Introduction
The long-term safety of acid blockers is a
relatively recent concern for general practitioners as well as
gastroenterologists. Vonoprazan (VPZ), a potassium-competitive acid
blocker, was made available in 2015 and has a stronger
acid-suppressing effect than proton pump inhibitors (PPI) (1). VPZ is widely used in Japan for the
long-term treatment of gastroesophageal reflux disease as well as
Helicobacter pylori (H. pylori) eradication therapy.
We previously reported the long-term effects of PPI and VPZ on
gastric morphological changes including fundic gland polyps,
gastric hyperplastic polyps, multiple white and flat elevated
lesions, and cobblestone-like gastric mucosa (2). Recently, a new morphological change
referred to as ‘stardust gastric mucosa’ was reported, which is
strongly related to long-term VPZ therapy (3).
In 2020, Kubo et al (4) reported a novel lesion which they
termed ‘gastric mucosal redness’ in patients undergoing VPZ therapy
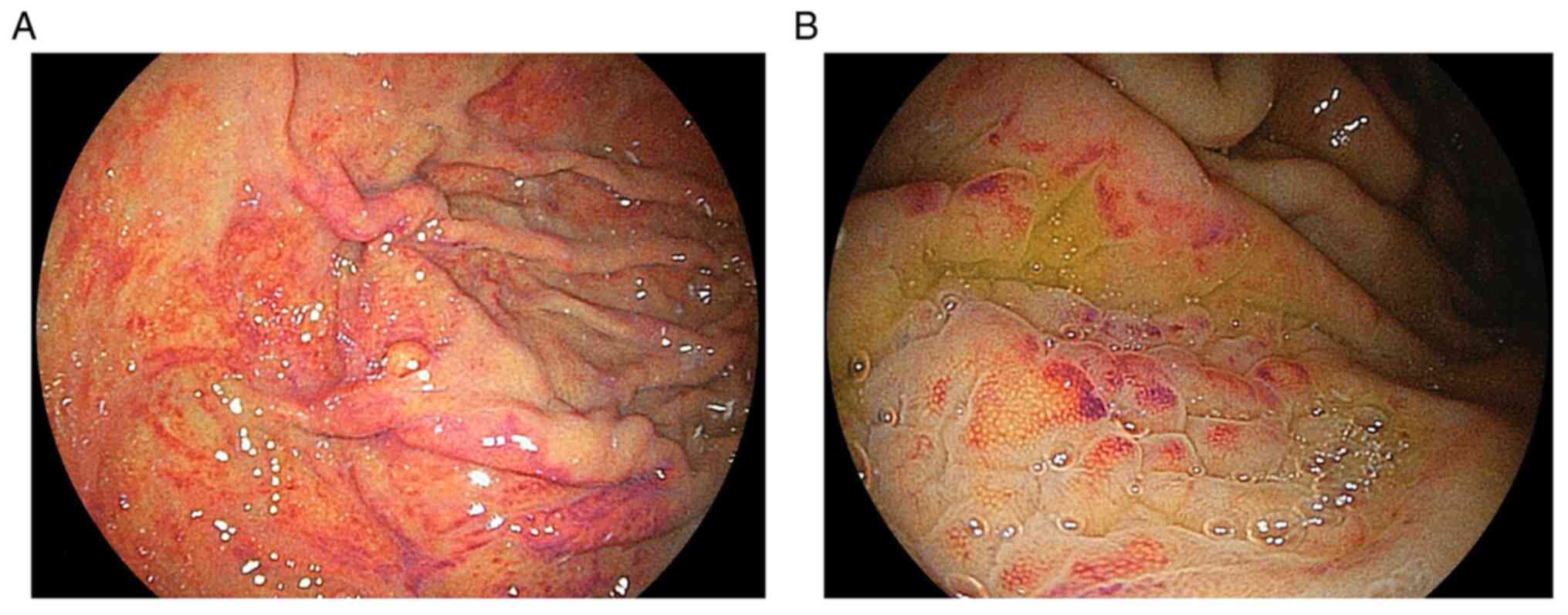
in a case series. Gastric mucosal redness was characterized by
linear or spotty red areas along the greater curvature of the
gastric body. The pathology was characterized by inflammatory cell
infiltration, oxyntic gland dilatation, and parietal cell
protrusion (5). However, the
prevalence of this finding and its association with acid blockers
were not reported. The aim of the present study was to investigate
the prevalence and risk factors for the development of gastric
mucosal redness.
