Introduction
Ischemic hepatitis, also known as hypoxic hepatitis
or shock liver, is a clinical syndrome frequently encountered in
critically ill patients, and represents a complication of
underlying cardiac, circulatory or respiratory failure without any
other known cause of acute hepatitis (1,2). The
most important cause of ischemic hepatitis is hypotension, such as
cardiogenic shock. However, a documented hypotensive event is seen
in only 50% of ischemic hepatitis cases (3). These cases of ischemic hepatitis
without documented hypotensive events are assumed to include both
cases in which transient subclinical hypotension was overlooked and
cases with hepatic tissue injury from hepatic hypoperfusion without
systemic hypotension (1). The
present report describes a rare case of ischemic hepatitis caused
by hepatic artery occlusion during the treatment of infectious
endocarditis. A case of acute hepatic injury during the follow-up
of infectious endocarditis was observed. The acute hepatic injury
occurred without any abdominal symptoms and showed severe hepatic
failure with <40% of prothrombin time (PT), and the hepatic
injury recovered spontaneously. Viral hepatitis or autoimmune
hepatic diseases were not detected, and the hepatic injury was
diagnosed as ischemic hepatitis that had derived from hepatic
hypoperfusion via hepatic artery occlusion with infectious
endocarditis. The spontaneous restoration of hepatic blood flow was
presumed to be supplied from the extrahepatic blood flow, not
recanalization of the hepatic artery. After the ischemic hepatitis
was cured, the patient did not exhibit any signs of hepatic
injuries.
Case report
A 58-year-old woman was referred to Suzuka General
Hospital with a primary complaint of fever. She had been treated
for systemic lupus erythematosus (SLE) with tacrolimus 2 mg/day per
os (PO) and prednisolone 5 mg/day PO. She had no rash or arthritis,
and her laboratory data on admission showed no SLE activity
(Table I). She had right-sided
hemiparesis caused by a cerebrovascular event 30 years earlier. On
admission, her temperature was >39˚C, and her laboratory data
showed a white blood cell count (WBC) of 15,000x104/µl,
C-reactive protein (CRP) levels of 10.55 mg/dl and procalcitonin
levels of 60.43 ng/ml (Table I).
Physical examination did not show any progression of paralysis. Her
systolic blood pressure was maintained at >100 mmHg, and she did
not have chest symptoms, tachypnea or mental status changes. Her
pulse rate was 80 beats per minute and regular. Her chest X-ray did
not show any abnormal findings. Her electrocardiogram did not show
atrial fibrillation, ST elevation and/or ST depression. Although
she underwent chest, abdominal, and pelvic computed tomography for
identification of the focus of the infection, it could not be
