Introduction
Allergic dermatitis caused by skin sensitizers is
one of the most important factors that should be taken into
consideration when evaluating the safety of chemical substances.
Skin sensitization is classified as type IV hypersensitivity
(delayed reaction), involving immune cells, such as T-cells
(1). In allergic dermatitis caused
by skin sensitization, inflammatory reactions peak 24 to 48 h after
contact with the causative agent. Allergic contact dermatitis
accounts for ~60% of all occupational skin diseases. Although skin
sensitization is not a life-threatening disease, it greatly affects
social life and can result in issues with regard to careers,
particularly in patients dealing with consumer products, reviewed
in (2).
An allergy consists of two aspects: The
sensitization stage, in which a person acquires reactivity to a
substance, and the elicitation stage, in which allergic symptoms
develop. In skin sensitization, when a chemical substance is
repeatedly absorbed transdermally, T-cells that specifically react
to a chemical substance proliferate in the body, and sensitization
is established. When the same chemical is transdermally absorbed
again, the reacting T-cells release cytokines and induce
inflammation at the contact site (triggering). Knowledge of the
chemical and biological mechanisms involved in skin sensitization
reactions is summarized in the adverse outcome pathway (AOP) that
includes initial events at the molecular level through intermediate
events to adverse effects (3). The
initial event at the molecular level, or the first key event (KE1),
is the covalent bonding of the nucleophilic center of a protein
with an electrophilic substance present in the skin. KE2 is the
covalent bonding of the electrophilic substance with a specific
cell signaling pathway, such as the inflammatory response in
keratinocytes or the antioxidant/electrophile response
sequence-dependent pathway. KE3 is the activation of dendritic
cells (DCs), and KE4 is the activation of T-cells. The skin
sensitization assay is an in vivo or in vitro
replication of all or part of the AOP. Animal experiments, such as
the guinea pig maximization test (4) and mouse local lymph node assay
(5), have been used to evaluate
skin sensitization.
Recent stricter regulations on animal experiments
aim to ensure animal welfare by following the basic principle of
animal testing defined by the 3Rs: Reduction, refinement and
replacement; replacement that signifies the complete elimination of
animal testing is the most important issue, reviewed in (6). In the EU, the leader in regulating
animal testing in the cosmetics industry, animal testing for
cosmetics started to be gradually banned in 2004. Since March 2013,
there has been a complete ban on the sale and import of all raw
materials, processed products containing raw materials, and
finished products tested on animals (7), and manufacturers exporting these raw
materials to the EU have been forced to respond. Not only for the
cosmetics industry, but also for other industries, including
pharmaceutical tests that likely accounts for the majority of
animal testing, reducing animal experiments would be preferred in
terms of animal welfare. Therefore, the development and use of
alternative methods for animal testing, such as in vitro and
in chemico testing are gaining importance. Currently, the
OECD Test Guidelines list the following alternative methods for
skin sensitization tests: The direct peptide reactivity assay
(DPRA), amino acid derivative reactivity assay (ADRA) for
KE1(8), KeratinoSens™ for
KE2(9), the human cell line
activation test (h-CLAT), U-SENS™ and IL-8 Luc assay for
KE3(10). However, an effective
alternative method for KE4 ‘T-cell activation’ has yet to be fully
developed, representing a challenge in this field.
KE4 in skin sensitization AOP involves activation of
naïve T-cells, recognition of antigen presentation by DCs, and
proliferation of antigen-specific effector T-cells and memory
T-cells; it plays an important role in the overall AOP (11). These responses occur through complex
biological processes. The activation of naïve T-cells requires two
signals that act synergistically. The first major signal results
from the binding of major histocompatibility complex (MHC)-antigen
peptide complexes presented by antigen-presenting cells (APCs),
such as DCs, to T-cell receptors (TCRs) on T-cells. The second
major signal is derived from the interaction of costimulatory
receptors on T-cells with ligands on APCs. A typical costimulatory
receptor is CD28 that interacts with the corresponding ligands on
APCs, CD80 and CD86. In addition to the two major signals,
cytokines are essential for T-cell activation. For example,
interleukins such as IL-1α, IL-1β, IL-18 and TNF-α are required for
the migration of APCs from the skin to lymph nodes and the
presentation of MHC-antigen peptide complexes, reviewed in
(12). Owing to the complexity of
these processes, it is difficult to fully reproduce these events
in vitro.
