1. Introduction
The quantity and composition of the daily salivary
production of the major (submandibular, sublingual and parotid) and
minor salivary glands is important for oral health. In a healthy
individual the salivary glands produce 0.5-1.5 l saliva per a day,
which is composed of 99.5% water 0.3% protein and 0.2% organic and
inorganic substances (1-3).
The salivary glands are composed of acinar cells, which are
responsible for the secretion and production of secretory granules
(3-5).
These granules contain amylase, mucins and immunoglobulins, which
are essential for the maintenance of a stable oral environment,
lubrication, digestion and in the immunity of the oral mucosa
(6).
There are two types of acinar cells, mucinous and
serous acinar cells, and each plays a different role in the proper
functioning of the salivary glands. Mucinous acinar cells are
characterized by an accumulation of a large number of mucinous
granules in the apical cytoplasmic region (7). These cells are responsible for the
production of mucins, which are essential for lubrication and oral
health (8). Serous acinar cells also
accumulate secretory granules, but are responsible for the
secretion of digestive amylase α, which aids primary food digestion
(7,8).
Sjögren's syndrome (SS) is an autoimmune disease
characterized by a dysfunction of the salivary glands. The primary
symptom is dryness of the mouth and eyes due to impaired salivary
and lacrimal gland function (9,10). It
has been reported that several factors may be responsible for this,
such as acinar cell atrophy and apoptosis (9,11),
glandular denervation (12),
inhibition of cytokine neurotransmitter release (13), Acetylcholine (ACh) depletion and
increased secretion of cholinesterase (14), presence of anti-muscarinic
autoantibodies blocking muscarinic M3 receptors (14), decreased nitric oxide production
[which in turn can disrupt calcium release induced by altered
levels of cyclic adenosine diphosphate-ribose (15)], altered calcium tunneling (16), and aberrant aquaporin (AQP)5
expression and localization (17).
Tea and its polyphenols, a product of the dried
leaves of Camellia sinensis, have been suggested to possess
several pharmacological properties (18). The green tea polyphenols (GTPs) are
useful in delaying or managing SS-like autoimmune disorders that
can affect multiple tissues or organs (19,20). The
prevalence of SS seems to be higher in the US population compared
to the Japanese and Chinese population (21,22). It
was reported that apoptosis, cytotoxicity and autoantigen
expression (23), as well as
oxidative stress (24), all of which
are involved in SS pathogenesis and the primary cellular mechanisms
underlying its development, are inhibited by GTPs (19,25,26).
The major GTP, Epigallocatechin-3-gallate (EGCG),
inhibits autoantigen expression in normal human keratinocytes and
immortalized normal human salivary acinar cells (26). Another study demonstrated that EGCG
can protect normal human salivary acinar cells from tumor necrosis
factor-α (TNF-α)-induced cytotoxicity, and the phosphorylation of
p38 mitogen-activated protein kinase (MAPK) serves a major role in
this protection (27).
EGCG undergoes modification by gut microbes
following oral administration (28).
Multiple resultant gut soluble metabolites have been reported to
possess pharmacological properties (29). EGCG reaches systemic circulation at
low concentrations, and excretion is complete within several hours
(30). Identification of the true
active component of EGCG, and conversely the true effect of EGCG on
the body, is a contentious topic (31). Nonetheless, whether the activity of
EGCG is carried out by the unaltered molecule, its microbial
metabolites, or its cellular metabolites, in the framework of SS
and the selected proteins, this activity may be beneficial.
The dysregulation of secretory granules in SS by
selected families of molecules, including soluble
N-ethylmaleimide-sensitive factor attachment protein receptors
(SNAREs), AQP5, actin, and tight junctions, are comprehensively
discussed. Furthermore, the possible mechanism of protection by
GTPs against the dysregulation of these molecules is reviewed.
2. Literature review
PRISMA guidelines were used to plan this
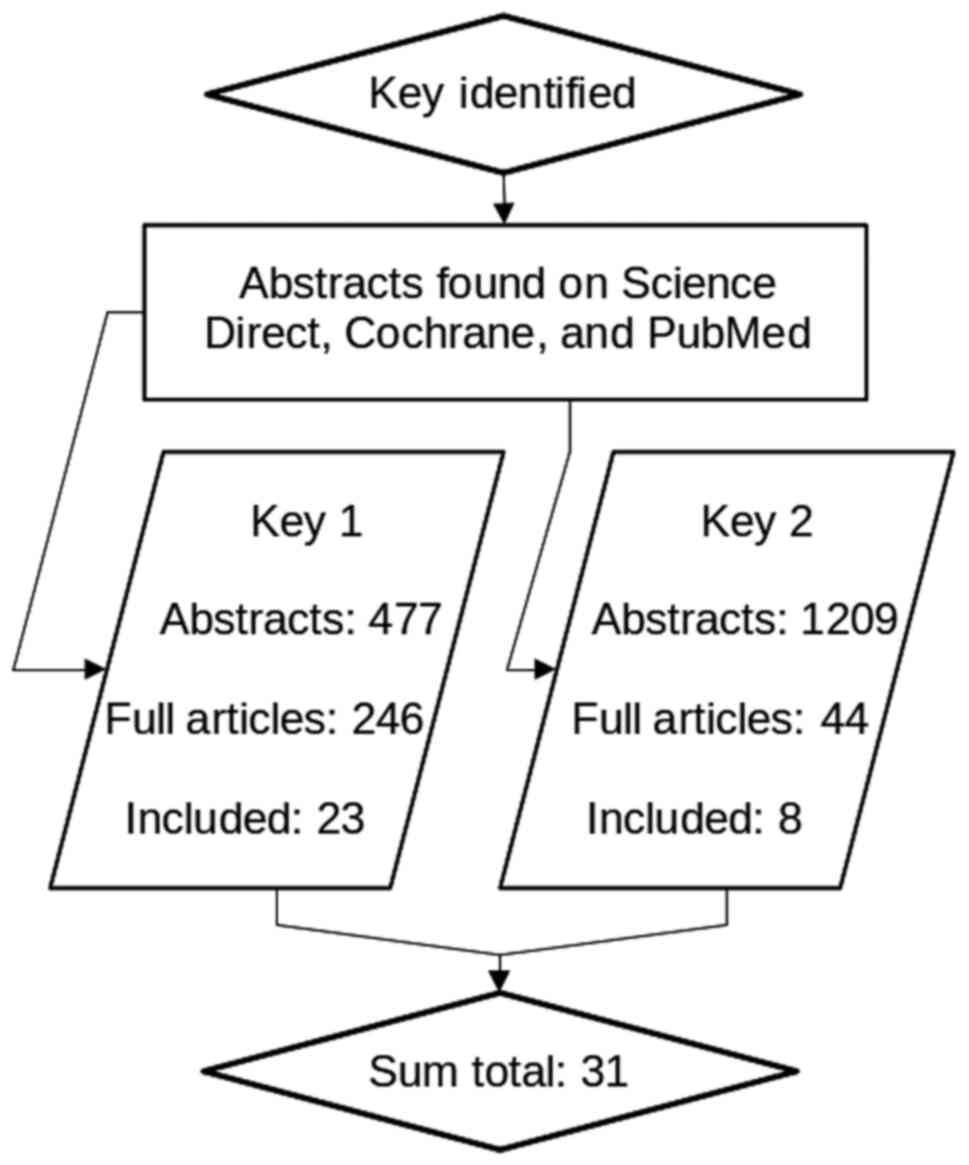
comprehensive review (32). Fig. 1 shows a flowchart summarizing the
results of the search strategy carried out for this review. The
review was split in to two bouts of data gathering, the molecular
action of selected molecules in SS covered by search key 1, and the
effect of EGCG on selected molecules covered by search key 2. Key
1: Sjogren AND salivary gland AND (aquaporin OR SNARE OR actin OR
tight junction); and Key 2: EGCG AND (aquaporin OR SNARE OR actin
OR tight junction).
The global inclusion criteria were: Published after
1998, the full text available, the article was written in the
English language and it described non-trivial involvement of
selected molecules. The PubMed, Science Direct and Cochrane
databases were searched, and Medical Subject Headings Terms used
where permitted by the search engine. Abstracts were screened for
the selection criteria, and the chosen full text articles were
screened again. Applicable results are summarized in Table I, Table
II, Table III and Table IV, and a pictorial representation is
shown in Figs. 2 and 3.
 | Table IKnown distribution of protein
involved in the mucosecretory mechanism between the salivary acini
and ducts, in healthy individuals and patients with SS. |
Table I
Known distribution of protein
involved in the mucosecretory mechanism between the salivary acini
and ducts, in healthy individuals and patients with SS.
| Location in
gland | Subject | Aquaporins | SNARE | Tight
Junctions | Actin |
|---|
| Acinus | Healthy | AQP1 (EC, MEC);
AQP3 (AC on the BM); AQP4 (NA); AQP5 (AC on the AM); AQP8 (NA) | SNAP23 (AC at the
AM and BM); VAMP8 (AC at the AM); Syntaxin-4 (AC at the AM and BM);
Syntaxin-3 (AC at the AM) | Claudins 1,3,4 and
5 (AC at the AM); Occludin (AC at the AM); ZO-1 (AC in the C) | F-actin (AC at the
AM) actin-α1/α2 (MEC) |
| | SS | AQP1 (MEC); AQP5
(at the AM and BM) | SNAP 23 (AC at the
LM); VAMP8 (AC in the C); Syntaxin-4 (AC in the C); Syntaxin-3 (AC
in the C and at the BM) | Claudins 3-5 (AC at
the BM); Occludin (AC at the AM); ZO-1 (AC in the C) | Cofilin-1,
α-enolase (NA) actin-α1/α2 (NA) |
| Duct | Healthy | AQP5 (NA); AQP3
(NA) | VAMP8 (NA);
Syntaxin-4 (NA) | Claudins 1,3 and 4
(DC, NA); Occludin (DC, NA); ZO-1 (DC, NA) | NA |
 | Table IIKnown distribution of proteins
involved in the mucosecretory mechanism between the salivary acini
and ducts, in healthy individuals and patients with Sjögren's
syndrome. |
Table II
Known distribution of proteins
involved in the mucosecretory mechanism between the salivary acini
and ducts, in healthy individuals and patients with Sjögren's
syndrome.