found. However, she was found to have a 12-mm-diameter vegetation
on the anterior mitral valve leaflet on echocardiography, and thus
was diagnosed with infectious endocarditis due to isolation of
Pseudomonas guariconensis from the blood culture on day 1.
Antibiotic therapy for her infectious endocarditis was started
(Fig. 1); ceftriaxone sodium
hydrate 2 g/day and daptomycin 175 mg/day were started empirically.
On day 2, the antibiotics were changed to meropenem hydrate 1 g/day
because of the detection of Gram-negative rods on Gram staining of
the patient's blood.
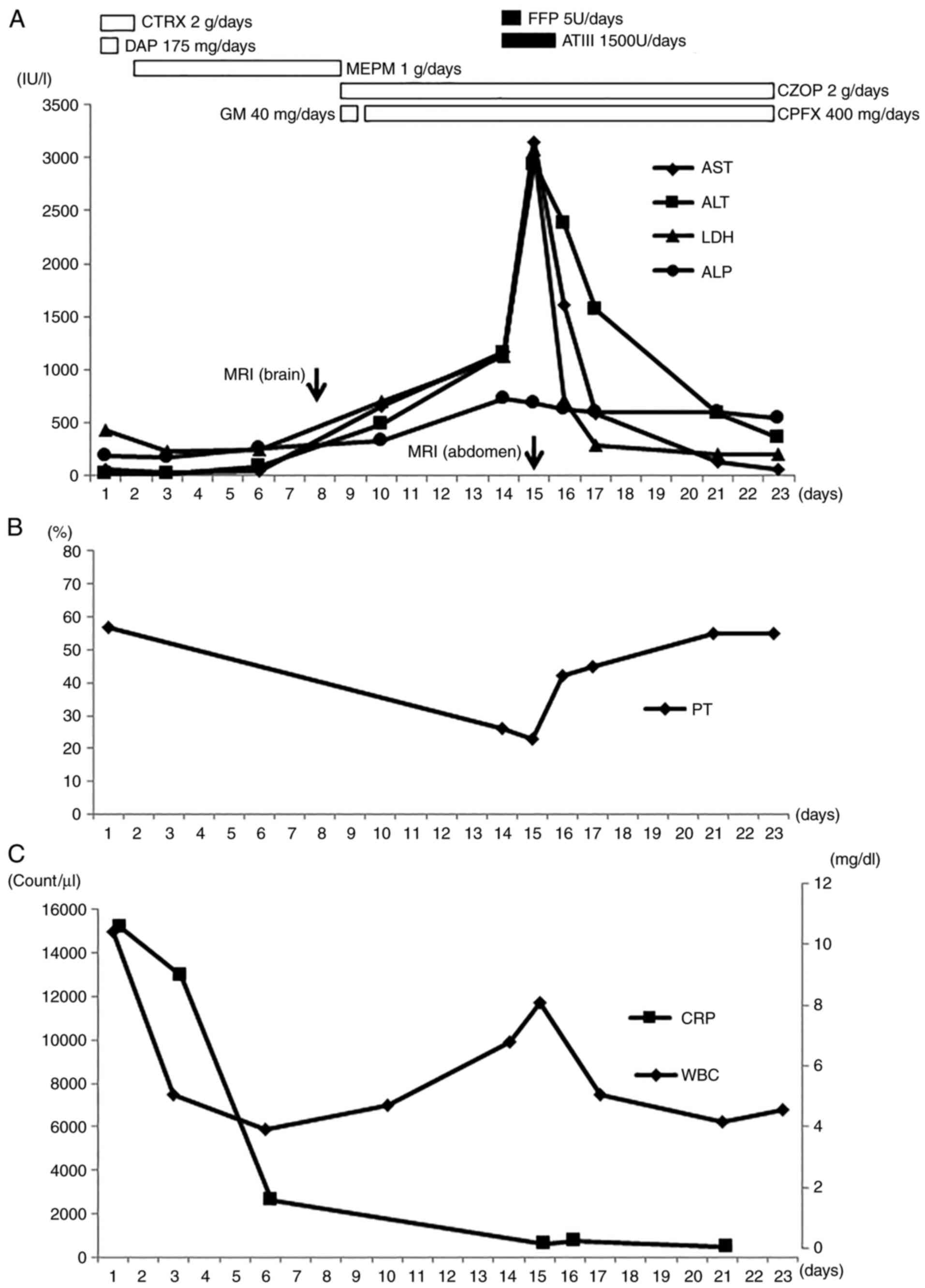 | Figure 1Clinical course of the patient. (A)
AST, ALT, LDH and ALP, (B) PT, (C) WBC and CRP levels over the
course of observation. AST, aspartate aminotransferase; ALT,
alanine aminotransferase; LDH, lactate dehydrogenase; ALP, alkaline
phosphatase; PT, prothrombin time; WBC, white blood cell count;
CRP, C-reactive protein; CTRX, ceftriaxone sodium hydrate; DAP,
daptomycin; MEPM, meropenem hydrate; GM, gentamicin sulfate; CZOP,
cefozopran hydrochloride; CPFX, ciprofloxacin; FFP, fresh frozen
plasma; ATIII, antithrombin III; MRI, magnetic resonance
imaging. |
 | Table ILaboratory data on admission. |
Table I
Laboratory data on admission.
| Factor | Value |
|---|
| Complete blood
count | Value |
|
White blood
cell count | 15,000/µl |
|
Segmented
cell | 90% |
|
Red blood
cell count |
305x106/µl |
|
Hemoglobin | 9.8 g/dl |
|
Hematocrit | 27.9% |
|
Platelet |
17.2x104/µl |
| Coagulation | |
|
Prothrombin
time | 57% |
|
Prothrombin
time-international | 1.38 |
|
normalized
ratio | |
|
Fibrin/fibrinogen
degradation products | 27.4 mg/ml |
|
D-dimer | 11.4 mg/ml |
|
Fibrinogen | 468 mg/dl |
| Immunochemistry | |
|
Anti nuclear
antibody | 40 |
|
Anti-DNA
Ab | <2.0 IU/ml |
|
Lupus
anti-coagulant | <0.7 |
|
Anti-cardiolipin
β2-glycoprotein 1 | 4.3 U/ml |
|
Anti-cardiolipin
Ab | 8.4 U/ml |
| Chemistry | |
|
Total
protein | 5.4 g/dl |
|
Albumin | 2.8 g/dl |
|
Aspartate
aminotransferase | 69 IU/l |
|
Alanine
aminotransferase | 26 IU/l |
|
Lactate
dehydrogenase | 434 IU/l |
|
Alkaline
phosphatase | 189 IU/l |
|
γ-glutamyltransferase | 93 IU/l |
|
Creatine
kinase | 1,271 IU/l |
|
Total
bilirubin | 0.8 mg/dl |
|
Direct
bilirubin | 0.1 mg/dl |
|
Cholinesterase | 199 IU/l |
|
Blood urea
nitrogen | 45.2 mg/dl |
|
Creatinine | 2.43 mg/dl |
|
Na+ | 130 mEq/l |
|
K+ | 5.0 mEq/l |
|
Cl- | 98 mEq/l |
|
Blood
sugar | 80 mg/dl |
|
C-reactive
protein | 10.55 mg/dl |
|
Procalcitonin | 60.43 ng/ml |
| Serology | |
|
IgG | 982 mg/dl |
|
IgA | 168 mg/dl |
|
IgM | 9 mg/dl |
After starting treatment with antibiotics, the blood
cultures on days 9 and 16 were negative. Her high fever (>38˚C)
continued for 9 days after starting antibiotics, with a peak
temperature of 39.6˚C; however, her temperature decreased to
<38˚C after day 10.