In the present study, an evaluation system for some
of these complex processes was established and proposed.
interleukin-2 (IL-2) expression in T-cells was used as an indicator
to evaluate the KE4 of skin-sensitizing AOPs. IL-2, a
pro-inflammatory cytokine, is released from activated T-cells and
plays an important role in the immune response by activating
immune-related cells, such as memory T-cells and natural killer
(NK) cells (13). Therefore, it was
hypothesized that the evaluation of IL-2 activity in T-cells could
provide important information for evaluating the KE4. The Jurkat
clone E6-1 human T-lymphocyte immortalized cell line is frequently
used in studies of human T-cell line activation in vitro
(14). In the present study, Jurkat
cells (IL-2p::Jurkat cells) that express the luciferase gene
downstream of the IL-2 promoter (IL-2p) were used. Using five skin
sensitizers, including a typical skin sensitizer
2,4-dinitrochlorobenzene (DNCB), and two non-sensitizers as test
substances, the activation of the IL-2 promoter as a marker of
T-cell activation was evaluated to determine the validity of this
assay.
Materials and methods
Cell culture
IL-2p::Jurkat cells (cat. no. J1651; Promega
Corporation; within 10-20 passages after obtaining the cell line)
(15) were maintained in RPMI-1640
medium supplemented with 10% FBS at 37˚C and 5% CO2. The
cell suspension was mixed with an equal volume of Trypan blue
solution (Nacalai Tesque Inc.), and the number of viable cells,
number of dead cells and cell viability were recorded using a
hemocytometer. Once every 2-3 days, the cells were seeded at a
density of 1-2x105 cells/ml, and a density of
0.1-1.0x106 cells/ml was maintained.
Determination of the chemical
concentration for 90% cell viability (CV90)
All chemicals used in the present study were
obtained from Sigma-Aldrich (Merck KGaA) or Tokyo Chemical
Industry. The concentrations of chemicals used for cell viability
studies were determined by referring to the exposure experiments on
Jurkat cells (11,16). Saline was used as a solvent for
NiSO4, glyoxal and lactic acid, and DMSO was used as a
solvent for the other chemicals.
IL-2p::Jurkat cells were transferred from flasks to
15 ml tubes, centrifuged (250 x g, 5 min, 25˚C), and the
supernatant was removed. The cell pellet was resuspended in fresh
medium to a concentration of 1x106 cells/ml. A total of
60 µl of the resuspended cells were added to a 96-well plate
(flat-bottom, transparent), and 30 µl PBS or test chemicals shown
in Table I were added to each well
containing the IL-2p::Jurkat cell suspension. WST-1 assay reagent
(3 µl; cat. no. MK400; Takara Bio, Inc.) was added and cells were
incubated for 1 h at 37˚C with 5% CO2. After incubation,
the absorbance at 450 nm (control wavelength 670 nm) was measured
using an iMark™ microplate reader (Bio-Rad Laboratories, Inc.), and
relative cell viability and CV90 were determined using Equations 1
and 2, respectively; Equation 1: Relative cell viability =
[(A450 of cells exposed to chemical)-(A670 of
cells exposed to chemical)]/[(A450 of cells exposed to
PBS)-(A670 of cells exposed to PBS)], and Equation 2:
LogCV90 = [(90-c)xLog(b)-(90-a)xLog(d)]/(a-c), where a is the
minimum viability at which the cell viability is >90%, c is the
maximum viability at which the cell viability was <90%, and b
and d are the concentrations at the cell viability of a and c,
respectively.
 | Table ICV90 of the chemicals used in this
study. |
Table I
CV90 of the chemicals used in this
study.