| Main protein
group | Examples | (Refs.) |
|---|
| Aquaporins | AQP1 | (54,55) |
| | AQP3 | (54,55) |
| | AQP4 | (54,55) |
| | AQP5 | (54-56,90,137) |
| | AQP8 | (138) |
| SNAREs | VAMP8 | (45) |
| | STX3 | (45) |
| | STX4 | (45) |
| | SNAP-23 | (45) |
| Tight
Junctions | Claudin-1 | (80,83) |
| | Claudin-3 | (80,139) |
| | Claudin-4 | (80) |
| | Claudin-5 | (140) |
| | Occludin | (80,85) |
| | ZO-1 | (80) |
| | JAM-1 | (83) |
| Actins | Moesin | (77) |
| | Cofilin-1 | (76) |
| | α-enolase | (76) |
| | Actin α1/α2 | (138) |
| | RGI2 | (76) |
 | Table IIIComparison of physiological and
pathological distribution and function of selected proteins
involved in SS. |
Table III
Comparison of physiological and
pathological distribution and function of selected proteins
involved in SS.
| Primary protein
group | Healthy
individuals | Patients with
SS | Most significant
dysregulation in patients with SS |
|---|
| AQPs | Expressed in the
apical membrane of acinar cells (parotid, submandibular, sublingual
and labial glands) (53,54). | Expressed in the
apical and basolateral membranes of acinar cells (minor salivary
glands) (54,102,103). | Presence of
anti-AQP5 IgG (62). Glandular
hypofunction (63). |
| SNARE | VAMP8: Expressed in
the apical cytoplasm (labial salivary glands) (38). STX4: Expressed in the apical and
basolateral plasma membranes (submandibular glands) (38). STX3: Expressed in the apical region
of acinar cells (labial salivary glands) (38). SNAP23: Expressed in the apical
membrane and in the basolateral plasma membrane (submandibular
glands, labial salivary glands) (38,43). | VAMP8: Expression
at the gene and protein level is decreased and is localized
throughout the entire cytoplasm (labial salivary glands) (38). STX3: Expression is increased and
localized throughout the entire cytoplasm and the basolateral
plasma membrane in patients (labial salivary glands) (38). STX4: Expression is decreased and
localized at the basal plasma membrane (labial salivary glands)
(38). SNAP23: Expression is absent
in the apical plasma membrane and decreased in the lateral plasma
membrane (labial salivary glands) (38). | Ectopic mucin
secretion (38). Fusion of secretory
granules (44). |
| Tight
junctions | Claudins: Expressed
in the apical plasma membrane (92).
Occludin: Expressed in the apical plasma membrane (90,91).
ZO-1: Cytosolic expression (90,91) | Claudin-1, 4 and 5:
Expression id increased (92,106).
Claudin-3, 4 and 5: Localized in the basal plasma membrane
(92,105,106).
Occludin: Expression is decreased (92). ZO-1: Expression is decreased
(92). | Alteration of
paracellular permeability in the salivary gland (92,105).
Presence of exosomes containing autoantigens from salivary gland
epithelial cell lines (99). |
| Actin | F-actin: Located
under the plasma membrane (parotid and subman dibular gland)
(75). Actin-α1/α2: Present in
myoepithe lial cells around the acini (104). | Expression of
(cofilin-1, α-enolase and RGI2) increased (81). Decrease in actin-α1/α2 levels
(104). | Presence of
anti-Moesin (82). The expression of
anti-cofilin-1, α-enolase and RGI2 increased (81). |
 | Table IVSummary of interactions of EGCG on
selected proteins in salivary glands. |
Table IV
Summary of interactions of EGCG on
selected proteins in salivary glands.
| Protein group | Interaction with
EGCG | (Refs.) |
|---|
| SNAREs | Stimulated
lysosomal activity | (108) |
| Aquaporins | Upregulated AQP5
and AQP3 expression | (105,116) |
| Actin | Maintenance of
apical F-actin structure and organization | (119) |
| Tight
Junctions | Stimulation of
Occludin and ZO-1 expression | (127-129) |
3. SNAREs
Physiological roles of SNAREs
SNARE proteins have been shown to serve a major role
in the regulation of exocytosis (33). Vesicle-associated membrane proteins
(VAMPs) are SNAREs that play a crucial role in the regulation of
secretory vesicle trafficking (34).
Indeed, these vesicles are transported from the trans-Golgi network
to the plasma membrane via the cytoskeleton (35). Once these vesicles reach the plasma
membrane, interactions between Ras-related protein (RAB), mammalian
uncoordinated-18 (MUNC18) and SNARE proteins promote the formation
of trans-SNARE (t-SNARE) (36).
During fusion between the plasma membrane and the secretory
vesicles, a small opening is created at the point of contact
between the two membranes (vesicles and plasma membrane), which
gradually increases in size until exocytosis (37,38).
In the salivary gland, mucous acinar cells contain a
large number of mucin granules aggregated in the apical cytoplasm
(39,40). Several studies have shown that the
exocytosis of mucins required precise localization of SNAREs and
other components (41). In acini
cells, the secretory pathway synthesizes, processes and exocytoses
mucins (42). Several families of
proteins, such as SNAREs, RAB and MUNC-18-bound proteins, are
involved in the assembly of the membrane fusion complex (42-44).
The localization of each unit of this molecular machinery is
essential for exocytosis (43).
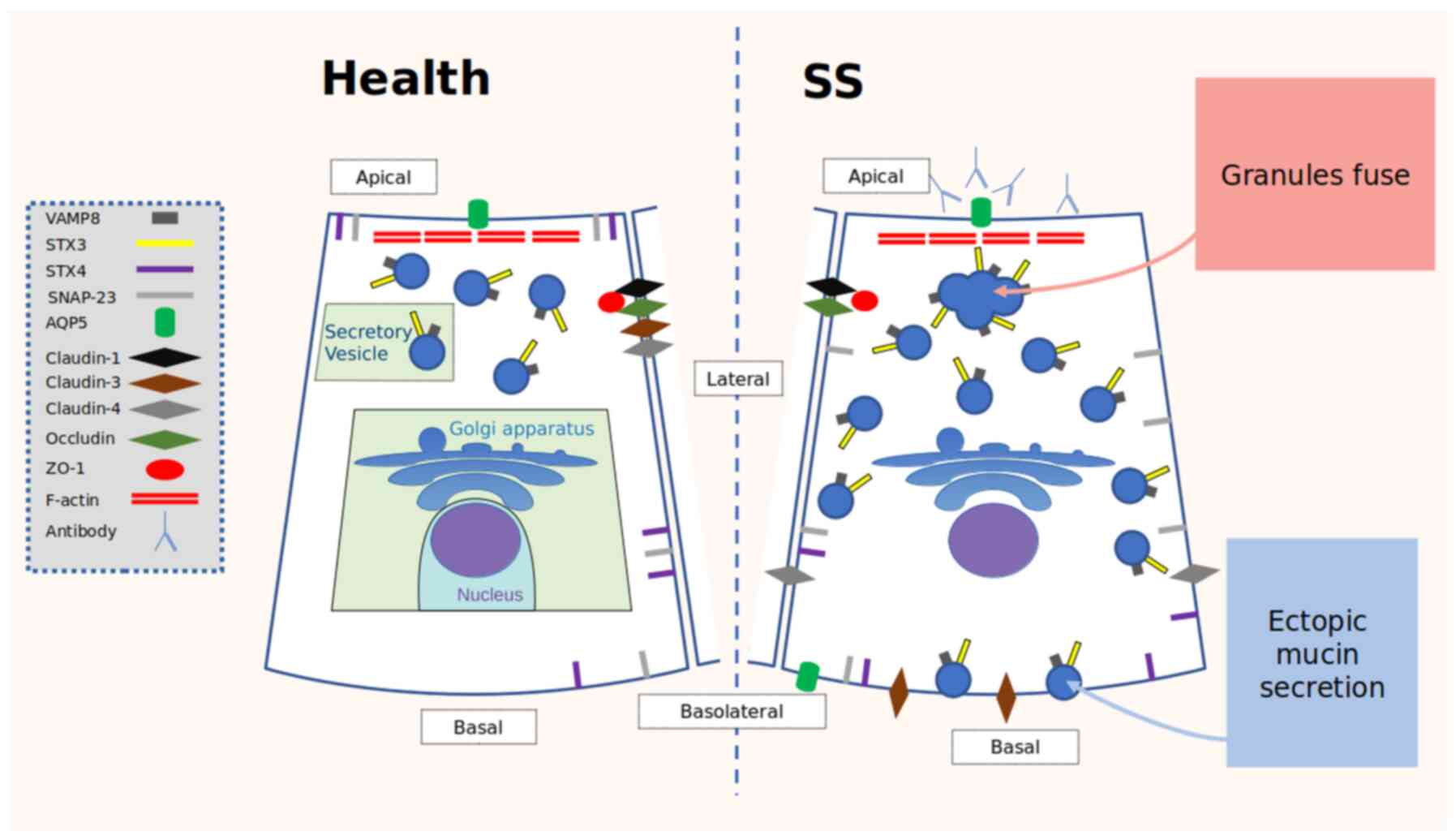
VAMP8 in patients with SS
Studies of endosomal compartments, notably
VAMP8(42), have indicated that it
is an important component in exocytosis (44). In SS, the expression and localization
of the VAMP8 protein in acinar cells has been shown to be
associated with dysfunction of the salivary labial glands. In
healthy individuals, the localization of VAMP8 is cytoplasmic in
the apical region of acinar cells. In contrast, in patients with
SS, it was observed that the expression of this protein is
decreased and localized throughout the entire cytoplasm. This
aberration in VAMP8 distribution is considered to be related to
ectopic mucin secretion (45).
Other studies in VAMP8-deficient (VAMP8-/-) mice
(normal at birth) have shown that the absence of interaction
between VAMP8 with t-SNARE, Syntaxin (STX)4, and soluble
N-ethylmaleimide-sensitive factor attachment protein (SNAP)23
induces abnormal accumulation of secretory granules, and increased
production of amylases and carbonic anhydrase VI in acinar cells of
the salivary glands (46,47). This suggests that the expression and
distribution of SNARE proteins serve an essential role in the
secretion of salivary gland proteins.
