Introduction
Essential hypertension (EHT) is the form of
hypertension that by definition has no identifiable secondary cause
and may be considered, according to the risk of ischemic cardiac
and cerebrovascular events in disease staging, when organ damage,
including left ventricular hypertrophy (LVH), is present (1-3).
The type of cardiac remodeling is influenced by the evolution of
EHT, associated pathologies [diabetes mellitus (DM) or chronic
kidney disease (CKD) in the dialysis stage], phenotype (age,
obesity and sex) and genetic factors (4-7).
Heart failure (HF) with a preserved ejection
fraction is associated with left ventricular diastolic dysfunction
(LVDD) (2). LVDD is defined as
increased resistance to LV filling (2). LVDD is influenced by humoral and
hemodynamic factors, and may represent a stage in the evolution of
hypertension (2). The causes can
include mechanical obstruction of filling (myocardial fibrosis),
impaired ventricular relaxation and compliance in LVH, or ischemic
heart disease (IHD) (8,9).
The Renin-Angiotensin-Aldosterone System (RAAS) is
involved in the pathophysiological process of EHT (10). The RAAS consists of angiotensinogen
(AGT), angiotensin I (AngI), angiotensin II (AngII), AngI to AngII
converting enzyme (ACE), AngII type 1 receptor (R1 AngII), AngII
type 2 receptor (R2 AngII) and renin (REN) (11). RAAS exerts its effects through
neurohumoral mechanisms to regulate blood pressure (BP) in the
pathophysiological process of cardiac remodeling (4,12), and
RAAS components have been implicated in functional LV remodeling in
the pathophysiological mechanism of LVDD (13,14).
AGT is involved in the pathogenesis of EHT, is a
growth factor for myocytes and it induces cardiac hypertrophy
(11). The M235T genetic
variant (chr 1q42-q43) is a point substitution in which methionine
is replaced by threonine at position 235(15). Hypertensive patients carrying the
M235T-AGT genotype have an increased risk of LVH and IHD, as
they exhibit increased plasma AGT levels (12,16).
The M235T-AGT genotype is associated with increased AGT
levels, TT homozygotes are at a high risk for LVD and IHD.
Male and female athletes carrying the TT-AGT genotype
exhibit LVH as an adaptive response to exercise (16).
ACE plays a role in converting AngI to AngII
(12,17). ACE and AngII are involved in the
pathophysiological process of LVH, independently of hemodynamic
factors (6). Insertion/deletion
(I/D) at position 16 of the ACE gene (chr 17q23.3)
influences the activity of ACE and AngII. ACE accelerates cardiac
fibrosis progression and the increase in LV mass, which is why
hypertensive homozygous carriers of the DD allele have an
increased risk for LVH and IHD (4,17,18).
This aspect of the increased risk of LVH associated with the
DD genotype differs depending on the geographical area and
race, with Caucasians being at highest risk (4). I/D-ACE polymorphisms are
associated with the increase in LV mass in hypertensive and
normotensive patients (2,6,17,19).
Normotensive athletes carrying the DD genotype have a
greater increase in heart muscle mass compared with athletes
carrying the I/I genotype (19). I/D-ACE polymorphisms are
associated with the increase in LV mass in hypertensive and
normotensive subjects, and the D allele is most frequently
associated with LVH (2,6,17,19).
R1 AngII and R2 AngII are part of the G-protein
coupled receptor family (20).
After binding R1, Ang II activates its role in stimulating the
release of aldosterone, regulating vasoconstriction, cell
proliferation and the increase in mitosis of smooth and striated
muscle fibers (6,20,21).
The A1166C-R1AngII genetic variant is a point mutation in
the R1 AngII gene (chr 3q21-q25) in which the adenine nucleotide at
position 1,166 of the gene is replaced by cytosine. It is
associated with the synthesis of type I collagen located in the
myocardium, which explains its role in the pathogenesis of LVH and
IHD (6,17,22).
The A3123C-R2AngII genetic variant is a point
mutation in the R2 AngII gene in which the adenine nucleotide is
replaced by cytosine at position 3,123 of the gene (23). R2 AngII expression can influence the
pathophysiological process of EHT and cardiac remodeling (24).
REN is an aspartate protease, which is involved in
the cleavage of the AGT precursor to Ang I, and activates the RAAS
cascade (25). REN activity is
associated with the BP response to salt consumption and
antihypertensive treatment (26).
The G83A-REN genetic variant is a point mutation in the
which guanine is replaced by adenine at position 83 of the renin
gene (chr 1q32). It is associated with an increase in REN activity
and EHT pathophysiology, as well as ischemic and hemorrhagic
cerebral events (25).
The association of at least 2 genes increases the
risk of cardiovascular complications, such as LVH, IHD and heart
failure, in hypertensive patients (4,27,28).
Although there are several factors that contribute
to hypertensive remodeling, the aim of the present study was to
identify the distribution of various RAAS genetic polymorphisms in
the patterns of cardiac structural and functional adaptation to
EHT.
Materials and methods
Study design and setting
The present study was a prospective cross-section
study performed on patients with EHT, who were being followed up,
were under treatment or were newly diagnosed, from three medical
centers in Cluj-Napoca, Romania June 2015 and December 2017. The
patients were selected on the occasion of ambulatory examinations
or hospitalization to internal medicine or cardiology services. The
study group consisted of 139 subjects, 71 females and 68 males,
with a median age (IQR) of 61 (49.5-67) and an age range of 23-86
years.
Written informed consent was obtained from all
participants, prior to their inclusion in the study. The Ethics
Committee of the ‘Iuliu Hatieganu’ University of Medicine and
Pharmacy approved the study (approval no. 333/2.06.2015).
Study group
To ensure homogeneity of the studied groups,
patients with secondary hypertension, left ventricular ejection
fraction (LVEF) <50%, with HF New York Heart Association
functional class III and IV, with unstable coronary disease, CKD
with a glomerular filtration rate <30 ml/min/1.72 m2
or in the dialysis stage were all excluded.
At 5 min prior to the beginning of the examination,
systolic BP (SBP) and diastolic BP (DBP) were measured as the
patient was sitting or lying down, with a manual BP monitor; three
successive measurements were taken 2-5 min apart. Newly diagnosed
EHT was defined according to the European Society of Hypertension
Guidelines as values ≥140/90 mmHg (1).
Detailed history was taken for each patient
including their age, sex, weight (kg) and height (cm). The body
mass index (BMI) was also calculated based on the formula:
BMI=weight (kg)/[height (m)]2.
Cardiac ultrasonography
All patients included in the present study underwent
cardiac ultrasound in M and 2D mode, using a Samsung H60 (Samsung
Medison) or Aloka Alpha 7 (Aloka Ltd.) ultrasound systems, with
transducers at frequencies ranging between 2-4 Mhz.
The examinations were performed in left lateral
decubitus. The end-diastolic interventricular septum (IVSd), the
end-diastolic LV posterior wall (PWd), as well as the LV
end-systolic diameter (LVESD) and LV end-diastolic diameter (LVEDD)
were measured in parasternal long axis incidence, perpendicular to
the LV long axis, and measured at the level of the mitral valve
leaflet tips, with the values being expressed in mm.
The LV mass (LVM), expressed in grams, was
calculated based on the Devereaux formula: LVM
(g)=0.8[1.04[([LVEDD+IVSd+PWd]3-LVEDD3)]]
+0.6. The LVM index (LVMI) was calculated as the ratio of the LVM
to the body surface area (g/m2).
LVH was defined based on the following criteria:
LVMI >131 g/m² in men, and >108 g/m² in women. To
differentiate concentric from eccentric LVH, the relative wall
thickness (RWT) was also calculated according to the formula:
RWT=2x PWD/LVEDD.
According to Ganau's criteria, 4 types of cardiac
remodeling were considered: i) normal geometry, with the absence of
LVH and with an RWT value <0.42; ii) concentric hypertrophy,
with the presence of LVH and an RWT value ≥0.42; iii) eccentric
hypertrophy, with the presence of LVH and an RWT value <0.42;
and iv) concentric remodeling, with the absence of LVH and an RWT
value ≥0.42(5).
