Introduction
Stuttering is a serious communication disorder,
characterized by involuntary disruptions in speech, and it
significantly affects or impedes the capacity of individuals to
communicate efficiently (1). A
higher prevalence rate of stuttering (~1.4%) is reported in
children and adolescents aged (2-19)
years old (2).
The occurrence of stuttering may be related to
cellular physiological, genetic, neurological and psychological
disorders, and this is significantly affected by environmental
factors (3-5).
For example, in previous studies, in 71% of patients who stuttered,
their condition could be attributed to genetic factors, whereas in
the other 29% of patients, their condition could be attributed to
environmental factors (6).
Stuttering has been shown to significantly affect behavioral,
cognitive, psychological and social aspects of a patient (3,4). In
the preadolescent population, 4-5% of individuals exhibit stuttered
speech, whereas in the adult, this decreases to ≤1% of individuals
(7-10).
Previous studies proposed that the
neuropathophysiology of stuttering may involve deficiencies in
connectivity amongst the cortical networks of regions that normally
allows left-sided engagement of the inferior frontal and premotor
cortices for efficient planning and execution of sound production,
and have also suggested that this deficiency in connectivity of the
left premotor/motor areas may be related to differences in the
right hemisphere and subcortical activity in individuals who
stutter (11). Stuttering
neurophysiology has been shown to be linked with other non-speech
related cognitive functions. For example, perception of speech is
known to activate the same sensorimotor mechanisms that are
involved in speech production (12-14),
with activation levels that typically correlate with task
difficulty (15). This leads to an
increase in sensorimotor activity, which appears to be associated
with cognitive resource allocation (e.g. attention and working
memory) and subsequently more difficult tasks, such as
discriminating specific speech in a noisy environment (16). In addition, a recent review provided
a valuable means of linking the cognitive differences associated
with stuttering to the underlying deficits that impact speech
(17).
Previous studies have shown that the core symptoms
of stuttering required abnormal function of the neural circuits,
whereas the individual manifestations of stuttering behavior can
vary (18-21).
However, other factors, such as emotional reactions and motor
compensatory behaviors and therapy, may interact with core neural
deficits. For example, stuttering severity can be exacerbated by
greater demands on attention, linguistic complexity and emotional
significance of the speech context (22).
Spontaneous normal recovery from stuttering ranges
from 40-80% in children <10 years old (23), and in some studies, up to 89% of
children who stutter recovered completely by adulthood (24). Adolescence is a unique period in
life, marking the transition from childhood to adulthood, when
individuals undergo physiological and physical maturation, and this
period of life is associated with complex cognitive and social
growth (25). It is conceivable
that the additional burden of a stutter in this complicated period
of development may increase the level of stress and negative
psychological states of individuals (26). Treating stuttering amongst
adolescents is likely to remove the additional associated-negative
psychological effects.
Adolescents and older adults who stutter exhibit
more severe negative psychological states, particularly depression
and anxiety, and score lower on mental health assessments (24,27-29).
Several studies have reported an association between cellular
physiological changes in the levels of stress hormones,
particularly in diseases associated psychological disorders
(30-32).
Different treatment approaches for stuttering have
shown to be most amenable for younger aged individuals compared
with individuals in adulthood who stutter. This may be related to
the greater degree of neuronal plasticity in individuals of a
younger age compared with the less tractable and far less
responsive to treatment neurons of adults, for whom it has often
been a long-term problem (27,33-35).
Although positive clinical results with traditional
drugs, such as biperiden, clomipramine and haloperidol have been
reported (36,37), the management of stuttering is
difficult and most of the times frustrating. In addition, several
therapeutic trials based on an improvement in speech fluency, means
of communication or targeting cognitive, behavioral or
psychologically associated factors provided limited information
regarding the exact mechanisms underlying the cause of stuttering
(27-29,34,35).
Alternative medicine strategies based on the use of
herbal medicines to treat stuttering or related consequences such
as depression, anxiety and other mental health problems are poorly
represented in the field of stuttering therapeutics.
Green tea (Camellia sinensis), is one of the
most popular beverage drinks consumed worldwide (36-38).
Epidemiological studies suggested that green tea consumption may be
associated with several beneficial effects on human health
(36-38).
It has been shown to beneficial in the management of several
diseases, such as adiposity, metabolic disorders, cardiovascular
diseases and cancer (36,38). Additionally, green tea has been to
shown to exert beneficial effects on an individual's psychological
state. It also possesses antioxidant, antimicrobial and
anti-inflammatory activities due to the polyphenolic compounds
present in green tea, such as theanine (also known as
γ-glutamylethylamide) a novel amino acid present in green tea that
is significantly associated with relaxation and a reduction in
cognitive stress, depression and anxiety amongst humans (39-43).
Recently, the safety, tolerability and
pharmacokinetic properties of the administration of EGCG as a
single dose (ranging from 50-1,600 mg) were assessed. The results
showed that administration of >1 g of purified EGCG or in green
tea has a mean terminal elimination half-life (t1/2z) of 1.9-4.6 h.
The data showed that doses of purified EGCG ≤1,600 mg were
generally well tolerated (44).
Hepatotoxicity of high doses of purified EGCG intake or
decaffeinated green tea were more pronounced in subjects with a
high body mass index (BMI), or when green tea extracts where used
in food supplements for weight control (45). In addition, it was reported that
consumption of ≥5 cups/day green tea did not elevate alanine
transferase (ALT) levels, a measure of hepatotoxicity (45). Catechins from green tea, prepared in
the traditional manner, as well is reconstituted drinks with an
equivalent composition to traditional green tea infusions were, in
general, considered safe. However, in rare cases, green tea
infusions have been shown to induce minor harmful effects on the
liver (45).
Epidemiological studies have identified an
association between caffeine intake and mental health stress and
lifestyle (22,46,47).
However, little is known regarding the effect of green tea
supplements on stuttering and/or the related psychological
consequences. Thus, in this study, the potential protective role of
green tea, as a natural source of polyphenols, on stuttering or its
related mental health consequences in adolescents with moderate
stuttering (MS) were assessed. In addition, the physiological
profile of stress hormones as a measure of the response to green
tea was measured amongst participants.