STX in patients with SS
Studies of STX in rat parotid glands have shown that
the localization of STX4 is abundantly expressed across the entire
plasma membrane, whereas STX2 and STX3 are expressed only at the
apical level of the plasma membrane (48,49). In
contrast, in the submandibular glands of humans, STX4 is expressed
in the apical and basolateral plasma membranes, and STX2 is
localized in both the apical plasma membrane and the cytoplasmic
vesicles (50).
In the labial salivary glands in patients with
primary SS, the pattern of expression and/or localization of STX3
and STX4 exhibits several differences compared with healthy
individuals. The localization of STX4 does not change compared with
healthy individuals, whereas its expression is decreased in
patients with SS. In contrast, whereas STX3 is normally localized
only at the cytoplasmic level in the apical region of acinar cells,
its expression was drastically increased in the entire cytoplasm in
patients (45). It has been reported
that increased STX3 expression in patients is related to the fusion
of secretory granules that were previously described as large
pleomorphic granules (51).
STX4-VAMP8 and STX3-SNAP23 complexes are
overabundant in acinar cells of the salivary labial glands in
patients with SS (45). It is
interesting to note that the interactions between STX4 and VAMP8
are observed only in the basolateral membrane of patients,
suggesting a major role of this complex in secretory vesicle
trafficking dysfunction and exocytosis (45). Additionally, it has been reported
that the co-localization of STX4 and RAB3D in mature secretory
vesicles in the subapical region is altered from the apical to
basolateral plasma membrane in patients with SS. This perturbation
of SNARE complexes results in altered interactions, distribution
and co-localization, which in-turn induce alterations in the
distribution of secretory mucins in patients with SS (45,51,52).
Altered expression and localization of SNARE
complexes with aberrant mucin exocytosis in the basal region could
induce mucin accumulation in the extracellular matrix. It has also
been suggested that the STX3/SNAP23/VAMP8 complex may serve a major
role in homotypic granule-granule fusion in Labial Salivary Gland
(LSG) acinar cells in patients with SS, as described in the
pancreas (44).
SNAPs in patients with SS
SNAP23 belongs to the SNAP25 (also known as Q-SNARE)
family and is anchored and localized in the plasma membrane
(53). Whilst SNAP25 is expressed in
the plasma membrane of neuronal and endocrine cells, SNAP23 which
is a non-neuronal homologue of SNAP25, is specifically expressed in
the apical and basolateral membranes of human submandibular glands
(50). In contrast, in rat parotid
glands, SNAP23 has been detected in the apical plasma membrane and
intracellular membranes, and forms complexes with STX3, STX4, VAMP2
and VAMP3(49).
Several differences have been noted in the
comparison of the expression and localization of SNAP23 in acinar
cells of the labial salivary glands between healthy individuals and
patients with SS. Whilst the expression of SNAP23 was detected
throughout the plasma membrane in healthy individuals, its
expression was absent in the apical plasma membrane and decreased
in the lateral plasma membrane in patients with SS. In contrast, no
changes were detected in the basal plasma membrane (45).
4. Aquaporins
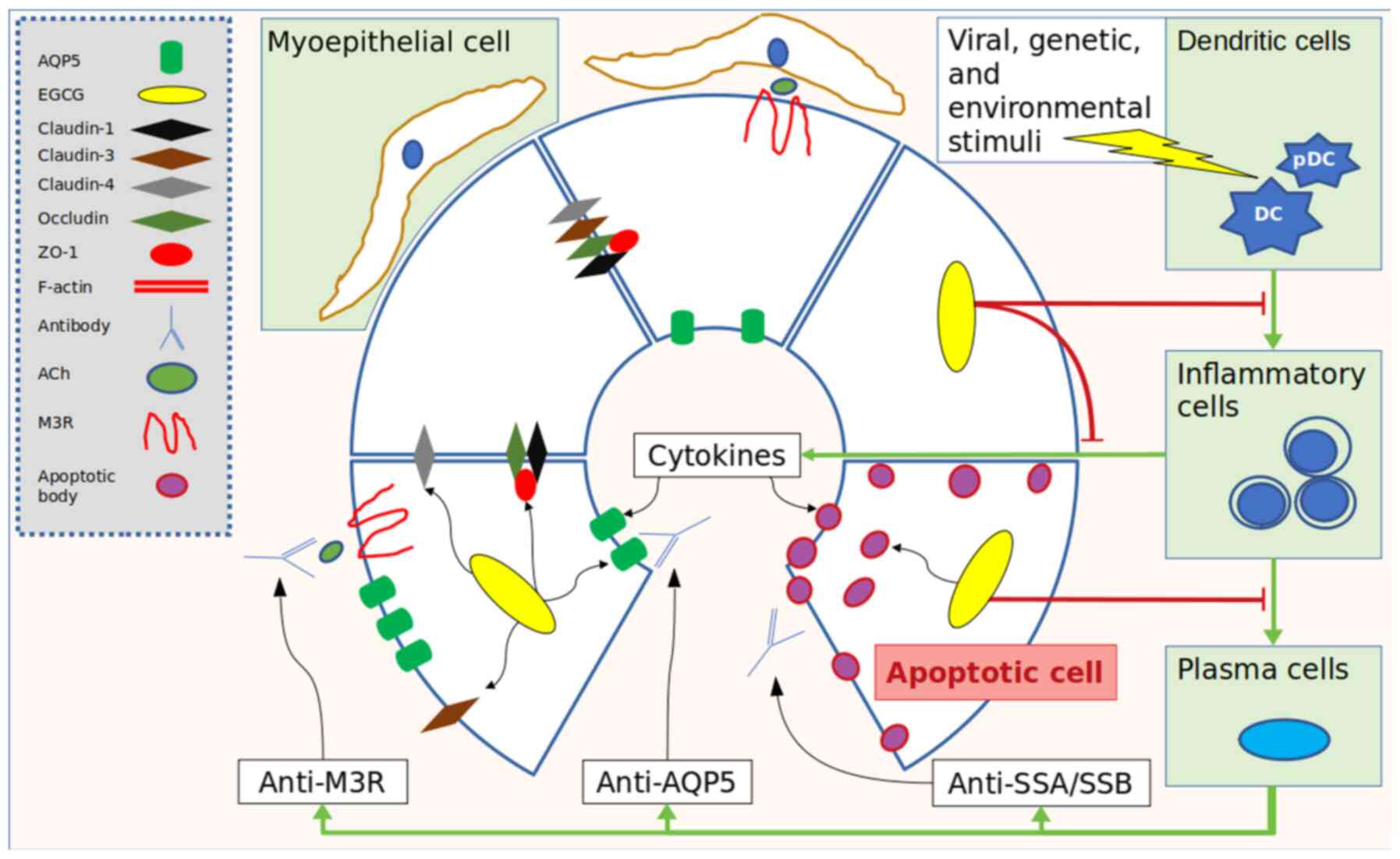
Physiological roles of AQP5
In human salivary glands, it is hypothesized that
AQP5 is the only AQP involved in salivary secretion (54). In the human parotid, submandibular,
sublingual and labial glands, AQP5 labelling was localized to the
apical membrane of acinar cells, and its expression was not
detectable in duct cells (55,56).
Proper expression and subcellular localization of AQP5 are required
to maintain homeostasis (56,57).
Parasympathetic innervation is essential for the expression and
distribution of AQP5 in the salivary gland (58,59).
Acetylcholine (ACh) acts on M3 muscarinic acetylcholine receptors
to induce translocation of AQP5 in the rat parotid glands (60), whereas in the rat submandibular
gland, cholinergic denervation reduces AQP5 expression (61).
AQP5 in patients with SS
Studies have shown defective function of AQP5 in
patients with SS (56,62). Abnormal localization of AQP5 has been
reported in patients with SS compared with patients without SS but
with xerostomia. AQP5 was shown to be expressed at both the apical
and basolateral membranes in the acinar cells of minor salivary
glands in patients with SS compared to healthy individuals, in whom
AQP5 was restricted to the apical membrane of acinar cells
(56).
Analysis of the saliva of transgenic mice lacking
AQP5 showed that it is viscous and hypertonic (63). Indirect immunofluorescence tests
performed in patients with SS detected the anti-AQP5 Immunoglobulin
G (IgG), which may explain the low rate of resting salivary flow
(64). The same authors demonstrated
in another study that these anti-AQP5 antibodies found in patients
with SS recognize different epitopes of AQP5, suggesting their role
in salivary gland dysfunction (65).
Treatments of SS AQP5 expression with plant extracts, such as
Dendrobium candidum, has been shown to yield positive
results when crude materials or the isolated phenolic active
compound chrystoxine (66,67).
5. Actin
Physiological role of actin. Cytoskeletal
components, such as actin filaments, play a major role in the
proliferation and differentiation of cells (68). They are also involved in salivary
gland protein secretion (69). It
has been reported that stimuli which promote salivation can
regulate F-actin activity within acinar cells of the salivary
glands. Indeed, when salivatory stimuli are absent, F-actin, which
is located under the plasma membrane and separates the secretory
granules from the luminal membrane, prevents the secretory granules
from reaching their exocytotic destination in the human parotid and
submandibular gland (70).
Following stimulation, the actin-cytoskeleton is
rearranged and disassembled, consequently allowing secretory
granules to reach their destination for exocytosis (71). F-actins are not only involved in the
regulation of the secretion granules trafficking, but also regulate
the formation of these vesicles and their movement to the cell
membrane (72). Similarly, it has
been reported that depolymerization of F-actin in the rat
submandibular gland prevents amylase release and exocytosis
(73,74).
It has been suggested in other studies that
proteins, such as cofilin, a protein necessary for the
depolymerization of actin and for controlling the renewal and
branching of microfilaments, may play a role in secretory vesicle
trafficking (75,76). In adrenal chromaffin cells, cofilin
is indispensable in the reorganization of the cortical actin
cytoskeleton, which is necessary to allow the movement of secretory
granules not yet attached to the plasma membrane (75).
Actin in patients with SS
F-actin in the human parotid and submandibular
glands serves to separate the secretory granules from the luminal
membrane, and to regulate the trafficking of secretory vesicles to
reach the sites of exocytosis (50,70).