LVEF was calculated using LVESD and LVEDD values
based on the Teicholz formula using an online tool (e-echocardiography.com) (29). To assess LV diastolic function,
pulsed Doppler was used. The LV diastolic filling mitral flow was
measured in the apical incidence. The peak of early passive filling
velocity (E wave) and late active filling velocity corresponding to
atrial contraction (A wave) was recorded and the E/A ratio was
calculated. Peak E-wave velocity (cm/sec) and peak A-wave velocity
(cm/sec) were measured in the apical four-chamber view, with color
flow imaging for optimal alignment of power Doppler; blood flow and
power Doppler sample volume was 1-3 mm axial size, between the
mitral leaflet tips. Peak E-wave velocity (cm/sec) was calculated
in peak modal velocity in early diastole (after ECG T wave) at the
leading edge of spectral waveform. Peak A-wave velocity (cm/sec)
was calculated in peak modal velocity in late diastole (after the
ECG P wave) at the leading edge of the spectral waveform.
Evaluations of 5 different diastoles were performed and their mean
was calculated. The isolated LVDD is expressed by E/A <1, and
was defined as abnormal relaxation.
Genetic determinations; identification
of M235T-AGT, I/D-ACE, A1166C-R1AngII, C3123A-R2AngII and G83A-REN
genotypes
For genetic determination, 5 ml venous blood was
collected in vacutainers with EDTA. Genomic DNA was isolated from
peripheral leukocytes using a Quick-DNA-Miniprep kit (Zymo
Research).
To identify M235T-AGT, I/D-ACE,
A1166C-R1AngII, A3123C-R2AngII and G83A-REN
genotypes, the methods described by Procopciuc et al
(15), as well as specific primers
(Table I) were used (Kaneka
Eurogentec S.A.) (15).
Amplification was performed in an iCycler (Bio-Rad Laboratories,
Inc.) in a 25 µl reaction mixture consisting of 20 ng DNA, 0.2 µM
primers, 2.0 mM MgCl2, 200 µM dNTPs and 2U Taq
polymerase in a specific buffer: 10X Taq buffer with
(NH4)2SO4 (750 mM tris-HCl (pH 8.8
at 25˚C), 200 mM (NH4)2SO4, 0.1%
(v/v) Tween-20).
 | Table IPCR-Restriction Fragment Length
Polymorphism conditions for M235T-AGT, I/D-ACE,
A1166C-R1AngII, A3123C-R2AngII and
G83A-REN. |
Table I
PCR-Restriction Fragment Length
Polymorphism conditions for M235T-AGT, I/D-ACE,
A1166C-R1AngII, A3123C-R2AngII and
G83A-REN.
| Genetic variants
(polymorphism) | Primer
sequence | PCR fragment,
bp | Annealing
temperature, ˚C | Restriction
enzyme | Enzymatic
digestion |
|---|
| M235T-AGT
(rs 699) | | 165 | 64 | Tth111I | M235 allele,
165 bp; T235 allele, |
|
Forward |
5'-CAGGGTGCTGTCCACACTGGACCCC-3' | | | | 141 and 24 bp |
|
Reverse |
5'-CCGTTTGTGCAGGGCCTGGCTCTCT-3' | | | | |
| I/D-ACE
(rs1799752) | | I allele, 390; | 69 | | |
|
Forward |
5'-CTGGAGACCACTCCCATCCTTTCT-3' | D allele, 290 | | | |
|
Reverse |
5'-GATGTGGCCATCACATTCGTCAGC-3' | | | | |
|
A1166C-R1AngII (rs
5186) | | 350 | 57 | DdeI | A1166
allele, 350 bp; C1166 allele, |
|
Forward |
5'-ATAATGTAAGCTCATCCACC-3' | | | | 211 and 139 bp |
|
Reverse |
5'-GAGATTGCATTTCTGTCAGT-3' | | | | |
|
A3123C-R2AngII (rs
11091046) | | 312 | 52 | AluI | A3123
allele, 215 and 107 bp; |
|
Forward |
5'-GGATTCAGATTCTCTTTGAA-3' | | | | C3123
allele, 312 bp |
|
Reverse |
5'-GCATAGGAGTATGATTTAATC-3' | | | | |
| G83A-REN
(rs 2368564) | | 250 | 66 | MboI | G83 allele,
171 and 79 bp; |
|
Forward |
5'-GAGGTTCGAGTCGGCCCCCT-3' | | | | A83 allele,
250 bp |
|
Reverse |
5'-TGCCCCAAACATGGCCACACAT-3' | | | | |
For M235T-AGT, I/D-ACE,
A1166C-R1AngII, A3123C-R2AngII, G83A-REN
genetic variants, the amplified fragments were subjected to
enzymatic digestion with specific restriction enzymes for 3 h.
The amplified and enzymatic digestion fragments were
visualized by UV light through migration in 2 and 3% agarose gels,
respectively, stained with 10 mg/ml ethidium bromide.
All PCR-Restriction Fragment Length Polymorphism
reagents were obtained from Fermentas (Thermo Fischer Scientific
Inc.) and Jena Bioscience (Analytik Jena AG), except for the
primers, which were from Eurogentec (Kaneka Eurogentec S.A.).
The amplification and enzymatic digestion conditions
are presented in Table I, and the
fragments obtained by enzymatic digestion for all the studied RAAS
genetic variants are presented in Fig.
1.
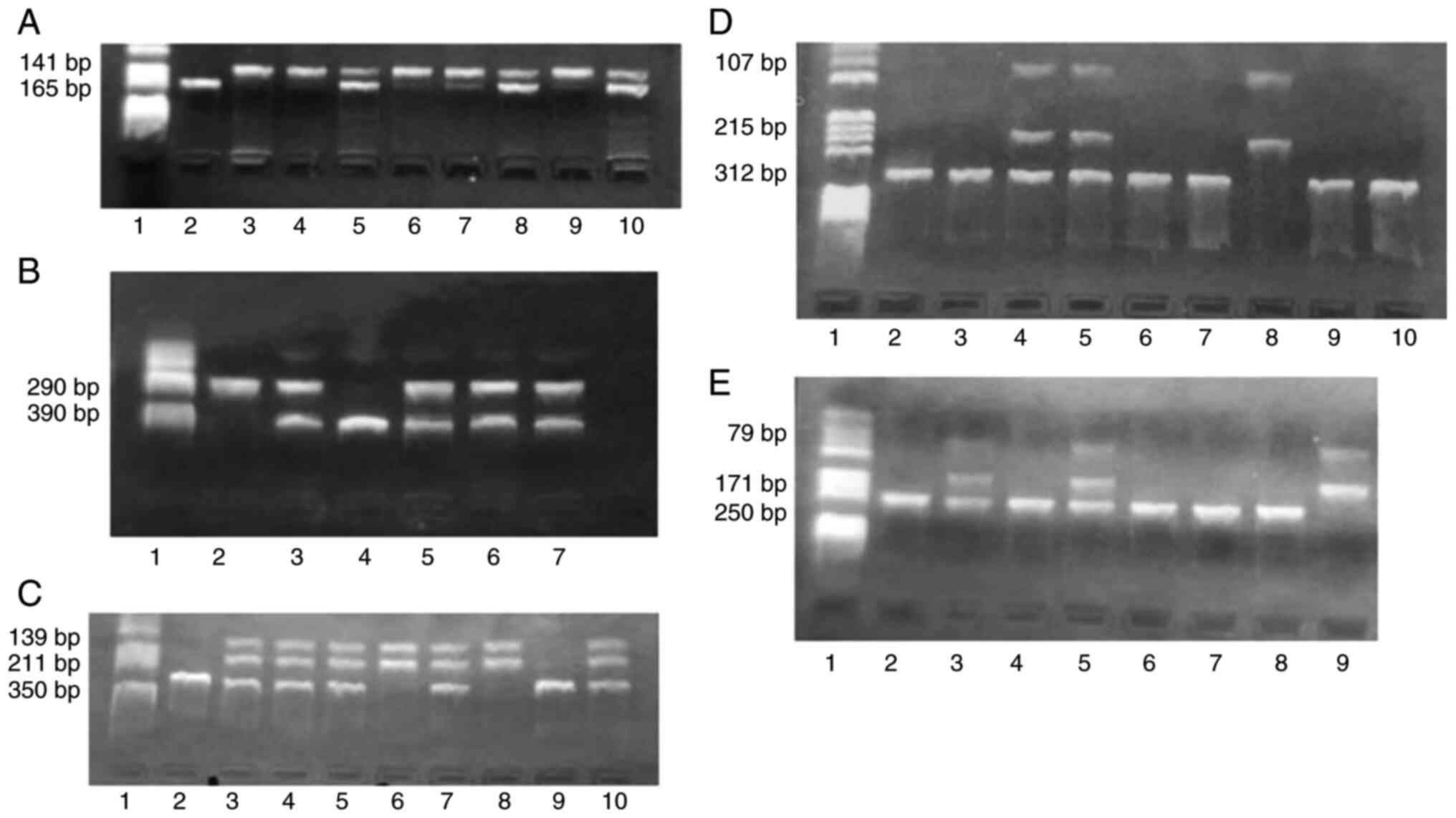 | Figure 1PCR amplification and enzymatic
digestion for identification of RAAS polymorphisms. (A) M235T-AGT:
lane 1-pBRHaeIII Digest DNA molecular marker; lane 2-M/M homozygous
genotype, 165 bp fragment; lanes 3, 4, 6 and 9-T/T homozygous
genotype, 141 bp fragment; lanes 5, 7, 8 and 10-M/T heterozygous
genotype, 165 and 141 bp fragments. (B) I/D-ACE: lane 1-pBRHaeIII
Digest DNA molecular marker; lane 2-D/D homozygous genotype, 290 bp
fragment; lanes 3, 5, 6 and 7-I/D heterozygous genotype, 390 and
290 bp fragments; lane 4-I/I homozygous genotype, 390 bp fragment.
