total of 110 patients with IS and 110 controls
were matched at 1:1, and their cytokine levels were measured. The
median (IQR) levels of IL-6 [patients with IS, 30.5 (14.0-81.8)
pg/ml vs. controls, 40.5 (17.0-110.3) pg/ml] and TNF-α [patients
with IS, 28.0 (7.8-59.5) pg/ml vs. controls, 24.0 (14.0-41.3)
pg/ml] were not significantly different between the two groups.
Furthermore, the plasma IL-6 and TNF-α levels were not different
amongst the various genotypes of patients with IS (Table VI). However, IL-6 levels were
significantly increased in the IL-6-174 GC genotype carriers
compared with the GG genotype carriers amongst controls (P=0.015).
Thus, the IL-6 -174 GC genotype appeared to be associated
with high levels of IL-6 in controls, but may not be associated
with the IL-6 levels in patients with IS.
 | Table VIPlasma IL-6 and TNF-α levels among
the genotypes of patients with IS and controls. |
Table VI
Plasma IL-6 and TNF-α levels among
the genotypes of patients with IS and controls.
| | Plasma level
(pg/ml), median (IQR, n) |
|---|
| Polymorphism | IS patients,
n=110 | P-value | Controls,
n=110 | P-value |
|---|
|
IL-6-174G/C | | | | |
|
GG | 32.5 (14.8-81.8,
n=106) | 0.307b | 36.0 (17.0-95.0,
n=103) | 0.015a,b |
|
GC | 14.0 (9.5-72.5,
n=4) | | 194.0 (81.0-195.0,
n=7) | |
|
CC | N/A | | N/A | |
|
TNF-α-308G/A | | | | |
|
GG | 29.0 (7.0-63.8,
n=90) | 0.668c | 24.0 (14.3-41.8,
n=100) | 0.803b |
|
GA | 18.0 (8.5-42.5,
n=17) | | 25.0 (11.8-42.3,
n=10) | |
|
AA | 26.0 (7.0-N/A,
n=3) | | N/A | |
Discussion
Inflammation is considered a significant contributor
to IS pathogenesis (7,8) and is proposed as a target of
pharmacological therapy for inflammatory diseases (17,18).
Inflammatory cytokines produced by immune and non-immune cells are
upregulated upon cerebral ischemia and injury (8,41).
Furthermore, polymorphisms of inflammatory cytokine genes at the
promoter region may predict IS risk factors and outcomes (14-16).
The present study evaluated the association of the genetic
polymorphisms of IL-6-174G/C and TNF-α-308G/A with
the risk of IS development. The current results indicated that
subjects carrying the TNF-α-308 GA and AA genotypes and the
A allele had a higher risk of IS development. In line with the
current findings, previous reports have shown that the
TNF-α-308 A allele was associated with a risk of cerebral
infarction in Korean populations (42), and that North Indian subjects
carrying TNF-α-308 GA and AA genotypes had a higher risk of
coronary artery disease (43). By
contrast, the TNF-α-308G/A polymorphism was not associated
with IS risk in a Chinese population (44) but was associated with protection
against IS in East Asians (31,32).
With regards to the IL-6-174G/C polymorphism, the present
study did not identify any association of IL-6-174G/C with
the risk of IS, although this SNP has been revealed as a risk
factor of IS in Asians in both the dominant mode (GG vs. GC + CC;
OR=0.74, 95% CI=0.62-0.88; P=0.0005) and recessive mode of
inheritance (CC vs. GG + GC; OR=1.61, 95% CI=1.17-2.21; P=0.003)
(14). In addition, subjects
carrying the IL-6-174 CC genotype were not observed in the
present study population, which was consistent with findings of
previous Chinese Han and Uyghur studies (28). The current finding demonstrated that
there was no association of the IL-6-174G/C polymorphism
with IS risk, which was in accordance with other studies of North
Indian (16) and Chinese
populations (28). Collectively, it
was suggested that the association of these two SNPs with IS
prevalence may be due to ethnic differences, methodological
differences and variations in sample sizes.
