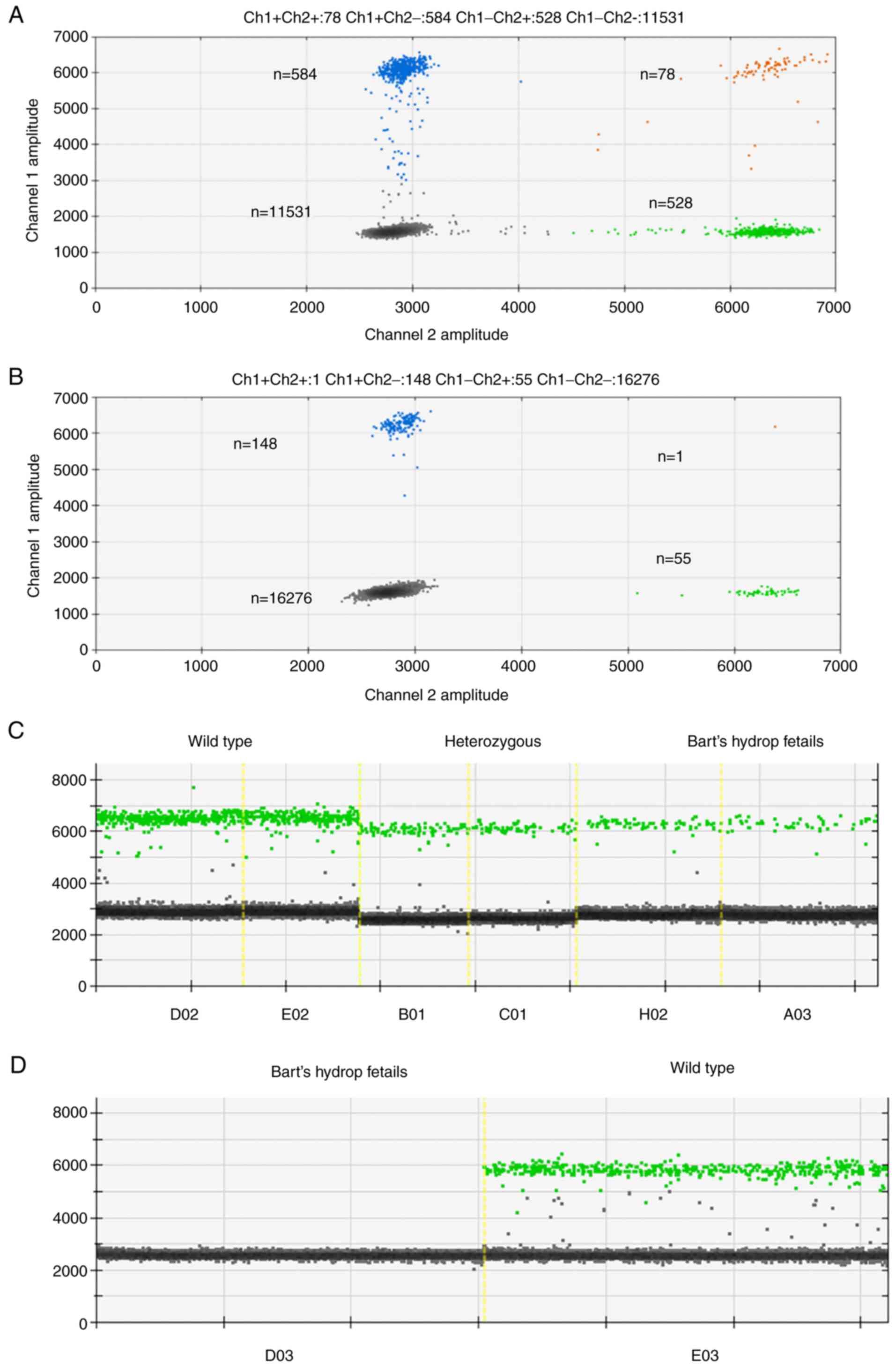
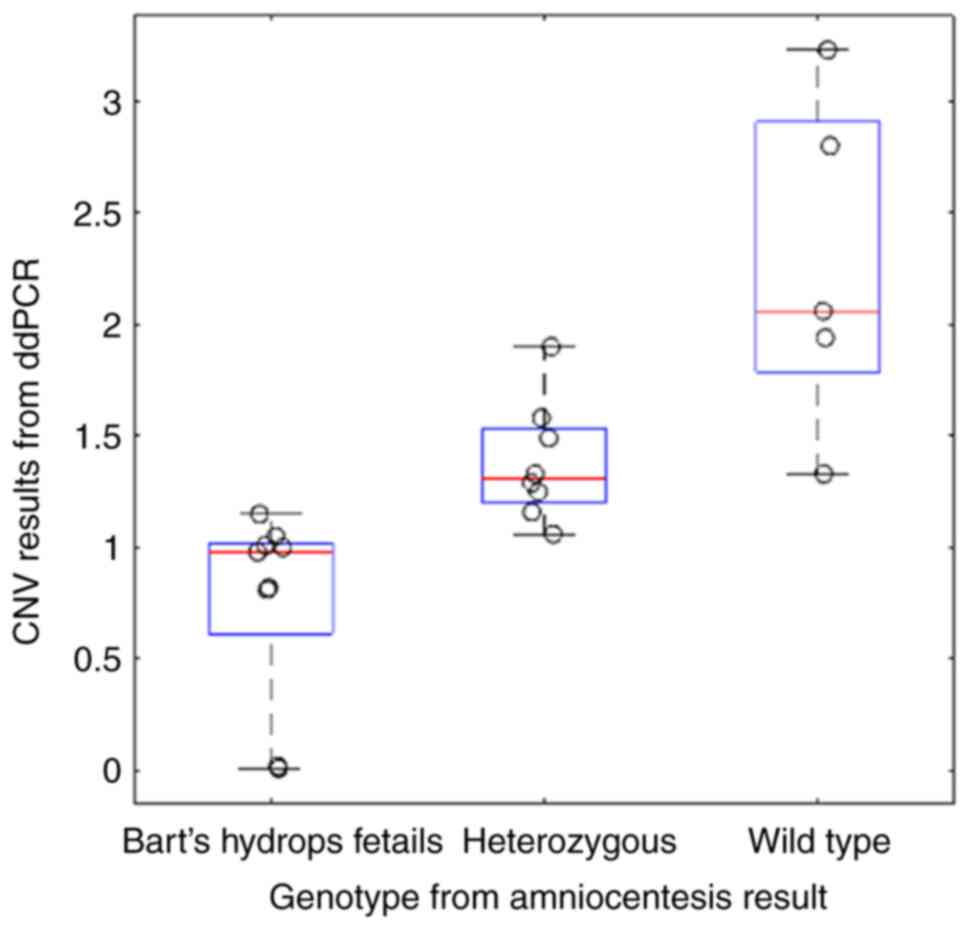
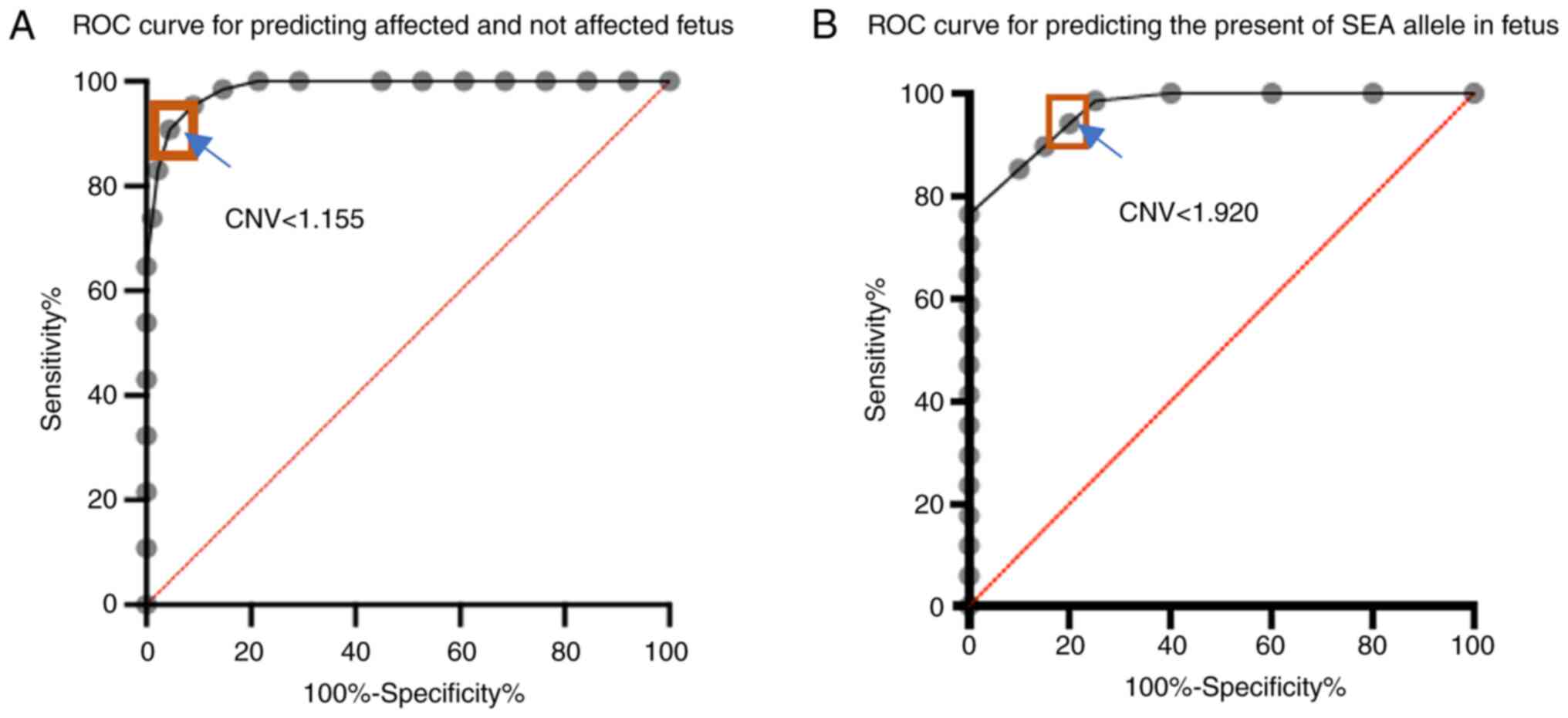