(C) A1166C-R1AngII: lane 1-pBRHaeIII Digest DNA molecular
marker; lanes 2 and 9-A/A homozygous genotype, 350 bp fragment;
lanes 3, 4, 5, 7 and 10-A/C heterozygous genotype, 350, 211 and 139
bp fragments; lanes 6 and 8-C/C homozygous genotype, 211 and 139 bp
fragments. (D) A3123C-R2AngII: lane 1-pBRHaeIII Digest DNA
molecular marker; lanes 2, 3, 6, 7, 9 and 10-C/C homozygous
genotype, 312 bp fragment; lanes 4 and 5-C/A heterozygous genotype,
312, 215 and 107 bp fragments; lane 8-A/A homozygous genotype, 215
and 107 bp fragments. (E) G83A-REN: lane 1-pBRHaeIII Digest
DNA molecular marker; lanes 2, 4, 6, 7 and 8-A/A homozygous
genotype, 250 bp fragment; lanes 3 and 5-A/G heterozygous genotype,
250, 171 and 79 bp fragments; lane 9-G/G homozygous genotype, 171
and 79 bp fragments. |
Statistical analysis
Categorical variables were reported as absolute and
relative frequencies. The associations between categorical
variables were tested using a χ2 test or a Fisher's
exact test (in cases of lower than expected frequencies). The
associations between binary data were assessed with odds ratios and
95% confidence intervals. An exact test was used to check the
Hardy-Weinberg equilibrium. Data that were normally distributed are
presented as the mean ± standard deviations, and compared using an
unpaired Student's t-test or one way ANOVA (30). Skewed data are presented as the
medians and interquartile ranges, and a Wilcoxon rank-sum test or a
Kruskal-Wallis test were used to compare the different groups.
To assess the associations between polymorphisms
with abnormal relaxation or RWT values >0.42, first, univariate
logistic regression analyses were used. Multivariate logistic
regression models where next used, adjusting each polymorphism for
age (years), BMI (kg/m2), SBP (mmHg) and DBP (mmHg). For
all multivariate logistic regression models, multicollinearity was
assessed for using the variance inflation factor. The linearity of
log odds in cases of continuous covariates were examined, using
splines terms in a generalized binomial additive model, and if the
assumption did not hold the continuous covariate was replaced, in
the logistic regression model, with a binary transformation using
the median as the cutoff. All logistic regression models are
presented using odds ratios, with 95% confidence intervals and
P-values.
For all statistical tests, a two-tailed P-value
<0.05 was considered to indicate a statistically significant
difference. All analyses were computed using R environment for
statistical computing and graphics (R Foundation for Statistical
Computing, Vienna, Austria), version 3.6.2(31). The Hardy-Weinberg equilibrium test
was computed using the package SNPassoc 1.9-2(32).
Results
Patients' characteristics according to
LVH status
The characteristics of the subjects in the study
cohort are presented in Tables II
and III. The most frequent LVH
was concentric remodeling, 59 patients (42.45%), followed by
concentric hypertrophy, 48 patients (34.53%) and eccentric
hypertrophy, 8 patients (5.75%).
 | Table IICharacteristics of the patients with
essential hypertension with regard to the left ventricular
pathophysiology. |
Table II
Characteristics of the patients with
essential hypertension with regard to the left ventricular
pathophysiology.
| | Left ventricular
geometry | |
|---|
| Characteristic | Normal geometry,
n=24 | Concentric
hypertrophy, n=48 | Eccentric
hypertrophy, n=8 | Concentric
remodeling, n=59 | P-value |
|---|
| Age, years, mean
(standard deviation) | 49.25 (13.48) | 59.9 (10.87) | 61.5 (13.33) | 59.46 (11.97) | 0.002a |
| Sex, n (%) | | | | | |
|
Female | 9 (37.5) | 27 (56.25) | 6(75) | 29 (49.15) | 0.253 |
|
Male | 15 (62.5) | 21 (43.75) | 2(25) | 30 (50.85) | |
| Weight status, n
(%) | | | | | 0.004a |
|
Healthy
weight | 4 (16.67) | 2 (4.17) | 0 (0) | 13 (22.03) | |
|
Overweight | 7 (29.17) | 26 (54.17) | 3 (37.5) | 13 (22.03) | |
|
Obesity | 13 (54.17) | 20 (41.67) | 5 (62.5) | 33 (55.93) | |
| Systolic blood
pressure, mmHg, median (IQR) | 160 (150-170) | 170 (160-180) | 160
(148.75-180) | 160 (150-170) | 0.001a |
| Diastolic blood
pressure, mmHg, median (IQR) | 90 (90-100) | 100 (90-100) | 87.5
(83.75-102.5) | 90 (90-100) | 0.087 |
| Abnormal
relaxation, n (%) | 8 (33.33) | 35 (72.92) | 6(75) | 38 (64.41) | 0.009a |
 | Table IIICharacteristics of the patients with
essential hypertension with regard to the E/A ratio. |
Table III
Characteristics of the patients with
essential hypertension with regard to the E/A ratio.
| | E/A ratio | |
|---|
| Characteristic | Abnormal
relaxation, n=87 | Absence of abnormal
relaxation, n=52 | P-value |
|---|
| Age, years, mean
(standard deviation) | 61.16 (10.21) | 52.62 (14.13) | 0.001b |
| Age ≥60 years, n
(%) | 54 (62.07) | 20 (38.46) | 0.007b |
| Sex, n (%) | | | |
|
Female | 44 (50.57) | 27 (51.92) | 0.878 |
|
Male | 43 (49.43) | 25 (48.08) | |
| Weight status, n
(%) | | | 0.138 |
|
Healthy
weight | 8 (9.2) | 11 (21.15) | |
|
Overweight | 32 (36.78) | 17 (32.69) | |
|
Obesity | 47 (54.02) | 24 (46.15) | |
| Systolic blood
pressure, mmHg, median (IQR) | 170 (150-180) | 160 (150-170) | 0.38 |
| Diastolic blood
pressure, mmHg, median (IQR) | 90 (90-100) | 90 (88.75-100) | 0.488 |
| Left ventricular
remodeling models, n (%) | | | 0.009b |
|
Normal
geometry | 8 (9.2) | 16 (30.77) | |
|
Concentric
hypertrophy | 35 (40.23) | 13(25) | |
|
Eccentric
hypertrophy | 6 (6.9) | 2 (3.85) | |
|
Concentric
remodeling | 38 (43.68) | 21 (40.38) | |
| RWT, median
(IQR) | 0.48
(0.44-0.53) | 0.44
(0.4-0.49) | 0.001b |
| RWT <0.42, n
(%) | 73 (83.91) | 32 (61.54) | 0.003b |
| LVM (g), median
(IQR) | 206 (176-241) | 181.5
(141.75-234) | 0.028a |
| LVMI,
g/m2, median (IQR) | 100 (92-120) | 92
(72.75-113.75) | 0.008b |
| Pathological LVMI,
n (%) | 41 (47.13) | 15 (28.85) | 0.033a |
The mean age was statistically significant
(P<0.002), a decade higher in subjects with LVH compared to
those with normal geometry. The weight status differed
significantly amongst groups (P<0.004), with individuals more
likely to be overweight in the concentric hypertrophy group
(54.17%), followed by the eccentric hypertrophy group (37.5%).