Several molecules have been reported to play a major role in this
regulation.
Recently, a study on moesin, a structural protein
involved in cytoskeletal organization and signaling pathways,
showed the presence of anti-moesin antibodies by ELISA and western
blotting in patients with SS (77).
Gland tissue samples from patients with primary-SS
and primary SS/mucosal associated lymphoid tissue (MALT) lymphoma
exhibited significantly upregulated expression of cofilin 1,
α-enolase and Rho GDP-dissociation inhibitor 2 compared to non-SS
controls. ELISA tests that were used to detect autoantibodies
against these proteins showed that three autoantibodies were
upregulated in patients with primary-SS/MALT lymphoma compared with
patients with primary-SS and the healthy controls, and that
patients with primary-SS also had higher levels compared with the
healthy controls (76). This
suggests that these autoantibodies may affect the functional role
of F-actin and may be indirectly involved in altered secretory
vesicle trafficking and exocytosis dysfunction. However, these
results require further research to understand the molecular
mechanism of the immune reaction against these proteins in
vivo.
6. Tight junctions
Physiological role of tight
junctions
Tight junctions are protein complexes that are
localized in the plasma membrane, such as claudin and occludin, or
in the cytosol, such as zonula occludens 1 (ZO1). The functional
role of tight junctions is to facilitate the transcellular
epithelial flow of ions and water (78,79).
Tight junctions in patients with
SS
Comparison of the expression and localization of
claudin-1, claudin-3, claudin-4, ZO1 and occludin in labial
salivary gland between patients with SS and healthy individuals
showed several differences. Whereas the expression of claudin-1 and
claudin-4 was significantly increased in patients with SS, the
levels of ZO1 and occludin were decreased compared to the healthy
individuals. In contrast, no changes were observed in the
expression of claudin-3, although a relocation of claudin-3 and
claudin-4 was observed in these patients, which may alter the
transcellular permeability of the salivary gland (80).
It has been shown in patients with SS that TNF and
interferon (IFN) levels are elevated, resulting in the alteration
of tight junctions and the dysfunction of the salivary epithelium
(41,81). In these patients, the alteration of
tight junctions induced by increased TNF and IFN may cause cell
alterations, increasing paracellular permeability (82), upregulated expression of claudin-1
and claudin-4, redistribution of claudin-3 and claudin-4 from the
apical to the basal plasma membrane, and a strong negative
regulation of occludin and ZO1 expression (80). The molecular mechanisms proposed for
TNF and IFN action on tight junctions (83,84) are
endocytosis of occluding (85),
claudin-1 and junctional adhesion molecule 1 by activation of Ras
homologue family member A (RhoA)/RhoA kinase (83), and promotion of accumulation of
myosin II-dependent vesicles in the apical region and
reorganization of the cytoskeleton (86). Other mechanisms have also been
suggested, such as an indirect effect of nuclear factor
κ-light-chain-enhancer of activated B cells (NF-κB), either by
modulating ZO1 mRNA translation or ZO1 protein degradation via the
proteasome (80).
It has been reported that exosomes that are released
by epithelial cells in salivary glands contain autoantigens
[anti-SS-related antigen A autoantibodies (SSA), SS type B antigen
(SSB) and Anti-Smith], which may induce an immune response in these
glands (87). Furthermore, a study
of the microvilli that are localized in the apical zone of the
acinar cells of labial salivary gland from patients with SS has
shown that they are disorganized, possibly due to alterations of
the tight junctions and modifications of the actin cytoskeleton
(88).
A recent study on AQP-null mice suggested that AQP5
and tight junctions are functionally related (89). Indeed, this previous study
demonstrated that alterations of the tight junctions were also
detected in these mice. Conversely, water transport was only
partially altered (51,89), suggesting that alteration of AQP5
allows water to pass through other routes.
As previously mentioned, AQP5 in patients with SS is
detected at both the apical and basolateral plasma membranes of
acinar cells, whereas normally these proteins are localized only to
the apical membrane (89,90). This indicates that the alteration in
AQP5 distribution may be related to the loss of integrity of tight
junction proteins. All proteins and their distribution in healthy
individuals and patients with SS are described in Tables II and III, and Fig.
2.
7. GTPs
EGCG in autoimmune disorders
EGCG, the ester of epigallocatechin and gallic acid,
is the most abundant catechin in green tea. Its potential effects
on human health and disease have been widely examined (91,92).
Targets for EGCG include phosphoinositide 3-kinase/protein kinase
B, Janus kinase/signal transducer and activator of transcription
proteins, MAPK, as well as proteases, such as metalloproteinases
and urokinase (93).
Autoantigen expression and apoptosis are the most
common factors that lead to salivary gland damage (19,27). It
has been shown that the administration of EGCG in drinking water in
non-obese diabetic mice had a protective effect against autoimmune
reactions in the salivary glands, as well as inhibiting apoptosis
and cell proliferation (27).
Inhibition of apoptosis (94), a cellular process in which cells
undergo controlled cell death, may be achieved through dampening of
proteolytic polymerases. This activity is dissimilar to the
pro-apoptotic results seen in cancer studies, and this difference
in behavior could be due to EGCG binding to sugars (95), such as ADP-ribose, is necessary for
the intrinsic metabolic apoptotic pathway (96) inhibiting their ability to bind to
Poly-(ADP-ribose) polymerases and carry out apoptosis in the
salivary glands. Additionally, EGCG itself can be an agonist of
receptor mediated apoptosis, particularly in cell lines were
expression of the TNF superfamily of receptors are upregulated
(97). EGCG also inhibits the
cellular process of proliferation (98). Both apoptosis and cellular
proliferation require intracellular remodeling, especially of
components such as actin filaments (99,100).
Inhibition of both of these processes may result in a suspended
state of the tissue in cell cycle arrest (101).
Other studies have shown that green tea extract can
also reduce the levels of autoantibodies in animal serum and that
EGCG affects TNF-α levels by preventing cytotoxicity in salivary
gland cells ex vivo (102,103).
EGCG can exert an anti-inflammatory effect by inhibiting
interleukin-1β, suppressing dendritic cell maturation and reducing
T cell activation (104). A summary
of EGCG interactions with the selected proteins is shown in
Table IV.
EGCG and the SS autoimmune
response
The study of the expression of autoantigens genes
[SSA, SSB, fodrin, centromere protein C, golgin-67, coilin and poly
(ADP-ribose) polymerase] in normal human epidermal keratinocytes
and immortalized salivary gland acinar cells has shown that
exposure to EGCG inhibits the expression of these genes (26), which may explain the low levels of
autoantibodies and salivary lymphocyte infiltration following GTP
administration in an accepted mouse model of SS [Non-Obese Diabetic
(NOD) mice] and in a mouse model of lupus erythematosus [Murphy
Roths Large (MRL) mice] (25).
An autoimmune sialadenitis model of MRL-Fas-lpr mice
demonstrated that AQP5 expression is reduced in the salivary glands
damaged by apoptosis of cells, and that EGCG was able to restore
AQP5 expression and improve the functionality of the glands. The
molecular mechanism of EGCG action has been suggested to involve
inactivating both the NF-κB and the N-terminal c-Jun kinase, as
well as preserving the activity of protein kinase A (105).
Anti-inflammatory activity of
EGCG
EGCG affects the submandibular gland in a NOD animal
by reducing the size of the SS foci (25). By using a MTT viability assay on the
human salivary gland cell line NS-SV-AC, it was shown that EGCG
could ameliorate the effects of TNF-α produced by inflammatory
cells, via the attenuation of the cytotoxic effect at the target
acinar cells. Similarly, the phosphorylation of p38 was shown to be
induced by EGCG via modulation of the MAPK signal transduction
pathways, suggesting the presence of another mechanism by which
GTPs can attenuate SS pathogenesis (27). p38 is a key modulator of
inflammation, regulating TNF-α and interleukin mediator secretion
(106).
In a dextran sulphate sodium mouse model of colitis,
EGCG has been found to be effective at reducing the severity of
inflammation and symptoms both preventatively and therapeutically
(107).
SNAREs and EGCG
EGCG, but not its metabolites, has been found to
marginally increase the lysosomal proteolysis and autophagy
(108-110).
Lysosomal function is dependent on cathepsins and inhibition of
Cathepsin S has been found to induce autophagy through reactive
oxygen species-mediated phosphatidylinositol 3-kinase and c-Jun
N-terminal kinase signaling pathways (111). Similarly, Cathepsin S activity was
found to be elevated in the lacrimal glands of the SS murine model
(112).
AQPs and EGCG
EGCG significantly inhibits the expression levels of
AQP4 in a traumatic brain injury model (113), as well as a rat spinal injury model
(114), reducing the associated
oedema. EGCG prevention of vasogenic oedema associated with status
epilepticus was also mediated through regulation of AQP4
expression, however this time via its upregulation (115).
AQP mediated moisture retention is increased with
EGCG; however, whilst moisturizing cream containing the additive
was found to cause cellular retention by increasing AQP3 mRNA
expression (116), a murine model
of EGCG induced liver failure found that the associated cellular
turgidity was concomitant to inhibition of AQP2(117).
Cellular apoptosis stimulated by EGCG in ovarian
cancer was also associated with inhibition of AQP5 production
(118), whereas a murine model of
sialadenitis exhibited upregulated secretin levels due to the
EGCG-mediated increase in AQP5 expression (105).
Actin and EGCG
Extracellular expression of α-enolase was inhibited
in a kidney model of calcium oxalate monohydrate (COM) crystal
binding, resulting in improved outcomes and reduced extracellular
crystallization (119).
Furthermore, EGCG was found to maintain and protect the apical
microvillar structure and F-actin organization of tubular
epithelial cells in a COM induced injury model (120).
Whilst EGCG can prevent IFN-γ mediated
disorganization and inhibition of moesin binding (121), at high enough concentrations, it
can begin to disrupt F-actin organization, which is associated with
reduced rates of proliferation (119).
Otherwise, commonly in the literature EGCG is found
to have an anti-fibrotic effect, associated with an inhibition of
α-smooth muscle actin expression (122-124).