| Chemical | LLNAa | h-CLATa | DPRAa | CV90c | Solvent used |
|---|
| DNCB | Strong | + | + | 1.059 | DMSO |
| NiSO4 | Moderate | + | + | 27.13 | Saline |
| Isoeugenol | Moderate | -b | + | 84.15 | DMSO |
|
Diethylenetriamine | Moderate | -b | + | 716.7 | DMSO |
| Glyoxal | Moderate | + | + | 30.20 | Saline |
| Benzyl benzoate | Weak | -b | - | 276.0 | DMSO |
| Lactic acid | Negative | - | - | 1203 | Saline |
Exposure of IL-2p::Jurkat cells to
chemicals and their evaluation
For the pre-activation of T-cells, anti-CD3
pre-coated 96-well plates were prepared. A total of 6 µl of a stock
solution of anti-CD3 antibody (Clone OKT-3; cat. no. ab86883;
Abcam) was diluted in 1,200 µl PBS. The diluted anti-CD3 antibody
solution was added to a 96-well plate (60 ng/well) and incubated at
4˚C overnight. After incubation, the solution was removed with an
aspirator, and 100 µl culture medium (RPMI-1640 + 10% FBS) was
added to each well. This process was repeated twice (insufficient
washing affects cell growth). A total of 30 µl IL-2p::Jurkat cell
suspension (1x106 cells/ml) was then added to each
96-well plate, and 15 µl PBS was added to the negative control, or
anti-CD28 antibody solution as a positive control (2 µg/ml; clone
CD28.2; cat. no. 12-577-C100; Exbio-Funakoshi, Co., Ltd.) and the
chemical solutions were added. The cells were then incubated for 9
h at 37˚C, and luminescence was measured using a GloMax®
Navigator Microplate Luminometer (Promega Corporation) after adding
7 µl Bio-Glo reagent (cat. no. G7941; Promega Corporation) to each
well according to the manufacturer's instructions. IL-2p (IL-2
promoter) induction was calculated using Equation 3; Equation 3:
IL-2p induction = [(luminescence of cells exposed to test
chemicals]-[luminescence of cells exposed to PBS)]
x100/[(luminescence of cells exposed to anti-CD28)-(luminescence of
cells exposed to PBS)].
Results
Determination of CV90
Reasonable alternative assays for the animal
experiments used for evaluation of KE4 of skin sensitizing AOP are
currently not available. In the present study, the proliferation of
an immortalized T-cell line, Jurkat cells, that stably express
luciferase under downstream of the IL-2 promoter (IL-2p::Jurkat)
were used to assist in method development. The skin sensitizers and
control chemicals used in this study are listed in Table I. Chemicals marked with ‘b’ exhibit
local lymph node assay-positive but h-CLAT-negative response. The
skin sensitivities were also verified using the assay developed in
this study, which was termed the IL-2p::Jurkat assay.
To determine the appropriate concentration of the
chemicals, cells were exposed to various concentrations of the
compounds, and CV90 was determined using Equations 1 and 2, as
shown in Table I.
Evaluation of skin sensitization by
IL-2p::Jurkat assay
IL-2p::Jurkat cells were pre-activated by plating
into anti-CD3 pre-coated 96-well plates. Test chemicals were then
added, incubated for 9 h, and luciferase activity corresponding to
IL-2 promoter activation was measured to determine cell
proliferation. IL-2p induction was determined using Equation 3.
Exposure to the skin sensitizers DNCB,
diethylenetriamine and glyoxal increased IL-2 promoter activity,
whereas exposure to the weak or non-sensitizing substances benzyl
benzoate and lactic acid did not. That is, the IL-2p::Jurkat assay
correctly reflected the skin sensitivity to these five substances
(Fig. 1 and Table II). However, this assay did not
detect skin sensitivity to NiSO4 and isoeugenol and
showed inhibition of T-cell proliferation.