Obesity was more common in the eccentric hypertrophy group (62.5%),
followed by the concentric remodeling group (55.93%). The SBP
differed significantly amongst groups (P<0.001), the highest
values were observed in the concentric hypertrophy group compared
to the other LV geometry groups.
Abnormal relaxation was statistically significantly
associated with LV geometries (P<0.009), being more frequent in
the eccentric hypertrophy group (75%), followed by concentric
hypertrophy (72.92%) and concentric remodeling (64.41%) (Table II).
Patients' characteristics according to
diastolic function (abnormal relaxation)
LVDD as expressed by abnormal relaxation was present
in 87 (62.58%) subjects. Compared to the group with absence of
abnormal relaxation, the mean age, SBP and DBP were higher in the
group of hypertensive patients with LVDD. The results obtained
showed a statistically significantly higher number of hypertensive
patients with LVDD aged >60 years (P<0.007).
There were more overweight subjects, 32 (36.78%) and
obese subjects, (54.02%), in the group with LVDD. The patients with
abnormal relaxation had abnormal 47 geometries, namely concentric
hypertrophy, 35 patients (40.23%), eccentric hypertrophy, 6
patients (6.9%), and concentric remodeling, 38 patients (43.68%),
compared with patients with absence of abnormal relaxation. The
differences between the two groups were only statistically
significant different with regard to age (P<0.001) and the
presence of LVH (P<0.009).
Hypertensive patients with LVDD had a median of RWT
value (P<0.001), LVM (g) (P<0.028), and LVMI
(g/m2) (P<0.008) in a statistically significantly
higher proportion than hypertensive patients without LVDD.
Accordingly, the frequency of RWT <0.42 (P<0.003) and of
pathological LVMI (g/m2) (P<0.033) was statistically
significantly lower in the group with LVDD (Table III).
Associations between the RAAS
polymorphisms and abnormal relaxation
There were statistically significant associations
between LVDD and genetic variants M235T-AGT (P<0.035),
A1166C-R1AngII (P<0.046), G83A-REN (P<0.02) in
the univariate logistic regression analysis. The association was
maintained even after adjustment for confounders (age, sex, BMI,
SBP and DBP), for patients carrying the M/T, T/T genotypes
(M235T-AGT polymorphism) (P<0.039) and for patients
carrying G/G-A/G genotypes (G83A-REN polymorphism)
(P<0.033), but not for patients carrying C/C, A/C
genotypes (A1166C-R1AngII polymorphism) (P<0.105).
Compared to the negative carriers, the chances of
hypertensive patients exhibiting LVDD was 2.22x higher in carriers
of the T/T, M/T genotypes without adjustment, and
2.42x higher after adjustment for confounders. The chances of
exhibiting LVDD was 2.32x higher in carriers of the G/G-A/G
genotypes compared to the A/A genotype without adjustment,
and 2.39x higher after adjustment for confounders.
There was no statistically significant associations
of I/D-ACE (P=0.977) and A3123C-R2AngII (P=0.202)
gene variants with abnormal relaxation (Table IV).
 | Table IVUnivariate and multivariate logistic
regressions assessing associations between E/A ratio and the
genetic variant in a cohort of patients with essential
hypertension. |
Table IV
Univariate and multivariate logistic
regressions assessing associations between E/A ratio and the
genetic variant in a cohort of patients with essential
hypertension.
| | E/A ratio | |
|---|
| Genetic variants, n
(%) | Abnormal
relaxation, n=87 | Absence of abnormal
relaxation, n=52 | OR unadjusted | 95% CI | P-value | OR
adjustedb | 95% CI | P-value |
|---|
| M235T-AGT;
T/T, M/T vs. M/M | 68 (78.16) | 32 (61.53) | 2.24 | (1.05-4.8) | 0.037a | 2.42 | (1.05-5.69) | 0.039a |
|
A1166C-R1AngII; C/C,
A/C vs. A/A | 35 (40.22) | 13(25) | 2.19 | (1.03-4.89) | 0.048a | 1.98 | (0.88-4.63) | 0.105 |
| G83A-REN;
G/G, A/G vs. A/A | 49 (56.32) | 19 (36.53) | 2.32 | (1.14-4.8) | 0.021a | 2.39 | (1.09-5.44) | 0.033a |
| I/D-ACE;
D/D, I/D vs. I/I | 32 (36.78) | 19 (36.53) | 0.99 | (0.48-2.01) | 0.977 | 1.05 | (0.47-2.3) | 0.911 |
|
A3123C-R2AngII; C/C, A/C vs.
A/A | 35 (40.22) | 16 (30.76) | 1.62 | (0.78-3.45) | 0.204 | 1.74 | (0.8-3.88) | 0.168 |
Associations of RAAS polymorphisms
with LV geometry
No statistically significant associations between
M235T-AGT, A1166C-R1AngII, G83A-REN,
I/D-ACE and A3123C-R2AngII polymorphisms and LV
geometry were identified.
The M235T-AGT (P<0.147) and
A3123C-R2AngII (P<0.156) polymorphisms were closest to
statistical significance regarding their association with LV
geometry. Compared with M/M genotype carriers, the
distribution of M235T-AGT genotypes in association with
concentric hypertrophy was higher for T/T, M/T
genotypes (72.92%), the distribution in association with eccentric
hypertrophy was higher only for T/T, M/T genotypes
(100%), and in association with concentric remodeling was higher
for T/T, M/T genotypes (72.88%). Regarding the
A3123C-R2AngII gene variant, the distribution of genotypes
in the group of hypertensive patients with concentric hypertrophy
was higher for C/C, A/C genotype carriers (43.75%)
(Table V).
 | Table VAssociations between left ventricular
geometry and the genetic variant in a cohort of patients with
EHT. |
Table V
Associations between left ventricular
geometry and the genetic variant in a cohort of patients with
EHT.
| | Left ventricular
geometry | |
|---|
| Genetic variants, n
(%) | Normal geometry,
n=24 | Concentric
hypertrophy, n=48 | Eccentric
hypertrophy, n=8 | Concentric
remodeling, n=59 | P-value |
|---|
| M235T-AGT;
T/T, M/T vs. M/M | 14 (58.33) | 35 (72.92) | 8(100) | 43 (72.88) | 0.147 |
|
A1166C-R1AngII; C/C,
A/C vs. A/A | 5 (20.83) | 18 (37.5) | 4(50) | 20 (34.48) | 0.387 |
| G83A-REN;
G/G, A/G vs. A/A | 8 (33.33) | 24 (51.06) | 4(50) | 30 (51.72) | 0.471 |
| I/D-ACE;
D/D, I/D vs. I/I | 11 (45.83) | 14 (29.17) | 3 (37.5) | 23 (38.98) | 0.538 |
|
A3123C-R2AngII; C/C, A/C vs.
A/A | 4 (16.67) | 21 (43.75) | 3 (37.5) | 22 (37.93) | 0.156 |
Associations of RAAS polymorphisms
with RWT
Given the lack of findings between the types of LV
geometry and the studied polymorphisms, next, the relationship
between the finer aspects of LV geometry with the studied
polymorphisms were assessed.
