Introduction
Androgenic alopecia, or male pattern hair loss, is a
hair loss disease mediated by dihydrotestosterone (DHT), an active
type of testosterone, which induces hair follicle (HF) shrinkage
and converts terminal hair into vellus hair (1). Without treatment, patients experience
gradual hair loss (2). Male-type
alopecia is the most common cause of hair loss and its incidence
increases with age. In the proportion of the population older than
80 years, ~73% of men and ~57% of women suffer from male-type
alopecia and ~58% of men over the age of 50 years suffer from
male-type alopecia (3-5).
Male pattern hair loss can cause negative psychological effects,
including obsessive self-doubt, aging-related anxiety and lethargy,
in both men and women. Furthermore, these effects are more
pronounced in women (6-8).
At present, minoxidil (MNX) and finasteride are the
only Food and Drug Administration (FDA)-approved drugs for hair
loss treatment that exhibit significant efficacy. However, MNX has
been reported to cause pruritus and contact dermatitis (9) and finasteride has been reported to
cause sexual dysfunction in a small number of patients (10). Patients are also reluctant to use
finasteride because of concerns regarding systemic side effects,
such as the headache, dizziness, skin rash and sexual dysfunction,
from oral administration (10-13).
Alternatively, low level laser therapy (LLLT) is an FDA-approved
hair loss treatment with significant hair loss inhibitory efficacy
(14). However, previous studies
have described the limitations of this treatment, including a small
number of responsive patients (15,16),
short experimental periods (17)
and lack of global phototrichograms (16). LLLT therefore has a lower success
rate compared with orally administered hair loss drugs.
Consequently, researchers are focusing on finding therapeutics that
are more effective and have fewer side effects.
Ultrasound is used in various ways in both the
medical and industrial fields. In medicine, ultrasounds are used as
an in vivo contrast diagnosis technology as well as for
increasing drug delivery efficiency (sonophoresis), fracture
treatment and neoplasm treatment (18-22).
In general, when ultrasounds are applied to the human body, a
medium such as a gel is used to increase their transmission
efficiency. However, Oohashi et al (23), demonstrated that an inaudible
frequency just above the audible sound frequency, which transmits
through air to subjects, may alter the brain waves and hormonal
levels of subjects (23-26).
Moreover, these effects occur not via the ears, but only when
inaudible sound is transmitted to the skin. Furthermore, a study by
Denda and Nakatani (27), indicated
that wound recovery was accelerated in mice when inaudible sound
directly reached the wound area. These data suggest that inaudible
sound can affect the skin surface and subsequently the whole
body.
Based on these observations, we hypothesized that
inaudible sound could also affect the scalp. In the present study,
the effect of inaudible sound on human dermal papilla cells (hDPCs)
was explored and whether inaudible sound could inhibit hair loss
signals derived from DHT treatment in hDPCs was investigated. The
efficacy of inaudible sound in treating the human scalp was
assessed by observing the changes in hair growth rate in HF organ
cultures treated with inaudible sound.
Materials and methods
Inaudible sound
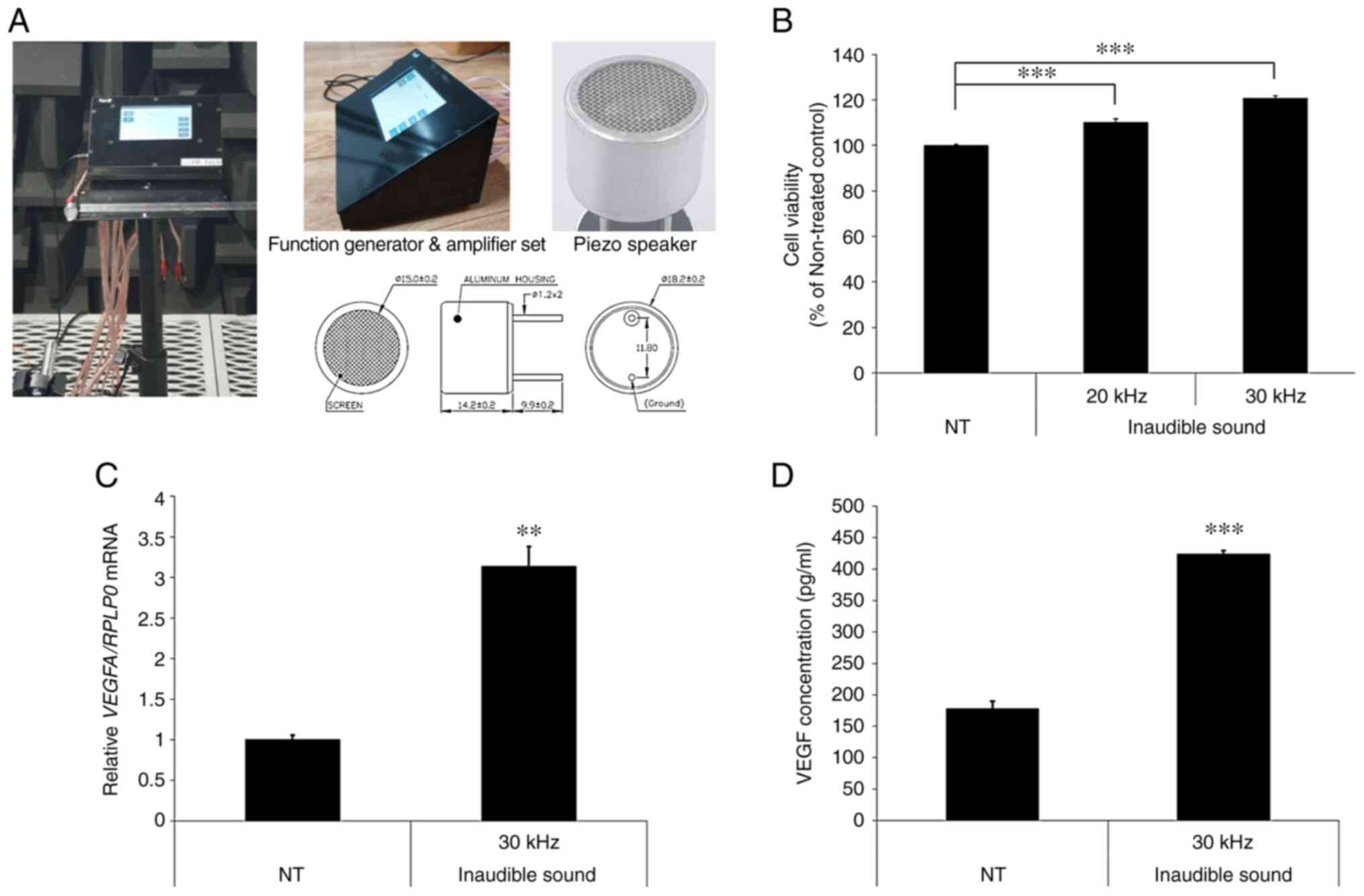
Electrical sine waves were generated using a
function generator and amplifier set (custom made; EM-Tech Co.,
Ltd.; Fig. 1A). Output power and
frequency were 24 Vrms and 30 kHz, respectively. Inaudible sound
was produced using the Piezo speaker 300ST18M (Pro-Wave Electronics
Corporation). The sound pressure level produced was 144 dB at 1 cm
distance. Cells were exposed to inaudible sound at 37˚C in a
humidified incubator with 95% O2 and 5% CO2
for 4 h in serum free low glucose DMEM supplemented with
L-glutamine and sodium pyruvate media (cat. no. SH30021.01;
HyClone). Cells were irradiated with inaudible sound from above the
media.