Patients and methods
Study population
This study was a retrospective observational study
based on the medical records and endoscopic reports of patients who
underwent esophagogastroduodenoscopy (EGD). Patients who underwent
EGD at the Shinozaki Medical Clinic (Utsunomiya, Tochigi, Japan)
between December 2020 and November 2021 were included. The
medications taken by each patient were verified before EGD by
checking the personal ‘medicine notebook’ issued by the Japan
Pharmaceutical Association and scrupulously maintained by each
patient that documents all prescriptions regardless of the medical
facility that prescribed it. When multiple EGDs were performed on
one patient during the study period, only the first EGD was
included. At least 1-year of continuous administration of VPZ, PPI,
and H2RA was confirmed using the medicine notebook. Patients with
current H. pylori infection status (n=58), acid suppression
therapy for <1-year despite being currently treated with
acid-suppressing drugs (n=41), previous esophageal or gastric
surgery (n=27), and gastric residue (n=7) were excluded.
Consequently, 1,101 patients were finally enrolled. We
retrospectively reviewed their medical records and abstracted all
pertinent information regarding acid blockers including H2RA, PPI,
and VPZ. The present study was approved by the Institutional Review
Board of the Shinozaki Medical Clinic (approval no. ID#31-R001).
The need for written informed consent was waived due to the
retrospective design of the study.
Endoscopic evaluation
All EGDs were performed by the first author and
recorded on video. In the case of unclear findings or lack of data,
the video was immediately checked. An ultrathin endoscope
(EG-L580NW7, Fujifilm Corporation) was used and endoscopic
observation was performed using linked color imaging throughout the
procedure (6). The standard
endoscopic report included the grade of gastric atrophy classified
by the Kimura-Takemoto system (7),
the presence/absence of fundic gland polyps, gastric hyperplastic
polyps, multiple white and flat elevated lesions (8), cobblestone-like mucosa (9), stardust gastric mucosa (3) and gastric mucosal redness (4) as compulsory items. ‘Gastric mucosal
redness’ was defined as multiple spotty and/or linear areas of
redness along the greater curvature of the gastric body or fundus
in H. pylori-negative individuals (Figs. 1 and 2). The first author assessed these
compulsory items during EGD and completed the standardized
endoscopic report form just after finishing the EGD. The H.
pylori infection status was evaluated by serum IgG,
13C-urea breath test, and/or a stool antigen test. H.
pylori eradication history was confirmed by an interview with
the patients and/or review of the medical record.
Statistical analysis
The frequency of gastric mucosal changes was
compared among the four groups in this study using a χ2
test. Univariate analysis was performed using a logistic regression
model. To diminish the influence of confounding factors,
multivariate logistic regression analysis was used. Factors for
multivariate analysis were selected based on clinical significance.
To compare continuous data between two groups, a Student's t-test
was used. Statflex version 7.0 software (Artech Co. Ltd.) was used
for all statistical analyses. P<0.05 was considered to indicate
a statistically significant difference.
Results
Prevalence of gastric mucosal
redness
Almost half of the patients (47%) were treated with
acid blockers (Table I). Gastric
atrophy was present in 57% of patients (631/1,101), and 46%
(502/1,101) previously underwent successful H. pylori
eradication. None of the patients were treated with any combination
of H2RA, PPI, or VPZ. The overall prevalence of gastric mucosal
redness was 4% (48/1,101).
 | Table IBaseline characteristics and
endoscopic findings of the recruited cohort. |
Table I
Baseline characteristics and
endoscopic findings of the recruited cohort.