With regard to the A3123C-R2AngII
polymorphism, the C/C, A/C genotypes were
statistically significantly associated with RWT <0.42
(P<0.033), and after adjustment for confounders (age, sex, BMI,
SBP and DBP), the association remained statistically significant
(P<0.008). Compared with the negative carriers, the chance of a
patient exhibiting an RWT value <0.42 was 2.72x higher for
C/C, A/C genotypes, and 4.02x higher after adjustment
for confounders.
No statistically significant associations between
M235T-AGT, I/D-ACE, A1166C-R1AngII and
G83A-REN with normal or pathological values of RWT were
identified, but homozygous and heterozygous carriers had a
>1-fold increased risk factor for pathological values of RWT in
all polymorphisms (Table VI).
 | Table VIUnivariate and multivariate logistic
regressions assessing associations between RWT and the genetic
variant in a cohort of patients with essential hypertension. |
Table VI
Univariate and multivariate logistic
regressions assessing associations between RWT and the genetic
variant in a cohort of patients with essential hypertension.
| | RWT | |
|---|
| Genetic variants, n
(%) | <0.42,
n=105 | >0.42, n=34 | OR unadjusted | 95% CI | P-value | OR
adjustedc | 95% CI | P-value |
|---|
| M235T-AGT;
T/T, M/T vs. M/M | 77 (73.33) | 23 (67.64) | 1.32 | (0.55-3) | 0.522 | 1.13 | (0.45-2.71) | 0.786 |
|
A1166C-R1AngII; C/C,
A/C vs. A/A | 39 (37.14) | 9 (26.47) | 1.6 | (0.7-3.95) | 0.285 | 1.56 | (0.64-4.06) | 0.342 |
| G83A-REN;
G/G, A/G vs. A/A | 55 (52.38) | 13 (38.23) | 1.71 | (0.78-3.86) | 0.183 | 1.87 | (0.79-4.57) | 0.159 |
| I/D-ACE;
D/D, I/D vs. I/I | 68 (64.76) | 20 (58.82) | 1.29 | (0.58-2.83) | 0.533 | 1.33 | (0.56-3.12) | 0.507b |
|
A3123C-R2AngII; C/C,
A/C vs. A/A | 44 (41.9) | 7 (20.58) | 2.72 | (1.13-7.29) | 0.033a | 4.02 | (1.52-12.13) | 0.008a |
Associations of RAAS polymorphisms
with pathological LVMI
No statistically significant associations between
M235T-AGT, A1166C-R1AngII, G83A-REN,
I/D-ACE and A3123C-R2AngII polymorphisms with LVMI
were identified; under these conditions; thus, the multivariate
analysis was not justified. Compared to the negative carriers, the
chance of having pathological LVMI was 1.51x higher in carriers of
the T/T, M/T genotypes (M235T-AGT
polymorphism), 1.47x higher in carriers of the C/C,
A/C genotypes (A1166C-R1AngII polymorphism), 1.2x
higher in carriers of the G/G-A/G genotypes
(G83A-REN polymorphism), and 1.58x higher in carriers of the
D/D, I/D genotypes (I/D-ACE polymorphism).
The polymorphisms that were closest to being
statistically significant regarding their association with LVMI
were MM235-AGT (P<0.297), AA1166-R1AngII
(P<0.284) and I/D-ACE (P<0.203) (Table VII).
 | Table VIIAssociations between pathological
LVMI and the genetic variant in a cohort of patients with essential
hypertension. |
Table VII
Associations between pathological
LVMI and the genetic variant in a cohort of patients with essential
hypertension.
| | LVMI | |
|---|
| Genetic variants, n
(%) | Yes, n=56 | No, n=83 | Odds ratio (95%
confidence interval) | P-value |
|---|
| M235T-AGT;
T/T, M/T vs. M/M | 43 (76.78) | 57 (68.67) | 1.51
(0.69-3.22) | 0.297 |
|
A1166C-R1AngII; C/C,
A/C vs. A/A | 22 (39.28) | 26 (31.32) | 1.47
(0.72-3.03) | 0.284 |
| G83A-REN;
G/G, A/G vs. A/A | 29 (51.78) | 39 (46.98) | 1.20
(0.60-2.38) | 0.6 |
| I/D-ACE;
D/D, I/D vs. I/I | 39 (69.64) | 49 (59.03) | 1.58
(0.77-3.22) | 0.203 |
|
C3123A-R2AngII; C/C,
A/C vs. A/A | 13 (23.21) | 21 (25.30) | 0.93
(0.42-2.08) | 0.874 |
The Hardy-Weinberg equilibrium was present for
M235T-AGT, A1166C-R1AngII and G83A-REN
polymorphisms, but not the I/D-ACE gene variant and
A3123C-R2AngII polymorphism, while eccentric hypertrophy was
present in the case of M235T-AGT.
Discussion
In the medical literature from the last 20 years,
data related to cardiac remodeling and LVDD in association with
RAAS gene polymorphisms have been inconsistent and contradictory.
Discrepancies are explained by interethnic differences, differences
in the methodology of recruitment, confounders and associated
pathologies (such as DM and CKD).
With regard to the relationship between LVH and sex
distribution, Radulescu et al (5) observed a higher prevalence of
concentric LVH in men and eccentric LVH in women. In 1999,
Perticone et al (33)
reported that male hypertensive patients are at higher risk of
developing LVH. Yang et al (7) explained that eccentric LVH is
predominantly found in the female sex due to the location of
estrogen receptors in cardiac myocytes and fibroblasts. Bahramali
et al (2), Sasu et al
(8) and Lalande and Johnson
(9) all reported that LVDD is more
frequently found in women In the present study, there was no
statistically significant association between cardiac morphological
and functional remodeling and sex distribution, although the
frequency of eccentric LVH and LVDD was higher in women, and the
frequency of normal cardiac geometry was higher in men.
Cardiac structural and functional changes occur with
aging. According to Radulescu (5)
and Lin et al (6),
hypertensive patients aged over 65 years old had LVH and
pathological RWT values According to Bahramali et al
(2), the prevalence of LVDD
increases in patients aged >65 years. In the present study,
there was a statistically significant correlation between LVDD, the
three types of cardiac remodeling and the age of hypertensive
patients >60 years.
Ji et al (34) and Song et al (35) observed that RAAS genetic variants
(AGT, ACE, R1 AngII and REN) were significantly associated with
high a BP in the Han Chinese population and Korean population
Radulescu et al (5) and Du
et al (36) showed that high
SBP and DBP values influence the type of cardiac remodeling In the
present study, only SBP significantly differed between the LV
geometry groups, having the highest median value (170 mmHg) in the
concentric hypertrophy group, and 160 mmHg in the other three
groups. DBP followed a similar pattern, being higher in the
concentric hypertrophy group (100 mmHg), and ~90 mmHg in the other
three groups, but the differences did not reach the level of
significance.
Yang et al (7) showed that SBP and DBP values influence
LV diastolic function, and with aging, they contribute to the
development of LVDD. In the present study, no statistically
significant correlation was found between the mean SBP and DBP
values with LVDD.
Obesity influences the pathophysiology of cardiac
morphological and functional remodeling in both hypertensive and
normotensive patients, as an independent factor or in association
with RAAS. According to Woodiwiss and Norton GR (37), obese patients can present with LVH
in the absence of high BP values. Rocha et al (38) reported that obese hypertensive
patients had LVDD in the absence of LVH. Alpert et al
(10) showed that LVDD in obese
patients was associated with LVH, and LVDD and eccentric LVH were
present in a higher proportion in obese subjects. In the present
study, weight status was statistically significantly correlated
with LVH, but not with LVDD. Obese and overweight patients had, in
a higher proportion, concentric LVH, concentric remodeling and
LVDD.
The study by Radulescu et al (5) showed that LVDD is associated with
concentric and eccentric LVH, especially if hypertension is of
secondary renal cause. Du et al (36) maintained that in hypertensive
patients with eccentric hypertrophy, LVDD is present. The results
of the present study demonstrated a statistical correlation between
cardiac remodeling, the pathological value of LVMI and LVDD.