Reagents
MNX and DMSO were purchased from Sigma-Aldrich
(Merck KGaA). The human vascular endothelial growth factor (VEGF),
Dickkopf-1 (DKK-1) and TGF-β1 levels were evaluated using Human
VEGF Quantikine ELISA Kit (cat. no. DVE00), Human DKK-1 Quantikine
ELISA kit (DKK100B) and Human TGF-β1 Quantikine ELISA Kit (cat. no.
DB100B), purchased from R&D Systems, Inc.
Isolation and culture of hDPCs and
human outer root sheath (ORS) keratinocytes
During the hair transplant procedure, performed
between October 2020 and May 2021 at Dankook University Hospital
(Cheonan, South Korea), hair biopsy specimens were obtained from
the non-balding occipital scalp region of three male patients (aged
32, 34 and 37) with androgenic alopecia during a hair transplant
procedure. Patients had received 1 mg/day oral finasteride
treatment for at least a year before the procedure. The Medical
Ethical Committee of Dankook Medical College (Cheonan, South Korea)
approved all the described protocols and informed written consent
was obtained from all patients (approval no. DKUH 2013-08-012-001).
HFs were isolated and cultured as described previously, with minor
modifications to the procedure (28-30).
Briefly, a subcutaneous fat of scalp skin including lower hair
follicles was dissected from the epidermis and dermis. The
follicles were then separated using a binocular microscope with
forceps and maintained at 6 follicle units/well in 24-well plates,
and grown in Williams E medium (Sigma-Aldrich; Merck KGaA) at 37˚C
in a humidified incubator with 5% CO2. Cultured hDPCs
and ORS keratinocytes of, no later than passage 3, were used for
the subsequent experiments and were maintained at 37˚C in a
humidified incubator with 5% CO2. hDPCs were grown in
low glucose DMEM supplemented with L-glutamine and sodium pyruvate,
which was supplemented with 10% FBS (Gibco; thermo Fisher
Scientific, Inc.). ORS keratinocytes were grown in Epilife medium
with 60 µM calcium (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with EpiLife Defined Growth Supplement (EDGS; Gibco;
Thermo Fisher Scientific, Inc.).
hDPC viability assay
Cell viability was determined using a Cell Counting
Kit-8 (CCK-8) assay. Briefly, hDPCs were seeded at a density of
1.5x105 cells/well into 6-well plates and cultured for
24 h at 37˚C in a humidified incubator with 5% CO2. The
cells were subsequently treated with an inaudible sound frequency
of 20 and 30 kHz for 4 h in FBS-free medium at 37˚C in a humidified
incubator with 5% CO2. After 48 h, cell viability was
assessed using a CCK-8 assay (Dojindo Molecular Technologies,
Inc.), and the absorbance at 450 nm was analyzed using a plate
reader after the CCK-8 solution was added and cells were incubated
at 37˚C in a humidified incubator with 5% CO2 for 2 h.
The cell viability rates were determined and are represented as
percentages of the control value (untreated cells).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was isolated using the RNeasy Mini Kit
(Qiagen GmbH) and 2 µg RNA was reverse-transcribed into
complementary DNA using SuperScript® III Reverse
Transcriptase (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. qPCR was performed using
the ABI 7500HT Fast System (Applied Biosystems; Thermo Fisher
Scientific, Inc.) and the Taqman™ Universal PCR Master Mix (Applied
Biosystems; Thermo Fisher Scientific, Inc.) to determine the
expression of the following genes: Bcl-2 (assay ID: Hs00608023_m1),
Bax (Hs00180269_m1), VEGFA (Hs00900055_m1) and ribosomal protein
lateral stalk subunit P0 (RPLP0, Hs00420895_gH). The following
thermocycling conditions were used: Initial denaturation at 95˚C
for 5 min; followed by 40 cycles of amplification at 95˚C for 15
sec, annealing at 65˚C for 30 sec, and extension at 72˚C for 30
sec. mRNA expression levels were quantified using the
2-∆∆Cq method (31).
RPLP0 was used as a housekeeping gene to normalize data.
Secretion factor analysis
VEGF, DKK-1 and TGF-β1 secretion was quantified
using commercial ELISA kits, according to the manufacturer's
protocol. For assessing the factors secreted by hDPCs treated with
inaudible sound, hDPCs (passage 3) were plated overnight at a
density of 1.5x105 cells/well into 6-well plates at 37˚C
in a humidified incubator with 5% CO2. The cells were
washed twice with PBS and then added to FBS-free media. For
quantification of VEGF, the cells were then incubated for 48 h
following treatment with inaudible sound of 30 kHz at 37˚C in a
humidified incubator with 5% CO2 for 4 h. In order to
quantify the involvement of DKK-1 and TGF-β1, cells were treated
with 100 nM DHT following the inaudible sound treatment. The
concentrations of VEGF, DKK-1 and TGF-β1 in the medium were
subsequently analyzed. Briefly, a capture antibody coated 96-well
microplate (included in the kit) was blocked with 1% BSA (Thermo
Fisher Scientific, Inc.) in PBS and incubated at room temperature
for 1 h. After washing three times with wash buffer, the
conditioned media, obtained from cells treated with inaudible sound
or the untreated control was added, and cells were further
incubated for 2 h at room temperature. The plate was then washed
three times with wash buffer and detection antibody was added and
cells were incubated for 2 h at room temperature. After washing
three times, streptavidin-HRP solution was added and samples were
incubated for 20 min at room temperature. The plate was washed
three times with wash buffer followed by the addition of substrate
solution and the plate was incubated for 20 min at room
temperature. Stop solution was added to stop the enzyme reaction.
Optical density was assessed using the Synergy™2 ELISA reader
(BioTek Instruments, Inc.) at 450 nm.
Antiapoptotic effect assay
DKK-1 and TGF-β1, known as hair loss inducers, are
known to induce apoptosis in ORS keratinocytes and to suppress cell
viability (32). To analyze the
antiapoptotic effect of ultrasound, ORS keratinocytes were seeded
at a density of 3x105 cells/well into 6-well plates and
cultured at 37˚C in a humidified incubator with 5% CO2
for 24 h. Prior to treatment, the culture medium was replaced with
a EDGS-free medium. After the cells were subsequently treated with
inaudible sound at 37˚C in a humidified incubator with 5%
CO2 for 4 h, 50 ng/ml DKK-1 and 50 ng/ml TGF-β1 were
added for 48 h. The efficacy of any antiapoptotic effects was
determined using the CCK-8 assay. The absorbance at 450 nm was
assessed using a plate reader after treating the cells with CCK-8
solution at 37˚C in a humidified incubator with 5% CO2
for 2 h. The cell viability rates were determined using the optical
density readings and are represented as percentages of the control
value (untreated cells).