On day 1, the laboratory data reflecting liver
function showed aspartate aminotransferase (AST) levels of 69 IU/l,
alanine aminotransferase (ALT) levels of 26 IU/l, lactate
dehydrogenase (LDH) levels of 434 IU/l and alkaline phosphatase
(ALP) levels of 189 IU/l (Table I),
and these values remained stable until day 6 (Fig. 1). However, on day 10, the hepatic
enzyme values of AST, ALT and LDH increased to 663, 492 and 708
IU/l, respectively. Hypotension had not been noted until these
hepatic enzyme elevations were seen. Anti-hepatitis A virus IgM
antibody, hepatitis B virus surface antigen, anti-hepatitis B virus
core IgM antibody, HCV RNA, hepatitis E virus IgA antibody,
anti-cytomegalovirus IgM antibody and anti-Epstein-Barr Virus IgM
antibody were negative.
Initially, it was suggested that the elevation of
these hepatic enzyme values was due to drug-induced liver injury,
and antibiotic therapy was thus changed to cefozopran hydrochloride
2 g/day and gentamicin sulfate (GM) 40 mg/day on day 10 (Fig. 1). On day 11, GM was changed to
ciprofloxacin 400 mg/day due to concerns regarding renal side
effects. However, the levels of hepatic enzymes, including AST,
ALT, LDH and ALP, continued to increase to 1,176, 1,150, 1,132 and
727 IU/l on day 14, respectively. On the other hand, she had no
abdominal symptoms and no mental status changes. On day 15, these
values increased to 3,157 IU/l for AST, 2,945 IU/l for ALT and
3,093 IU/l for LDH. With respect to fibrin/fibrinogen degradation
products (FDP) and D-dimer, these values decreased (FDP, 12 mg/ml
on day 16; D-dimer, 8.2 µg/ml on day 16).
At the time, it was hypothesized that the hepatic
injury was caused by hepatic artery occlusion, as multiple emboli
to her brain had been seen on the diffusion-weighted imaging of
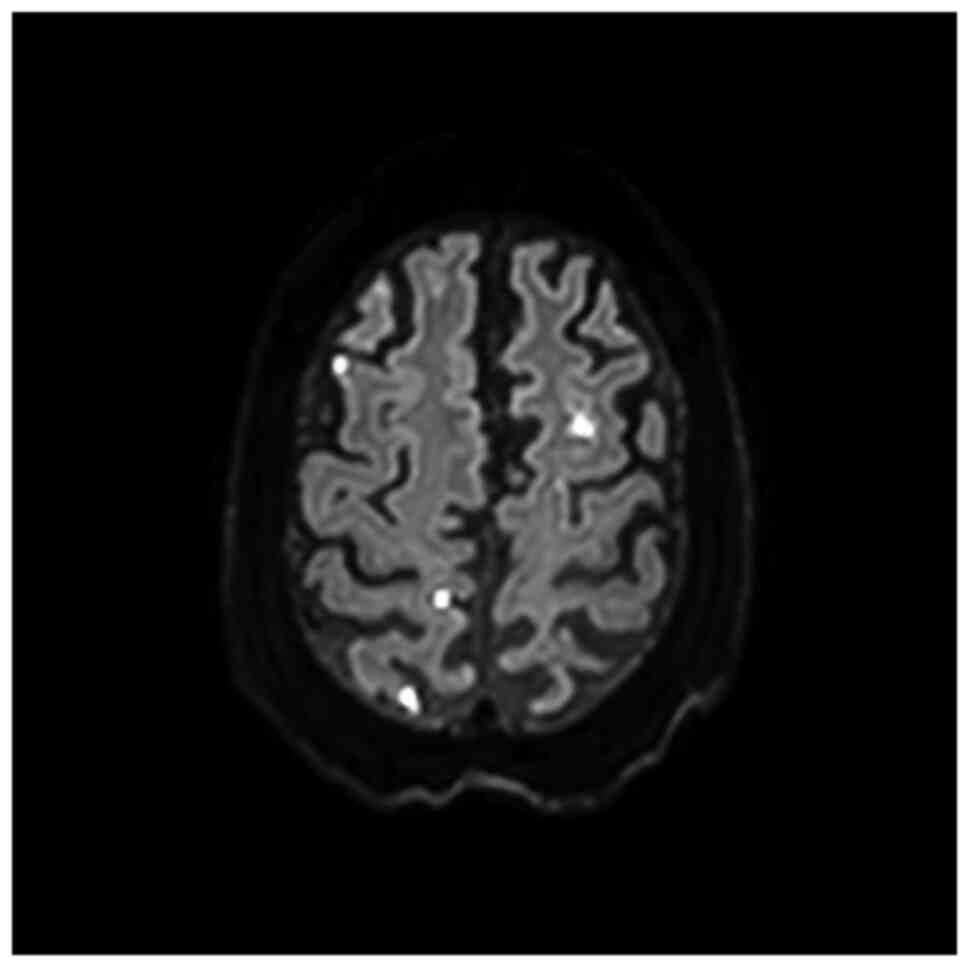
magnetic resonance imaging (MRI) on day 8 as multiple
high-intensity spots (Fig. 2).