Patients with LVDD had, in an increased proportion of eccentric and
concentric hypertrophy, and in a smaller percentage, cardiac
remodeling.
Chahal et al (39) reported that the E/A ratio decreases
to <1 with the increase in the RWT value, and Bamaiyi (40) showed that pathological RWT is
statistically significantly associated with LVDD. In the present
study, no statistical correlation was found between LVDD and the
pathological value of RWT.
In hypertensive Chinese and Uzbek Central Asian
subjects, without other associated pathologies, M235T-AGT
polymorphisms were associated with LVH according to Tang et
al (12) and Kurbanova and
Eliseyeva (41) Tran et al
(42), Eliseeva et al
(43), Jeng (44) and Iwai et al (45) showed that Vietnamese, Uzbek, Chinese
and Japanese carriers of the T/T genotype exhibited
increased AGT concentrations, which explains the implication of the
genetic variant in the development of LVH in patients without other
associated diseases. Wang et al (4) demonstrated that the T/T
genotype was associated with LVH in Chinese hypertensive patients
with CKD in the hemodialysis stage. In contrast, Olcay et al
(46) and Shilyakhto et al
(47) found no association between
LVH and M235T-AGT genetic variants in Turkish and Russian
patients with EHT In the present study, there was a tendency to
association, but without statistical significance, between the
M235T-AGT genetic polymorphism and cardiac remodeling;
carriers of the T/T, M/T genotype have a risk factor
for developing LVH compared to carriers of the negative M/M
genotype.
Rani et al (22) described the association between the
T/T genotype and the increase in RWT value in Indian
hypertensive patients. In the present study, there was no
statistical association between the M235T-AGT polymorphism
and RWT, although the risk of having pathological RWT values
increases for hypertensive carriers of M/T, T/T
genotypes.
There was a statistically significant association
between LVDD and the M235T-AGT polymorphism identified in
the present study. However no previous literature confirming or
refuting this result could be found.
According to studies conducted by Kurbanova and
Eliseyeva (41) and Eliseeva et
al (43), the D allele
(I/D-ACE) is associated with the severity of the disease,
with an unfavorable progression of LVH in Uzbek hypertensive
patients. Lynch et al (27)
using the data from Hypertension Genetic Epidemiology Network
observed that the I/D-ACE genetic variant was implicated in
the development of LVH amongst white, but not African-American
hypertensive participants. Wang et al (4), Shilyakhto et al (47) and West et al (48) did not identify an association
between LVH and the I/D-ACE polymorphism in hypertensive
Chinese, Russian and Australian subjects
Bahramali et al (2) and Cosenso-Martin et al
(3) showed that the presence of the
D allele could be a predictor for the development of LVH in
hypertensive patients with LVDD from Iran and Brazil. Smilde et
al (13) and Li et al
(19) identified the contribution
of the I/D-ACE genetic variant to the development of LVH in
individuals from Netherlands and China, especially if EHT is
associated with other diseases, such as CKD In the present study,
there was no statistical association between the I/D-ACE
genotype and cardiac anatomical remodeling; however, patients
carrying the D/D and I/D genotypes did exhibit an
increased risk of developing LVH.
Studies by Rani et al (22) and Ueno et al (49) reported that the D allele is
significantly associated with pathological RWT more frequently in
D/D homozygotes than in I/D heterozygotes in Indian
and Japanese hypertensive patients. There was no significant
correlation between the I/D-ACE polymorphism and the
pathological RWT value identified in the present study.
Bahramali et al (2) and Rani et al (22) observed that LVDD is associated with
the D allele in hypertensive Iranian and Indian patients
carrying the; however, no correlation was identified between LVDD
and the I/D-ACE polymorphism in the present study.
Kuznetsova et al (23) and Shilyakhto et al (47) did not identify any association
between LVH and A1166C-R1AngII in hypertensive patients from
Poland, Russia and Italy. In contrast, Mishra et al
(50) reported that Indian patients
carrying the C/C and A/C genotypes were at higher
risk of LVH and LVDD. In the present study, carriers of the
C/C and A/C genotypes had a n increased risk of
developing LVH compared to carriers of the A/A genotype,
although the difference was not significant.
Lin et al (6) and Smilde et al (13) described in studies conducted in
China and Netherlands, respectively, an association between CKD,
aged >60 years old with the A1166C-R1AngII genetic
polymorphism with the risk of development of LVH. In the present
study, only patients with EHT were selected, excluding CKD, a
potential bias factor related to the primary or secondary nature of
hypertension.
In a study by Jin et al (51) amongst patients randomly recruited in
a white European population, homozygous patients for the C/C
genotype had increased values of LVMI and RWT compared to carriers
of the A allele). The results of the present study showed
that patients carrying the C/C and A/C genotypes had
an increased risk of exhibiting pathological RWT and LVMI values,
although the correlation was not significant.
Bahramali et al (2) and Wu et al (52) showed that the A1166C-R1AngII
genetic variant is associated with LVDD in Iranian and Chinese
hypertensive patients. Rani et al (22) and Jin et al (51) found a statistical association
between this genetic variant and LVH, but not LVDD in Indian and
white European populations There was a significant association
between LVDD and the C/C and A/C genotypes in the
univariate analysis, but in the multivariate analysis this
association was not present.
Schmieder et al (53) and Herrmann et al (54) showed that the A3123C-R2AngII
genotype was involved in the pathophysiology of LVH in white male
German and British subjects. However, in the present study, there
was no statistical significance between this genetic variant and
LVH.
From a pathophysiological point of view, Xiao et
al (24) reported that
electrophysiological stimulation of R2 AngII improves LV cardiac
remodeling and influences cellular hypertrophy in experimental
animal models. Schmieder et al (53) showed that white male German
hypertensive patients carrying the A allele had pathological values
of LVMI and RWT. In the present study, in both univariate and
multivariate analysis, it was observed that the C/C and
A/C genotypes (A3123C-R2AngII) were associated with
non-concentric cardiac remodeling, but not with LVDD. No previous
studies have demonstrated this aspect to the best of our knowledge.
Thus the present study may be the first to confirm this
hypothesis.
West et al (48) found there was no association between
the G83A-REN genetic polymorphism in the occurrence of LVH
in Australian hypertensive patients. Similarly, in the present
study, no statistical correlation between the G83A-REN
polymorphism and cardiac anatomy was identified; however, carriers
of the G/G and A/G genotypes were at increased risk
of LVH and had pathological RWT values. Additionally, in the
present study, the A/A genotype and LVDD association was
close to the level of statistical significance, and no previous
studies assessed this association, to the best of our
knowledge.
The limitations of the present study were: i)
Characterization of LVDD based on abnormal relaxation alone
(without assessing the possible impact of heart rate). It would
have been useful to analyze the other components of transmitral
flow. Unfortunately, there were no available data for the entire
group subjected to genetic analysis. ii) The determined levels of
SBP and DBP were influenced, in the case of hypertensive patients
on treatment, by the use of the previously recommended
medication.
The strengths of the present study are: i)
Identification of the preferential distribution of certain RAAS
gene variants in the subpopulation with non-concentric remodeling
and/or LVDD; and ii) hypertensive patients carrying the
M235T-AGT and G83A-REN genetic variants can develop
LVDD when assessing abnormal relaxation, this association
emphasizes the significant effect of this genetic polymorphism in
the adaptative functional response to EHT. To the best of our
knowledge, there are no studies to establish this aspect, making
the present study first to determine the association between
genetic variants in Romanian patients diagnosed with EHT.
In conclusion, the results confirmed the hypothesis
of the adaptive functional association of the variable response to
EHT, conditioned by RAAS genetic polymorphisms. The
A3123C-R2AngII genetic variant was associated with
non-concentric cardiac remodeling as an adaptive response to EHT.
Apart from the aforementioned limitations, the present study
provides evidence for a differentiated approach for management of
hypertensive patients, depending not only on classes of risk, but
also on individual vulnerability.
Acknowledgements
Not applicable.