Materials and methods
Participants
A total of 60 school students (median age, 12.1; age
range, 12-18 years old; female/male, 9/51) who following the same
academic curricula were selected from different public schools and
randomly invited to participate in this study. A sample size of 60
was selected from the public schools to give an estimated power of
96% and a significance level of 0.05, with an expected frequency of
8.3%. For all participants, to estimate the severity of the
stuttering, one standardized questionnaire (depression anxiety
stress scale-42; DASS-42) and one standardized test material
(Stuttering Severity Instrument, 4th Edition; SSI-4) were used as
previously reported (48-50)
Then, the participants were clearly diagnosed by an Expert Speech
Language Pathologist with >15 years of experience in his
respective field, and the students were classified into two groups;
normal healthy group (n=30) and a MS group (n=30). None of the
selected participants had any chronic diseases, an abnormal iron
status, iron or vitamin deficiency, or mental diseases that could
prevent them from participating in this study. In addition,
participants classed as obese (BMI≥25) or who received prescribed
drugs (including hormonal contraceptives), or who had medical or
psychiatric disorders that may cause or exacerbate the stuttering,
or a negative emotional state were excluded from this study. All
subjects were free of neurological or medical disorders. None of
the studied subjects were under active behavioral or
pharmacological stuttering therapy at the time of study
participation. During the entire study, the supervision and follow
up of all participants was performed at an outpatient clinic for
rehabilitation: The Medical Rehabilitation Department, King Khalid
University. The aims and methodology of this study as well as a
complete description of the potential effects of green tea were
explained to all participants. Informed consent was obtained from
the parents of all students recruited in the present study.
Research involving human subjects complied with all relevant
national regulations and institutional policies, and were approved
by the Review Board (HAPO-06-B-001) of The Deanship of Scientific
Research, King Khalid University (Abha, Saudi Arabia; approval no.
ECM#2019-117). All studies complied with the guidelines described
in the 1975 Declaration of Helsinki (51,52).
The demographic and baseline data of the participants are shown in
Table I.
 | Table IDemographics and baseline
characteristics of the participantsc. |
Table I
Demographics and baseline
characteristics of the participantsc.
| Parameters | Control group | Moderate stuttering
group | Degree of
freedom | t (P-value) |
|---|
| N | 30 | 30 | - | - |
| Male/female | 25/5 | 26/4 | - | - |
| Age, years, | 12.3±4.1 | 11.9±5.8 | 198 | -0.231 (0.82) |
| Body mass index,
kg/m2 | 22.8±3.2 | 23.7±1.8 | 198 | -1.57
(0.01a) |
| Waist-hip
ratio | 0.82±0.09 | 1.38±0.05 | 198 | -26.1
(0.001b) |
Assessment of stuttering severity
The SSI-4 quantifies disfluency duration, frequency
and physical features in preschool children through to adulthood
(48-50).
The SSI-4 enables the assessment of behavioral severity levels in
both readers and non-readers. Classification of stuttering severity
based on the total score and percentile ranks was as follows: No
(total score, 10-17; percentile rank, 1-11), mild (total score,
18-24; percentile rank, 12-40), moderate (total score, 25-31;
percentile rank, 41-77), severe (total score, 32-36; percentile
rank, 78-95), and very severe (total score, 37-46; percentile rank,
96-99) (48-50).
Assessment of green tea phenolic
compounds
Commercial decaffeinated green tea bags produced by
Kao Ltd., were purchased from a convenience store. Green tea leaves
(tea bags) were analyzed for the presence of catechins and caffeine
constituents in green tea (100 mg) according to previously reported
preparation techniques (53,54).
A HP1050 Knauer liquid chromatograph equipped with a
200x4.6 mm, dp 5 µm, Hypersil ODS column, a UV-visible detector
(230 nm), and a Rheodyne injector with a 20-µl loop was used to
estimate the contents of catechins and caffeine constituents in the
leaves of decaffeinated green tea bags. A total of 5 µl aqueous
extract was injected. Total run time for the separation was 15 min
at a flow rate of 0.60 ml/min and the mobile phase yielded results
of 40% methanol: 60% distilled water. The phenolic contents of the
extracts were determined by use of calibration plots constructed
for every standard. Finally, the peaks of each phenolic content was
identified and measured at 520 nm. For all the phenolic acids,
calibration plots were linear in a range of 0.0625-1 mg ml-1
(R2>0.9980) (53,54).
Preparation and uses of green tea
supplement
Previous research studies have showed that
individuals who drank 2-3 cups of green tea a day benefitted the
most from the full antioxidant benefits of the polyphenols in green
tea (53). However, in order to
drink more cups of tea (6-8),
decaffeinated green tea was used to prevent any effects of caffeine
present in green tea leaves (54,55),
and 5-7 cups of decaffeinated green tea a day significantly
promoted cardiovascular and metabolic health (56). Thus, in the present study,
decaffeinated green tea bags were steeped in boiled water (300
ml/cup) for 10 min. No sugar or milk was added to the tea, but
artificial sweetener was used in accordance with the preference of
the participants. The contents of polyphenols per each cup of green
tea are provided in Table II. In
this study; participants were provided with 6 cups of green tea in
a container and were asked to consume the entire amount within 6-8
h every day for 6 weeks. From previous studies that recommend that
administration of >5 cups of decaffeinated green tea, which
contained a total phenolic compound dose of 50-1,600, it was shown
that these doses were well tolerated and did not have any notable
side effects (44,45,53-56).
Thus, in the present study, it was proposed that administration of
6 cups of decaffeinated green tea bags was safe and may have
considerable potency for improving the negative emotional states of
participants. In addition, the HPLC results showed that 6 cups of
decaffeinated green tea had a safe amount of EGCG (548 mg) and
total catechin content (1,536 mg) (Table III). To avoid any changes in the
proper constituents of green tea, participants were told not to
reheat the tea during the day, and to drink it straight from the
container (54,55). Although all participants had no iron
disorders or anemia, they were advised to wait at least 1 h after
eating before drinking green tea to avoid the influence of green
tea on iron absorption and iron status. During the entire study
period, all participants were under medical observation by
qualified medical doctors to detect any hepato- or renal
cytotoxicity as a result of green tea consumption. All participants
were subjected for the routine analysis of serum aspartate
transferase (AST), alanine transferase (ALT), total bilirubin and
creatinine levels following treatment with tea (six cups/6 weeks).