TUNEL assay
A TUNEL kit (In Situ Cell Death Detection
Kit, Fluorescein; Roche Diagnostics GmbH) was used according to the
manufacturer's protocol to evaluate apoptotic cells. Briefly, ORS
keratinocytes at 2x104 cells/200 µl were seeded into
8-chamber slides (Thermo Fisher Scientific, Inc.). After adherence,
the cell medium was replaced with a growth supplement-free medium.
Subsequently, the cells were treated with inaudible sound at 37˚C
in a humidified incubator with 5% CO2 for 4 h and
treated with 50 ng/ml DKK-1 and 50 ng/ml TGF-β1 at 37˚C in a
humidified incubator with 5% CO2 for 24 h. These cells
were then fixed in 4% paraformaldehyde for 10 min at room
temperature. After washing with PBS, the cells were incubated with
0.1% Triton X-100 in 0.1% sodium citrate for 1 h at room
temperature. After washing with PBS, the cells were treated with
TUNEL reagent and incubated in a humidified incubator for 1 h at
37˚C in the dark. After washing with PBS, the cells were stained
with DAPI and mounted using Fluoroshield with DAPI (cat. no. F6057;
Sigma-Aldrich, USA), which was applied to the solution by addition
of 3-4 drops, after which, the cover glass was mounted and the
samples were left at room temperature for 5 min. DAPI staining was
used to visualize the nuclei. Representative images were captured
using a fluorescence microscope (Olympus Corporation;
magnification, x100).
HF organ culture and assessment of
hair elongation
Anagen HFs were obtained from human scalp skin
specimens. Six HFs per well in 24-well plates were cultured in 37˚C
in a humidified incubator with 5% CO2, with 500 µl of
Williams E medium supplemented with 10 µg/ml insulin
(Sigma-Aldrich; Merck KGaA), 10 ng/ml hydrocortisone
(Sigma-Aldrich; Merck KGaA), 2 mM L-glutamine (Sigma-Aldrich; Merck
KGaA), 0.1% fungizone (Gibco; Thermo Fisher Scientific, Inc.), 10
µg/ml streptomycin and 100 U/ml penicillin (Gibco; Thermo Fisher
Scientific, Inc.) according to Philpott's method (33). Each experimental group contained at
least 30 anagen HFs derived from three different patients. HFs were
treated with 30 kHz inaudible sound at 37˚C in a humidified
incubator with 5% CO2 for 4 h, once a day. The
incubation medium was renewed every two days. HF elongation was
analyzed directly at 2 and 5 days of culture using a
stereomicroscope (Olympus Corporation).
Ki-67 immunohistochemistry
Ki-67 staining was performed as previously described
(34,35). To confirm the effect of ultrasound
treatment on hair, HFs were treated with 30 kHz inaudible sound at
37˚C in a humidified incubator with 5% CO2 for 4 h, once
a day for 3 days. After 3 days, HFs were embedded in optimal
cutting temperature (OCT) freezing compound (Thermo Fisher
Scientific, Inc.) and frozen 6-µm sections were prepared using a
CM1850 cryostat (Leica Microsystems GmbH). The cryostat temperature
was maintained between-15 and -23˚C. The follicles were then
cryo-sectioned and fixed in 10% paraformaldehyde for 10 min at room
temperature. The samples were blocked using 4% BSA in TBS with 0.1%
Tween-20 (TBST) for 30 min at room temperature, followed by washing
with TBST. The samples were then incubated with Ki-67 monoclonal
antibody (1:50; cat. no. 14-5698-82, Invitrogen; Thermo Fisher
Scientific, Inc.) at 4˚C overnight. After washing, Alexa Fluor™ 488
anti-rat secondary antibody (1:50; cat. no. A-11006, Invitrogen;
Thermo Fisher Scientific, Inc.) was added and incubated for 1 h at
room temperature. The samples were subsequently mounted and
counterstained with DAPI as above. The samples were imaged using a
fluorescence microscope (IX-73 with U-HGLGPS; Olympus Corporation;
magnification, x100).
Statistical analysis
Data are presented as the mean ± SD. Comparisons
among three or more groups were analyzed using a one-way ANOVA
followed by the Tukey's honestly significant difference test.
Comparisons between two groups were analyzed using a paired
two-tailed Student's t-test. Results were processed using SPSS for
Windows, version 22.0 (IBM Corp.). P<0.05 was considered to
indicate a statistically significant difference.
Results
Inaudible sound stimulates cell
proliferation and the expression of hair growth related factors in
hDPCs
To determine the potential effect of ultrasound on
the proliferation of hDPCs, the CCK-8 assay was performed 2 days
after treatment with 20 and 30 kHz inaudible sound for 4 h. The
results demonstrated that ultrasound significantly enhanced hDPC
proliferation in a frequency-dependent manner compared with the
non-treated control (NT) (Fig. 1B).
A frequency 30 kHz was chosen for subsequent experiments because it
elevated hDPC proliferation more than 20 kHz. To confirm the effect
of inaudible sound on hDPC proliferation, RT-qPCR and ELISA were
performed. VEGF is a typical growth factor that enhances hair
growth and the effect of MNX on VEGF expression has previously been
investigated (36). The results
showed that treatment with inaudible sound significantly increased
the mRNA expression levels and concentration of VEGF in hDPCs
compared with the NT (Fig. 1C and
D).