Funding
This study was funded by the ‘Iuliu Hatieganu’ University of
Medicine and Pharmacy Cluj-Napoca [grant nos. Doctoral Research
Projects (DRP) 7690/26 and DRP 5200/24].
Availability of data and materials
The datasets used and/or analyzed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
OM contributed to the conception and design of the
study, data collection, data analysis and interpretation, and
writing the manuscript. DR contributed to the conception and design
of the study and final proofreading. EB contributed to the
conception and design of the study, data collection and final
proofreading. DCL was involved in data analysis and interpretation.
AC contributed to data collection and the critical review of the
manuscript. SAB participated in performing the genetic
determinations performed a critical review of the manuscript. LMP
contributed to the conception and design of the study, data
collection, in performing the genetic determinations and gave final
approval of the manuscript. All authors read and approved the final
manuscript. All authors confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
Written informed consent was obtained from all
participants, prior to their inclusion in the study. The Ethics
Committee of the ‘Iuliu Hatieganu’ University of Medicine and
Pharmacy approved the study (approval no. 333/2.06.2015).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Williams B, Mancia G, Spiering W, Rosei
EA, Azizi M, Burnier M, Clement D, Coca A, Simone GD, et al: 2018
practice guidelines for the management of arterial hypertension of
the European society of cardiology and the European society of
hypertension ESC/ESH task force for the management of arterial
hypertension. J Hypertens. 36:76–88. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bahramali E, Rajabi M, Jamshidi J, Mousavi
SM, Zarghami M, Manafi A and Firouzabadi N: Association of ACE gene
D polymorphism with left ventricular hypertrophy in patients with
diastolic heart failure: A case-control study. BMJ Open.
6(e010282)2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Cosenso-Martin LN, Vaz-De-Melo RO, Pereira
LR, Cesarino CB, Yugar-Toledo JC, Cipullo JP, de Souza Pinhel MA,
Souza DR and Vilela-Martin JF: Angiotensin-converting enzyme
insertion/deletion polymorphism, 24-h blood pressure profile and
left ventricular hypertrophy in hypertensive individuals: A
cross-sectional study. Eur J Med Res. 20(74)2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wang AYM, Chung-Ngor Chan J, Wang M, Poon
E, Lui SF, Kam-Tao Li P and Sanderson J: Cardiac hypertrophy and
remodeling in relation to ACE and angiotensinogen genes genotypes
in Chinese dialysis patients. Kidney Int. 63:1899–1907.
2003.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Radulescu D, Stoicescu L, Buzdugan E and
Donca V: Patterns of left ventricular remodeling among patients
with essential and secondary hypertension. Rev Med Chil.
141:1520–1527. 2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lin TH, Chiu HC, Lee YT, Su HM, Voon WC,
Liu HW, Lai WT and Sheu SH: Association between functional
polymorphisms of renin-angiotensin system, left ventricular mass,
and geometry over 4 years in a healthy Chinese population aged 60
years and older. J Gerontol A Biol Sci Med Sci. 62:1157–1163.
2007.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yang Y, Zhu LM, Xu JZ, Tang XF and Gao PJ:
Comparison of left ventricular structure and function in primary
aldosteronism and essential hypertension by echocardiography.
Hypertens Res. 40:243–250. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Sasu D, Popescu L and Carauş A: Diastolic
dysfunction and heart failure with preserved ejection
fraction-general physiopathology and management. Arta Medica.
4:45–48. 2011.
|
|
9
|
Lalande S and Johnson BD: Diastolic
dysfunction: A link between hypertension and heart failure. Drugs
Today (Barc). 44:503–513. 2008.
|
|
10
|
Alpert MA, Karthikeyan K, Abdullah O and
Ghadban R: Obesity and cardiac remodeling in adults: Mechanisms and
clinical implications. Prog Cardiovasc Dis. 61:114–123.
2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Fountain JH and Lappin SL: Physiology,
Renin Angiotensin System. In: StatPearls. StatPearls Publishing,
Treasure Island, FL, 2019.
|
|
12
|
Tang W, Devereux RB, Rao DC, Oberman A,
Hopkins PN, Kitzman DW and Arnett DK: Associations between
angiotensinogen gene variants and left ventricular mass and
function in the HyperGEN study. Am Heart J. 143:854–860.
2002.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Smilde TDJ, Zuurman MW, Hillege HL, van
Veldhuisen DJ, van Gilst WH, van der Steege G, Voors AA, Kors JA,
de Jong PE and Navis G: Renal function dependent association of
AGTR1 polymorphism (A1166C) and electrocardiographic
left-ventricular hypertrophy. Am J Hypertens. 20:1097–1103.
2007.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Gumprecht J, Domek M, Lip GYH and
Shantsila A: Invited review: Hypertension and atrial fibrillation:
Epidemiology, pathophysiology, and implications for management. J
Hum Hypertens. 33:824–836. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Procopciuc LM, Caracostea G, Zaharie G,
Puscas M, Iordache G, Popa M, Colcear D, Olteanu I and Stamatian F:
Maternal/newborn genotype contribution of the renin-angiotensin
system (Met235Thr, Thr174Met, I/D-ACE, A2350G-ACE, A1166C-AT2R1,
C3123A-AT2R2, 83A/G-REN) to the risk of pre-eclampsia: A Romanian
study. J Reni Angiotensin Aldosterone Syst. 12:539–548.
2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Alves GB, Oliveira EM, Alves CR, Rached
HRS, Mota GFA, Pereira AC, Rondon MU, Hashimoto NY, Azevedo LF,
Krieger JE and Negrão CE: Influence of angiotensinogen and
angiotensin-converting enzyme polymorphisms on cardiac hypertrophy
and improvement on maximal aerobic capacity caused by exercise
training. Eur J Cardiovasc Prev Rehabil. 16:487–492.
2009.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Bahramali E, Firouzabadi N, Rajabi M,
Manafi A, Zarghami M, Mousavi SM and Jamshidi J: Association of
renin-angiotensin-aldosterone system gene polymorphisms with left
ventricular hypertrophy in patients with heart failure with
preserved ejection fraction: A case-control study. Clin Exp
Hypertens. 39:371–376. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Li S, Wang Z, Yang X, Hu B, Huang Y and
Fan S: Association between circulating angiotensin-converting
enzyme 2 and cardiac remodeling in hypertensive patients. Peptides.
90:63–68. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Li X, Li Y, Jia N, Guo S, Chu S and Niu W:
Angiotensin-converting enzyme gene deletion allele increases the
risk of left ventricular hypertrophy: Evidence from a
meta-analysis. Mol Biol Rep. 39:10063–10075. 2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Arumugam S, Sreedhar R, Thandavarayan RA,
Karuppagounder V, Krishnamurthy P, Suzuki K, Nakamura M and
Watanabe K: Angiotensin receptor blockers: Focus on cardiac and
renal injury. Trends Cardiovasc Med. 26:221–228. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Jaźwiec P, Gać P, Chaszczewska-Markowska
M, Bogunia-Kubik K, Mazur G and Poręba R: Genetically determined
enlargement of carotid body evaluated using computed
angiotomography. Respir Physiol Neurobiol. 254:10–15.
2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Rani B, Kumar A, Bahl A, Sharma R, Prasad
R and Khullar M: Renin-angiotensin system gene polymorphisms as
potential modifiers of hypertrophic and dilated cardiomyopathy
phenotypes. Mol Cell Biochem. 427:1–11. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kuznetsova T, Staessen JA, Thijs L, Kunath
C, Olszanecka A, Ryabikov A, Tikhonoff V, Stolarz K, Bianchi G,
Casiglia E, et al: Left ventricular mass in relation to genetic
variation in angiotensin II receptors, renin system genes, and
sodium excretion. Circulation. 110:2644–2650. 2004.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Xiao Y, Long WQ, Guan KP, Long M, Lu GH
and Huang ZB: Role of angiotensin II type 2 receptor during
electrophysiological remodeling of left ventricular hypertrophic
myocardium in spontaneously hypertensive rats. J Am Soc Hypertens.