Tight junctions and EGCG
Reports have shown that whilst EGCG is unable to
easily cross the intestinal or blood-brain barrier, there is some
mechanism involving tight junctions, which, in vivo allows
it (125,126). On a molecular level, EGCG was
associated with stimulated secretion of tight junction proteins
zonula occludens-1 (ZO-1) and occluding (127-129)
in response to bacterial and TNF-α stimulation.
The protective role of EGCG against inflammation
stimulated by cytokine release extends to inhibition of tight
junction dysfunction induced by IFN-γ (130,131).
These findings have been replicated in a colitis mouse model,
showing a significant increase in expression of occludin, claudin-1
and ZO-1, whilst inhibiting claudin-4, all of which were associated
with EGCG mediated inhibition of IL-6 and 12, as well as IFN-γ
(107).
8. Discussion and conclusions
Selecting publications on EGCG with a narrow
pharmacokinetic focus is challenging. Epidemiological and
longitudinal studies on the health benefits in large populations
are far outnumbered by cellular, histological and pharmacological
investigations. This problem is further exacerbated by the complex
interactions EGCG can have, which depend on its route of
administration, the gut microflora and its cellular metabolism,
which appears to be organ specific. Nonetheless, some signs of
structured analysis of EGCG are appearing in the literature,
allowing a comprehensive look at its role in salivary
physiology.
It is striking to observe the action of the
autoimmune response on AQP5, actin and tight junction directly or
indirectly. It seems that the alteration of the localization and
expression of AQP5, SNAREs, actin and tight junctions can lead to
salivary gland dysfunction in SS (Table
I; Fig. 1). An attractive
hypothesis is that SNAREs act as a regulator of the distribution
and movement of all these components through the cell, and the
autoimmune reaction is the result of the alterations of these
proteins. However, as mentioned above GTPs, such as EGCG, have
multiple protective effects for autoimmune disease. They maintain
total serum autoantibody production at moderate levels due to an
inhibitory effect on antigen expression (26) and suppress the increase in
TNF-α-induced apoptotic activity in human salivary gland acinar
cells in vitro (27).
However, the mechanisms for such multi-level protection by
polyphenols are still poorly understood and warrant further
investigation of this unique phytochemical with regard to its
potential human benefits. The apical plasma membrane water channel
AQP5 plays an important role in transporting water across the
apical surface of the salivary gland epithelia (58,59).
Multiple studies have demonstrated the presence of
anti-AQP5 IgG antibodies in patients with primary-SS (132,133).
Others have demonstrated that EGCG (592 µg/mouse in drinking water,
for 57 days) was able to restore AQP5 expression levels and improve
gland functionality (105). These
findings suggest a central role of AQP5 in SS, without excluding
the importance of SNAREs (134,135),
actin (136) and tight junctions
(51,89), in the regulation of AQP trafficking,
activity and distribution. It is also necessary to underline the
involvement of these molecules in the regulation of secretory
vesicles and exocytosis. Alterations of the molecules involved in
the regulation of AQP5 trafficking and activity may lead to
dysfunction in secretion of vesicles. However, the direct link
between the alterations of the trafficking activity of AQP5, the
presence of the anti-AQP5 antibodies and the impact on the
secretory granule trafficking dysfunction requires further
investigation.
Acknowledgements
Not applicable.
Funding
The present review was supported by internal funding from Poznan
University of Medicinal Sciences and University Catholique of
Louvain. There is no associated grant no.
Availability of data and materials
Not applicable.
Authors' contributions
MWS and AE conceived the topic of study. AE
performed the methodology. MWS validated the content. MWS, AE and
MN performed the investigation. AE curated the data. AE drafted the
manuscript. AE and MWS edited the manuscript. All authors have read
and approved the final manuscript. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liu J and Duan Y: Saliva: A potential
media for disease diagnostics and monitoring. Oral Oncol.
48:569–577. 2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Chiappin S, Antonelli G, Gatti R and De
Palo EF: Saliva specimen: A new laboratory tool for diagnostic and
basic investigation. Clin Chim Acta. 383:30–40. 2007.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Karnati R, Laurie DE and Laurie GW:
Lacritin and the tear proteome as natural replacement therapy for
dry eye. Exp Eye Res. 117:39–52. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sitaramamma T, Shivaji S and Rao GN:
Effect of storage on protein concentration of tear samples. Curr
Eye Res. 17:1027–1035. 1998.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wilmarth PA, Riviere MA, Rustvold DL,
Lauten JD, Madden TE and David LL: Two-dimensional liquid
chromatography study of the human whole saliva proteome. J Proteome
Res. 3:1017–1023. 2004.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Humphrey SP and Williamson RT: A review of
saliva: Normal composition, flow, and function. J Prosthet Dent.
85:162–169. 2001.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tucker AS: Salivary gland development.
Semin Cell Dev Biol. 18:237–244. 2007.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Br H and Mp H: Regulatory mechanisms
driving salivary gland organogenesis. Curr Top Dev Biol.
115:111–130. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Voulgarelis M and Tzioufas AG:
Pathogenetic mechanisms in the initiation and perpetuation of
Sjögren's syndrome. Nat Rev Rheumatol. 6:529–537. 2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mavragani CP and Moutsopoulos HM: The
geoepidemiology of Sjögren's syndrome. Autoimmun Rev. 9:A305–A310.
2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Busamia B, Gonzalez-Moles MA, Ruiz-Avila
I, Brunotto M, Gil-Montoya JA, Bravo M, Gobbi C and Finkelberg A:
Cell apoptosis and proliferation in salivary glands of Sjögren's
syndrome. J Oral Pathol Med. 40:721–725. 2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Pedersen AM, Dissing S, Fahrenkrug J,
Hannibal J, Reibel J and Nauntofte B: Innervation pattern and
Ca2+ signalling in labial salivary glands of healthy
individuals and patients with primary Sjögren's syndrome (pSS). J
Oral Pathol Med. 29:97–109. 2000.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zoukhri D and Kublin CL: Impaired
neurotransmitter release from lacrimal and salivary gland nerves of
a murine model of Sjögren's syndrome. Invest Ophthalmol Vis Sci.
42:925–932. 2001.PubMed/NCBI
|
|
14
|
Dawson LJ, Stanbury J, Venn N, Hasdimir B,
Rogers SN and Smith PM: Antimuscarinic antibodies in primary
Sjögren's syndrome reversibly inhibit the mechanism of fluid
secretion by human submandibular salivary acinar cells. Arthritis
Rheum. 54:1165–1173. 2006.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Caulfield VL, Balmer C, Dawson LJ and
Smith PM: A role for nitric oxide-mediated glandular hypofunction
in a non-apoptotic model for Sjogren's syndrome. Rheumatology
(Oxford). 48:727–733. 2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Dawson LJ, Fox PC and Smith PM: Sjogrens
syndrome-the non-apoptotic model of glandular hypofunction.
Rheumatology (Oxford). 45:792–798. 2006.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Soyfoo MS, Vriese CD, Debaix H,
Martin-Martinez MD, Mathieu C, Devuyst O, Steinfeld SD and Delporte
C: Modified aquaporin 5 expression and distribution in
submandibular glands from NOD mice displaying autoimmune
exocrinopathy. Arthritis Rheum. 56:2566–2574. 2007.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Aktas O, Prozorovski T, Smorodchenko A,
Savaskan NE, Lauster R, Kloetzel PM, Infante-Duarte C, Brocke S and
Zipp F: Green tea epigallocatechin-3-gallate mediates T cellular
NF-kappa B inhibition and exerts neuroprotection in autoimmune
encephalomyelitis. J Immunol. 173:5794–5800. 2004.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Gillespie K, Kodani I, Dickinson DP,
Ogbureke KU, Camba AM, Wu M, Looney S, Chu TC, Qin H, Bisch F, et
al: Effects of oral consumption of the green tea polyphenol EGCG in
a murine model for human Sjogren's syndrome, an autoimmune disease.
Life Sci. 83:581–588. 2008.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Dickinson D, Yu H, Ohno S, Thomas C,
Derossi S, Ma YH, Yates N, Hahn E, Bisch F, Yamamoto T and Hsu S:
Epigallocatechin-3-gallate prevents autoimmune-associated down-
regulation of p21 in salivary gland cells through a p53-independent
pathway. Inflamm Allergy Drug Targets. 13:15–24. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Carsons S: A review and update of
Sjögren's syndrome: Manifestations, diagnosis, and treatment. Am J
Manag Care. 7:S433–443. 2001.PubMed/NCBI
|
|
22
|
Zhang NZ, Shi CS, Yao QP, Pan GX, Wang LL,
Wen ZX, Li XC and Dong Y: Prevalence of primary Sjögren's syndrome
in China. J Rheumatol. 22:659–661. 1995.PubMed/NCBI
|
|
23
|
Ohno S, Yu H, Dickinson D, Chu TC,
Ogbureke K, Derossi S, Yamamoto T and Hsu S:
Epigallocatechin-3-gallate modulates antioxidant and DNA
repair-related proteins in exocrine glands of a primary Sjogren's
syndrome mouse model prior to disease onset. Autoimmunity.
45:540–546. 2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Saito K, Mori S, Date F and Ono M:
Epigallocatechin gallate inhibits oxidative stress-induced DNA
damage and apoptosis in MRL-Fas(lpr) mice with autoimmune
sialadenitis via upregulation of heme oxygenase-1 and Bcl-2.
Autoimmunity. 47:13–22. 2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Hsu S and Dickinson D: A new approach to
managing oral manifestations of Sjogren's syndrome and skin
manifestations of lupus. J Biochem Mol Biol. 39:229–239.
2006.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hsu S, Dickinson DP, Qin H, Lapp C, Lapp
D, Borke J, Walsh DS, Bollag WB, Stöppler H, Yamamoto T, et al:
Inhibition of autoantigen expression by
(-)-epigallocatechin-3-gallate (the major constituent of green tea)
in normal human cells. J Pharmacol Exp Ther. 315:805–811.
2005.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Hsu SD, Dickinson DP, Qin H, Borke J,
Ogbureke KU, Winger JN, Camba AM, Bollag WB, Stöppler HJ, Sharawy
MM and Schuster GS: Green tea polyphenols reduce autoimmune
symptoms in a murine model for human Sjogren's syndrome and protect
human salivary acinar cells from TNF-alpha-induced cytotoxicity.