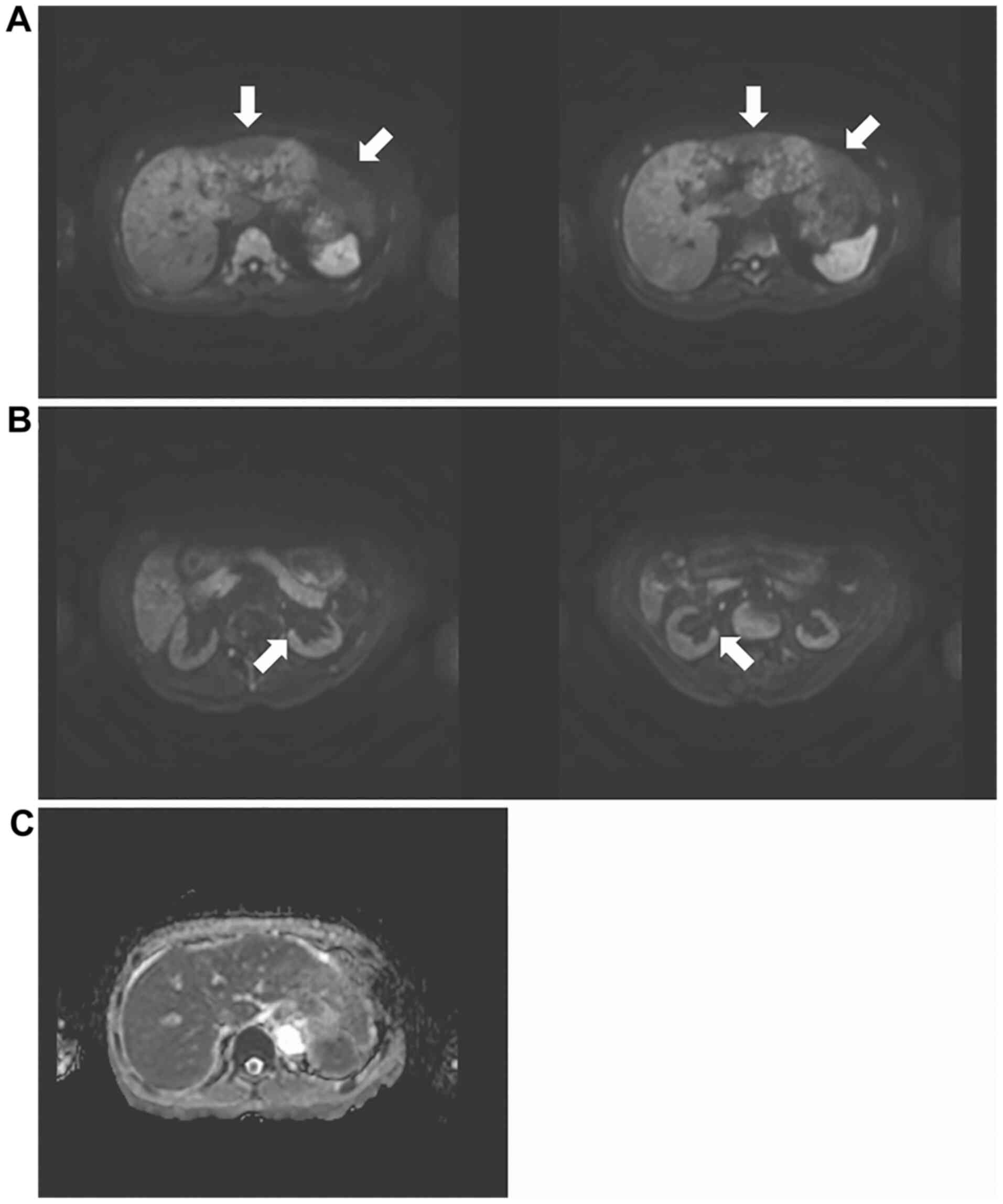
Abdominal MRI showed a diffuse high-intensity signal of the whole
liver, except for the left hepatic lobe on day 15 (Fig. 3A). Simultaneously high-intensity
spots were seen in bilateral kidneys (Fig. 3B). The apparent diffusion
coefficient (ADC) map showed low signal intensity of the right
hepatic lobe (Fig. 3C). Unlike the
MRI of the brain and kidney, the hepatic MRI showed no
high-intensity spots. Because the bacteremia improved with
antibiotic treatment, and WBC and CRP levels decreased at the time
of hepatitis onset, hepatic artery embolization caused by
destruction of the vegetation on the mitral valve was suspected.