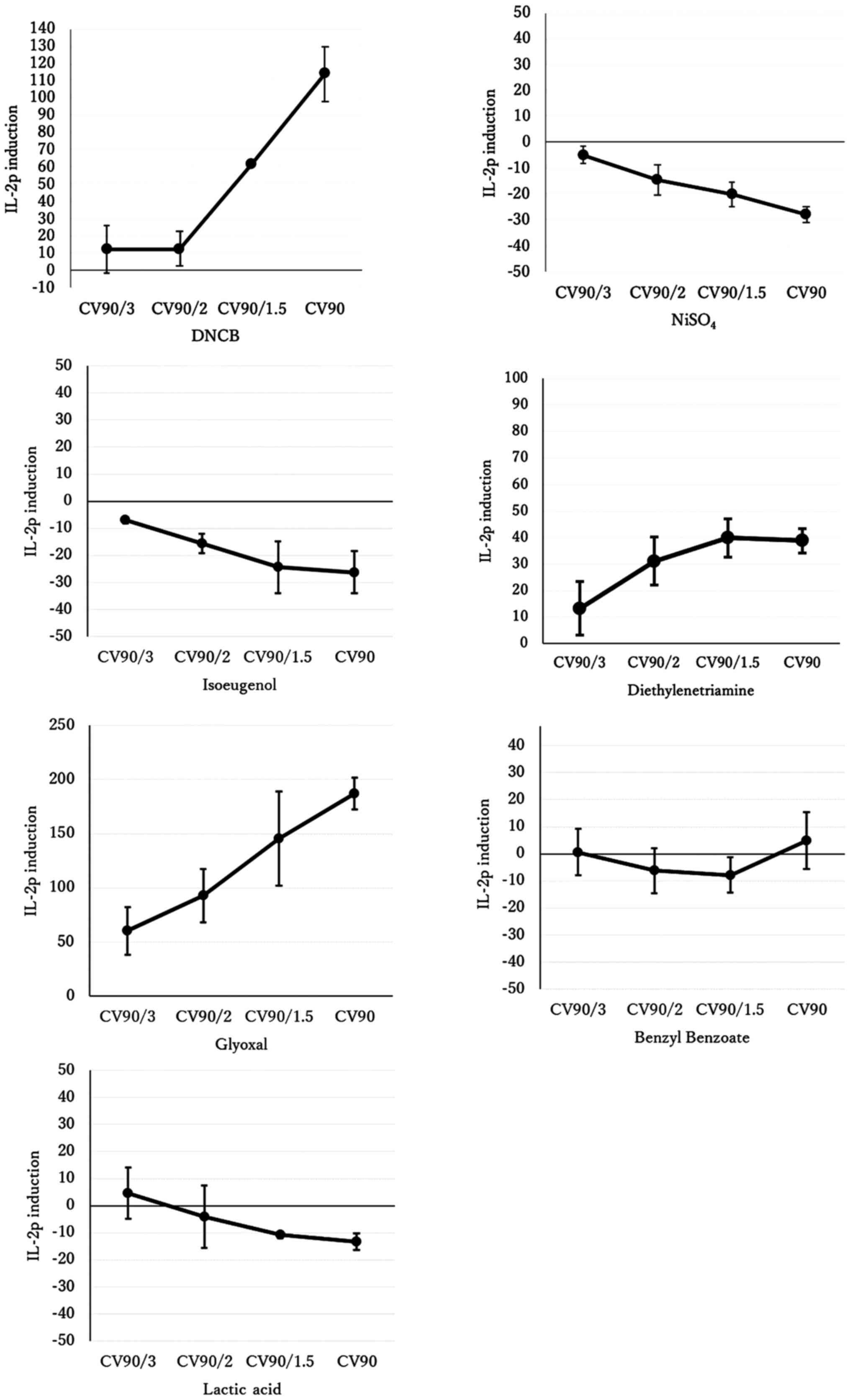 | Figure 1Relative IL-2p induction.