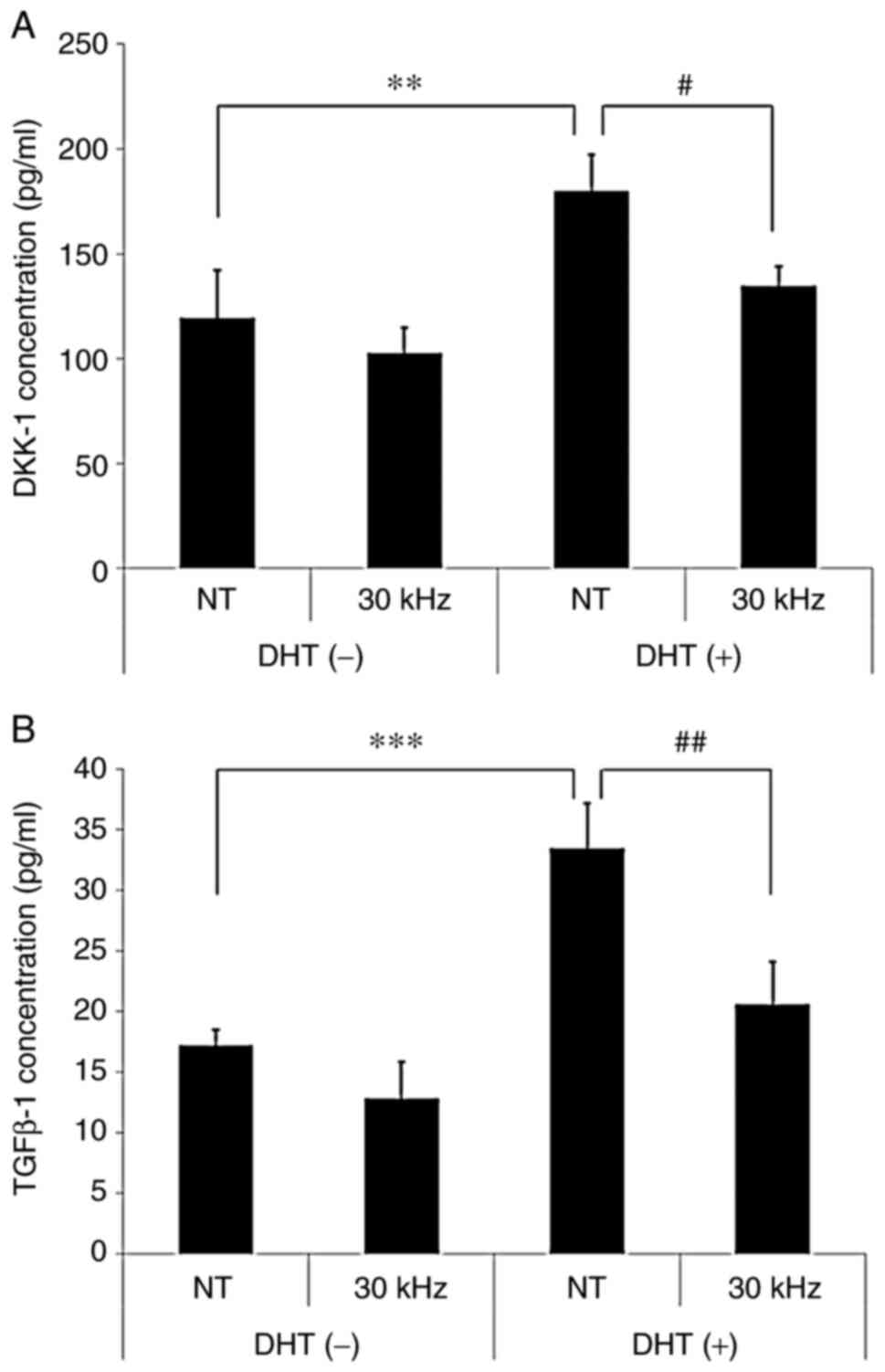
Inaudible sound abrogates DHT-induced
secretion of catagen-related factors in hDPCs
To investigate the potential role of inaudible sound
frequencies on the inhibition of catagen-related secretion factors,
such as DKK-1 and TGF-β1, in hDPCs, ELISAs were performed 2 days
following treatment in the presence or absence of DHT and 30 kHz
inaudible sound. The results demonstrated that DHT significantly
increased the concentrations of catagen-related secretion factors,
DKK-1 and TGFβ1, in hDPCs, whereas inaudible sound significantly
reduced the concentrations of DKK-1 and TGFβ1 compared with the NT
group (Fig. 2A and B). These results suggested that the
inhibition of catagen-related secretion factors may be likely
mechanism responsible for increased hair loss following treatment
with inaudible sound in DHT-treated hDPCs, as opposed to increased
hair growth.
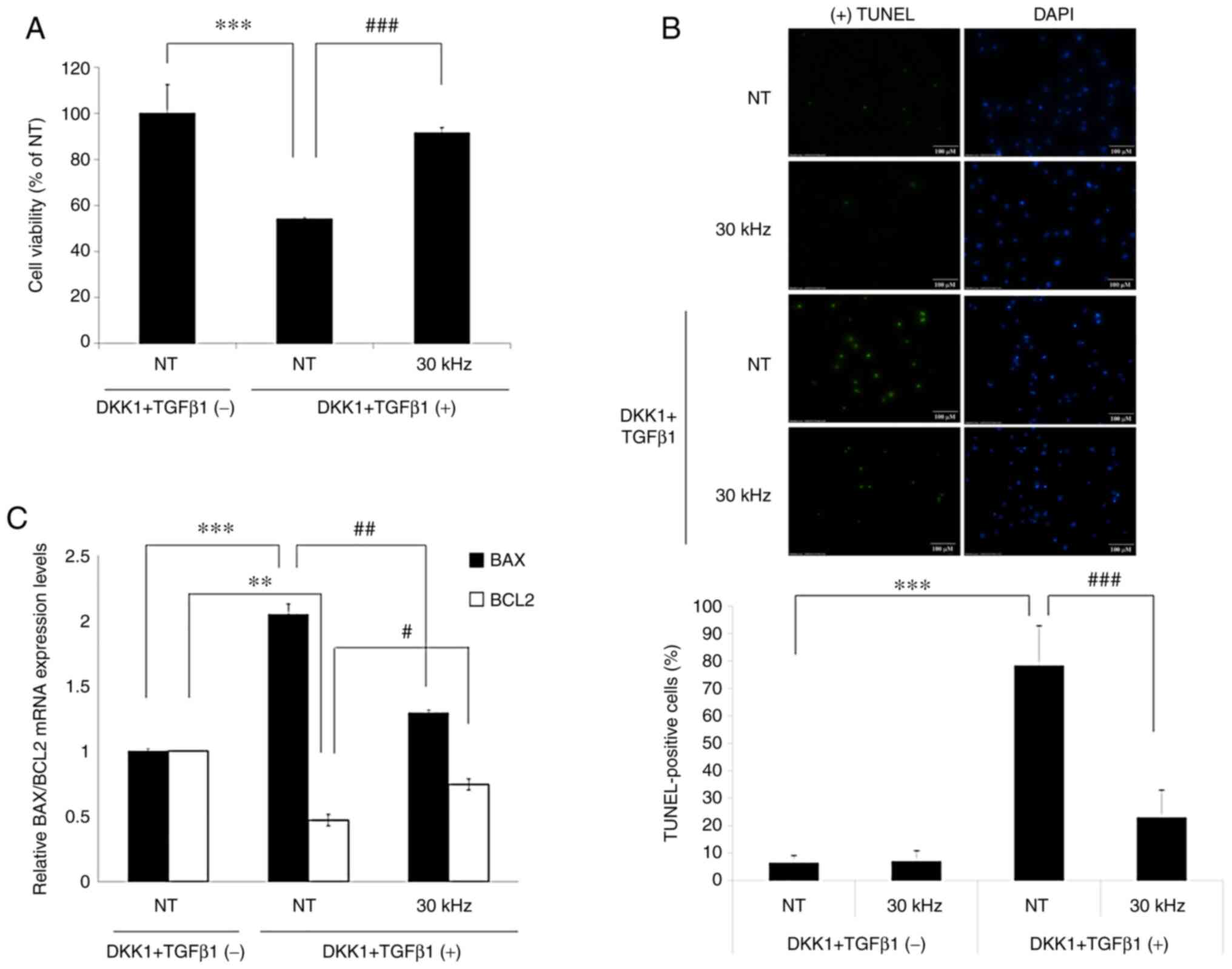
Inaudible sound inhibits apoptosis
mediated by DKK-1 and TGF-β1 and regulates the expression of
apoptosis-related genes in ORS keratinocytes
To investigate the potential role of inaudible sound
on the inhibition of apoptosis in ORS keratinocytes, the CCK-8
assay was performed 1 day after treatment in the presence or
absence of DKK-1, TGF-β1 and inaudible sound. The DKK-1 and TGF-β1
concentrations used were determined in preliminary tests (Fig. S1). Treatment with DKK-1 (50 ng/ml)
and TGF-β1 (50 ng/ml) significantly inhibited the viability of ORS
keratinocytes compared with the untreated NT group. The inhibitory
effect of apoptosis by DKK-1 and TGFβ-1 on ORS keratinocyte
viability was significantly reversed by 30 kHz inaudible sound
compared with the NT and DKK1 + TGFβ-1 group (Fig. 3A).