In addition, the participants were instructed not to change their
normal habits or use any other soft or hot drinks containing
caffeine or drugs that may have affected the results during the
period of study.
 | Table IIDaily dosage of catechins from green
teaa. |
Table II
Daily dosage of catechins from green
teaa.
| Phenolic compound,
mg | 1 cup | 4 cups | 5 cups | 6 cups |
|---|
| EGCG | 96 | 396 | 476 | 548 |
| EGC | 54 | 218 | 287 | 332 |
| ECG | 39 | 156 | 210 | 238 |
| EC | 31 | 128 | 156 | 189 |
| Total
catechinb | 256 | 986 | 1350 | 1536 |
| Theanine | 0.03 | 0.13 | 0.16 | 0.18 |
 | Table IIIHepatotoxic and renal effects of
administration of drinking six cups of green tea/day for 6
weeksa. |
Table III
Hepatotoxic and renal effects of
administration of drinking six cups of green tea/day for 6
weeksa.
| | Control group | Moderate stuttering
group |
|---|
| Parameters | Before | After | P-value | Before | After | P-value |
|---|
| AST, U/l | 13.8±3.6 | 13.9±2.9 | 0.142 | 14.3±2.5 | 14.5±1.9 | 0.18 |
| ALT, U/l | 11.5±5.7 | 12.6±5.2 | 0.381 | 13.3±2.5 | 13.7±2.8 | 0.36 |
|
Total-bilirubin | 0.45±0.12 | 0.50±0.34 | 0.145 | 0.52±0.15 | 0.58±0.18 | 0.16 |
| Creatinine | 0.57±0.18 | 0.62±0.12 | 0.134 | 0.61±0.21 | 0.68±0.31 | 0.14 |
Assessment of negative emotional
states
All participants were subjected to the estimation of
negative emotional states pre- and post-green tea administration
(six cups/day for 6 weeks). Before and after green tea
administration, all participants were under careful follow up by an
Expert Speech Language Pathologist with >15 years of experience
in their respective field. The initial changes in the scores of
depression, anxiety, stress and mental health, as measures of the
individuals psychological state, were evaluated before and after
green tea supplementation as follows.
Assessment of depression, anxiety and
stress
All participants underwent estimation of negative
emotional states of depression, anxiety and stress using DASS, as
previously reported (56). The DASS
questionnaire consists of 42 items designed to assess three related
undesirable emotional states: Depression, anxiety and
tension/stress (56). Each item has
a four-point rating scale: Did not apply to me at all (score 0)-to
applied to me very much (score 3). DASS has 14 items each for
depression, anxiety and stress (14+14+14=42). The score for each of
the respondents over each of the sub-scales (depression, anxiety
and stress) were then estimated as per the severity-rating index:
Normal, mild, moderate, severe and extremely severe (56).
Assessment of mental health
status
Mental health status was evaluated in all
participants using the 12-item General Health Questionnaire (GHQ
12) as previously reported (42,57).
In general practice, the GHQ 12 is recommended as a screening tool
for assessing mental ill-health (42,56).
In this questionnaire, responses to each item were coded on
(0-0-1-1). The sum of all the questions is the final GHQ score, and
is in an integer between 0-12. Participants with a GHQ score ≥4 are
considered to have mental ill-health problems (58,59).
Assessment of adrenal hormones
Blood samples were taken once from for all
participants at 8.30 AM pre and post green tea consumption (all 6
cups). After centrifugation, serum samples of all tests were stored
at -80˚C until required. Several adrenal hormones were measured in
order to detect several major adrenal pathways. Both cortisol and
acetylcholine (ACTH) were measured in serum samples using RIA-ELISA
(cat. no. KIPI28000; DPC Inc.) and SIA-ELISA kits (cat. no.
M046006; MD Biosciences Inc.), respectively according to the
manufacturer's protocol. Serum levels of DHEA were measured using
an immunoassay according to the manufacturer's protocol (cat. no.
DHA31-K01; IMMULITE 1000, Diagnostic Products Corporation), with an
intra-assay coefficient of variation of <5.2%. Serum levels of
corticosterone were measured using a quantitative competitive
enzyme immunoassay technique that measures corticosterone levels
according to the manufacturer's protocol (cat. no. EC3001-1; Assay
pro LLC.).
Statistical analysis
Statistical analysis was performed using SPSS
version 17 (SPSS Inc.). Data are presented as the mean ± SD. For
all continuous variables, such as the levels of stress hormones,
and the renal and hepatotoxicity parameters, a paired t-test was
used. A Wilcoxon signed rank sum test was performed for
non-parametric data (depression, anxiety, stress and mental health
scores). Pearson's correlation analysis was performed to calculate
the correlations between the levels of adrenal hormones and the
scores of stuttering, stress, anxiety and depression in adolescents
with moderate stuttering. P<0.05 was considered to indicate a
statistically significant difference.
Results
In this study, the beneficial effects of green tea
supplementation on stuttering and its associated negative
psychological states were evaluated in adolescents. A total of 60
adolescents aged 12-18 years old of both sexes were enrolled in the
present study, and classified using SSI-4 for estimation of the
severity of the stuttering. The participants were classified into
two groups; normal healthy group (n=30) and the MS group (n=30), as
shown in Table I. The data showed
that adolescents in the experimental group were significantly more
obese, as measured by BMI, (P=0.01) and had a larger waist-hip
ratio (P=0.001) compared with the healthy controls (Table I).
Assessment of active constituents in
decaffeinated green tea bags
Green tea polyphenolic compounds were evaluated
using liquid chromatography analysis as shown in Table II. The results showed that the
total catechin content in one cup of decaffeinated green tea bags
was 256 mg. The data showed that drinking six cups of green tea
contained a total catechin content of 1,580 mg as well as
containing thiamine and other related polyphenols (Table II).
Assessments of the cumulative side
effects of consumption of six cups of decaffeinated green tea
The cumulative side effects of administration of six
cups of decaffeinated green tea containing EGCG and other catechins
for 6 weeks on both the liver and kidneys were estimated (Table III). The results showed no hepato-
or renal toxicity in all participants following administration of
decaffeinated green tea. In this experiment, the concentrations of
serum AST, ALT, total bilirubin and creatinine did not increase
notably following treatment with tea (six cups/6 weeks). The data
obtained after 6 six did not differ significantly from that
obtained prior to initiation of the study (Table III).
Assessment of the effects of green tea
on depression, anxiety and stress
The correlation between negative psychological
states and the incidence of stuttering amongst adolescents was
evaluated using DASS and GHQ 12 tests (Fig. 1). The data showed that there was a
significant reduction in the DASS scores for depression (Fig. 1A), anxiety (Fig. 1B) and stress (Fig. 1C), as well as a decrease in GHQ-12
scores (Fig. 1D) for mental health
in both control (P=0.01) and adolescents with MS (P=0.001) at the
end of the study procedure. The data showed that the administration
of six cups of green tea for 6 weeks significantly improved
depression, anxiety, stress and mental health status amongst
adolescents with MS whilst also improving the mental health status
of healthy subjects as well. As show in Table IV, a significant improvement in the
scores of depression, anxiety, stress and mental health status were
observed among adolescents with MS. Depression, anxiety, stress and
mental health status among subjects with MS were significantly
reduced.