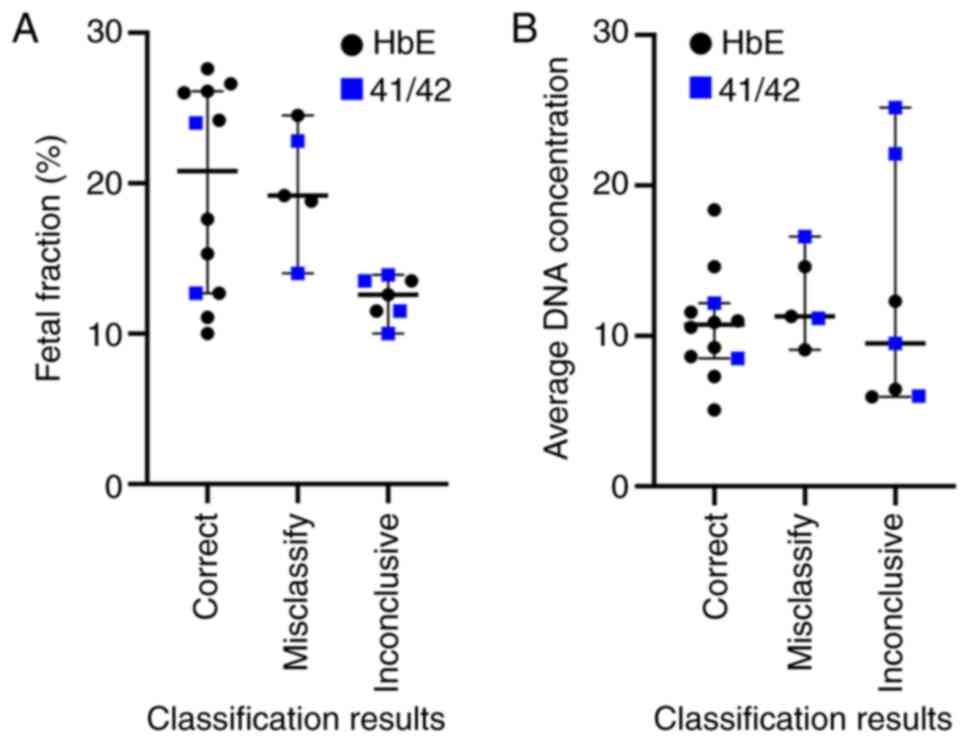
|
1
|
Modell B and Darlison M: Global
epidemiology of haemoglobin disorders and derived service
indicators. Bull World Health Organ. 86:480–487. 2008.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Galanello R and Cao A: Gene test review.