In the present study, elevated IL-6 levels were
associated with IL-6-174 GC genotypes in controls; however,
no association between the TNF-α-308G/A polymorphism and
TNF-α levels was found in patients with IS when compared with the
controls. The functional SNP IL-6-174G/C in the GC genotype
is associated with higher serum IL-6 levels, increased IS severity
and poorer outcomes in patients with IS from North India (16). However, the IL-6-174G/C
polymorphism is not associated with elevated serum IL-6 levels in
young Indian patients with IS (aged 18-45 years) and controls
(29). A meta-analysis revealed
that TNF-α levels were significantly increased in patients with IS
compared with control subjects (15). Moreover, amongst healthy
individuals, TNF-α-308 GA and AA genotypes had lower TNF-α
levels compared with the GG genotype (15). Inconclusive cytokine expression
results may depend on multiple non-genetic and genetic factors, as
well as their interaction, leading to variability in the expression
levels (15,29). The interactions of other SNPs
located in the inflammatory gene's promoter region (15) or gene-gene interactions (45) may affect the overall transcriptional
activity of gene promoters. However, other SNPs located in the
promoter region of the IL-6 and TNF-α genes and the
interactions of each SNP are yet to be verified.
In previous studies, it was reported that
inflammatory gene transcripts were upregulated following ischemic
injury of the astrocytes in vitro (46) and the brain in vivo (47). The upregulation of inflammatory
genes is mediated via the induction of the Toll-like receptor/NF-κB
signaling pathway, which is the major contributor induced by
cerebral ischemia/reperfusion (48,49).
NF-κB acts as a critical transcription factor contributing to the
transcription of various inflammatory genes, including IL-6
and TNF-α after cerebral ischemia-reperfusion injury
(50). The DNA binding ability and
transcriptional activity of NF-κB depend on the variants at the
binding site of the NF-κB target gene, which modulate gene
expression and influence disease risk (51). The molecular mechanism of how
IL-6 and TNF-α gene polymorphisms influence the
occurrence of IS remains to be elucidated. It has been suggested
that functional polymorphisms at the promoter region of the
IL-6 and TNF-α genes may increase the promoters'
transcriptional activation by enhancing its responsiveness to
inflammatory stimuli-mediated activation (52,53).
Thus, modulation of gene expression may be the mechanism via which
IL-6 and TNF-α genetic variations influence IS
occurrence.
In the present analysis, the interactions between
IL-6-174 GG and hypertension, hyperlipidemia and alcohol
consumption were associated with a higher risk of IS. Notably,
patients with IS had a higher prevalence of hypertension,
hyperlipidemia and alcohol consumption compared with the controls.
In a Chinese population, the IL-6-174G/C polymorphism was
found to interact with hypertension and obesity, but not smoking,
to elevate the risk of IS development (54). The genotypes in the current analysis
differ from those in a Chinese study (54), in that the IL-6-174 GG
genotype, rather than the CC genotype, interacted with hypertension
and hyperlipidemia. Consistent with the current findings, it has
been demonstrated that the combination of alcohol consumption and
the IL-6-174G/C interaction increases the risk of carotid
atherosclerosis, a risk factor for stroke in Western Germany
(55). For the TNF-α-308G/A
polymorphism, it was discovered that the GA and AA genotypes
interacted with hypertension and hyperlipidemia to increase the
risk of developing IS. The TNF-α-308 GA + AA genotypes were
found to be associated with the risk factor of hypertension in the
Asian population, which is consistent with the current findings
(30). The TNF-α-308 A
allele also appeared to have a higher risk of stroke when patients
also had fever (56) and showed an
increased risk of MI when combined with obesity (57). A previous study reported that serum
high-density lipoprotein cholesterol levels in TNF-α-308 A
allele carriers were negatively associated with polyunsaturated
fatty acid intake (58). These
studies support the current findings indicating increased risk of
IS occurrence when the TNF-α-308 GA and AA genotypes were
combined with hypertension and hyperlipidemia. The present study
also identified no interaction between the TNF-α-308 GA and
AA genotypes and smoking for IS risk. Consistent with the current
results, the interaction of TNF-α-308G/A with smoking was
not associated with the risk of MI (57). Thus, the current results provide the
potential clinical utility of genetic variants as one of the risk
predictions for IS development. This study can help to inform
implementation strategies and support future research for IS
prevention, lower stroke severity and improve therapeutic options
to reduce IS incidence.