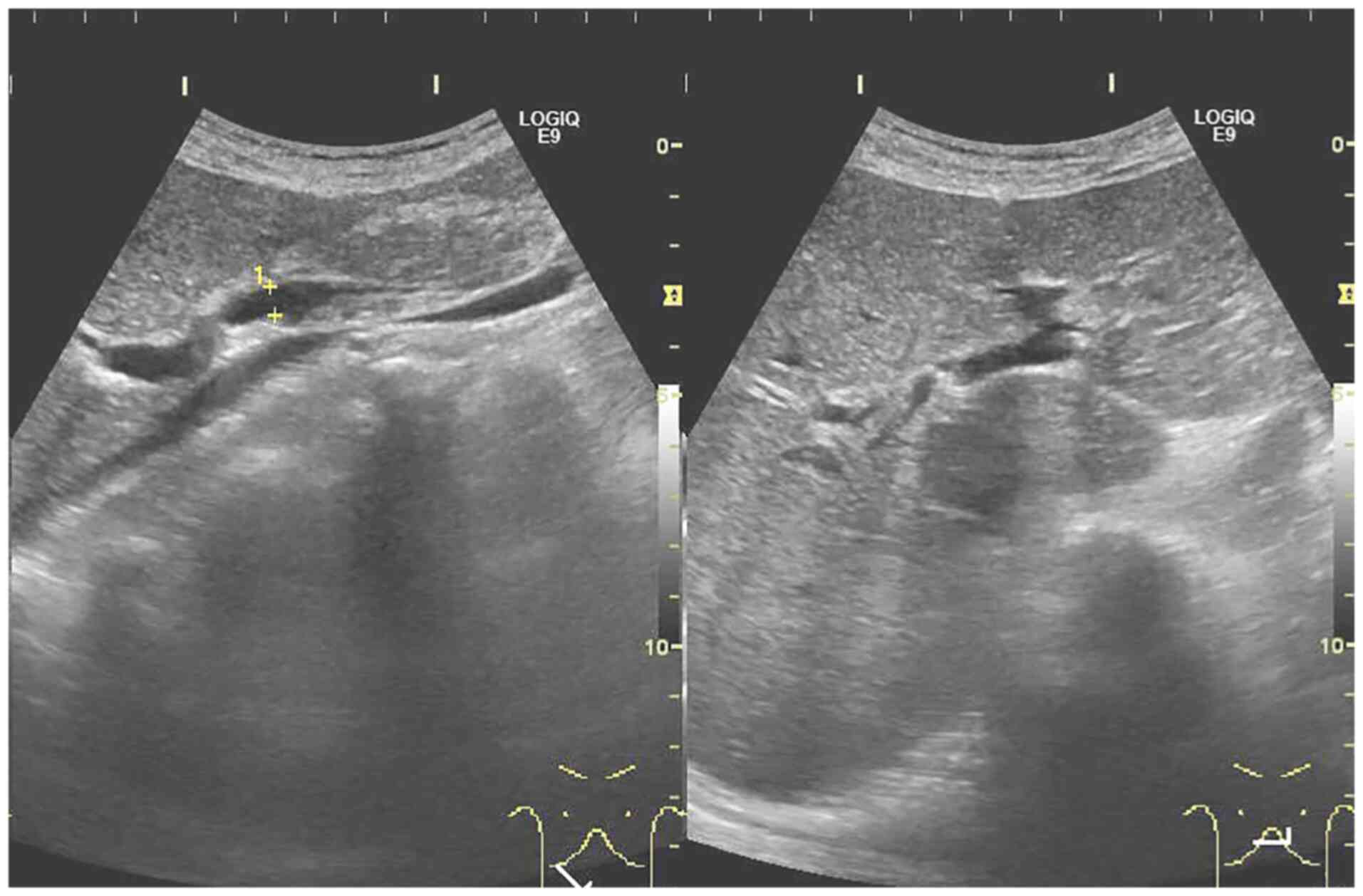
Abdominal ultrasonography showed no stenosis, mass, obstruction
and/or collapse of the hepatic portal vein (Fig. 4).
In addition, prolonged PT and PT-international
normalized ratio were observed (Fig.
1 and Table II). Although the
WBC was >10,000x104/µl, CRP was within the normal
range. The disseminated intravascular coagulation (DIC) scoring
showed that the total score was 1, and DIC was not diagnosed based
on JSTH's provisional draft DIC diagnostic criteria (4).
 | Table IIThe laboratory data associated with
DIC as well as DIC scoring on day 14. |
Table II
The laboratory data associated with
DIC as well as DIC scoring on day 14.
| Measurement | Value | DIC
scorea |
|---|
| Platelet |
52.2x104/µl | 0 |
| Fibrin/fibrinogen
degradation products | 15.3 mg/ml | 1 |
| Prothrombin time
international normalized ratio | 2.29 | 2 |
| Antithrombin
III | 61% | 1 |
|
Thrombin-antithrombin Ⅲ complex | 2.6 ng/ml | 0 |
| Liver failure | (+) | -3 |
| Total DIC
score | | 1 |
Ischemic hepatitis due to hepatic artery
embolization and acute liver failure were diagnosed. Fulminant
hepatitis was not diagnosed due to the lack of hepatic
encephalopathy. She was treated with fresh frozen plasma and
antithrombin III for the acute liver failure. Steroid pulse
treatment was not administered given the infectious disease. On day
16, the transaminase values decreased to 1,615 IU/l for AST, 2,374
IU/l for ALT, 711 IU/l for LDH and 626 IU/l for ALP, and PT was
restored. These transaminase values decreased further to 59 IU/l
for AST, 355 IU/l for ALT, 206 IU/l for LDH and 550 IU/l for ALP by
day 23.