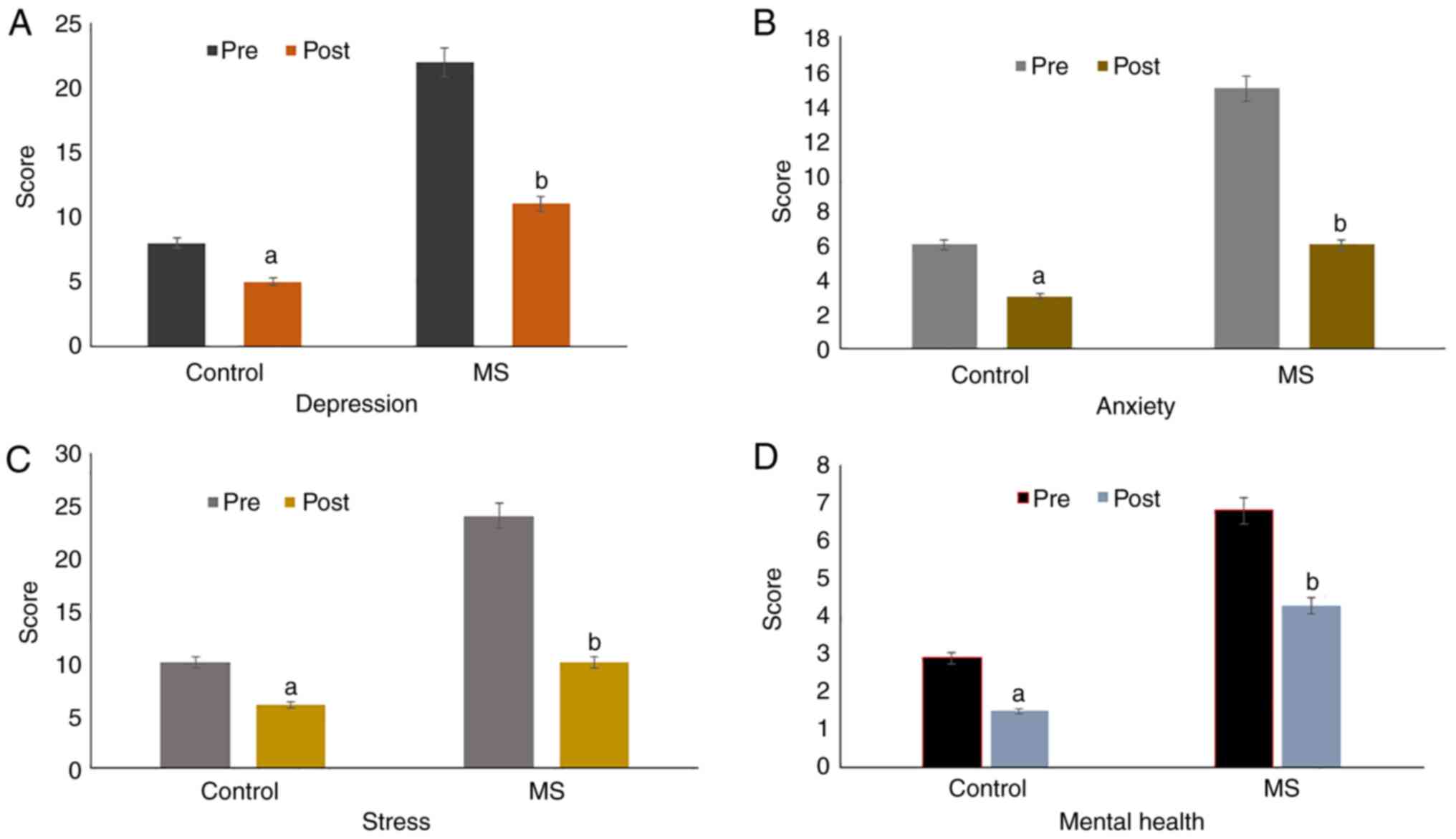 | Figure 1Graphical representation of the
scores obtained for DASS and GHQ-12 scores for estimating the
negative psychological states (A) depression, (B) anxiety, (C)
stress and (D) and mental health in the control and MS patients
pre- and post-green tea consumption (six cups/day for 6 weeks). The
results showed a significant decrease in the negative psychological
traits of depression, anxiety, stress and mental health status in
the Control (P=0.01; pre vs. post) and adolescents with MS
(P=0.001; pre vs. post). The results are presented as the mean ±
SD. A Wilcoxon signed rank sum test was performed for analysis of
non-parametric data, including depression, anxiety, stress and
mental health scores. P<0.05 was considered to indicate a
statistically significant difference. DASS, depression anxiety
stress scale; GHQ, General Health Questionnaire; MS, moderate
stuttering. |
 | Table IVImprovement of stuttering and mental
status in adolescents with moderate stuttering before and after the
green tea regimenc. |
Table IV
Improvement of stuttering and mental
status in adolescents with moderate stuttering before and after the
green tea regimenc.