| Characteristic | Value |
|---|
| Age, years, mean ±
standard deviation | 62.9±14.8 |
| Sex, n (%) | |
|
Male | 492 (45%) |
|
Female | 609 (55%) |
| Acid suppression
drug, n (%) | |
|
None | 580 (53%) |
|
Histamine-2
receptor antagonist | 65 (6%) |
|
Proton pump
inhibitor | 146 (13%) |
|
Vonoprazan | 310 (28%) |
| Degree of gastric
atrophy, n (%) | |
|
None | 470 (43%) |
|
Closed
type | 296 (27%) |
|
Open
type | 335 (30%) |
| Fundic gland polyps,
n (%) | 366 (33%) |
| Gastric hyperplastic
polyps, n (%) | 48 (4%) |
| Multiple white and
flat elevated lesions, n (%) | 235 (21%) |
| Cobblestone-like
mucosa, n (%) | 52 (5%) |
| Stardust gastric
mucosa, n (%) | 168 (15%) |
| Gastric mucosal
redness, n (%) | 48 (4%) |
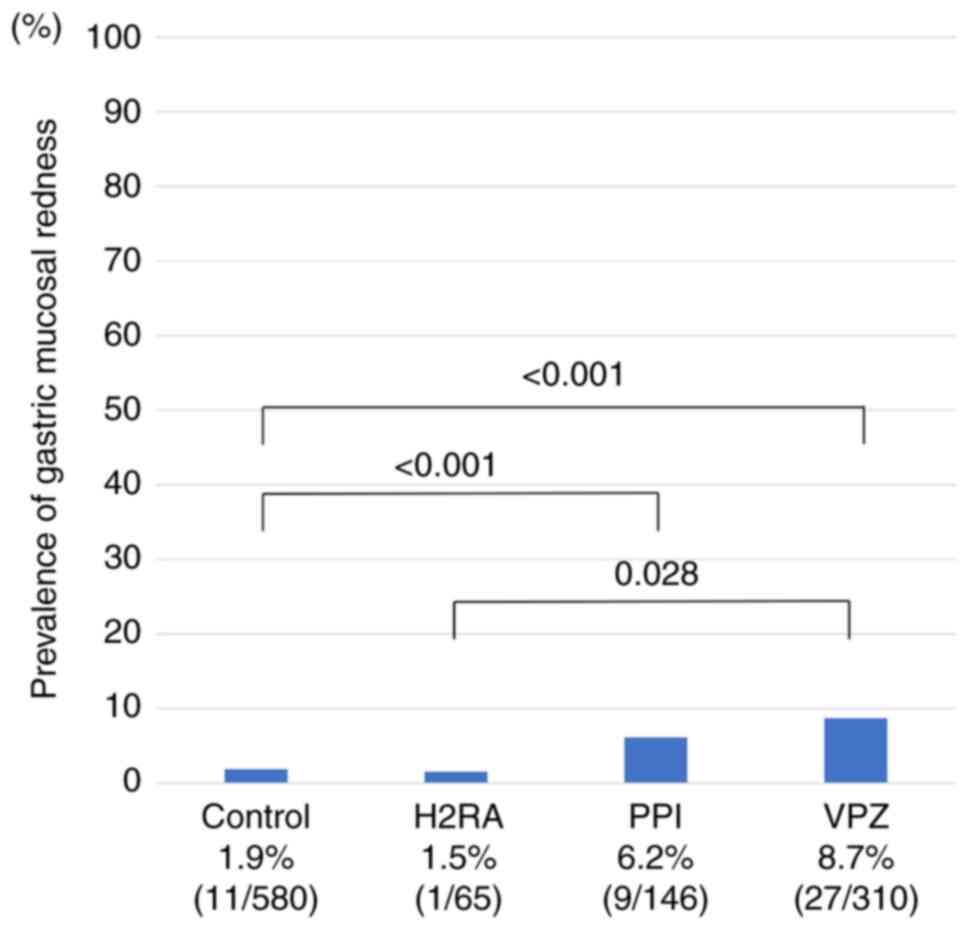
The 1,101 included patients were divided into four
groups: Controls, H2RA, PPI, and VPZ groups (Fig. 3). Even in patients receiving PPI or
VPZ, the prevalence of gastric mucosal redness was not high (6-9%).
Both the PPI and VPZ groups had a significantly higher prevalence
of gastric mucosal redness than the control group (both
P<0.001). The VPZ group also had a significantly higher
prevalence of mucosal redness than the H2RA group (P=0.028). The
reddish finding associated with portal hypertensive gastropathy
should be differentiated from gastric mucosal redness, but 8
patients with cirrhosis had neither gastric mucosal redness nor
portal hypertensive gastropathy.