A limitation of the present study was that not all
variants at the promoter regions of IL-6 and TNF-α
genes were assessed. Complete sequencing may enable the
identification of potentially causative mutations in the whole-gene
function region of inflammatory genes. An interaction study of
other variants at the promoter regions of IL-6 and
TNF-α genes and the two variants presented in the current
study may be required to determine the gene promoters'
transcriptionally-related transcripts and the association with IS
susceptibility. As the TNF-α-308G/A polymorphism has been
proposed as a predictive risk factor of IS development in the
current study, the mechanism of how the TNF-α-308G/A
polymorphism regulates IS susceptibility should be confirmed in the
future. Another limitation was the relatively small sample size
used to analyze plasma IL-6 and TNF-α levels. Further
investigations with additional samples from various study
populations should be performed to confirm the current findings in
the southern Thai population and other ethnic groups.
In conclusion, the present study demonstrated that
the promoter polymorphism of TNF-α-308G/A was associated
with a higher risk of IS occurrence, while there was no association
between the IL-6-174G/C polymorphism and the risk of IS in
this study population. It was suggested that the IL-6-174G/C
and TNF-α-308G/A polymorphisms, combined with the three
clinical variables of hypertension, hyperlipidemia and alcohol
consumption, may enhance the risk of developing IS. Moreover, the
high expression levels of IL-6 appeared to be associated with
IL-6-174 GC genotype carriers in the control group.
Collectively, to the best of our knowledge, the current findings
provide the first report on the association of inflammatory
cytokine gene polymorphism with the risk of IS susceptibility in a
southern Thai population.
Acknowledgements
We are grateful to Assistant Professor Dr Wanida
Limmun, Department of Mathematics and Statistics, School of
Science, Walailak University for statistical analysis
recommendations.
Funding
The present study was supported by the Walailak University Fund
(grant no. WU-IRG-62-034), Thailand Science Research and Innovation
Fund (grant no. WU-FF64102) and the Development and Promotion of
Science and Technology Talents Project (DPST).
Availability of data and materials
All data generated and/or analyzed during the
current study are available in the GenBank Nucleotide Database
repository [ncbi.nlm.nih.gov/genbank; accession nos.
MZ379749-MZ379813 for IL-6-174G/C (rs1800795) (n=20) and
MZ379814-MZ379833 for TNF-α-308G/A (rs1800629) (n=20)].
Authors' contributions
KK, NP, OM and WP performed the experiments and
analyzed the data. KK, NP and PP contribute to data curation and
validated the study. MN, PJ and WC participated in the study design
and supervised the study. NK and JT aided in the experimental
design and refined the manuscript. WC wrote and edited the
manuscript, and was the corresponding author during the manuscript
submission and revision. All the authors have read and approve to
the final version of the manuscript. KK, NP, MN, PJ and WC confirm
the authenticity of all the raw data.
Ethics approval and consent to
participate
The study was performed in accordance with the
Declaration of Helsinki guidelines and approved by the Human
Research Ethics Committee of Walailak University (approval no.
WUEC-19-189-01).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kim J, Thayabaranathan T, Donnan GA,
Howard G, Howard VJ, Rothwell PM, Feigin V, Norrving B, Owolabi M,
Pandian J, et al: Global stroke statistics 2019. Int J Stroke.
15:819–838. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Boot E, Ekker MS, Putaala J, Kittner S, De
Leeuw FE and Tuladhar AM: Ischaemic stroke in young adults: A
global perspective. J Neurol Neurosurg Psychiatry. 91:411–417.