| | Stuttering and
psychological status | Clinical
response |
|---|
| Parameters | Before | After | P-value | Before | After | P-value |
|---|
| Depression
score | 22±3 | 11±2b |
<0.001b | Moderate | Mildb |
<0.001b |
| Stress score | 24±5 | 10±2b |
<0.001b | Moderate | Mildb |
<0.001b |
| Anxiety score | 15±3 | 8±2a |
<0.01a | Moderate | Milda |
<0.01a |
| Mental health
scores | 6.8±3.5 |
4.3±2.8a |
<0.01a | Moderate | Milda |
<0.01a |
| Total stuttering
score (F+DSP+PC) | 32±6 | 23±5b |
<0.001b | Moderate | Mildb |
<0.001b |
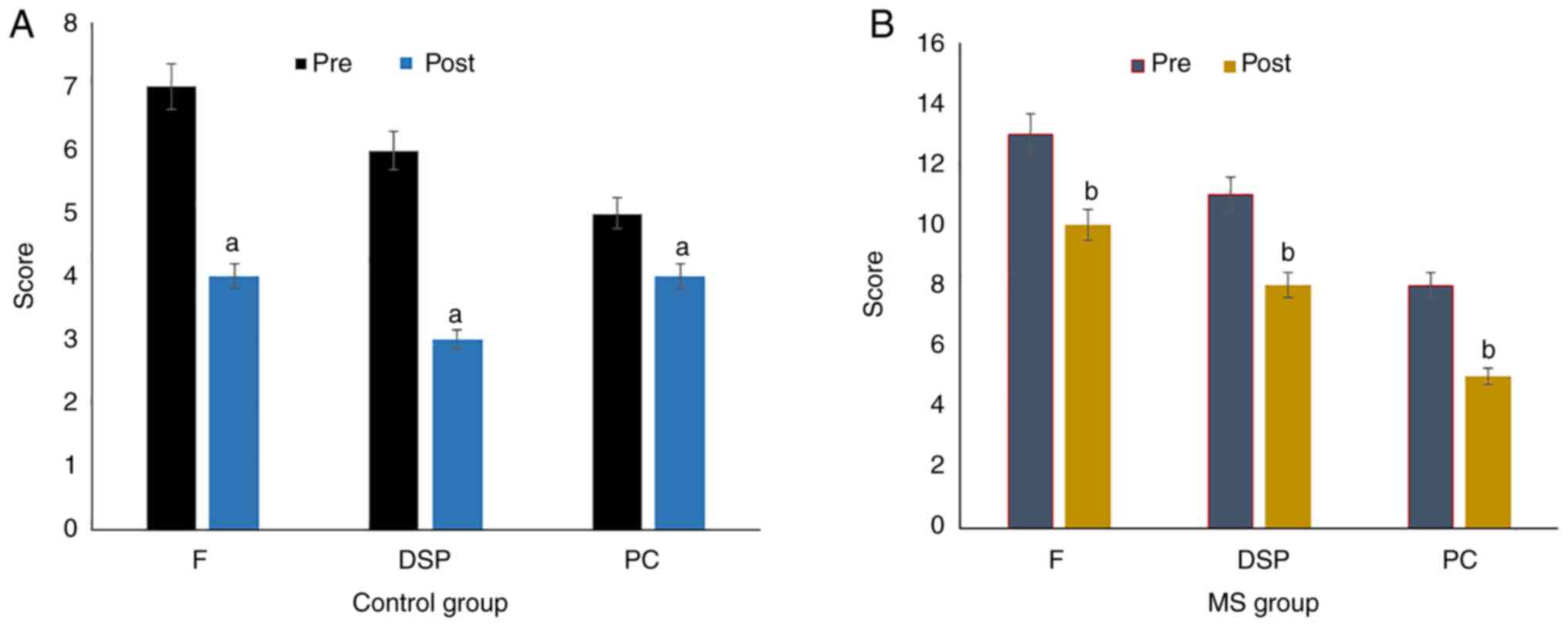
Assessment of the effects of green tea
on stuttering
The effect of green tea supplement on the severity
of stuttering among adolescents was evaluated using the SSI-4 test
(Fig. 2). Frequency, duration of
speech behavior and physical concomitants as measures of stuttering
showed significant improvements in the control subjects ((P=0.01;
Fig. 2A) as well as in the
adolescents with MS (P=0.001; Fig.
2B) following the 6 weeks of green tea consumption. In
addition, a positive clinical response to green tea supplementation
was reported in the adolescents with MS. The data showed a
significant reduction in the total score of the SSI-4 test to a
mild status (Table IV), indicating
the probable positive curative effects of green tea against
stuttering.
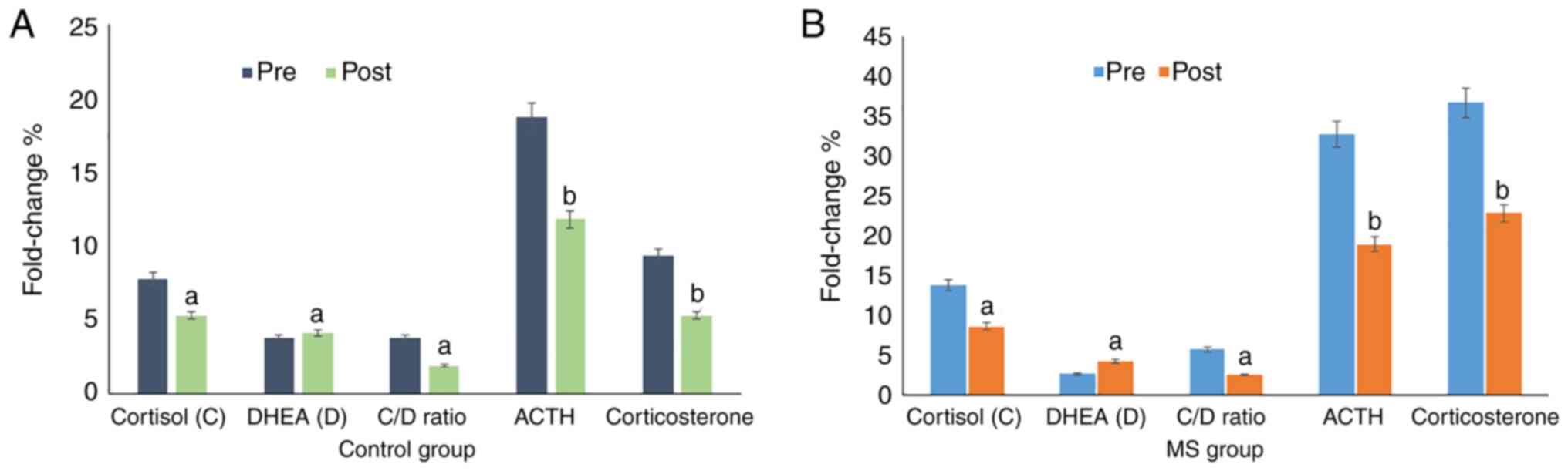
Assessment of the effects of green tea
on adrenal stress hormones
To study the relationship between the physiological
response of adrenal stress hormones with both stuttering and a
negative psychological status, the expression levels of cortisol,
DHEA, ACTH, corticosterone and the cortisol:DHEA ratio were assayed
in all participants (Table V;
Fig. 3). All subjects in the
control group (Fig. 3A) and MS
group (Fig. 3B) showed a
significant decrease (P=0.01 and P=0.001, respectively) in the
expression levels of cortisol, DHEA, ACTH and corticosterone, with
an increase in the cortisol:DHEA ratio following the 6 weeks of
green tea administration.
 | Table VCorrelation analysis of adrenal
hormones with stuttering, stress, anxiety, and depression scores in
adolescents with moderate stuttering following the green tea
regimenc. |
Table V
Correlation analysis of adrenal
hormones with stuttering, stress, anxiety, and depression scores in
adolescents with moderate stuttering following the green tea
regimenc.