12:58–65. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Vamsi UM, Swapna N, Usha G, Vishnupriya S
and Padma T: Contribution of REN gene MBbo I polymorphism in
conferring risk for essential hypertension: A case control study
from South India. J Renin Angiotensin Aldosterone Syst. 14:242–247.
2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Sun B, Williams JS, Pojoga L, Chamarthi B,
Lasky-Su J, Raby BA, Hopkins PN, Jeunemaitre X, Brown NJ, Ferri C
and Williams GH: Renin gene polymorphism: Its relationship to
hypertension, renin levels and vascular responses. J Renin
Angiotensin Aldosterone Syst. 12:564–571. 2011.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Lynch AI, Tang W, Shi G, Devereux RB,
Eckfeldt JH and Arnett DK: Epistatic effects of ACE I/D and AGT
gene variants on left ventricular mass in hypertensive patients:
The HyperGEN study. J Hum Hypertens. 26:133–140. 2012.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Borai IH, Hassan NS, Shaker OG, Ashour E,
Badrawy MEl, Fawzi OM and Mageed L: Synergistic effect of ACE and
AGT genes in coronary artery disease. Beni Suef University J Basic
Appl Sci. 7:111–117. 2018.
|
|
29
|
e-Echocardiography: Ejection Fraction. JLS
Interactive, LLC, Lincoln, NE, 2020. https://e-echocardiography.com/page/page.php?UID=175615301.
Accessed February 1, 2020.
|
|
30
|
Wigginton JE, Cutler DJ and Abecasis GR: A
note on exact tests of Hardy-Weinberg equilibrium. Am J Hum Genet.
76:887–893. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
31
|
R Core Team: R: A language and environment
for statistical computing. R foundation for Statistical Computing,
Vienna, 2019. http://www.R-project.org/.
|
|
32
|
González JR, Armengol L, Solé X, Guino E,
Mercader JM, Estivill X and Moreno V: SNPassoc: SNPassoc: an R
package to perform whole genome association studies.
Bioinformatics. 23:654–655. 2007.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Perticone F, Maio R, Cosco C, Ceravolo R,
Iacopino S, Chello M, Mastroroberto P, Tramontano D and Mattioli
PL: Hypertensive left ventricular remodeling and ACE-gene
polymorphism. Cardiovasc Res. 43:192–199. 1999.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Ji L, Cai X, Zhang L, Fei L, Wang L, Su J,
Lazar L, Xu J and Zhang Y: Association between polymorphisms in the
reninangiotensin-aldosterone system genes and essential
hypertension in the Han Chinese population. PLoS One.
28(e72701)2013.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Song SB, Jin HS, Hong KW, Lim JE, Moon JY,
Jeong KH, Ihm CG, Lee TW, Oh B and Lee SH: Association between
renin-angiotensin-aldosterone system-related genes and blood
pressure in a Korean population. Blood Press. 20:204–210.
2011.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Du GQ, Li HR, Xue JY, Chen S, Du P, Wu Y
and Tian JW: Wave intensity analysis can identify eccentric cardiac
hypertrophy in hypertensive patients with varied left ventricular
configurations. J Ultrasound Med. 34:2019–2027. 2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Woodiwiss AJ and Norton GR: Obesity and
left ventricular hypertrophy: The hypertension connection. Curr
Hypertens Rep. 17(539)2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Rocha IEGM, Victor EG, Braga MC, Silva OB
and de Moraes Chaves Becker M: Echocardiography evaluations for
asymptomatic patients with severe obesity. Arq Bras Cardiol.
88:52–58. 2007.PubMed/NCBI View Article : Google Scholar : (In English,
Portuguese).
|
|
39
|
Chahal NS, Lim TK, Jain P, Chambers JC,
Kooner JS and Senior R: New insights into the relationship of left
ventricular geometry and left ventricular mass with cardiac
function: A population study of hypertensive subjects. Eur Heart J.
31:588–594. 2010.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Bamaiyi AJ, Norton GR, Peterson V,
Libhaber CD, Sareli P and Woodiwiss AJ: Limited contribution of
left ventricular mass and remodelling to the impact of blood
pressure on diastolic function in a community sample. J Hypertens.
37:1191–1199. 2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Kurbanova D and Eliseyeva M: Genetic
background of left ventricular hypertrophy in Uzbek hypertensive
men. Turk Kardiyol Dern Ars. 38:466–472. 2010.PubMed/NCBI
|
|
42
|
Tran T, Mai TP, Tran HCB, Le LHG, Vu HA,
Tran TK, Hoang SV, Chau HN and Do MD: Association between AGT M235T
and left ventricular mass in vietnamese patients diagnosed with
essential hypertension. Front Cardiovasc Med.
8(608948)2021.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Eliseeva MR, Srozhidinova NZ,
Khamidullaeva GA and Abdullaeva GZh: Genetic determinants of
cardiovascular remodeling in uzbek patients with essential
hypertension. Ter Arkh. 81:64–69. 2009.PubMed/NCBI2017. (In Russian).
|
|
44
|
Jeng JR: Left ventricular mass, carotid
wall thickness, and angiotensinogen gene polymorphism in patients
with hypertension. Am J Hypertens. 12:443–450. 1999.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Iwai N, Shimoike H, Ohmichi N and
Kinoshita M: Angiotensinogen gene and blood pressure in the
japanese population. Hypertension. 25 (4 Pt 2):688–693.
1995.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Olcay A, Nişanci Y, Ekmekçi CG, Ozbek U,
Sezer M, Umman B and Buğra Z: Angiotensinogen M235T polymorphism
and left ventricular indices in treated hypertensive patients with
normal coronary arteries. Anadolu Kardiyol Derg. 7:257–261.
2007.PubMed/NCBI
|
|
47
|
Shilyakhto EV, Shwartz EI, Nefedova YB,
Zukova AV, Vinnic TA and Conrady AO: Lack of association of the
renin-angiotensin system genes polymorphisms and left ventricular
hypertrophy in hypertension. Blood Press. 10:135–141.
2001.PubMed/NCBI View Article : Google Scholar
|
|
48
|
West MJ, Summers KM, Burstow DJ, Wong KK
and Huggard PR: Renin and angiotensin-converting enzyme genotypes
in patients with essential hypertension and left ventricular
hypertrophy. Clin Exp Pharmacol Physiol. 21:207–210.
1994.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Ueno H, Takata M, Yasumoto K, Tomita S and
Inoue H: Angiotensin-converting enzyme gene polymorphism and
geometric patterns of hypertensive left ventricular hypertrophy.
Jpn Heart J. 40:589–98. 1999.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Mishra A, Srivastava A, Kumar S, Mittal T,
Garg N, Agarwal SK, Pande S and Mittal B: Role of angiotensin II
type 1 (AT1 A1166C) receptor polymorphism in susceptibility of left
ventricular dysfunction. Indian Heart J. 67:214–221.
2015.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Jin Y, Kuznetsova T, Thijs L, Schmitz B,
Liu Y, Asayama K, Brand SM, Heymans S, Brand E, Fagard R and
Staessen JA: Association of left ventricular mass with the AGTR1
A1166C polymorphism. Am J Hypertens. 25:472–478. 2012.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Wu CK, Tsai CT, Chang YC, Luo JL, Wang YC,
Hwang JJ, Lin JL, Tseng CD and Chiang FT: Genetic Polymorphism of
the angiotensin II type 1 receptor gene and diastolic heart
failure. J Hypertens. 27:502–507. 2009.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Schmieder RE, Erdmann J, Delles C, Jacobi
J, Fleck E, Hilgers K and Regitz-Zagrosek V: Effect of the
angiotensin II type 2-receptor gene (+1675 G/A) on left ventricular
structure in humans. J Am Coll Cardiol. 37:175–182. 2001.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Herrmann SM, Nicaud V, Schmidt-Petersen K,
Pfeifer J, Erdmann J, McDonagh T, Dargie HJ, Paul M and
Regitz-Zagrosek V: Angiotensin II type 2 receptor gene polymorphism
and cardiovascular phenotypes: the GLAECO and GLAOLD studies. Eur J
Heart Fail. 4:707–712. 2002.PubMed/NCBI View Article : Google Scholar
|