2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Avan A, Digaleh H, Di Napoli M, Stranges
S, Behrouz R, Shojaeianbabaei G, Amiri A, Tabrizi R, Mokhber N,
Spence JD and Azarpazhooh MR: Socioeconomic status and stroke
incidence, prevalence, mortality, and worldwide burden: An
ecological analysis from the Global Burden of Disease Study 2017.
BMC Med. 17(191)2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
The Thai Ministry of Public Health: Public
Health Statistics A.D. 2019, 2020. Available from: https://bps.moph.go.th/new_bps/sites/default/files/statistic62.pdf.
|
|
5
|
Yamada Y, Kato K, Oguri M, Horibe H,
Fujimaki T, Yasukochi Y, Takeuchi I and Sakuma J: Identification of
nine genes as novel susceptibility loci for early-onset ischemic
stroke, intracerebral hemorrhage, or subarachnoid hemorrhage.
Biomed Rep. 9:8–20. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lee J, Park A, Mun S, Kim HJ, Son H, Choi
H, Kim D, Lee SJ, Kim JG and Kang HG: Proteomics-based
identification of diagnostic biomarkers related to risk factors and
pathogenesis of ischemic stroke. Diagnostics (Basel).
10(340)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Genchi A, Semerano A, Gullotta GS, Strambo
D, Schwarz G, Bergamaschi A, Panni P, Simionato F, Scomazzoni F,
Michelozzi C, et al: Cerebral thrombi of cardioembolic etiology
have an increased content of neutrophil extracellular traps. J
Neurol Sci. 423(117355)2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Clausen BH, Wirenfeldt M, Høgedal SS,
Frich LH, Nielsen HH, Schrøder HD, Østergaard K, Finsen B,
Kristensen BW and Lambertsen KL: Characterization of the TNF and
IL-1 systems in human brain and blood after ischemic stroke. Acta
Neuropathol Commun. 8(81)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Mo Y, Sun YY and Liu KY: Autophagy and
inflammation in ischemic stroke. Neural Regen Res. 15:1388–1396.
2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Campbell BCV, De Silva DA, Macleod MR,
Coutts SB, Schwamm LH, Davis SM and Donnan GA: Ischaemic stroke.
Nat Rev Dis Primers. 5(70)2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Meng H, Zhao H, Cao X, Hao J, Zhang H, Liu
Y, Zhu MS, Fan L, Weng L, Qian L, et al: Double-negative T cells
remarkably promote neuroinflammation after ischemic stroke. Proc
Natl Acad Sci USA. 116:5558–5563. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Martha SR, Cheng Q, Fraser JF, Gong L,
Collier LA, Davis SM, Lukins D, Alhajeri A, Grupke S and
Pennypacker KR: Expression of cytokines and chemokines as
predictors of stroke outcomes in acute ischemic stroke. Front
Neurol. 10(1391)2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zheng PF, Liao FJ, Yin RX, Chen LZ, Li H,
Nie RJ, Wang Y and Liao PJ: Genes associated with inflammation may
serve as biomarkers for the diagnosis of coronary artery disease
and ischaemic stroke. Lipids Health Dis. 19(37)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chen M and Yang Y: A meta-analysis on
associations of IL-6 and IL-10 polymorphisms with
susceptibility to ischemic stroke. J Neuroimmunol.