On day 23, the patient was transferred to another
advanced treatment hospital and underwent resection of the
vegetation on the anterior leaflet of the mitral valve. The
resected vegetation was the size of a rice grain. The pathological
analysis was not performed because the specimen of vegetation was
inadequate for pathological analysis due to the small size. On day
245, she was stable with no fever, no symptoms and no hepatic
enzyme abnormalities (AST, 18 IU/l; ALT, 12 IU/l; LDH, 233 IU/l;
and ALP, 271 IU/l) after treatment.
Oral informed consent, including a statement of
agreement to the use of the samples in scientific research, was
obtained from the patient in the Outpatient Department of Suzuka
General Hospital. She did not consent to saving of this consent as
a document, which was respected and Suzuka General Hospital Ethics
Committee agreed to having consent being obtained orally in this
case.
Discussion
Ischemic hepatitis is an hepatic tissue injury that
manifests as a result of hypoxia caused by hepatic hypoperfusion.
Hepatic hypoperfusion is most commonly caused by acute cardiac
failure, toxic shock states and respiratory failure (5), but hepatic artery occlusion is a rare
cause of significant hepatic ischemia (6). Hepatic artery thrombosis, which can
induce hepatic artery occlusion and/or a hepatic artery blood
inflow disturbance, is the most common complication following liver
transplantation or other surgical procedures (7). However, the current patient had not
undergone these operations before onset of the present event.
SLE causes chronic inflammation in multiple organs
(8). As SLE is associated with an
accelerated atherosclerotic process (8), and patients with SLE show inflammatory
involvement of vessels of all sizes (9), SLE may affect hepatic perfusion and
induce liver injury due to vasculopathy of hepatic arteries.
However, the patient had continued treatment for SLE with
prednisolone and tacrolimus during the treatment for the infectious
endocarditis, and the elevated hepatic enzyme levels were resolved
without increasing the dosage or changing the medication for SLE.
Although she had a cerebrovascular event ~30 years earlier, she did
not have any atherosclerotic-associated disease events since then.
Thus, SLE may not have been associated with the hepatic injury. Lu
et al (10) reported that
hepatic arterial aneurysms are associated with SLE; however, the
patient was not found to possess any arterial aneurysms involving
the hepatic artery.
Although sepsis accounts for 23.4% of cases of
ischemic hepatitis (3), the patient
did not develop septic shock during the clinical course, and the
infectious endocarditis had been controlled with antibiotic
treatment, as shown by the decreasing CRP values and fever. The WBC
also decreased until the onset of hepatic injury (Fig. 1). Given the above clinical course,
the rebound increase of WBC was assumed to be due to ischemic
hepatic tissue injury rather than aggravation of the infectious
endocarditis. In this case, multiple cerebral and kidney
infarctions developed due to the emboli, which were seen as
multiple high-intensity spots on diffusion-weighted imaging of MRI.
However, the diffusion-weighted imaging of hepatic MRI did not show
multiple high-intensity spots, but a diffuse high intensity for the
whole liver except for the left hepatic lobe. Angiography of the
hepatic artery could not be performed at the time of hepatic injury
because of renal dysfunction. As DWI is a widely accepted technique
for detecting early ischemic change, especially in neuroradiology
(11), it can be assumed that
hepatic artery occlusion, probably involving the common hepatic
artery, occurred due to embolization, and ischemic hepatitis
developed as a result. Based on the theory that restricted or
impeded water diffusion is seen in tissues with high cellularity,
such as in cytotoxic edema (11),
the diffuse high intensity of the whole liver was presumed to
reflect the swelling of the hepatic cells by ischemia. An isolated
hepatic artery occlusion is usually considered an unlikely cause of
significant hepatic ischemia in non-transplant patients and is
considered an unlikely cause of significant hepatic ischemia, as
there is a dual blood supply to the liver that comes from the
portal vein and hepatic artery (7).
However, Béland et al (6)
reported a case of fulminant hepatitis caused by common hepatic
artery occlusion in a patient with a history of atrial fibrillation
(6). As the values of FDP and
D-dimer decreased after starting treatment, thrombosis was unlikely
to have caused ischemic hepatitis in the present case. Von Glinski
et al (12) reported a case
of ischemic hepatitis that developed secondary to celiac trunk
stenosis as a result of cephalad displacement of the celiac trunk
and compression of the artery by the diaphragmatic ligament. Zhang
et al (13) reported 19
cases of transient liver enzyme elevation alone after complete
occlusion of hepatic arterial flow. Thus, the present case was
likely ischemic hepatitis caused by hepatic artery occlusion
despite a non-portal venous blood flow disturbance. In addition,
thrombosis was unlikely to have caused ischemic hepatitis in the
present case as the values of FDP and D-dimer decreased after
starting treatment.