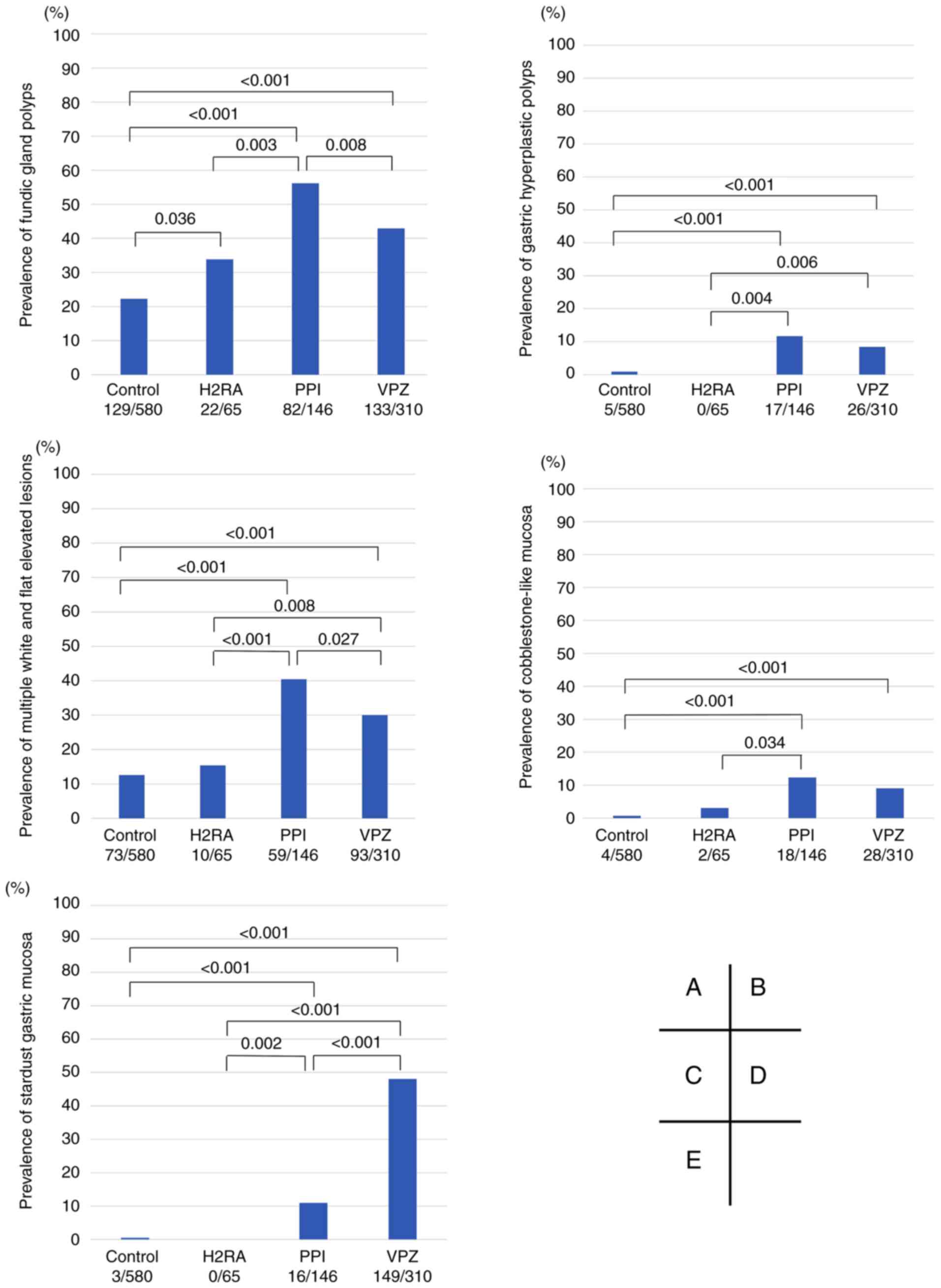
Additionally, the prevalence of five representative
changes associated with acid blockers including fundic gland
polyps, gastric hyperplastic polyps, multiple white and flat
elevated lesions, cobblestone-like mucosa, and stardust gastric
mucosa were compared (Fig. 4). Both
the PPI and VPZ groups had a significantly higher prevalence of all
five representative changes compared with the control group.