335(577004)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Cui G, Wang H, Li R, Zhang L, Li Z, Wang
Y, Hui R, Ding H and Wang DW: Polymorphism of tumor necrosis factor
alpha (TNF-alpha) gene promoter, circulating TNF-alpha level, and
cardiovascular risk factor for ischemic stroke. J
Neuroinflammation. 9(235)2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chakraborty B, Chowdhury D, Vishnoi G,
Goswami B, Kishore J and Agarwal S: Interleukin-6 gene -174 G/C
promoter polymorphism predicts severity and outcome in acute
ischemic stroke patients from north India. J Stroke Cerebrovasc
Dis. 22:683–689. 2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Fabris M, Quartuccio L, Fabro C, Sacco S,
Lombardi S, Ramonda R, Biasi D, Punzi D, Adami S, Olivieri I, et
al: The -308 TNFα and the -174 IL-6 promoter polymorphisms
associate with effective anti-TNFα treatment in seronegative
spondyloarthritis. Pharmacogenomics J. 16:238–242. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Bank S, Julsgaard M, Abed OK, Burisch J,
Broder Brodersen J, Pedersen NK, Gouliaev A, Ajan R, Nytoft
Rasmussen D, Honore Grauslund C, et al: Polymorphisms in the NFkB,
TNF-alpha, IL-1beta, and IL-18 pathways are associated with
response to anti-TNF therapy in Danish patients with inflammatory
bowel disease. Aliment Pharmacol Ther. 49:890–903. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Rincon M: Interleukin-6: From an
inflammatory marker to a target for inflammatory diseases. Trends
Immunol. 33:571–577. 2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Klimiec-Moskal E, Piechota M, Pera J,
Weglarczyk K, Slowik A, Siedlar M and Dziedzic T: The specific ex
vivo released cytokine profile is associated with ischemic stroke
outcome and improves its prediction. J Neuroinflammation.
17(7)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Reiche EMV, Gelinksi JR, Alfieri DF,
Flauzino T, Lehmann MF, de Araújo MCM, Lozovoy MAB, Simão ANC, de
Almeida ERD and Maes M: Immune-inflammatory, oxidative stress and
biochemical biomarkers predict short-term acute ischemic stroke
death. Metab Brain Dis. 34:789–804. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Choi DW, Kim TS, Kim YS and Kim DJ:
Elevated plasma biomarkers of inflammation in acute ischemic stroke
patients with underlying dementia. BMC Neurol.
20(293)2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kim JW, Park MS, Kim JT, Kang HJ, Bae KY,
Kim SW, Shin MG, Cho KH and Kim JM: The impact of tumor necrosis
factor-α and interleukin-1β levels and polymorphisms on long-term
stroke outcomes. Eur Neurol. 79:38–44. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Chauhan G and Debette S: Genetic risk
factors for Ischemic and Hemorrhagic stroke. Curr Cardiol Rep.
18(124)2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ramazi S, Heydari-Zarnagh H, Goudarzian M,
Khalaj-Kondori M and Bonyadi M: Thromboxane A synthase 1 gene
expression and promotor haplotypes are associated with risk of
large artery-atherosclerosis stroke in Iranian population. J Cell
Biochem. 120:15222–15232. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Fan Y, Chen H, Li A, Shi Y, Zhang Y, Feng
Q, Sun Y, Zheng H and He Y: A promoter polymorphism (rs17222919,
-1316T/G) of ALOX5AP gene is associated with decreased risk of
ischemic stroke in two independent Chinese populations. PLoS One.
10(e0122393)2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Banerjee I, Gupta V, Ahmed T, Faizaan M,
Agarwal P and Ganesh S: Inflammatory system gene polymorphism and
the risk of stroke: A case-control study in an Indian population.
Brain Res Bull. 75:158–165. 2008.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Tong Y, Wang Z, Geng Y, Liu J, Zhang R,
Lin Q, Li X, Huang D, Gao S, Hu D, et al: The association of
functional polymorphisms of IL-6 gene promoter with ischemic
stroke: Analysis in two Chinese populations. Biochem Biophys Res
Commun. 391:481–485. 2010.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Akhter MS, Biswas A, Abdullah SM, Hobani
Y, Ranjan R, Behari M and Saxena R: Influence of interleukin-6
(IL-6) promoter gene polymorphisms (-174G>C, -572G>C, and
-597G>A) on IL-6 plasma levels and their impact in the
development of acute ischemic stroke in young Indians. Clin Appl
Thromb Hemost. 25(1076029619854136)2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Yao YS, Chang WW and Jin YL: Association
between TNF-α promoter -308G/A polymorphism and essential
hypertension in the Asian population: A meta-analysis. J Renin
Angiotensin Aldosterone Syst. 18(1470320317741066)2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Tong Y, Geng Y, Xu J, Wang Z, Zhang Y, Lin
L, Zhang R, Deng P, Li Y, Hou W, et al: The role of functional
polymorphisms of the TNF-alpha gene promoter in the risk of
ischemic stroke in Chinese Han and Uyghur populations: Two
case-control studies. Clin Chim Acta. 411:1291–1295.