The incidence of embolic events is high in patients
with infective endocarditis (14).
Embolic events occur after a median time of 7 days following the
start of adequate antibiotic therapy (15), and 65-71.4% of cases, occur within
~2 weeks (15,16). Certain organ arterial emboli in
infective endocarditis can cause lethal and/or severe outcomes
(17-19).
In the present case, the hepatic artery embolic event occurred in
the second week after starting antibiotic treatment. The antibiotic
treatment was effective at the time of hepatitis onset, and thus it
can be assumed that the source of embolization was not so much
septic emboli, but the destruction of the mitral valve vegetation,
as septic emboli were unlikely to occur unless bacteremia
persisted. The emboli of the brain and kidney may have been caused
by septic emboli that occurred before antibiotic treatment, and
thus the MRI of the liver may have been different from that of the
brain and kidneys.
In the present case, the patient also had severe
hepatic dysfunction with PT <40%, but fulminant hepatitis did
not develop. The case of Béland et al (6) showed severe hepatic enzyme elevations
due to hepatic artery occlusion, with AST >15,000 IU/l and ALT
>6,000 IU/l. On the other hand, the peak AST and ALT values were
only 3,157 and 2,945 IU/l in the present case, respectively. The
difference in hepatitis severity between the present case and that
of Béland et al (6) may be
due to the difference in the cause of arterial occlusion
(embolization derived from destruction of vegetation and
thrombosis). Furthermore, the diffusion-weighted imaging of hepatic
MRI showed a low-intensity area in part of the liver in the present
case. These low-intensity areas were assumed to reflect the area
that had not been affected by hepatic hypoperfusion. These areas
were in the left lobe of the liver, and blood perfusion via
extrahepatic collateral arteries including the left inferior
phrenic artery or the left gastric artery (20,21)
might have resulted in rescue of hepatic tissue from the
hypoperfusion damage caused by the hepatic artery occlusion. Cho
et al (22) reported that no
ischemic liver injuries developed after hepatic artery embolization
with no portal vein stenosis and bilobar hepatic arterial flow via
the left hepatic artery aberrantly arising from the left gastric
artery or from the common hepatic artery. In addition, Sato et
al (23) suggested an
association with hepatic failure related to hepatic arterial
embolization for hemostasis and the absence of hepatic collaterals.
The ischemic hepatitis resolved without any treatment for hepatic
occlusion, and the reason for the spontaneous resolution of the
hepatic hypoperfusion is unknown; however, the spontaneous recovery
of hepatitis in the present case may have reflected hepatic
perfusion recovery via extrahepatic collateral blood inflow rather
than recanalization of the hepatic artery. No hepatic injury may
occur under intact portal venous blood flow and efficient
collateral extrahepatic arterial blood flow at the time of hepatic
arterial occlusion. Although an attempt was made to visualize the
blood supply from extrahepatic collaterals after recovery, it was
impossible to detect the collateral vessels by magnetic resonance
angiography due to limitations of its resolving power.
In conclusion, a case of ischemic hepatitis that
developed as a complication of infectious endocarditis is reported
on. Although the patient developed acute liver failure, the
hepatitis did not progress to fulminant hepatitis, and instead
resolved spontaneously. The possibility of ischemic hepatitis due
to hepatic artery hypoperfusion caused by embolization when
hepatitis occurs during the follow-up of patients with infectious
endocarditis should thus be considered. Additionally, the potential
for whole-organ ischemic damage caused by vessel occlusion when
treating a case of infectious endocarditis should also be taken
into consideration.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HO was involved in the conception and design of the
study, writing the manuscript and preparing the tables. RO, HA, KN,
ST, TT, HK, TSakuno, YI, HT, SM, TSase, TSaito, KM and AN collected
the data. HI operated on the patient and collected the data. All
authors revised the manuscript. All authors have read and approved
the final manuscript. HO and AN confirm the authenticity of all of
the raw data.
Ethics approval and consent to
participate
Oral informed consent, including a statement of
agreement to the use of the samples in scientific research, was
obtained from the patient in the Outpatient Department of Suzuka
General Hospital. She did not consent to saving of this consent as
a document, which was respected and Suzuka General Hospital Ethics
Committee agreed to having consent being obtained orally in this
case.
Patient consent for publication
The patient gave consent for the publication of this
study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lightsey JM and Rockey DC: Current
concepts in ischemic hepatitis. Curr Opin Gastroenterol.
33:158–163. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Shen BQ, Dong LQ and Ma Y: Research
progress of ischemic hepatitis. Zhonghua Gan Zang Bing Za Zhi.