To confirm the inhibitory effect of inaudible sound
on apoptosis in ORS keratinocytes, the TUNEL assay was performed.
The results demonstrated that TUNEL-positive cells undergoing
apoptosis were significantly increased when ORS keratinocytes were
treated with DKK-1 and TGFβ-1 compared with the NT group (Fig. 3B). TUNEL-positive cells were
significantly decreased by 30 kHz inaudible sound despite
co-treatment with DKK-1 and TGFβ1, compared with the NT DKK1 +
TGFβ-1 group.
To further investigate the antiapoptotic effects of
inaudible sound on DKK-1 and TGFβ-1, changes in mRNA expression
levels of apoptosis-related genes were investigated via RT-qPCR.
Bcl-2 and Bax genes, which are related to DKK-1 and TGF-β1 have
previously been demonstrated to induce apoptosis in numerous cell
types, including HF cells (30,37,38)
and were therefore used to measure apoptosis. ORS keratinocytes
were treated with 30 kHz and/or a combination of DKK-1 and TGFβ-1.
In ORS keratinocytes, combination treatment with DKK-1 and TGFβ-1
significantly decreased the mRNA expression levels of antiapoptotic
factor, Bcl-2, compared with the NT group without treatment.
Furthermore, treatment with 30 kHz significantly reversed the
inhibition of Bcl-2 mRNA expression induced by DKK-1 and TGFβ-1,
compared with the NT DKK1 + TGFβ-1 group. DKK-1 and TGF-β1 also
significantly induced the mRNA expression of the proapoptotic
factor Bax compared with the untreated NT group. Moreover,
treatment with 30 kHz significantly inhibited Bax mRNA expression
levels in ORS keratinocytes compared with the NT DKK1 + TGFβ-1
group (Fig. 3C), despite the
presence of DKK-1 and TGFβ-1. These results therefore indicated t
that inaudible sound may promote the survival of ORS keratinocytes
and increase the ratio of Bcl-2/Bax to inhibit cell death.
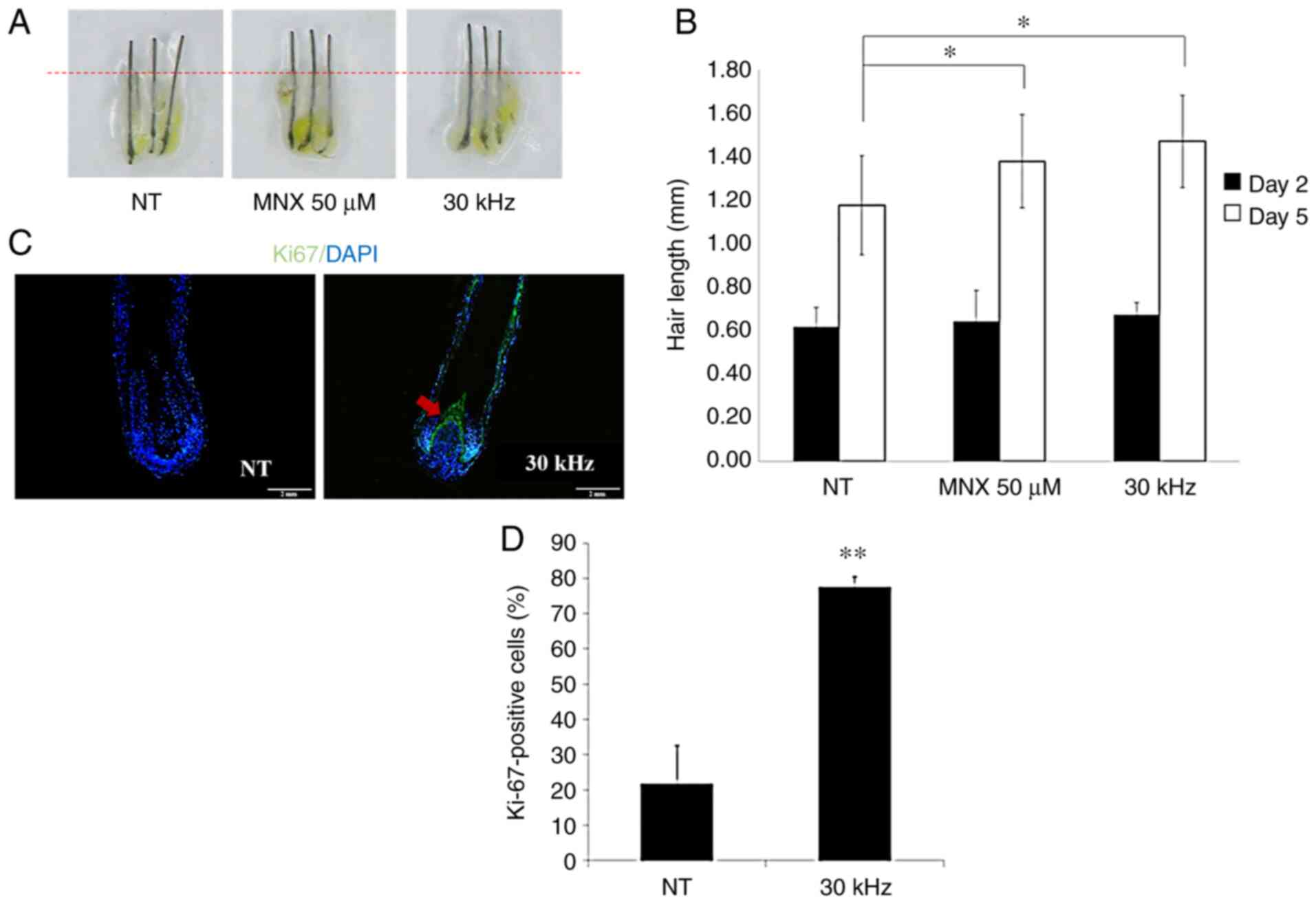
Inaudible sound promotes HF elongation
and hair matrix keratinocyte proliferation in human HF organ
culture
To examine the hair growth effect of inaudible sound
at the organ level, ex vivo cultures of whole human scalp
HFs were investigated. MNX and vehicle served as the positive and
negative controls, respectively. It was observed that HFs treated
with 30 kHz inaudible sound grew significantly longer compared with
the NT HFs at 5 days, which was similar to the growth of HFs
treated with MNX (Fig. 4A and
B).