2010.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Song D and Cheng D: Associations of
TNFα-308G/A and TNFα-238G/A polymorphisms with ischemic stroke in
East Asians and non-East Asians: A meta-analysis. Genet Test Mol
Biomarkers. 21:10–16. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Faul F, Erdfelder E, Lang AG and Buchner
A: G*Power 3: A flexible statistical power analysis program for the
social, behavioral, and biomedical sciences. Behav Res Methods.
39:175–191. 2007.PubMed/NCBI View Article : Google Scholar
|
|
34
|
World Medical Association (WMA): WMA
Declaration of Helsinki-ethical principles for medical research
involving human subjects, 2018. Available from: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/.
|
|
35
|
Williams B, Mancia G, Spiering W, Agabiti
Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G,
Dominiczak A, et al: 2018 ESC/ESH Guidelines for the management of
arterial hypertension. Eur Heart J. 39:3021–3104. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Morgan JM and Capuzzi DM:
Hypercholesterolemia. The NCEP Adult Treatment Panel III
Guidelines. Geriatrics. 58:33–38. 2003.PubMed/NCBI
|
|
37
|
American Diabetes Association. 2.
Classification and diagnosis of diabetes. Diabetes Care. 40 (Suppl
1):S11–S24. 2017.
|
|
38
|
Expert Dyslipidemia Panel. Grundy SM: An
International atherosclerosis society position paper: Global
recommendations for the management of dyslipidemia. J Clin Lipidol.
7:561–565. 2013.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Pongchaiyakul C, Nguyen TV, Wanothayaroj
E, Karusan N and Klungboonkrong V: Prevalence of metabolic syndrome
and its relationship to weight in the Thai population. J Med Assoc
Thai. 90:459–467. 2007.PubMed/NCBI
|
|
40
|
Ranganathan P, Pramesh CS and Aggarwal R:
Common pitfalls in statistical analysis: Logistic regression.
Perspect Clin Res. 8:148–151. 2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Erta M, Quintana A and Hidalgo J:
Interleukin-6, a major cytokine in the central nervous system. Int
J Biol Sci. 8:1254–1266. 2012.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Um JY and Kim HM: Tumor necrosis factor
alpha gene polymorphism is associated with cerebral infarction.
Brain Res Mol Brain Res. 122:99–102. 2004.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Kumari R and Kumar S, Ahmad MK, Singh R,
Kant Kumar S, Pradhan A, Chandra S and Kumar S: Promoter variants
of TNF-α rs1800629 and IL-10 rs1800871 are independently associated
with the susceptibility of coronary artery disease in north Indian.
Cytokine. 110:131–136. 2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Gu L, Wu G, Su L, Yan Y, Liang B, Tan J,
Cai H, Jiang H, Wei Q, Shen T and Wei A: TNF-a (-238G/A and
-308G/A) gene polymorphisms may not contribute to the risk of
ischemic stroke. Int J Neurosci. 126:219–226. 2016.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Linderson Y, Bresso F, Buentke E,
Pettersson S and D'Amato M: Functional interaction of CARD15/NOD2
and Crohn's disease-associated TNFalpha polymorphisms. Int J
Colorectal Dis. 20:305–311. 2005.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Yu AC and Lau LT: Expression of
interleukin-1 alpha, tumor necrosis factor alpha and interleukin-6
genes in astrocytes under ischemic injury. Neurochem Int.