| | Cortisol | DHEA | Cortisol; DHEA
ratio | Acetylcholine | Corticosterone |
|---|
| Parameter | Before | After | P-value | Before | After | P-value | Before | After | P-value | Before | After | P-value | Before | After | P-value |
|---|
| Depression
score | 0.12 | 0.231 |
<0.01a | 0.125 | 0.29 |
<0.001b | 0.35 | 0.28 |
<0.01a | 0.22 | 0.58 |
<0.001b | 0.38 | 0.36 |
<0.001b |
| Stress score | 0.23 | 0.258 |
<0.01a | 0.326 | 0.35 |
<0.001b | 0.36 | 0.38 |
<0.01a | 0.32 | 0.56 |
<0.001b | 0.45 | 0.47 |
<0.001b |
| Anxiety score | 0.125 | 0.321 |
<0.01a | 0.258 | 0.38 |
<0.001b | 0.27 | 0.32 |
<0.01a | 0.28 | 0.86 |
<0.001b | 0.65 | 0.65 |
<0.001b |
| Mental health
score | 0.231 | 0.145 |
<0.01a | 0.231 | 0.26 |
<0.001b | 0.82 | 0.78 |
<0.01a | 0.31 | 0.27 |
<0.001b | 0.54 | 0.47 |
<0.001b |
| Stuttering
score | 0.114 | 0.256 |
<0.01a | 0.321 | 0.37 |
<0.001b | 0.89 | 0.34 |
<0.01a | 0.28 | 0.31 |
<0.001b | 0.47 | 0.61 |
<0.001b |
Together, the results highlight the beneficial
effects of decaffeinated green tea on stuttering and mental health.
In addition, the changes in expression of adrenal stress hormones
were significantly associated with the improvements in depression,
anxiety, stress and mental health status amongst adolescents who
stuttered (Table V).
Discussion
In this study the administration of six cups/day of
green tea for 6 weeks significantly reduced depression, anxiety,
stress and the mental health consequences associated with
stuttering in adolescents. The severity of stuttering was
significantly reduced to a mild status amongst adolescents who
suffered from MS.
Approximately 4-5% preadolescents suffer from some
degree of stuttering compared with ≤1% in adults (9,33,35),
and this has compounding effects on the behavioral, cognitive,
psychological and social aspects for a patient (2-4).
In addition, more severe negative psychological states are
significantly more commonly reported in adolescents and adults who
stutter, particularly in relation to mental health (31,32,39).
In this study, depression, anxiety, stress and
mental health status were significantly reduced in both the control
group and adolescents who stuttered following green tea
supplementation. The data significantly correlated with the
reduction in the severity of stuttering from moderate to mild,
highlighting the significant improvement in clinical response to
green tea. This indicates an interesting association between green
tea consumption in everyday life and mental health problems
associated with stuttering. It has been widely reported that mental
stress, anxiety and depression have a significant influence on the
psychological status of people who stutter (3,4,47-54).
Previous studies showed that the polyphenolic compounds present in
green tea, particularly catechins and theanine, enhanced relaxation
in situations where an individual were in a negative mood,
depressed or stressed (40-42,60,61).
Several studies have reported on the association
between changes associated with cellular physiological changes and
the levels of stress hormones, particularly in individuals with
psychological disorders (38-41).
Thus, in the present study, adrenal stress hormones, including
cortisol, DHEA, ACTH, corticosterone and the cortisol:DHEA ratio
were evaluated in the control and experimental group.
The levels of stress hormones were significantly
improved and this was positively correlated with the reduction in
the scores of stuttering and the related depression, anxiety,
stress and mental health consequences. The data confirmed the
potential protective role of green tea against the severity of
stuttering, particularly among younger individuals. This may be due
to the relaxing effect as well as other biological activities of
the polyphenolic compounds present in green tea (35,36,52-57).
In experimental animals, green tea polyphenols have been shown to
have a significant antidepressant-like effect on experimental
animals (40,41,60,61)].
Green tea polyphenols have been shown to reduce serum
corticosterone and ACTH levels in mice exposed to severe stressing
factors. These studies showed that green tea polyphenols exert
antidepressant-like effects in mouse behavioral models of
depression, where the mechanism likely involves inhibition of the
hypothalamic-pituitary-adrenal axis (58-61).
Recent clinical trials have shown that higher
consumption of green tea significantly lowers the prevalence
depression-like symptoms in elderly Japanese individuals (60,61).
In addition, it was reported that green tea polyphenols inhibit
monoamine oxidase enzyme activity, consequently increasing
monoamine levels in glial cells (60,61).
Conventional and newer antidepressants exert their effects
predominantly through an increase in the synaptic concentrations of
monoamines, indicating that monoamine systems play an essential
role in the mechanism of action of antidepressants and the
pathophysiology of depression (59,61-66).
In conclusion, the present study showed that green
tea as a source of natural polyphenols improved psychological
disorders associated with stuttering in adolescents. Higher
consumption of green tea (six cups/day) significantly improved the
severity of stuttering and the related negative psychological
states; depression, anxiety, stress and general mental health
status in adolescents with MS. In addition, the improvement in
stuttering and other consequences correlated positively with the
reduction in the expression levels of adrenal stress hormones.
However, additional studies with larger cohorts are required for a
more thorough evaluation of the biological effects of green tea on
stuttering.
Acknowledgements
The authors are grateful to the Deanship of
Scientific Research at King Khalid University, Abha, Saudi Arabia
for funding this work through a Research Group Project under grant
number (RGP2/205/43).
Funding
Funding: This project was funded by the Deanship of Scientific
Research at King Khalid University (Abha, Saudi Arabia; grant no.
RGP2/205/43). The funding body played no role in the study design,
manuscript writing, or decision to submit the manuscript for
publication.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
AA and SAG conceived and designed the study,
performed the practical work, analyzed the data, and wrote and
edited the manuscript. AA and SAG have read and approved the final
manuscript. AA and SAG confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
The aims and methodology of this study as well as a
complete description of the potential effects of green tea were
explained to all participants. Informed consent was obtained from
the parents of all students recruited in the present study.
Research involving human subjects complied with all relevant
national regulations and institutional policies, and were approved
by the Review Board (HAPO-06-B-001) of The Deanship of Scientific
Research, King Khalid University (Abha, Saudi Arabia; approval no.
ECM#2019-117). All studies complied with the guidelines described
in the 1975 Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bloodstein O and Ratner NB: A Handbook on
Stuttering. 6th edition. Thomson Delmar, Clifton Park, NY,
2008.
|
|
2
|
Craig A, Hancock K and Cobbin D: Managing
adolescents who relapse following treatment for stuttering. Asia
Pac J Speech Lang Hear. 7:79–91. 2002.
|
|
3
|
Yaruss JS and Quesal RW: Stuttering and
the international classification of functioning, disability, and
health: An update. J Commun Disord. 37:35–52. 2004.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Guitar B: Stuttering: An Intergrated
Approach to Its Nature and Management. Lippincott Williams &
Wilkins, Baltimore, MD, 2006.
|
|
5
|
Howell P: Signs of developmental
stuttering up to age eight and at 12 plus. Clin Psychol Rev.