Autoimmunity. 40:138–147. 2007.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Guo T, Song D, Cheng L and Zhang X:
Interactions of tea catechins with intestinal microbiota and their
implication for human health. Food Sci Biotechnol. 28:1617–1625.
2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Chiou YS, Wu JC, Huang Q, Shahidi F, Wang
YJ, Ho CT and Pan MH: Metabolic and colonic microbiota
transformation may enhance the bioactivities of dietary
polyphenols. J Funct Foods. 7:3–25. 2014.
|
|
30
|
Pervin M, Unno K, Takagaki A, Isemura M
and Nakamura Y: Function of green tea catechins in the brain:
Epigallocatechin gallate and its metabolites. Int J Mol Sci.
20(3630)2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kim HS, Quon MJ and Kim J: New insights
into the mechanisms of polyphenols beyond antioxidant properties;
lessons from the green tea polyphenol, epigallocatechin 3-gallate.
Redox Biology. 2:187–195. 2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: An updated guideline for
reporting systematic reviews. BMJ. 372(n71)2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Hong W: SNAREs and traffic. Biochim
Biophys Acta. 1744:120–144. 2005.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Grote E, Hao JC, Bennett MK and Kelly RB:
A targeting signal in VAMP regulating transport to synaptic
vesicles. Cell. 81:581–589. 1995.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Alberts B, Johnson A, Lewis J, Raff M,
Roberts K and Walter P: Transport from the ER through the Golgi
Apparatus. Molecular Biology of the Cell 4th edition, 2002.
|
|
36
|
Whyte JRC and Munro S: Vesicle tethering
complexes in membrane traffic. J Cell Sci. 115:2627–2637.
2002.PubMed/NCBI
|
|
37
|
Chen YA and Scheller RH: SNARE-mediated
membrane fusion. Nat Rev Mol Cell Biol. 2:98–106. 2001.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Han J, Pluhackova K and Böckmann RA: The
multifaceted role of SNARE proteins in membrane fusion. Front
Physiol. 8(5)2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Srivanitchapoom P, Pandey S and Hallett M:
Drooling in Parkinson's Disease: A review. Parkinsonism Relat
Disord. 20:1109–1118. 2014.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Yu GY, Zhu ZH, Mao C, Cai ZG, Zou LH, Lu
L, Zhang L, Peng X, Li N and Huang Z: Microvascular autologous
submandibular gland transfer in severe cases of
keratoconjunctivitis sicca. Int J Oral Maxillofac Surg. 33:235–239.
2004.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Ewert P, Aguilera S, Alliende C, Kwon YJ,
Albornoz A, Molina C, Urzúa U, Quest AF, Olea N, Pérez P, et al:
Disruption of tight junction structure in salivary glands from
Sjögren's syndrome patients is linked to proinflammatory cytokine
exposure. Arthritis Rheum. 62:1280–1289. 2010.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Wong SH, Zhang T, Xu Y, Subramaniam VN,
Griffiths G and Hong W: Endobrevin, a novel synaptobrevin/VAMP-like
protein preferentially associated with the early endosome. Mol Biol
Cell. 9:1549–1563. 1998.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Lang T and Jahn R: Core proteins of the
secretory machinery. Handb Exp Pharmacol. 107–127. 2008.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Cosen-Binker LI, Binker MG, Wang CC, Hong
W and Gaisano HY: VAMP8 is the v-SNARE that mediates basolateral
exocytosis in a mouse model of alcoholic pancreatitis. J Clin
Invest. 118:2535–2551. 2008.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Barrera MJ, Sánchez M, Aguilera S,
Alliende C, Bahamondes V, Molina C, Quest AF, Urzúa U, Castro I,
González S, et al: Aberrant localization of fusion receptors
involved in regulated exocytosis in salivary glands of Sjögren's
syndrome patients is linked to ectopic mucin secretion. J
Autoimmun. 39:83–92. 2012.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Wang CC, Shi H, Guo K, Ng CP, Li J, Gan
BQ, Chien Liew H, Leinonen J, Rajaniemi H, et al: VAMP8/endobrevin
as a general vesicular SNARE for regulated exocytosis of the
exocrine system. Mol Biol Cell. 18:1056–1063. 2007.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Wang CC, Ng CP, Lu L, Atlashkin V, Zhang
W, Seet LF and Hong W: A role of VAMP8/endobrevin in regulated
exocytosis of pancreatic acinar cells. Dev Cell. 7:359–371.
2004.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Takuma T, Arakawa T and Tajima Y:
Interaction of SNARE proteins in rat parotid acinar cells. Arch
Oral Biol. 45:369–375. 2000.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Imai A, Nashida T, Yoshie S and Shimomura
H: Intracellular localisation of SNARE proteins in rat parotid
acinar cells: SNARE complexes on the apical plasma membrane. Arch
Oral Biol. 48:597–604. 2003.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Stoeckelhuber M, Scherer EQ, Janssen KP,
Slotta-Huspenina J, Loeffelbein DJ, Rohleder NH, Nieberler M,
Hasler R and Kesting MR: The human submandibular gland:
Immunohistochemical analysis of SNAREs and cytoskeletal proteins. J
Histochem Cytochem. 60:110–120. 2012.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Goicovich E, Molina C, Pérez P, Aguilera
S, Fernández J, Olea N, Alliende C, Leyton C, Romo R, Leyton L and
González MJ: Enhanced degradation of proteins of the basal lamina
and stroma by matrix metalloproteinases from the salivary glands of
Sjögren's syndrome patients: Correlation with reduced structural
integrity of acini and ducts. Arthritis Rheum. 48:2573–2584.
2003.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Coursey TG, Tukler Henriksson J, Barbosa
FL, de Paiva CS and Pflugfelder SC: Interferon-γ-induced unfolded
protein response in conjunctival goblet cells as a cause of mucin
deficiency in Sjögren syndrome. Am J Pathol. 186:1547–1558.
2016.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Holt M, Varoqueaux F, Wiederhold K,
Takamori S, Urlaub H, Fasshauer D and Jahn R: Identification of
SNAP-47, a novel Qbc-SNARE with ubiquitous expression. J Biol Chem.
281:17076–17083. 2006.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Wang W, Hart PS, Piesco NP, Lu X, Gorry MC
and Hart TC: Aquaporin expression in developing human teeth and
selected orofacial tissues. Calcif Tissue Int. 72:222–227.
2003.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Gresz V, Kwon TH, Hurley PT, Varga G,
Zelles T, Nielsen S, Case RM and Steward MC: Identification and
localization of aquaporin water channels in human salivary glands.
Am J Physiol Gastrointest Liver Physiol. 281:G247–G254.
2001.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Steinfeld S, Cogan E, King LS, Agre P,
Kiss R and Delporte C: Abnormal distribution of aquaporin-5 water
channel protein in salivary glands from Sjögren's syndrome
patients. Lab Invest. 81:143–148. 2001.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Krane CM, Melvin JE, Nguyen HV, Richardson
L, Towne JE, Doetschman T and Menon AG: Salivary acinar cells from
aquaporin 5-deficient mice have decreased membrane water
permeability and altered cell volume regulation. J Biol Chem.
276:23413–23420. 2001.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Ishikawa Y, Cho G, Yuan Z, Inoue N and
Nakae Y: Aquaporin-5 water channel in lipid rafts of rat parotid
glands. Biochim Biophys Acta. 1758:1053–1060. 2006.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Ishikawa Y, Cho G, Yuan Z, Skowronski MT,
Pan Y and Ishida H: Water channels and zymogen granules in salivary
glands. J Pharmacol Sci. 100:495–512. 2006.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Ishikawa Y, Eguchi T, Skowronski MT and
Ishida H: Acetylcholine acts on M3 muscarinic receptors and induces
the translocation of aquaporin5 water channel via cytosolic Ca2+
elevation in rat parotid glands. Biochem Biophys Res Commun.
245:835–840. 1998.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Xiang B, Zhang Y, Li YM, Zhang K, Zhang
YY, Wu LL and Yu GY: Effects of phenylephrine on transplanted
submandibular gland. J Dent Res. 85:1106–1111. 2006.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Tsubota K, Hirai S, King LS, Agre P and
Ishida N: Defective cellular trafficking of lacrimal gland
aquaporin-5 in Sjögren's syndrome. Lancet. 357:688–689.
2001.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Ma T, Song Y, Gillespie A, Carlson EJ,
Epstein CJ and Verkman AS: Defective secretion of saliva in
transgenic mice lacking aquaporin-5 water channels. J Biol Chem.
274:20071–20074. 1999.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Alam J, Koh JH, Kim N, Kwok SK, Park SH,
Song YW, Park K and Choi Y: Detection of autoantibodies against
aquaporin-5 in the sera of patients with primary Sjögren's
syndrome. Immunol Res. 64:848–856. 2016.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Alam J, Koh JH, Kwok SK, Park SH, Park K
and Choi Y: Functional Epitopes for Anti-Aquaporin 5 Antibodies in
Sjögren Syndrome. J Dent Res. 96:1414–1421. 2017.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Xiao L, Ng TB, Feng YB, Yao T, Wong JH,
Yao RM, Li L, Mo FZ, Xiao Y, Shaw PC, et al: Dendrobium candidum
extract increases the expression of aquaporin-5 in labial glands
from patients with Sjögren's syndrome. Phytomedicine. 18:194–198.
2011.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Lin X, Shaw PC, Sze SCW, Tong Y and Zhang
Y: Dendrobium officinale polysaccharides ameliorate the abnormality
of aquaporin 5, pro-inflammatory cytokines and inhibit apoptosis in
the experimental Sjögren's syndrome mice. Int Immunopharmacol.
11:2025–2032. 2011.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Sart S, Errachid A, Schneider YJ and
Agathos SN: Modulation of mesenchymal stem cell actin organization
on conventional microcarriers for proliferation and differentiation
in stirred bioreactors. J Tissue Eng Regen Med. 7:537–551.
2013.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Nashida T, Yoshie S, Imai A and Shimomura
H: Presence of cytoskeleton proteins in parotid glands and their
roles during secretion. Arch Oral Biol. 49:975–982. 2004.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Segawa A, Riva A, Loffredo F, Congiu T,
Yamashina S and Testa Riva F: Cytoskeletal regulation of human
salivary secretion studied by high resolution electron microscopy
and confocal laser microscopy. Eur J Morphol. 36 (Suppl):S41–S45.