26:707–709. 2018.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
3
|
Tapper EB, Sengupta N and Bonder A: The
incidence and outcomes of ischemic hepatitis: A systematic review
with meta-analysis. Am J Med. 128:1314–1321. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Asakura H, Takahashi H, Uchiyama T, Eguchi
Y, Okamoto K, Kawasugi K, Madoiwa S and Wada H: DIC subcommittee of
the Japanese society on thrombosis and hemostasis. Proposal for new
diagnostic criteria for DIC from the Japanese Society on thrombosis
and hemostasis. Thromb J. 14(42)2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Waseem N and Chen PH: Hypoxic hepatitis: A
review and clinical update. J Clin Transl Hepatol. 4:263–268.
2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Béland M, Despatis MA and Gahide G:
Hepatic artery emboli causing fulminant hepatitis. J Vasc Interv
Radiol. 30(1613)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Elsayes KM, Shaaban AM, Rothan SM, Javadi
S, Madrazo BL, Castillo RP, Casillas VJ and Menias CO: A
comprehensive approach to hepatic vascular disease. Radiographics.
37:813–836. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zanatta E, Colombo C, D'Amico G,
d'Humières T, Dal Lin C and Tona F: Inflammation and coronary
microvascular dysfunction in autoimmune rheumatic diseases. Int J
Mol Sci. 20(E5563)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Barile-Fabris L, Hernández-Cabrera MF and
Barragan-Garfias JA: Vasculitis in systemic lupus erythematosus.
Curr Rheumatol Rep. 16(440)2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lu M, Weiss C, Fishman EK, Johnson PT and
Verde F: Review of visceral aneurysms and pseudoaneurysms. J Comput
Assist Tomogr. 39:1–6. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kele PG and van der Jagt EJ: Diffusion
weighted imaging in the liver. World J Gastroenterol. 16:1567–1576.
2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Von Glinski KS, Krettek C, Blauth M and
Oldhafer KJ: Hepatic ischemia as a complication after correction of
post-traumatic gibbus at the thoracolumbar junction. Spine (Phila
Pa 1976). 25:1040–1044. 2000.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhang J, Qian HG, Leng JH, Qiu H, Wu JH,
Liu BN, Li CP, Wei M, Liu Q, Lv A and Hao CY: Ischemic liver injury
after complete occlusion of hepatic artery in the treatment of
delayed postoperative arterial bleeding. J Gastrointest Surg.
19:2235–2242. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Fabri J Jr, Issa VS, Pomerantzeff PM,
Grinberg M, Barretto AC and Mansur AJ: Time-related distribution,
risk factors and prognostic influence of embolism in patients with
left-sided infective endocarditis. Int J Cardiol. 110:334–339.
2006.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Thuny F, Di Salvo G, Belliard O, Avierinos
JF, Pergola V, Rosenberg V, Casalta JP, Gouvernet J, Derumeaux G,
Iarussi D, et al: Risk of embolism and death in infective
endocarditis: Prognostic value of echocardiography: A prospective
multicenter study. Circulation. 112:69–75. 2005.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Vilacosta I, Graupner C, San Román JA,
Sarriá C, Ronderos R, Fernández C, Mancini L, Sanz O, Sanmartín JV
and Stoermann W: Risk of embolization after institution of
antibiotic therapy for infective endocarditis. J Am Coll Cardiol.
39:1489–1495. 2002.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Castelli JB, Almeida G and Siciliano RF:
Sudden death in infective endocarditis. Autops Case Rep. 6:17–22.
2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Oestreich BA, Sommer P and Armstrong EJ:
Coronary artery embolism from infectious endocarditis treated with
catheter thrombectomy using a GuideLiner catheter. Catheter
Cardiovasc Interv. 87:E197–E201. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Schmidt D and Zehender M: Arterial
occlusion of the eye in infectious endocarditis. Ophthalmologe.
96:264–266. 1999.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
20
|
Gwon DI, Ko GY, Yoon HK, Sung KB, Lee JM,
Ryu SJ, Seo MH, Shim JC, Lee GJ and Kim HK: Inferior phrenic
artery: Anatomy, variations, pathologic conditions, and
interventional management. Radiographics. 27:687–705.
2007.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Miyayama S, Yamashiro M, Okuda M, Aburano
H, Shigenari N, Morinaga K and Matsui O: Anastomosis between the
hepatic artery and the extrahepatic collateral or between
extrahepatic collaterals: Observation on angiography. J Med Imaging
Radiat Oncol. 53:271–282. 2009.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Cho SK, Kim SS, Do YS, Park KB, Shin SW,
Park HS, Choo SW and Choo IW: Ischemic liver injuries after hepatic
artery embolization in patients with delayed postoperative
hemorrhage following hepatobiliary pancreatic surgery. Acta Radiol.
52:393–400. 2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Sato A, Yamada T, Takase K, Matsuhashi T,
Higano S, Kaneda T, Egawa S, Takeda K, Ishibashi T and Takahashi S:
The fatal risk in hepatic artery embolization for hemostasis after
pancreatic and hepatic surgery: Importance of collateral arterial
pathways. J Vasc Interv Radiol. 22:287–293. 2011.PubMed/NCBI View Article : Google Scholar
|