To confirm the cell proliferation promoting effect
of inaudible sound in HF matrix keratinocytes, immunofluorescence
staining for Ki-67 was performed after human HFs were exposed to 30
kHz inaudible sound for 4 h every day, over 3 days. HF sections
derived from three different individuals were analyzed for the
proliferation of follicular matrix keratinocytes in the hair bulb
(green fluorescence; Fig. 4C).
Nuclei were counterstained with DAPI (blue fluorescence). For
quantitative analyses, the number of Ki-67+ cells were
counted and normalized to the number of DAPI-stained cells
(Fig. 4D). The Ki-67+
signal was especially strong in the upper part of the matrix above
the hDPCs. After 3 days of culture, numerous NT HFs switched to the
catagen phase, thus losing proliferative follicular matrix
keratinocytes in the process. However, 30 kHz inaudible
sound-treated cells displayed significantly enhanced cell
proliferation levels in HFs compared with the NT and maintained the
anagen phase.
Discussion
In the present study, the effects of inaudible sound
on hair growth in hDPCs and HFs, and whether it can inhibit hair
loss induced by DHT at the cellular level, were investigated.
Although the molecular pathogenic mechanism of DHT is unclear, its
role in male-pattern baldness has been well-documented (1,39).
Circulating androgens such as DHT enter the follicle via
capillaries, bind with the androgen receptors (ARs) on hDPCs and
subsequently activate target genes, including DKK-1 and
TGFβ1(40). Therefore, in the
present study hair loss signals were induced by treating hDPCs with
DHT and it was then observed whether inaudible sound inhibited the
secretion level of the hair loss-inducing factors, DKK1 and TGF-β1,
which are induced by DHT. Furthermore, the results demonstrated
that inaudible sound could significantly inhibit apoptosis induced
by DKK1 and TGF-β1 in ORS keratinocytes, which was determined using
a cell viability assay, TUNEL assay and the mRNA expression levels
of apoptosis-related genes, Bcl-2 and Bax. Moreover, the results
demonstrated that the direct treatment of human HFs with inaudible
sound accelerated the growth rate of hair. The protein expression
of Ki-67, a proliferation marker of matrix cells, was also
significantly increased in the hair bulb in response to inaudible
sound.
To the best of our knowledge, the present study is
the first to report this phenomenon, which can be explained by two
hypotheses. The first hypothesis in the present study was that
promotion of hair growth occurred due to activation of the
Wnt/β-catenin signaling pathway caused by inaudible sound
vibrations. Our lab observed that inaudible sound altered Wnt
related genes expression in hDPCs (data not shown). Canonical Wnt
signaling is transmitted via allosteric modulation induced by a
structural change in the Wnt receptor when it binds to Wnt ligands
(41). Weak vibrations such as
inaudible sound, may lead to structural changes in membrane
receptors (42). Structural changes
caused by inaudible sound in the Wnt receptor may mimic the
structural change caused by Wnt ligand binding, resulting in
allosteric modulation. The present study provides the first report
of hair growth promotion by inaudible sound; however, it is well
known that signal changes caused by vibrations exert effects such
as stem cell activity, wound healing and bone fracture healing in a
number of cell types (27,43,44).
Therefore`, in future studies it will be necessary to verify if
inaudible sound exhibits hair growth effects via allosteric
modulation of the Wnt receptor.
The second hypothesis is that promotion of hair
growth occurs as a result of mechanotransduction from the cell
membrane of HF cells, which is modified by inaudible sound. Organs
and cells routinely face mechanical stress such as muscle
contraction, blood flow, or stretching. Within the cell, forces
such as those generated by the actin-myosin cytoskeleton or surface
tension generated by the membrane are present. These forces
themselves act as signals to cells and are known to affect cell
fate or organ formation (45-47).
Recently, Thompson et al (48) reported the activation of mesenchymal
stem cells (MSC) by low-intensity vibration (LIV). LIV is a
micro-vibration that occurs upon light exercise or blood flow. This
study reported that when LIV is applied to bone marrow,
Yes-associated protein (YAP), a mechanotransducer, is translocated
into the nucleus from the cytosol of MSCs. YAP enters the MSC
nucleus where it acts as a transcriptional co-activator and
transcribes various genes associated with bone production. It has
been hypothesized that this is the mechanism by which bone
stiffness increases upon exercise (48). In the present study, inaudible sound
was used as a scalp stimulator, which was transmitted to the scalp
via the air. Moreover, the inaudible sound transmitted to the scalp
may transmit LIV to the HF. Transferred LIV has been reported to
affect HF stem cells (HFSC) of the HF bulge, which may promote the
expression of various genes to promote hair growth by translocating
YAP from the HFSC cytosol into the nucleus. In the present study it
has been demonstrated that inaudible sound may inhibit hair loss by
blocking DHT-induced apoptotic signaling and promoting follicle
cell proliferation; however, further studies are needed to verify
the aforementioned hypotheses for determining the mechanisms
underlying this phenomenon.