IL-2p::Jurkat cells were exposed to four concentrations (CV90,
CV90/1.5, CV90/2, CV90/3) of the indicated chemicals, incubated for
9 h, and then IL-2p induction was measured. The vertical axis is
the IL-2p induction where cell exposure to PBS was set to 0 as a
negative and background control, and cell exposure to anti-CD28 was
set to 100 as a positive control. The values in the graph represent
the mean ± standard deviation of three independent experiments. The
concentrations and CVs that indicate maximum and minimum induction
are shown in Table II.
Importantly, CV90/1.5, CV90/2, or CV90/3 refer to CV90 divided by
1.5, 2 or 3, respectively. CV, cell viability; IL-2p, interleukin-2
promoter. |
 | Table IIMaximum/minimum interleukin-2 promoter
induction at the indicated CV90. |
Table II
Maximum/minimum interleukin-2 promoter
induction at the indicated CV90.
| Chemical | Maximuma (CV90 fraction) | Minimuma (CV90 fraction) | Prediction using
Table III |
|---|
| DNCB | 113.9 (CV90) | 12.5 (CV90/3) |
Hyper-immunogenic |
| NiSO4 | -4.9 (CV90/3) | -27.9 (CV90) |
Immunosuppressive |
| Isoeugenol | -7.0 (CV90/3) | -26.3 (CV90) |
Immunosuppressive |
|
Diethylenetriamine | 39.9 (CV90/1.5) | 13.3 (CV90/3) |
Hyper-immunogenic |
| Glyoxal | 187.2 (CV90) | 60.3 (CV90/3) |
Hyper-immunogenic |
| Benzyl
benzoate | 4.9 (CV90) | -7.8
(CV90/1.5) | Negative |
| Lactic acid | 4.7 (CV90/3) | -13.2 (CV90) | Negative |
Possibility of simultaneous evaluation
of skin sensitization and immunosuppressiveness
There are two types of immunotoxicity:
Immunosuppressive and hyper-immunogenic (17,18).
Initially, the aim was to develop an assay to evaluate skin
sensitization, a feature of hyper-immunotoxicity. However, as
mentioned above, NiSO4 and isoeugenol, which exert an
immunosuppressive effect on T-cells, suppressed the expression of
IL-2. Therefore, in the present study, it may be possible to
simultaneously evaluate immunotoxicity in terms of both
immunosuppressive and hyper-immunogenic properties based on the
degree of suppression and enhancement of IL-2 expression. The
maximum and minimum values of IL-2p induction for each chemical in
this study are listed in Table
II.
As shown in Table
II, the weak or non-sensitizing substances benzyl benzoate and
lactic acid had maximum and minimum values of IL-2p induction
within ± 20. Therefore, the test developed in the present study may
be used as a simple screening test for immunotoxicity of chemical
substances by defining ‘IL-2p induction ≥20’ as
hyper-immunogenic/immunotoxic (a skin sensitizer), 20> IL-2p
induction >-20 or as negatively immunotoxic, and ‘IL-2p
induction ≤-20’ as immunosuppressive/immunotoxic (Table III).
 | Table IIIProposed new index for T cell
activation. |
Table III
Proposed new index for T cell
activation.
| Interleukin-2
promoter induction | Prediction |
|---|
| Induction ≥20 | Hyper-immunogenic
(skin sensitizer) |
| |Induction|
<20 | Negative |
| Induction ≤-20 |
Immunosuppressive |
Discussion
In the present study, an IL-2p::Jurkat assay for
determining the skin sensitization AOP KE4 T-cell activation was
designed and evaluated. The inflammatory cytokine IL-2 is a typical
cytokine produced by activated T-cells; this assay has been shown
to correctly evaluate the activation of T-cells by chemicals.
However, it is unclear at which stage of the skin sensitization
response Jurkat cells reflect T-cell activation in vivo. The
two major time points when T-cells are activated in the skin
sensitization response are as follows: First, when naïve T-cells
initially recognize antigens presented by DCs, and second, when
antigen-specific memory T-cells are already sensitized and
proliferating and recognize antigens presented by DCs. Normally, it
takes repeated exposure to a chemical and some time for naïve
T-cells to recognize it and for sensitized T-cells to proliferate
(19). In the test system used in
this study, the time of exposure of the cells to the chemical
substance was relatively short (9 h) and required pre-activation by
anti-CD3, so the activation of antigen-specific memory T-cells that
have already been sensitized was likely being evaluated rather than
naïve T-cells.