Alpha-thalassemia. Genet Med. 13:83–88. 2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Fucharoen S, Winichagoon P, Siritanaratkul
N, Chowthaworn J and Pootrakul P: Alpha- and beta-thalassemia in
Thailand. Ann N Y Acad Sci. 850:412–414. 1998.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Cao A and Galanello R: Beta-thalassemia.
Genet Med. 12:61–76. 2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Origa R: β-thalassemia. Genet Med.
19:609–619. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Chaibunruang A, Sornkayasit K,
Chewasateanchai M, Sanugul P, Fucharoen G and Fucharoen S:
Prevalence of thalassemia among newborns: A re-visited after 20
years of a prevention and control program in Northeast Thailand.
Mediterr J Hematol Infect Dis. 10(e2018054)2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Fucharoen S and Winichagoon P: Thalassemia
in SouthEast Asia: Problems and strategy for prevention and
control. Southeast Asian J Trop Med Public Health. 23:647–655.
1992.PubMed/NCBI
|
|
8
|
Cao A and Kan YW: The prevention of
thalassemia. Cold Spring Harb Perspect Med.
3(a011775)2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Old J: Chapter 71 - Hemoglobinopathies and
thalassemias. In: Emery and rimoin's principles and practice of
medical genetics. 6th edition. Academic Press, Cambridge, MA,
pp1-44, 2013.
|
|
10
|
Evans MI and Wapner RJ: Invasive prenatal
diagnostic procedures 2005. Semin Perinatol. 29:215–218.
2005.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lo YM, Corbetta N, Chamberlain PF, Rai V,
Sargent IL, Redman CW and Wainscoat JS: Presence of fetal DNA in
maternal plasma and serum. Lancet. 350:485–487. 1997.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Jenkins LA, Deans ZC, Lewis C and Allen S:
Delivering an accredited non-invasive prenatal diagnosis service
for monogenic disorders and recommendations for best practice.
Prenat Diagn. 38:44–51. 2018.PubMed/NCBI View
Article : Google Scholar
|
|
13
|
Rabinowitz T and Shomron N: Genome-wide
noninvasive prenatal diagnosis of monogenic disorders: Current and
future trends. Comput Struct Biotechnol J. 18:2463–2470.
2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Baysal E and Huisman TH: Detection of
common deletional alpha-thalassemia-2 determinants by PCR. Am J
Hematol. 46:208–213. 1994.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Bowden DK, Vickers MA and Higgs DR: A
PCR-based strategy to detect the common severe determinants of
alpha thalassaemia. Br J Haematol. 81:104–108. 1992.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sirichotiyakul S, Charoenkwan P and
Sanguansermsri T: Prenatal diagnosis of homozygous
alpha-thalassemia-1 by cell-free fetal DNA in maternal plasma.
Prenat Diagn. 32:45–49. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Tungwiwat W, Fucharoen S, Fucharoen G,
Ratanasiri T and Sanchaisuriya K: Development and application of a
real-time quantitative PCR for prenatal detection of fetal
alpha(0)-thalassemia from maternal plasma. Ann N Y Acad Sci.
1075:103–107. 2006.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yan TZ, Mo QH, Cai R, Chen X, Zhang CM,
Liu YH, Chen YJ, Zhou WJ, Xiong F and Xu XM: Reliable detection of
paternal SNPs within deletion breakpoints for non-invasive prenatal
exclusion of homozygous α-thalassemia in maternal plasma. PLoS One.
6(e24779)2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Pornprasert S, Sukunthamala K, Kunyanone
N, Sittiprasert S, Thungkham K, Junorse S, Pongsawatkul K,
Pattanaporn W, Jitwong C and Sanguansermsri T: Analysis of
real-time PCR cycle threshold of alpha-thalassemia-1 Southeast
Asian type deletion using fetal cell-free DNA in maternal plasma
for noninvasive prenatal diagnosis of bart's hydrops fetalis. J Med
Assoc Thai. 93:1243–1248. 2010.PubMed/NCBI
|
|
20
|
Norton ME, Brar H, Weiss J, Karimi A,
Laurent LC, Caughey AB, Rodriguez MH, Williams J III, Mitchell ME,
Adair CD, et al: Non-invasive chromosomal evaluation (NICE) study:
Results of a multicenter prospective cohort study for detection of
fetal trisomy 21 and trisomy 18. Am J Obstet Gynecol.