27:287–306. 2007.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Peters TJ and Guitar B: Stuttering: An
Integrated Approach to Its Nature and Treatment. Williams and
Wilkins, Maryland, MD, 1991.
|
|
7
|
Yairi E, Ambrose N and Cox N: Genetics of
stuttering: A critical review. J Speech Hear Res. 39:771–784.
1996.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Månsson H: Childhood stuttering: Incidence
and development. J Fluency Disord. 25:47–57. 2000.
|
|
9
|
Guitar B: Stuttering: An integrated
approach to its nature and treatmen. Lippincott Williams &
Wilkins, 2013.
|
|
10
|
Weston CG and Riolo SA: Childhood and
adolescent precursors to adult personality disorders. Psychiatr
Ann. 37:114–120. 2007.
|
|
11
|
Chang SE, Horwitz B, Ostuni J, Reynolds R
and Ludlow CL: Evidence of left inferior frontal-premotor
structural and functional connectivity deficits in adults who
stutter. Cereb Cortex. 21:2507–2518. 2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Callan D, Callan A, Gamez M, Sato MA and
Kawato M: Premotor cortex mediates perceptual performance.
NeuroImage. 51:844–858. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Bowers A, Saltuklaroglu T, Jenson D,
Harkrider A and Thornton D: Power and phase coherence in
sensorimotor mu and temporal lobe alpha components during covert
and overt syllable production. Exp Brain Res. 237:705–721.
2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Bowers A, Saltuklaroglu T, Harkrider A and
Cuellar M: Suppression of the µ rhythm during speech and non-speech
discrimination revealed by independent component analysis:
Implications for sensorimotor integration in speech processing.
PLoS One. 8(e72024)2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Szenkovits G, Peelle JE, Norris D and
Davis MH: Individual differences in premotor and motor recruitment
during speech perception. Neuropsychologia. 50:1380–1392.
2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Jenson D, Thornton D, Harkrider AW and
Saltuklaroglu T: Influences of cognitive load on sensorimotor
contributions to working memory: An EEG investigation of mu rhythm
activity during speech discrimination. Neurobiol Learn Mem.
166(107098)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Jenson D, Bowers AL, Hudock D and
Saltuklaroglu T: The application of EEG Mu rhythm measures to
neurophysiological research in stuttering. Front Hum Neurosci.
13(458)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chang SE, Garnett EO, Etchell A and Chow
HM: Functional and neuroanatomical bases of developmental
stuttering: Current insights. Neuroscientist. 25:566–582.
2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chang SE: Research updates in neuroimaging
studies of children who stutter. Semin Speech Lang. 35:67–79.
2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lu C, Chen C, Ning N, Ding G, Guo T, Peng
D, Yang Y, Li K and Lin C: The neural substrates for atypical
planning and execution of word production in stuttering. Exp
Neurol. 221:146–156. 2010.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Alm PA: Stuttering and the basal ganglia
circuits: A critical review of possible relations. J Commun Disord.
37:325–369. 2004.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Smith A: Effects of caffeine on human
behavior. Food Chem Toxicol. 40:1243–1255. 2002.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ham RE: Therapy of Stuttering: Preschool
Through Adolescence. Prentice-Hall, Englewood Cliffs, NJ, 1990.
|
|
24
|
Yairi E, Ambrose NG, Paden EP and
Throneburg RN: Predictive factors of persistence and recovery:
Pathways of childhood stuttering. J Commun Disord. 29:51–77.
1996.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Spear LP: The adolescent brain and
age-related behavioral manifestations. Neurosci Biobehav Rev.
24:417–463. 2000.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kelly KB: Promoting adolescent health.
Acta Paediatr. 96:1389–1391. 2007.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Lincoln M, Packman A and Onslow M: Altered
auditory feedback and the treatment of stuttering: A review. J
Fluency Disord. 31:71–89. 2006.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lowe R, Guastella AJ, Chen NT, Menzies RG,
Packman A, O'Brian S and Onslow M: Avoidance of eye gaze by adults
who stutter. J Fluency Disord. 37:263–274. 2012.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Bricker-Katz G, Lincoln M and McCabe P: A
life-time of stuttering: How emotional reactions to stuttering
impact activities and participation in older people. Disabil
Rehabil. 31:1742–1752. 2009.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Skosnik PD, Chatterton RT Jr, Swisher T
and Park S: Modulation of attentional inhibition by norepinephrine
and cortisol after psychological stress. Int J Psychophysiol.
36:59–68. 2000.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Nejtek VA: High and low emotion events
influence emotional stress perceptions and are associated with
salivary cortisol response changes in a consecutive stress
paradigm. Psychoneuroendocrinology. 27:337–352. 2002.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Agha-Hosseini F, Mirzaii-Dizgah I,
Mansourian A and Zabihi-Akhtechi G: Serum and stimulated whole
saliva parathyroid hormone in menopausal women with oral dry
feeling. Oral Surg Oral Med Oral Pathol Oral Radiol Endod.
107:806–810. 2009.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Prins D and Ingham RJ: Evidence-based
treatment and stuttering-historical perspective. J Speech Lang Hear
Res. 52:254–263. 2009.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Prado-Velasco M and Fernández-Peruchena C:
An advanced concept of altered auditory feedback as a
prosthesis-therapy for stuttering founded on a non-speech etiologic
paradigm. In: Handbook of research on personal autonomy
technologies and disability informatics. IGI Global: pp76-118,
2011.
|
|
35
|
Blomgren M: Behavioral treatments for
children and adults who stutter: A review. Psychol Res Behav Manag.
6:9–19. 2013.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kumar A and Balan S: Fluoxetine for
persistent developmental stuttering. Clin Neuropharmacol. 30:58–59.
2007.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Shaygannejad V, Khatoonabadi SA, Shafiei
B, Ghasemi M, Fatehi F, Meamar R and Dehghani L: Olanzapine versus
haloperidol: Which can control stuttering better? Int J Prev Med. 4
(Suppl 2):S270–S273. 2013.PubMed/NCBI
|
|
38
|
Kobayashi K, Nagato Y, Aoi N, Juneja LR,
Kim M, Yamamoto T and Sugimoto S: Effects of L-theanine on the
release of α-brain waves in human volunteers. Nippon Nogeikagaku
Kaishi. 72:153–157. 1998.(In Japanese).
|
|
39
|
Maron DJ, Lu GP, Cai NS, Wu ZG, Li YH,
Chen H, Zhu JQ, Jin XJ, Wouters BC and Zhao J: Cholesterol-lowering
effect of a theaflavin-enriched green tea extract: A randomized
controlled trial. Arch Intern Med. 163:1448–1453. 2003.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Jian L, Xie LP, Lee AH and Binns CW:
Protective effect of green tea against prostate cancer: A
case-control study in southeast China. Int J Cancer. 108:130–155.