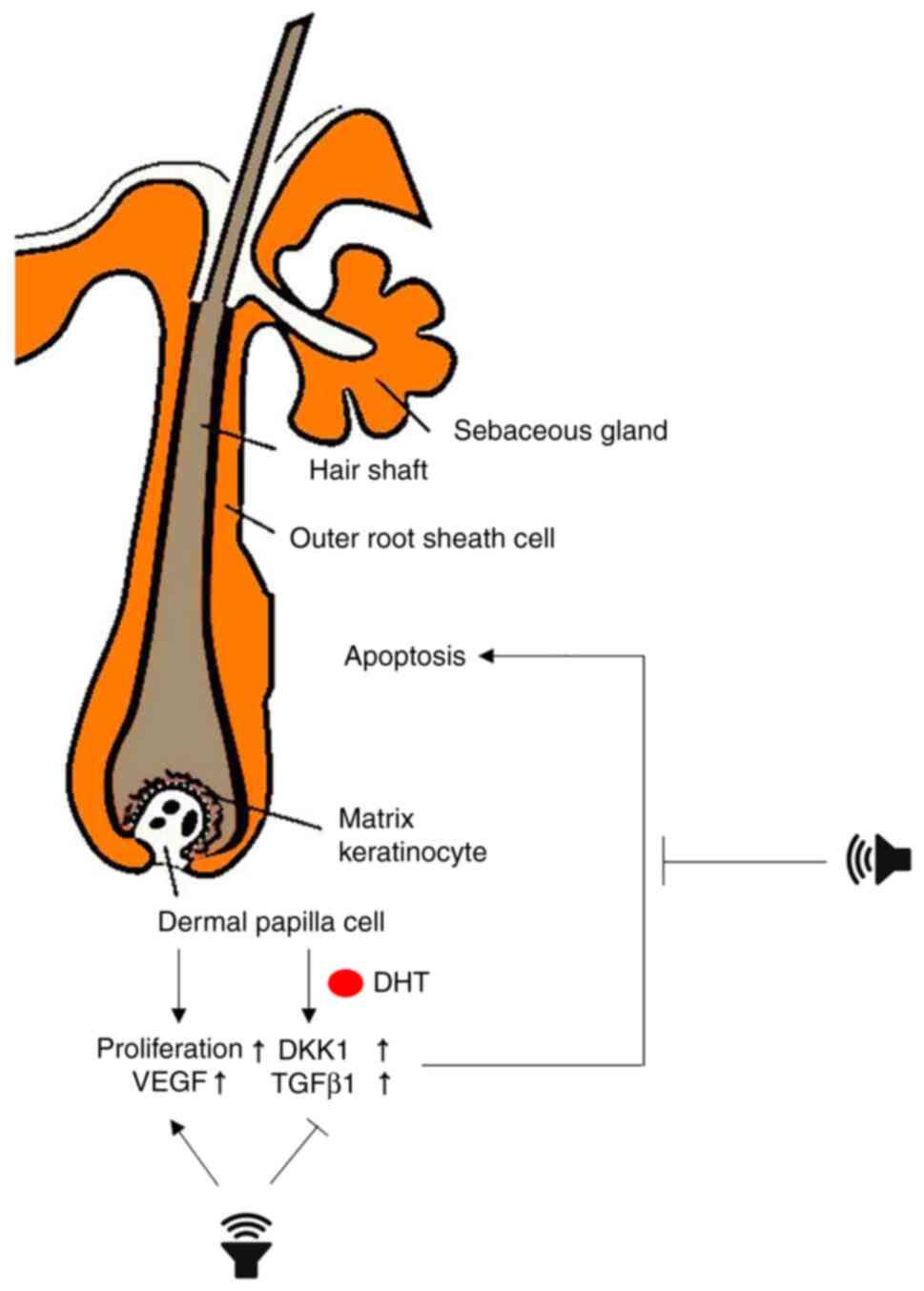
In conclusion, the present study demonstrated that
inaudible sound promoted the proliferation of HF cells and that
this effect inhibited hair loss signals induced by DHT (Fig. 5). Although the mechanism of action
remains to be elucidated, the present study has provided the basis
for a new therapeutic approach that can replace or complement
existing hair loss treatments.
Supplementary Material
Combination of DKK-1 and TGF-β1
inhibited cell viability by stimulating apoptosis in the outer root
sheath of keratinocytes. Cells were treated with 50 ng/ml DKK-1, 50
ng/ml TGF-β1, or a combination of DKK-1 and TGF-β1 for 48 h. A
CCK-8 assay was subsequently performed on day 2. Data are presented
as the mean ± SD of three independent experiments. Statistically
significant differences were determined using a one-way ANOVA
followed by a Tukey's honestly significant difference test.
***P<0.001. DKK-1, Dickkopf-1; NT, non-treated
control.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HC and BJK were responsible for the
conceptualization of the present study. HC, YL and SHS designed the
methodology and HC, JN, WSP and BJK validated the data. Data was
collected by HC, YL and SHS and the investigation and
interpretation of the data was performed by HC, YL, SHS, BCP and
BJK. Statistical analysis was performed by HC. HC prepared the
original draft manuscript and HC, YL and BJK edited and wrote the
manuscript. HC, WSP and BJK supervised the project. HC, JN, WSP and
BJK edited the manuscript. WSP and BJK confirmed the authenticity
of all the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The Medical Ethical Committee of Dankook Medical
College (Cheonan, South Korea) approved all the described protocols
and informed written consent was obtained from all patients (IRB
approval no. DKUH 2020-11-004).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Imperato-McGinley J, Guerrero L, Gautier T
and Peterson RE: Steroid 5alpha-reductase deficiency in man: An
inherited form of male pseudohermaphroditism. Science.
186:1213–1215. 1974.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kaufman KD, Girman CJ, Round EM,
Johnson-Levonas AO, Shah AK and Rotonda J: Progression of hair loss
in men with androgenetic alopecia (male pattern hair loss):
Long-term (5-year) controlled observational data in placebo-treated
patients. Eur J Dermatol. 18:407–411. 2008.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hamilton JB: Patterned loss of hair in
man: Types and incidence. Ann NY Acad Sci. 53:708–728.
1951.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Gan DC and Sinclair RD: Prevalence of male
and female pattern hair loss in Maryborough. J Investig Dermatol
Symp Proc. 10:184–189. 2005.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Shankar DK, Chakravarthi M and Shilpakar
R: Male androgenetic alopecia: Population-based study in 1,005
subjects. Int J Trichology. 1:131–133. 2009.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Cash TF: The psychological effects of
androgenetic alopecia in men. J Am Acad Dermatol. 26:926–931.
1992.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Cash TF, Price VH and Savin RC:
Psychological effects of androgenetic alopecia on women:
Comparisons with balding men and with female control subjects. J Am
Acad Dermatol. 29:568–575. 1993.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ludwig E: Classification of the types of
androgenetic alopecia (common baldness) occurring in the female
sex. Br J Dermatol. 97:247–254. 1977.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Friedman ES, Friedman PM, Cohen DE and
Washenik K: Allergic contact dermatitis to topical minoxidil
solution: Etiology and treatment. J Am Acad Dermatol. 46:309–312.
2002.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Irwig MS and Kolukula S: Persistent sexual
side effects of finasteride for male pattern hair loss. J Sex Med.
8:1747–1753. 2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Samplaski MK, Lo K, Grober E and Jarvi K:
Finasteride use in the male infertility population: Effects on
semen and hormone parameters. Fertil Steril. 100:1542–1546.
2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Amory JK, Wang C, Swerdloff RS, Anawalt
BD, Matsumoto AM, Bremner WJ, Walker SE, Haberer LJ and Clark RV:
The effect of 5alpha-reductase inhibition with dutasteride and
finasteride on semen parameters and serum hormones in healthy men.
J Clin Endocrinol Metab. 92:1659–1665. 2007.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Anita KG, Neetu S and Prashant S: Atypical
post-finasteride syndrome: A pharmacological riddle. Indian J
Pharmacol. 48:316–317. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Egger A, Resnik S, Aickara D, Maranda E,
Kaiser M, Wikramanayake TC and Jimenez JJ: Examining the safety and
efficacy of low-level laser therapy for male and female pattern
hair loss: A review of the literature. Skin Appendage Disord.
6:259–267. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kim H, Choi JW, Kim JY, Shin JW, Lee SJ
and Huh CH: Low-level light therapy for androgenetic alopecia: A
24-week, randomized, double-blind, sham device-controlled
multicenter trial. Dermatol Surg. 39:1177–1183. 2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Jimenez JJ, Wikramanayake TC, Bergfeld W,
Hordinsky M, Hickman JG, Hamblin MR and Schachner LA: Efficacy and
safety of a low-level laser device in the treatment of male and
female pattern hair loss: A multicenter, randomized, sham
device-controlled, double-blind study. Am J Clin Derm. 15:115–127.