207(137)2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Bianchi DW, Platt LD, Goldberg JD,
Abuhamad AZ, Sehnert AJ and Rava RP: MatErnal BLood IS Source to
Accurately diagnose fetal aneuploidy (MELISSA) Study Group.
Genome-wide fetal aneuploidy detection by maternal plasma DNA
sequencing. Obstet Gynecol. 119:890–901. 2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Rava RP, Srinivasan A, Sehnert AJ and
Bianchi DW: Circulating fetal cell-free DNA fractions differ in
autosomal aneuploidies and monosomy X. Clin Chem. 60:243–250.
2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Chiu RW, Chan KC, Gao Y, Lau VY, Zheng W,
Leung TY, Foo CH, Xie B, Tsui NB, Lun FM, et al: Noninvasive
prenatal diagnosis of fetal chromosomal aneuploidy by massively
parallel genomic sequencing of DNA in maternal plasma. Proc Natl
Acad Sci USA. 105:20458–20463. 2008.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Xiong L, Barrett AN, Hua R, Ho S, Jun L,
Chan K, Mei Z and Choolani M: Non-invasive prenatal testing for
fetal inheritance of maternal β-thalassaemia mutations using
targeted sequencing and relative mutation dosage: A feasibility
study. BJOG. 125:461–468. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Lam KW, Jiang P, Liao GJ, Chan KC, Leung
TY, Chiu RW and Lo YM: Noninvasive prenatal diagnosis of monogenic
diseases by targeted massively parallel sequencing of maternal
plasma: Application to β-thalassemia. Clin Chem. 58:1467–1475.
2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lo YM, Chan KC, Sun H, Chen EZ, Jiang P,
Lun FM, Zheng YW, Leung TY, Lau TK, Cantor CR and Chiu RWK:
Maternal plasma DNA sequencing reveals the genome-wide genetic and
mutational profile of the fetus. Sci Transl Med.
2(61ra91)2010.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Orhant L, Anselem O, Fradin M, Becker PH,
Beugnet C, Deburgrave N, Tafuri G, Letourneur F, Goffinet F, El
Khattabi LA, et al: Droplet digital PCR combined with
minisequencing, a new approach to analyze fetal DNA from maternal
blood: Application to the non-invasive prenatal diagnosis of
achondroplasia. Prenat Diagn. 36:397–406. 2016.PubMed/NCBI View
Article : Google Scholar
|
|
28
|
Hudecova I: Digital PCR analysis of
circulating nucleic acids. Clin Biochem. 48:948–956.
2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
van Ginkel JH, Huibers MMH, van Es RJJ, de
Bree R and Willems SM: Droplet digital PCR for detection and
quantification of circulating tumor DNA in plasma of head and neck
cancer patients. BMC Cancer. 17(428)2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Gruber A, Pacault M, El Khattabi LA,
Vaucouleur N, Orhant L, Bienvenu T, Girodon E, Vidaud D, Leturcq F,
Costa C, et al: Non-invasive prenatal diagnosis of paternally
inherited disorders from maternal plasma: Detection of NF1 and CFTR
mutations using droplet digital PCR. Clin Chem Lab Med. 56:728–738.
2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Hudecova I and Chiu RWK: Non-invasive
prenatal diagnosis of thalassemias using maternal plasma cell free
DNA. Best Pract Res Clin Obstet Gynaecol. 39:63–73. 2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Pornprasert S and Prasing W: Detection of
alpha(0)-thalassemia South-East Asian-type deletion by droplet
digital PCR. Eur J Haematol. 92:244–248. 2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lun FM, Tsui NB, Chan KC, Leung TY, Lau
TK, Charoenkwan P, Chow KCK, Lo WYW, Wanapirak C, Sanguansermsri T,
et al: Noninvasive prenatal diagnosis of monogenic diseases by
digital size selection and relative mutation dosage on DNA in
maternal plasma. Proc Natl Acad Sci USA. 105:19920–19925.