Three possible reasons for the false-negative result
in which the assay did not detect skin sensitivity to
NiSO4 and isoeugenol, and showed inhibition of T-cell
proliferation, are as follows: i) The immunosuppressive effect on
T-cells: Nickel and isoeugenol have been reported to exert
immunosuppressive effects on T-cells and suppress IL-2 expression
(20,21). Note that the word
‘immunosuppression’ is used here according to the previous
literature (20,21). Whether the expression of other
cytokines and cell surface antigens were altered by these agents to
determine their suppression or regulation was not performed in the
present study. ii) The lack of DCs: Nickel has been reported to
exert an immunosuppressive immunotoxic effect on T-cells but
induces cell maturation via NF-κB and MAPK signaling pathways in
antigen-presenting DCs (20). DCs
were absent in the in vitro test; nickel and isoeugenol,
having an immunosuppressive effect on T-cells alone, may exert
hyperimmunogenicity when interacting with DCs; hence, the absence
of DCs may have caused this false-negative result. iii)
Pre/pro-hapten issue; isoeugenol is a pre/pro-hapten chemical that
is prone to air oxidation and metabolism (22,23); a
pre/pro-hapten such as isoeugenol could be a false negative in the
test in the absence of a metabolic system.
The IL-2 expression of T-cells evaluated in this
study is a part of the skin sensitization AOP KE4 ‘T-cell
activation’. To complement KE4, other indicators, such as the
expression of IFN-γ and other molecules, the proliferation of
antigen-specific memory T-cells, expression of granzyme B in
CD8+ T-cells, and other assay systems, such as adopting
stimulation from DCs, oxidation and metabolism need to be
considered.
The concentration of chemicals tested with
IL-2p::Jurkat cells in the present study were determined based on
the CV90 value; this correctly determined the skin sensitization of
the five chemicals better than CV75 used in KeratinoSens and hCLAT
(12); therefore, the use of CV90
is considered appropriate. However, if false-negative results are
obtained when testing weaker skin sensitizers in the future, it
will be necessary to re-examine the effect of exposure
concentrations. It is important to note that exposure to high
concentrations of highly cytotoxic chemicals as well as skin
sensitizers, such as DNCB, may cause cell death, producing
unreliable results; the duration of chemical exposure may be
another factor to be re-examined in the case of false-negative
results when testing weaker skin sensitizers.
In the future, the accuracy and validity of the test
will be assessed by evaluating more chemicals using IL-2p::Jurkat
cells. Agents that are unstable in the atmosphere or water were not
assessed to avoid inaccuracies in the evaluations, although it
would be better to have a greater number of test chemicals
evaluated for the further validation. In addition, evaluation
indices other than IL-2 mentioned above will be used to complement
the skin sensitization evaluation data related to KE4 by combining
the test system with the test system using CD69 as an index, as
this has already been examined (11), and/or the THP-1 cell and
CD4+ T-lymphocyte co-culture system (24,25).
As described in the Materials and methods, FBS for
Jurkat cell culture was used, although it was minimal. FBS is
widely used for cell culture; therefore, there is a large amount of
accumulated data. Thus, it was decided that it would be beneficial
and necessary to compare and confirm our data obtained using past
accumulated data. Since FBS is derived from animals, there is a
potential concern that its performance depends on its batch
(26,27). Furthermore, there have been ethical
concerns regarding FBS and discussions about a fully chemically
defined medium to replace FBS (26,27).
Although limited, FBS alternatives have been developed and used for
particular purposes, for example, non-allergenic purposes.