2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lanzafame RJ, Blanche RR, Bodian AB,
Chiacchierini RP, Fernandez-Obregon A and Kazmirek ER: The growth
of human scalp hair mediated by visible red light laser and LED
sources in males. Lasers Surg Med. 45:487–495. 2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Woodcock JP: Doppler ultrasound in
clinical diagnosis. Br Med Bull. 36:243–248. 1980.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Vranić E: Sonophoresis-mechanisms and
application. Bosn J Basic Med Sci. 4:25–32. 2004.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Boucaud A: Trends in the use of
ultrasound-mediated transdermal drug delivery. Drug Discov Today.
9:827–828. 2004.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Gebauer D, Mayr E, Orthner E and Ryaby JP:
Low-intensity pulsed ultrasound: Effects on nonunions. Ultrasound
Med Biol. 31:1391–1402. 2005.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kremkau FW: Cancer therapy with
ultrasound: A historical review. J Clin Ultrasound. 7:287–300.
1979.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Oohashi T, Nishina E, Honda M, Yonekura Y,
Fuwamoto Y, Kawai N, Maekawa T, Nakamura S, Fukuyama H and
Shibasaki H: Inaudible high-frequency sounds affect brain activity:
Hypersonic effect. J Neurophysiol. 83:3548–3558. 2000.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Oohashi T, Kawai N, Nishina E, Honda M,
Yagi R, Nakamura S, Morimoto M, Maekawa T, Yonekura Y and Shibasaki
H: The role of biological system other than auditory air-conduction
in the emergence of the hypersonic effect. Brain Res.
1073-1074:339–347. 2006.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kawai N, Honda M, Nakamura S, Samatra P,
Sukardika K, Nakatani Y, Shimojo N and Oohashi T: Catecholamines
and opioid peptides increase in plasma in humans during possession
trances. Neuroreport. 12:3419–3423. 2001.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Yagi R, Nishina E, Honda M and Oohashi T:
Modulatory effect of inaudible high-frequency sounds on human
acoustic perception. Neurosci Lett. 351:191–195. 2003.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Denda M and Nakatani M: Acceleration of
permeability barrier recovery by exposure of skin to 10-30 kHz
sound. Br J Dermatol. 162:503–507. 2010.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Magerl M, Kauser S, Paus R and Tobin DJ:
Simple and rapid method to isolate and culture follicular papillae
from human scalp hair follicles. Exp Dermatol. 11:381–385.
2002.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Messenger AG: The culture of dermal
papilla cells from human hair follicles. Br J Dermatol.
110:685–689. 1984.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Philpott MP, Sanders DA and Kealey T:
Effects of insulin and insulin-like growth factors on cultured
human hair follicles: IGF-I at physiologic concentrations is an
important regulator of hair follicle growth in vitro. J Invest
Dermatol. 102:857–861. 1994.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kwack MH, Sung YK, Chung EJ, Im SU, Ahn
JS, Kim MK and Kim JC: Dihydrotestosterone-inducible dickkopf 1
from balding dermal papilla cells causes apoptosis in follicular
keratinocytes. J Invest Dermatol. 128:262–269. 2008.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Philpott MP, Green MR and Kealey T: Human
hair growth in vitro. J Cell Sci. 97:463–471. 1990.PubMed/NCBI
|
|
34
|
Miyauchi S, Hashimoto K and Miki Y: The
innermost cell layer of the outer root sheath is positive with
Ki-67. J Invest Dermatol. 95(393)1990.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Commo S and Bernard BA:
Immunohistochemical analysis of tissue remodelling during the
Anagen-catagen transition of the human hair follicle. Br J
Dermatol. 137:131–38. 1997.PubMed/NCBI
|
|
36
|
Messenger AG and Rundegren J: Minoxidil:
Mechanisms of action on hair growth. Br J Dermatol. 150:186–194.
2004.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Kwack MH, Kim MK, Kim JC and Sung YK:
Dickkopf 1 promotes regression of hair follicles. J Invest
Dermatol. 132:1554–1560. 2012.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Foitzik K, Lindner G, Mueller-Roever S,
Maurer M, Botchkareva N, Botchkarev V, Handjiski B, Metz M, Hibino
T, Soma T, et al: Control of murine hair follicle regression
(catagen) by TGF-beta1 in vivo. FASEB J. 14:752–760.
2000.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Kuttenn F, Mowszowicz I, Wright F, Baudot
N, Jaffiol C, Robin M and Mauvais-Jarvis P: Male
pseudohermaphroditism: A comparative study of one patient with 5
alpha-reductase deficiency and three patients with the complete
form of testicular feminization. J Clin Endocrinol Metab.
49:861–865. 1979.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Randall VA, Thornton MJ, Hamada K and
Messenger AG: Androgen action in cultured dermal papilla cells from
human hair follicles. Skin Pharmacol. 7:20–26. 1994.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Riccio G, Bottone S, La Regina G, Badolati
N, Passacantilli S, Rossi GB, Accardo A, Dentice M, Silvestri R,
Novellino E and Stornaiuolo M: A negative allosteric modulator of
WNT receptor Frizzled 4 switches into an allosteric agonist.
Biochemistry. 57:839–851. 2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Abramavičius S, Volkevičiūtė A, Tunaitytė
A, Venslauskas M, Bubulis A, Bajoriūnas V and Stankevičius E:
Low-Frequency (20 kHz) ultrasonic modulation of drug action.
Ultrasound Med Biol. 46:3017–3031. 2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Zhao Q, Lu Y, Gan X and Yu H: Low
magnitude high frequency vibration promotes adipogenic
differentiation of bone marrow stem cells via P38 MAPK signal. PLoS
One. 12(e0172954)2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Nikukar H, Reid S, Tsimbouri PM, Riehle
MO, Curtis AS and Dalby MJ: Osteogenesis of mesenchymal stem cells
by nanoscale mechanotransduction. ACS Nano. 7:2758–2767.
2013.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Mammoto A, Mammoto T and Ingber DE:
Mechanosensitive mechanisms in transcriptional regulation. J Cell
Sci. 125:3061–3073. 2012.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Ricca BL, Venugopalan G and Fletcher DA:
To pull or be pulled: Parsing the multiple modes of
mechanotransduction. Curr Opin Cell Biol. 25:558–564.
2013.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Engler AJ, Sen S, Sweeney HL and Discher
DE: Matrix elasticity directs stem cell lineage specification.
Cell. 126:677–689. 2006.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Thompson M, Woods K, Newberg J, Oxford JT
and Uzer G: Low-intensity vibration restores nuclear YAP levels and
acute YAP nuclear shuttling in mesenchymal stem cells subjected to
simulated microgravity. NPJ Microgravity. 6(35)2020.PubMed/NCBI View Article : Google Scholar
|