36:369–377. 2000.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Yamaguchi A, Jitsuishi T, Hozumi T,
Iwanami J, Kitajo K, Yamaguchi H, Mori Y, Mogi M and Sawai S:
Temporal expression profiling of DAMPs-related genes revealed the
biphasic post-ischemic inflammation in the experimental stroke
model. Mol Brain. 13(57)2020.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Ye X, Kong D, Wang J, Ishrat T, Shi H,
Ding X, Cui G and Hua F: MyD88 contributes to neuroinflammatory
responses induced by cerebral ischemia/reperfusion in mice. Biochem
Biophys Res Commun. 480:69–74. 2016.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Hao T, Yang Y, Li N, Mi Y, Zhang G, Song
J, Liang Y, Xiao J, Zhou D, He D and Hou Y: Inflammatory mechanism
of cerebral ischemia-reperfusion injury with treatment of
stepharine in rats. Phytomedicine. 79(153353)2020.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Zhai Y, Zhu Y, Liu J, Xie K, Yu J, Yu L
and Deng H: Dexmedetomidine post-conditioning alleviates cerebral
ischemia-reperfusion injury in rats by inhibiting high mobility
group protein B1 group (HMGB1)/Toll-Like Receptor 4 (TLR4)/Nuclear
Factor kappa B (NF-κB) Signaling Pathway. Med Sci Monit.
26(e918617)2020.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Yang JH, Chen WT, Lee MC, Fang WH, Hsu YJ,
Chin-Lin Chen HC, Chang HL, Chen CF, Tu MY, et al: Investigation of
the variants at the binding site of inflammatory transcription
factor NF-κB in patients with end-stage renal disease. BMC Nephrol.
20(300)2019.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Chen J, Liu RY, Yang L, Zhao J, Zhao X, Lu
D, Yi N, Han B, Chen XF, Zhang K, et al: A two-SNP IL-6 promoter
haplotype is associated with increased lung cancer risk. J Cancer
Res Clin Oncol. 139:231–242. 2013.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Kiss-Toth E, Harlock E, Lath D,
Quertermous T and Wilkinson JM: A TNF variant that associates with
susceptibility to musculoskeletal disease modulates thyroid hormone
receptor binding to control promoter activation. PLoS One.
8(e76034)2013.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Yang X, Feng L, Li C and Li Y: Association
of IL-6-174G>C and -572C>G polymorphisms with risk of young
ischemic stroke patients. Gene. 539:258–262. 2014.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Jerrard-Dunne P, Sitzer M, Risley P,
Steckel DA, Buehler A, von Kegler S and Markus HS: Carotid
Atherosclerosis Progression Study. Interleukin-6 promoter
polymorphism modulates the effects of heavy alcohol consumption on
early carotid artery atherosclerosis: The carotid atherosclerosis
progression study (CAPS). Stroke. 34:402–407. 2003.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Lalouschek W, Schillinger M, Hsieh K,
Endler G, Greisenegger S, Marculescu R, Lang W, Wagner O, Cheng S
and Mannhalter C: Polymorphisms of the inflammatory system and risk
of ischemic cerebrovascular events. Clin Chem Lab Med. 44:918–923.
2006.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Padovani JC, Pazin-Filho A, Simões MV,
Marin-Neto JA, Zago MA and Franco RF: Gene polymorphisms in the TNF
locus and the risk of myocardial infarction. Thromb Res.
100:263–269. 2000.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Fontaine-Bisson B, Wolever TM, Chiasson
JL, Rabasa-Lhoret R, Maheux P, Josse RG, Leiter LA, Rodger NW, Ryan
EA, Connelly PW, et al: Genetic polymorphisms of tumor necrosis
factor-alpha modify the association between dietary polyunsaturated
fatty acids and fasting HDL-cholesterol and apo A-I concentrations.
Am J Clin Nutr. 86:768–774. 2007.PubMed/NCBI View Article : Google Scholar
|