2004.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Juneja LR, Chu DC, Okubo T, Nagato Y and
Yokogoshi H: L-theanine-a unique amino acid of green tea and its
relaxation effect in humans. Trends Food Sci Technol. 10:199–204.
1999.
|
|
42
|
Caspi A, Sugden K, Moffitt TE, Taylor A,
Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A
and Poulton R: Influence of life stress on depression: Moderation
by a polymorphism in the 5-HTT gene. Science. 301:386–389.
2003.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Yagyu T, Wackermann J, Kinoshita T, Hirota
T, Kochi K, Kondakor I, Koenig T and Lehmann D: Chewing-gum flavor
affects measures of global complexity of multichannel EEG.
Neuropsychobiology. 35:46–50. 1997.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Ullmann U, Haller J, Decourt JP, Girault
N, Girault J, Richard-Caudron AS, Pineau B and Weber P: A single
ascending dose study of epigallocatechin gallate in healthy
volunteers. J Int Med Res. 31:88–101. 2003.PubMed/NCBI View Article : Google Scholar
|
|
45
|
EFSA Panel on Food Additives and Nutrient
Sources added to Food (ANS). Younes M, Aggett P, Aguilar F,
Crebelli R, Dusemund B, Filipič M, Frutos MJ, Galtier P, Gott D, et
al: Scientific opinion on the safety of green tea catechins. EFSA
J. 16(e05239)2018.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Quinlan PT, Lane J, Moore KL, Aspen J,
Rycroft JA and O'Brien DC: The acute physiological and mood effects
of tea and coffee: The role of caffeine level. Pharmacol Biochem
Behav. 66:19–28. 2000.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Loke WH: Effects of caffeine on mood and
memory. Physiol Behav. 44:367–372. 1988.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Kaplan GB, Greenblatt DJ, Ehrenberg BL,
Goddard JE, Cotreau MM, Harmatz JS and Shader RI: Dose-dependent
pharmacokinetics and psychomotor effects of caffeine in humans. J
Clin Pharmacol. 37:693–703. 1997.PubMed/NCBI View Article : Google Scholar
|
|
49
|
O'Brian S, Jones M, Packman A, Menzies R
and Onslow M: Stuttering severity and educational attainment. J
Fluency Disord. 36:86–92. 2011.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Riley GD and Bakker K: SSI-4: Stuttering
severity instrument. Pro-Ed, 2009.
|
|
51
|
Declaration of Helsinki History Website.
Ethical Principles For Medical Research. The JAMA Network.
Retrieved 26 July 2015.
|
|
52
|
Vanderpool HY: The Ethics of Research
Involving Human Subjects: Facing the 21st century. University
Publishing Group, Inc., Frederick ML, pp85, 1996.
|
|
53
|
Waksmundzka M, Oniszczuk A, Szewczyk K and
Wianowska D: Effect of sample-preparation methods on the HPLC
quantitation of some phenolic acids in plant materials. Acta
Chromatogr. 19:227–237. 2007.
|
|
54
|
Gabr SA, Alghadir AH, Ghoniem GA, Zhang
XW, Choe YH, Park YJ and Kim BS: Regulation of cartilage and
inflammatory biomarkers in rheumatoid arthritis patients treated
with green tea therapy. Afr J Pharmacy Pharmacol. 8:263–273.
2014.
|
|
55
|
Pang J, Zhang Z, Zheng TZ, Bassig BA, Mao
C, Liu X, Zhu Y, Shi K, Ge J, Yang YJ, et al: Green tea consumption
and risk of cardiovascular and ischemic related diseases: A
meta-analysis. Int J Cardiol. 202:967–974. 2016.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Kuriyama S, Shimazu T, Ohmori K, Kikuchi
N, Nakaya N, Nishino Y, Tsubono Y and Tsuji I: Green tea
consumption and mortality due to cardiovascular disease, cancer,
and all causes in Japan: The Ohsaki study. JAMA. 296:1255–1265.
2006.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Wolfram S: Effects of green tea and EGCG
on cardiovascular and metabolic health. J Am Coll Nutr.
26:373S–388S. 2007.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Lovibond PF and Lovibond SH: The structure
of negative emotional states: Comparison of the depression anxiety
stress scales (DASS) with the beck depression and anxiety
inventories. Behav Res Ther. 33:335–343. 1995.PubMed/NCBI View Article : Google Scholar
|
|
59
|
McDowell I and Newell C: Measuring health:
A guide to rating scales and questionnaires. 2nd edition.
University Press, New York, Oxford, pp225-237, 1996.
|
|
60
|
Goldberg DP: The detection of psychiatric
illness by questionnaire. London: Oxford University Press,
1972.
|
|
61
|
Niu K, Hozawa A, Kuriyama S, Ebihara S,
Guo H, Nakaya N, Ohmori-Matsuda K, Takahashi H, Masamune Y, Asada
M, et al: Green tea consumption is associated with depressive
symptoms in the elderly. Am J Clin Nutr. 90:1615–1622.
2009.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Zhu WL, Shi HS, Wei YM, Wang SJ, Sun CY,
Ding ZB and Lu L: Green tea polyphenols produce antidepressant-like
effects in adult mice. Pharmacol Res. 65:74–80. 2012.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Mazzio EA, Harris N and Soliman KF: Food
constituents attenuate monoamine oxidase activity and peroxide
levels in C6 astrocyte cells. Planta Med. 64:603–606.
1998.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Haenisch B and Bönisch H: Depression and
antidepressants: Insights from knockout of dopamine, serotonin or
noradrenaline re-uptake transporters. Pharmacol Ther. 129:352–368.
2011.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Garrone G and Dick P: Monoamine oxidase
inhibitors in the treatment of depressive states. Psychiatr Neurol
(Basel). 140:107–114. 1960.PubMed/NCBI(In French).
|
|
66
|
Sagen J, Sortwell CE and Pappas GD:
Monoaminergic neural transplants prevent learned helplessness in a
rat depression model. Biol Psychiatry. 28:1037–1048.
1990.PubMed/NCBI View Article : Google Scholar
|