1998.PubMed/NCBI
|
|
71
|
Perrin D, Möller K, Hanke K and Söling HD:
cAMP and Ca(2+)-mediated secretion in parotid acinar cells is
associated with reversible changes in the organization of the
cytoskeleton. J Cell Biol. 116:127–134. 1992.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Valentijn KM, Gumkowski FD and Jamieson
JD: The subapical actin cytoskeleton regulates secretion and
membrane retrieval in pancreatic acinar cells. J Cell Sci.
112:81–96. 1999.PubMed/NCBI
|
|
73
|
Muallem S, Kwiatkowska K, Xu X and Yin HL:
Actin filament disassembly is a sufficient final trigger for
exocytosis in nonexcitable cells. J Cell Biol. 128:589–598.
1995.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Busch L, Sterin-Borda L and Borda E:
Differences in the regulatory mechanism of amylase release by rat
parotid and submandibular glands. Arch Oral Biol. 47:717–722.
2002.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Birkenfeld J, Kartmann B, Betz H and Roth
D: Cofilin activation during Ca(2+)-triggered secretion from
adrenal chromaffin cells. Biochem Biophys Res Commun. 286:493–498.
2001.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Cui L, Elzakra N, Xu S, Xiao GG, Yang Y
and Hu S: Investigation of three potential autoantibodies in
Sjogren's syndrome and associated MALT lymphoma. Oncotarget.
8:30039–30049. 2017.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Zhang Y, Hussain M, Yang X, Chen P, Yang
C, Xun Y, Tian Y and Du H: Identification of moesin as a novel
autoantigen in patients with Sjögren's syndrome. Protein Pept Lett.
25:350–355. 2018.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Mitic LL, Van Itallie CM and Anderson JM:
Molecular physiology and pathophysiology of tight junctions I.
Tight junction structure and function: Lessons from mutant animals
and proteins. Am J Physiol Gastrointest Liver Physiol.
279:G250–G254. 2000.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Beguin P, Errachid A, Larondelle Y and
Schneider YJ: Effect of polyunsaturated fatty acids on tight
junctions in a model of the human intestinal epithelium under
normal and inflammatory conditions. Food Funct. 4:923–931.
2013.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Flynn AN, Itani OA, Moninger TO and Welsh
MJ: Acute regulation of tight junction ion selectivity in human
airway epithelia. Proc Natl Acad Sci USA. 106:3591–3596.
2009.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Fox RI, Kang HI, Ando D, Abrams J and Pisa
E: Cytokine mRNA expression in salivary gland biopsies of Sjögren's
syndrome. J Immunol. 152:5532–5539. 1994.PubMed/NCBI
|
|
82
|
Fox PC, Grisius MM, Bermudez DK and Sun D:
Cytokine mRNA expression in labial salivary glands and cytokine
secretion in parotid saliva in Sjögren's syndrome. Adv Exp Med
Biol. 438:909–915. 1998.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Baker OJ, Camden JM, Redman RS, Jones JE,
Seye CI, Erb L and Weisman GA: Proinflammatory cytokines tumor
necrosis factor-alpha and interferon-gamma alter tight junction
structure and function in the rat parotid gland Par-C10 cell line.
Am J Physiol Cell Physiol. 295:C1191–C1201. 2008.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Youakim A and Ahdieh M: Interferon-gamma
decreases barrier function in T84 cells by reducing ZO-1 levels and
disrupting apical actin. Am J Physiol. 276:G1279–G1288.
1999.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Ma TY, Iwamoto GK, Hoa NT, Akotia V,
Pedram A, Boivin MA and Said HM: TNF-alpha-induced increase in
intestinal epithelial tight junction permeability requires NF-kappa
B activation. Am J Physiol Gastrointest Liver Physiol.
286:G367–376. 2004.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Mankertz J, Tavalali S, Schmitz H,
Mankertz A, Riecken EO, Fromm M and Schulzke JD: Expression from
the human occludin promoter is affected by tumor necrosis factor
alpha and interferon gamma. J Cell Sci. 113:2085–2090.
2000.PubMed/NCBI
|
|
87
|
Utech M, Ivanov AI, Samarin SN, Bruewer M,
Turner JR, Mrsny RJ, Parkos CA and Nusrat A: Mechanism of
IFN-gamma-induced endocytosis of tight junction proteins: Myosin
II-dependent vacuolarization of the apical plasma membrane. Mol
Biol Cell. 16:5040–5052. 2005.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Manoussakis MN and Kapsogeorgou EK: The
role of epithelial cells in the pathogenesis of Sjögren's syndrome.
Clin Rev Allergy Immunol. 32:225–230. 2007.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Kawedia JD, Nieman ML, Boivin GP, Melvin
JE, Kikuchi K, Hand AR, Lorenz JN and Menon AG: Interaction between
transcellular and paracellular water transport pathways through
Aquaporin 5 and the tight junction complex. Proc Natl Acad Sci USA.
104:3621–3626. 2007.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Ichiyama T, Nakatani E, Tatsumi K,
Hideshima K, Urano T, Nariai Y and Sekine J: Expression of
aquaporin 3 and 5 as a potential marker for distinguishing dry
mouth from Sjögren's syndrome. J Oral Sci. 60:212–220.
2018.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Chow HH, Cai Y, Hakim IA, Crowell JA,
Shahi F, Brooks CA, Dorr RT, Hara Y and Alberts DS:
Pharmacokinetics and safety of green tea polyphenols after
multiple-dose administration of epigallocatechin gallate and
polyphenon E in healthy individuals. Clin Cancer Res. 9:3312–3319.
2003.PubMed/NCBI
|
|
92
|
Fürst R and Zündorf I: Plant-derived
anti-inflammatory compounds: Hopes and disappointments regarding
the translation of preclinical knowledge into clinical progress.
Mediators Inflamm. 2014(146832)2014.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Wyganowska-Świątkowska M,
Matthews-Kozanecka M, Matthews-Brzozowska T, Skrzypczak-Jankun E
and Jankun J: Can EGCG alleviate symptoms of down syndrome by
altering proteolytic activity? Int J Mol Sci.
19(248)2018.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Yan X, Li Y, Yu H, Wang W, Wu C, Yang Y,
Hu Y, Shi X and Li J: Epigallocatechin-3-gallate inhibits
H2O2-induced apoptosis in mouse vascular
smooth muscle cells via 67kD laminin receptor. Sci Rep.
7(7774)2017.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Wyganowska-Swiatkowska M, Nohawica M,
Grocholewicz K and Nowak G: Influence of herbal medicines on HMGB1
release, SARS-CoV-2 viral attachment, acute respiratory failure,
and sepsis. A literature review. Int J Mol Sci.
21(4639)2020.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Soldatenkov VA and Smulson M:
Poly(ADP-ribose) polymerase in DNA damage-response pathway:
Implications for radiation oncology. Int J Cancer. 90:59–67.
2000.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Zhang Y, Duan W, Owusu L, Wu D and Xin Y:
Epigallocatechin-3-gallate induces the apoptosis of hepatocellular
carcinoma LM6 cells but not non-cancerous liver cells. Int J Mol
Med. 35:117–124. 2015.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Harakeh S, Abu-El-Ardat K, Diab-Assaf M,
Niedzwiecki A, El-Sabban M and Rath M: Epigallocatechin-3-gallate
induces apoptosis and cell cycle arrest in HTLV-1-positive and
-negative leukemia cells. Med Oncol. 25:30–39. 2008.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Lancaster OM and Baum B: Shaping up to
divide: Coordinating actin and microtubule cytoskeletal remodelling
during mitosis. Semin Cell Dev Biol. 34:109–115. 2014.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Desouza M, Gunning PW and Stehn JR: The
actin cytoskeleton as a sensor and mediator of apoptosis.
Bioarchitecture. 2:75–87. 2012.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Mayr C, Wagner A, Neureiter D, Pichler M,
Jakab M, Illig R, Berr F and Kiesslich T: The green tea catechin
epigallocatechin gallate induces cell cycle arrest and shows
potential synergism with cisplatin in biliary tract cancer cells.
BMC Complement Altern Med. 15(194)2015.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Yang CS, Lee MJ and Chen L: Human salivary
tea catechin levels and catechin esterase activities: Implication
in human cancer prevention studies. Cancer Epidemiol Biomarkers
Prev. 8:83–89. 1999.PubMed/NCBI
|
|
103
|
Wheeler DS, Catravas JD, Odoms K,
Denenberg A, Malhotra V and Wong HR: Epigallocatechin-3-gallate, a
green tea-derived polyphenol, inhibits IL-1 beta-dependent
proinflammatory signal transduction in cultured respiratory
epithelial cells. J Nutr. 134:1039–1044. 2004.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Ahn SC, Kim GY, Kim JH, Baik SW, Han MK,
Lee HJ, Moon DO, Lee CM, Kang JH, Kim BH, et al:
Epigallocatechin-3-gallate, constituent of green tea, suppresses
the LPS-induced phenotypic and functional maturation of murine
dendritic cells through inhibition of mitogen-activated protein
kinases and NF-kappaB. Biochem Biophys Res Commun. 313:148–155.
2004.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Saito K, Mori S, Date F and Hong G:
Epigallocatechin gallate stimulates the neuroreactive salivary
secretomotor system in autoimmune sialadenitis of MRL-Fas(lpr) mice
via activation of cAMP-dependent protein kinase A and inactivation
of nuclear factor κB. Autoimmunity. 48:379–388. 2015.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Schieven GL: The biology of p38 kinase: A
central role in inflammation. Curr Top Med Chem. 5:921–928.