Therefore, FBS alternatives for Jurkat cell culture should be
considered in the future. Additionally, as described in the
Materials and methods, anti-CD3 and anti-CD28 mouse monoclonal
antibodies obtained from pharmaceutical companies were used.
Although information on their origins is unavailable, they were
likely purified from ascites. These antibodies should be replaced
with those that can be confirmed to have been purified from
hybridomas expressing anti-CD3 (OKT-3) and anti-CD28 (28.2), which
would further reduce the use of animal-derived materials. Ideally,
these antibodies should be replaced with identical recombinant
antibodies purified using animal-free materials if such antibodies
are available in the future.
In conclusion, the alternative method to evaluate
KE4 of skin sensitization proposed in this study, if further
developed, may eliminate the need for conventional skin
sensitization experiments that cause suffering and pain in animals,
thus contributing to the reduction of animal testing.
Supplementary Material
Relative IL-2p::Jurkat cell viability
when exposed to the chemicals at the indicated concentrations.
These values were used to calculate the chemical concentration for
90% cell viability 90 as shown in Table
I.
Acknowledgements
The authors would like to thank Dr Mayuko Koreishi
(Okayama University, Okayama, Japan) for cell culture and technical
assistance.
Funding
This work was supported in part by MEXT/JSPS KAKENHI (grant no.
18K06133) and the Okayama Prefecture Tokubetsu Dengen.
Availability of data and materials
All data generated and/or analyzed during this study
are included in this published article.
Authors' contributions
TN and AS performed the experiments and analyzed the
data. TN, YT, ET, HH and AS contributed to the conception and
design of the study. TN and AS contributed to data acquisition and
analysis. All authors read and approved the final manuscript. YT,
ET and HH confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
van Vliet E, Kühnl J, Goebel C,
Martinozzi-Teissier S, Alépée N, Ashikaga T, Blömeke B, Del Bufalo
A, Cluzel M, Corsini E, et al: State-of-the-art and new options to
assess T cell activation by skin sensitizers. ALTEX. 35:179–192.
2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Qin R and Lampel HP: Review of
occupational contact dermatitis - Top allergens, best avoidance
measures. Curr Treat Options Allergy. 2:349–364. 2015.
|
|
3
|
Organisation for Economic Co-operation and
Development: The Adverse Outcome Pathway for Skin Sensitisation
Initiated by Covalent Binding to Proteins. Available from:
https://doi.org/10.1787/9789264221444-en.
|
|
4
|
Organisation for Economic Co-operation and
Development: Test No. 406: Skin Sensitisation. 1992. Available
from: https://doi.org/10.1787/9789264070660-en.
|
|
5
|
Organisation for Economic Co-operation and
Development: Test No. 429: Skin Sensitisation. 2010. Available
from: https://doi.org/10.1787/9789264071100-en.
|
|
6
|
Herrmann K, Pistollato F and Stephens ML:
Beyond the 3Rs: Expanding the use of human-relevant replacement
methods in biomedical research. ALTEX. 36:343–352. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
European Commision: Ban on Animal Testing:
Internal Market, Industry, Entrepreneurship and SMEs. Available
from: https://ec.europa.eu/growth/sectors/cosmetics/animal-testing_en.
|
|
8
|
Organisation for Economic Co-operation and
Development: Test No. 442C: In Chemico Skin Sensitisation. 2020.
Available from: https://doi.org/10.1787/9789264229709-en.
|
|
9
|
Organisation for Economic Co-operation and
Development: Test No. 442D: In Vitro Skin Sensitisation. 2018.
Available from: https://doi.org/10.1787/9789264229822-en.
|
|
10
|
Organisation for Economic Co-operation and
Development: Test No. 442E: In Vitro Skin Sensitisation. 2018.