2008.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Kho SL, Chua KH, George E and Tan JA: A
novel gap-PCR with high resolution melting analysis for the
detection of alpha-thalassaemia Southeast Asian and Filipino
β˚-thalassaemia deletion. Sci Rep. 5(13937)2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Chan KC, Ding C, Gerovassili A, Yeung SW,
Chiu RW, Leung TN, Lau TK, Chim SS, Chung GT, Nicolaides KH and Lo
YM: Hypermethylated RASSF1A in maternal plasma: A universal fetal
DNA marker that improves the reliability of noninvasive prenatal
diagnosis. Clin Chem. 52:2211–2218. 2006.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Manokhina I, Singh TK, Peñaherrera MS and
Robinson WP: Quantification of cell-free DNA in normal and
complicated pregnancies: Overcoming biological and technical
issues. PLoS One. 9(e101500)2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
El Karoui N, Zhou W and Whittemore AS:
Getting more from digital SNP data. Stat Med. 25:3124–3133.
2006.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Pornprasert S, Phusua A, Suanta S, Saetung
R and Sanguansermsri T: Detection of alpha-thalassemia-1 Southeast
Asian type using real-time gap-PCR with SYBR Green1 and high
resolution melting analysis. Eur J Haematol. 80:510–514.
2008.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Tsui NB, Kadir RA, Chan KC, Chi C, Mellars
G, Tuddenham EG, Leung TY, Lau TK, Chiu RW and Lo YM: Noninvasive
prenatal diagnosis of hemophilia by microfluidics digital PCR
analysis of maternal plasma DNA. Blood. 117:3684–3691.
2011.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Lun FM, Chiu RW, Chan KC, Leung TY, Lau TK
and Lo YM: Microfluidics digital PCR reveals a higher than expected
fraction of fetal DNA in maternal plasma. Clin Chem. 54:1664–1672.
2008.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Amicucci P, Gennarelli M, Novelli G and
Dallapiccola B: Prenatal diagnosis of myotonic dystrophy using
fetal DNA obtained from maternal plasma. Clin Chem. 46:301–302.
2000.PubMed/NCBI
|
|
42
|
Saito H, Sekizawa A, Morimoto T, Suzuki M
and Yanaihara T: Prenatal DNA diagnosis of a single-gene disorder
from maternal plasma. Lancet. 356(1170)2000.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Chiu RW, Lau TK, Leung TN, Chow KC, Chui
DH and Lo YM: Prenatal exclusion of beta thalassaemia major by
examination of maternal plasma. Lancet. 360:998–1000.
2002.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Ding C, Chiu RW, Lau TK, Leung TN, Chan
LC, Chan AY, Charoenkwan P, Ng IS, Law HY, Ma ES, et al: MS
analysis of single-nucleotide differences in circulating nucleic
acids: Application to noninvasive prenatal diagnosis. Proc Natl
Acad Sci USA. 101:10762–10767. 2004.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Hudecova I, Jiang P, Davies J, Lo YMD,
Kadir RA and Chiu RWK: Noninvasive detection of F8 int22h-related
inversions and sequence variants in maternal plasma of hemophilia
carriers. Blood. 130:340–347. 2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Taylor SC, Carbonneau J, Shelton DN and
Boivin G: Optimization of droplet digital PCR from RNA and DNA
extracts with direct comparison to RT-qPCR: Clinical implications
for quantification of Oseltamivir-resistant subpopulations. J Virol
Methods. 224:58–66. 2015.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Sirichotiyakul S, Charoenkwan P and
Sanguansermsri T: Prenatal diagnosis of homozygous
alpha-thalassemia-1 by cell-free fetal DNA in maternal plasma.
Prenat Diagn. 32:45–49. 2012.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Whale AS, Cowen S, Foy CA and Huggett JF:
Methods for applying accurate digital PCR analysis on low copy DNA
samples. PLoS One. 8(e58177)2013.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Vermeulen J, Derveaux S, Lefever S, De
Smet E, De Preter K, Yigit N, De Paepe A, Pattyn F, Speleman F and
Vandesompele J: RNA pre-amplification enables large-scale RT-qPCR
gene-expression studies on limiting sample amounts. BMC Res Notes.
2(235)2009.PubMed/NCBI View Article : Google Scholar
|