2005.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Stillman A, Connors M, Miller M, Qazzaz H
and Dryden G: P-145 oral administration of EGCG, a green tea
polyphenol, both suppresses and rescues mice from DSS-induced
colitis. Inflamm Bowel Dis. 22:S54. 2016.
|
|
108
|
Sakai M, Ohnishi K, Masuda M, Ohminami H,
Yamanaka-Okumura H, Hara T and Taketani Y: Isorhamnetin, a
3'-methoxylated flavonol, enhances the lysosomal proteolysis in
J774.1 murine macrophages in a TFEB-independent manner. Biosci
Biotechnol Biochem. 84:1221–1231. 2020.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Holczer M, Besze B, Zámbó V, Csala M,
Bánhegyi G and Kapuy O: Epigallocatechin-3-Gallate (EGCG) promotes
autophagy-dependent survival via influencing the balance of
mTOR-AMPK pathways upon endoplasmic reticulum stress. Oxid Med Cell
Longev. 2018(e6721530)2018.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Zhang S, Cao M and Fang F: The role of
Epigallocatechin-3-Gallate in autophagy and endoplasmic reticulum
stress (ERS)-induced apoptosis of human diseases. Med Sci Monit.
26(e924558)2020.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Zhang L, Wang H, Xu J, Zhu J and Ding K:
Inhibition of cathepsin S induces autophagy and apoptosis in human
glioblastoma cell lines through ROS-mediated PI3K/AKT/mTOR/p70S6K
and JNK signaling pathways. Toxicol Lett. 228:248–259.
2014.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Hamm-Alvarez SF, Janga SR, Edman MC,
Madrigal S, Shah M, Frousiakis SE, Renduchintala K, Zhu J, Bricel
S, Silka K, et al: Tear cathepsin S as a candidate biomarker for
Sjögren's syndrome. Arthritis Rheumatol. 66:1872–1881.
2014.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Zhang B, Wang B, Cao S and Wang Y:
Epigallocatechin-3-Gallate (EGCG) attenuates traumatic brain injury
by inhibition of edema formation and oxidative stress. Korean J
Physiol Pharmacol. 19:491–497. 2015.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Ge R, Zhu Y, Diao Y, Tao L, Yuan W and
Xiong X: Anti-edema effect of epigallocatechin gallate on spinal
cord injury in rats. Brain Res. 1527:40–46. 2013.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Kim JE, Park H, Jeong MJ and Kang TC:
Epigallocatechin-3-Gallate and PEDF 335 peptide, 67LR activators,
attenuate vasogenic edema, and astroglial degeneration following
status epilepticus. Antioxidants (Basel). 9(854)2020.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Nakamura Y, Tsuchiya T, Hara-Chikuma M,
Yasui M and Fukui Y: Identification of compounds in red wine that
effectively upregulate aquaporin-3 as a potential mechanism of
enhancement of skin moisturizing. Biochem Biophys Rep.
24(100864)2020.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Wang X, Yang L, Wang J, Zhang Y, Dong R,
Wu X, Yang CS, Zhang Z and Zhang J: A mouse model of subacute liver
failure with ascites induced by step-wise increased doses of
(-)-epigallocatechin-3-gallate. Sci Rep. 9(18102)2019.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Yan C, Yang J, Shen L and Chen X:
Inhibitory effect of Epigallocatechin gallate on ovarian cancer
cell proliferation associated with aquaporin 5 expression. Arch
Gynecol Obstet. 285:459–467. 2012.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Tepedelen BE, Soya E and Korkmaz M:
Epigallocatechin-3-gallate reduces the proliferation of benign
prostatic hyperplasia cells via regulation of focal adhesions. Life
Sci. 191:74–81. 2017.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Fong-Ngern K, Vinaiphat A and
Thongboonkerd V: Microvillar injury in renal tubular epithelial
cells induced by calcium oxalate crystal and the protective role of
epigallocatechin-3-gallate. FASEB J. 31:120–131. 2017.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Magro F, Fraga S and Soares-da-Silva P:
Interferon-gamma-induced STAT1-mediated membrane retention of NHE1
and associated proteins ezrin, radixin and moesin in HT-29 cells.
Biochem Pharmacol. 70:1312–1319. 2005.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Meng M, Li YQ, Yan MX, Kou Y and Ren HB:
Effects of epigallocatechin gallate on
diethyldithiocarbamate-induced pancreatic fibrosis in rats. Biol
Pharm Bull. 30:1091–1096. 2007.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Higashi N, Kohjima M, Fukushima M, Ohta S,
Kotoh K, Enjoji M, Kobayashi N and Nakamuta M:
Epigallocatechin-3-gallate, a green-tea polyphenol, suppresses Rho
signaling in TWNT-4 human hepatic stellate cells. J Lab Clin Med.
145:316–322. 2005.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Asaumi H, Watanabe S, Taguchi M, Tashiro
M, Nagashio Y, Nomiyama Y, Nakamura H and Otsuki M: Green tea
polyphenol (-)-epigallocatechin-3-gallate inhibits ethanol-induced
activation of pancreatic stellate cells. Eur J Clin Invest.
36:113–122. 2006.PubMed/NCBI View Article : Google Scholar
|
|
125
|
Cano A, Ettcheto M, Chang JH, Barroso E,
Espina M, Kühne BA, Barenys M, Auladell C, Folch J, Souto EB, et
al: Dual-drug loaded nanoparticles of Epigallocatechin-3-gallate
(EGCG)/Ascorbic acid enhance therapeutic efficacy of EGCG in a
APPswe/PS1dE9 Alzheimer's disease mice model. J Control Release.
301:62–75. 2019.PubMed/NCBI View Article : Google Scholar
|
|
126
|
Qiu J, Kitamura Y, Miyata Y, Tamaru S,
Tanaka K, Tanaka T and Matsui T: Transepithelial transport of
theasinensins through Caco-2 cell monolayers and their absorption
in Sprague-Dawley rats after oral administration. J Agric Food
Chem. 60:8036–8043. 2012.PubMed/NCBI View Article : Google Scholar
|
|
127
|
Lagha AB and Grenier D: Tea polyphenols
protect gingival keratinocytes against TNF-α-induced tight junction
barrier dysfunction and attenuate the inflammatory response of
monocytes/macrophages. Cytokine. 115:64–75. 2019.PubMed/NCBI View Article : Google Scholar
|
|
128
|
Li J, Ye L, Wang X, Liu J, Wang Y, Zhou Y
and Ho W: (-)-Epigallocatechin gallate inhibits endotoxin-induced
expression of inflammatory cytokines in human cerebral
microvascular endothelial cells. J Neuroinflammation.
9(161)2012.PubMed/NCBI View Article : Google Scholar
|
|
129
|
Lagha AB, Groeger S, Meyle J and Grenier
D: Green tea polyphenols enhance gingival keratinocyte integrity
and protect against invasion by Porphyromonas gingivalis. Pathog
Dis. 76:2018.PubMed/NCBI View Article : Google Scholar
|
|
130
|
Watson JL, Ansari S, Cameron H, Wang A,
Akhtar M and McKay DM: Green tea polyphenol (-)-epigallocatechin
gallate blocks epithelial barrier dysfunction provoked by IFN-gamma
but not by IL-4. Am J Physiol Gastrointest Liver Physiol.
287:G954–961. 2004.PubMed/NCBI View Article : Google Scholar
|
|
131
|
Suzuki T and Hara H: Role of flavonoids in
intestinal tight junction regulation. J Nutr Biochem. 22:401–408.
2011.PubMed/NCBI View Article : Google Scholar
|
|
132
|
Amerongen AV, Bolscher JG and Veerman EC:
Salivary mucins: Protective functions in relation to their
diversity. Glycobiology. 5:733–740. 1995.PubMed/NCBI View Article : Google Scholar
|
|
133
|
Alliende C, Kwon YJ, Brito M, Molina C,
Aguilera S, Pérez P, Leyton L, Quest AF, Mandel U, Veerman E, et
al: Reduced sulfation of muc5b is linked to xerostomia in patients
with Sjögren syndrome. Ann Rheum Dis. 67:1480–1487. 2008.PubMed/NCBI View Article : Google Scholar
|
|
134
|
Xu H, Shan XF, Cong X, Yang NY, Wu LL, Yu
GY, Zhang Y and Cai ZG: Pre- and post-synaptic effects of botulinum
toxin A on submandibular glands. J Dent Res. 94:1454–1462.
2015.PubMed/NCBI View Article : Google Scholar
|
|
135
|
Besserer A, Burnotte E, Bienert GP,
Chevalier AS, Errachid A, Grefen C, Blatt MR and Chaumont F:
Selective regulation of maize plasma membrane aquaporin trafficking
and activity by the SNARE SYP121. Plant Cell. 24:3463–3481.
2012.PubMed/NCBI View Article : Google Scholar
|
|
136
|
Noda Y, Horikawa S, Kanda E, Yamashita M,
Meng H, Eto K, Li Y, Kuwahara M, Hirai K, Pack C, et al: Reciprocal
interaction with G-actin and tropomyosin is essential for
aquaporin-2 trafficking. J Cell Biol. 182:587–601. 2008.PubMed/NCBI View Article : Google Scholar
|
|
137
|
Beroukas D, Hiscock J, Jonsson R, Waterman
SA and Gordon TP: Subcellular distribution of aquaporin 5 in
salivary glands in primary Sjögren's syndrome. Lancet.
358:1875–1876. 2001.PubMed/NCBI View Article : Google Scholar
|
|
138
|
Nashida T, Yoshie S, Haga-Tsujimura M,
Imai A and Shimomura H: Atrophy of myoepithelial cells in parotid
glands of diabetic mice; detection using skeletal muscle actin, a
novel marker. FEBS Open Bio. 3:130–134. 2013.PubMed/NCBI View Article : Google Scholar
|
|
139
|
Mei M, Xiang RL, Cong X, Zhang Y, Li J, Yi
X, Park K, Han JY, Wu LL and Yu GY: Claudin-3 is required for
modulation of paracellular permeability by TNF-α through
ERK1/2/slug signaling axis in submandibular gland. Cell Signal.
27:1915–1927. 2015.PubMed/NCBI View Article : Google Scholar
|
|
140
|
Cong X, Zhang XM, Zhang Y, Wei T, He QH,
Zhang LW, Hua H, Lee SW, Park K, Yu GY and Wu LL: Disruption of
endothelial barrier function is linked with hyposecretion and
lymphocytic infiltration in salivary glands of Sjögren's syndrome.
Biochim Biophys Acta Mol Basis Dis. 1864:3154–3163. 2018.PubMed/NCBI View Article : Google Scholar
|