Available from: https://doi.org/10.1787/9789264264359-en.
|
|
11
|
Hou F, Xing C, Li B, Cheng J and Chen W:
Performance of a novel in vitro assay for skin sensitization based
on activation of T lymphocytes. ALTEX. 37:451–468. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Croft M and Dubey C: Accessory molecule
and costimulation requirements for CD4 T cell response. Crit Rev
Immunol. 37:261–290. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Abbas AK, Trotta E, Simeonov DR, Marson A
and Bluestone JA: Revisiting IL-2: Biology and therapeutic
prospects. Sci Immunol. 3(eaat1482)2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Claesson MH, Dissing S, Tscherning T and
Geisler C: T-cell activation. V. Anti-major histocompatibility
complex class I antibody-induced activation and clonal abortion in
Jurkat T-leukaemic cells. Immunology. 78:444–448. 1993.PubMed/NCBI
|
|
15
|
Stecha P, Grailer J, Cheng JJ, Hartnett J,
Fan F and Stecha MC: American Association of Cancer Research
(AACR): Abstract 5439: Development of a robust reporter-based
T-cell activation assay for bispecific therapeutic antibodies in
immunotherapy. Cancer Ref: Aug 12, 2015 (Epub ahead of print).
|
|
16
|
Takenouchi O, Fukui S, Okamoto K, Kurotani
S, Imai N, Fujishiro M, Kyotani D, Kato Y, Kasahara T, Fujita M, et
al: Test battery with the human cell line activation test, direct
peptide reactivity assay, and DEREK based on a 139 chemical data
set for predicting skin sensitizing potential and potency of
chemicals. J Appl Toxicol. 35:1318–1332. 2015.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Exon JH, Koller LD, Talcott PA, O'Reilly
CA and Henningsen GM: Immunotoxicity testing: an economical
multiple-assay approach. Fundam Appl Toxicol. 7:387–397.
1986.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Rooney AA, Luebke RW, Selgrade MK and
Germolec DR: Immunotoxicology and its application in risk
assessment. Exp Suppl. 101:251–287. 2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Esser PR and Martin SF: Pathomechanisms of
contact sensitization. Curr Allergy Asthma Rep.
17(83)2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Saito R, Hirakawa S, Ohara H, Yasuda M,
Yamazaki T, Nishii S and Aiba S: Nickel differentially regulates
NFAT and NF-κB activation in T cell signaling. Toxicol Appl
Pharmacol. 254:245–255. 2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Park KR, Lee JH, Choi CY, Liu KH, Seog DH,
Kim YH, Kim DE, Yun CH and Yea SS: Suppression of interleukin-2
gene expression by isoeugenol is mediated through down-regulation
of NF-AT and NF-kappaB. Int Immunopharmacol. 7:1251–1258.
2007.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ahn J, Avonto C, Chittiboyina AG and Khan
IA: Is isoeugenol a prehapten? Characterization of a Thiol-reactive
oxidative byproduct of isoeugenol and potential implications for
skin sensitization. Chem Res Toxicol. 33:948–954. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Barratt MD and Basketter DA: Possible
origin of the skin sensitization potential of isoeugenol and
related compounds. (I).Preliminary studies of potential reaction
mechanisms. Contact Dermatitis. 27:98–104. 1992.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Clouet E, Bechara R, Raffalli C, Damiens
MH, Groux H, Pallardy M, Ferret PJ and Kerdine-Römer S: The THP-1
cell toolbox: A new concept integrating the key events of skin
sensitization. Arch Toxicol. 93:941–951. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Organisation for Economic Co-operation and
Development: Guideline No. 497D: Defined Approaches on Skin
Sensitisation. 2021. Available from: https://www.oecd.org/env/guideline-no-497-defined-approaches-on-skin-sensitisation-b92879a4-en.htm.
|
|
26
|
van der Valk J, Bieback K, Buta C,
Cochrane B, Dirks WG, Fu J, Hickman JJ, Hohensee C, Kolar R,
Liebsch M, et al: Fetal Bovine Serum (FBS): Past-Present-Future.
ALTEX. 35:99–118. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Weber T and Wagner K: Replacing fetal
bovine serum (FBS) in research and testing. ALTEX. 38:163–164.
2021.PubMed/NCBI View Article : Google Scholar
|