Factors associated with gastric
mucosal redness
Risk factors for gastric mucosal redness were
investigated (Table II). In the
multivariate analysis, PPI and VPZ use were significantly
associated with the prevalence of gastric mucosal redness. The VPZ
group had a slightly stronger association with the development of
gastric mucosal redness compared with the PPI group (odds ratio:
3.415 vs. 2.665). VPZ was not the only risk factor for gastric
mucosal redness; PPI use was also identified as a risk factor.
 | Table IIFactors associated with gastric
mucosal redness. |
Table II
Factors associated with gastric
mucosal redness.
| | Univariate
analysis | Multivariate
analysis |
|---|
| Factor | Odds ratio | 95% confidence
interval | P-value | Odds ratio | 95% confidence
interval | P-value |
|---|
| Age ≥60 y | 2.102 | 1.035-4.266 | 0.039a | 1.327 | 0.616-2.861 | 0.470 |
| Male sex | 1.146 | 0.642-2.044 | 0.645 | | | |
| Histamine-2 receptor
antagonist use | 0.329 | 0.045-2.422 | 0.274 | | | |
| Proton pump inhibitor
use | 1.530 | 0.725-3.228 | 0.264 | 2.665 | 1.061-6.692 | 0.036a |
| Vonoprazan use | 3.498 | 1.946-6.288 |
<0.001c | 3.415 | 1.477-7.899 | 0.004b |
| Open type gastric
atrophy | 1.673 | 0.929-3.015 | 0.086 | 1.553 | 0.825-2.924 | 0.172 |
| Fundic gland
polyps | 1.596 | 0.890-2.864 | 0.116 | | | |
| Gastric hyperplastic
polyps | 4.214 | 1.783-9.961 | 0.001b | 2.384 | 0.975-5.828 | 0.056 |
| Multiple white and
flat elevated lesions | 0.968 | 0.475-1.974 | 0.929 | | | |
| Cobblestone-like
mucosa | 1.903 | 0.657-5.514 | 0.235 | | | |
| Stardust gastric
mucosa | 3.612 | 1.964-6.643 |
<0.001c | 1.650 | 0.781-3.485 | 0.189 |
We previously reported four representative gastric
mucosal changes associated with acid blocker use (2). In the current study, multivariate
analyses were performed on the five gastric mucosal changes.
Consequently, all five changes including fundic gland polyps
(Table III), gastric hyperplastic
polyps (Table IV), multiple white
and flat elevated lesions (Table
V), cobblestone-like mucosa (Table
VI) and stardust gastric mucosa (Table VII) were significantly associated
with PPI and VPZ use.
 | Table IIIFactors associated with fundic gland
polyps. |
Table III
Factors associated with fundic gland
polyps.
| | Univariate
analysis | Multivariate
analysis |
|---|
| Factor | Odds ratio | 95% confidence
interval | P-value | Odds ratio | 95% confidence
interval | P-value |
|---|
| Age ≥60 y | 0.807 | 0.622-1.048 | 0.107 | | | |
| Male sex | 0.598 | 0.462-0.773 |
<0.001c | 0.669 | 0.498-0.898 | 0.007b |
| Histamine-2 receptor
antagonist use | 1.029 | 0.606-1.748 | 0.915 | 2.161 | 1.169-3.995 | 0.013a |
| Proton pump
inhibitor use | 2.976 | 2.089-4.240 |
<0.001c | 5.932 | 3.744-9.401 |
<0.001c |
| Vonoprazan use | 1.800 | 1.371-2.362 |
<0.001c | 2.817 | 2.019-3.931 |
<0.001c |
| Open type gastric
atrophy | 0.086 | 0.055-0.136 |
<0.001c | 0.069 | 0.043-0.112 |
<0.001c |
| Gastric mucosal
redness | 1.596 | 0.890-2.864 | 0.116 | | | |
| Gastric
hyperplastic polyps | 1.460 | 0.811-2.628 | 0.207 | | | |
| Multiple white and
flat elevated lesions | 1.749 | 1.301-2.351 |
<0.001c | 1.389 | 0.965-1.999 | 0.077 |
| Cobblestone-like
mucosa | 2.085 | 1.193-3.646 | 0.009b | | | |
| Stardust gastric
mucosa | 1.449 | 1.034-2030 | 0.031a | | | |
 | Table IVFactors associated with gastric
hyperplastic polyps. |
Table IV
Factors associated with gastric
hyperplastic polyps.
| | Univariate
analysis | Multivariate
analysis |
|---|
| Factor | Odds ratio | 95% confidence
interval | P-value | Odds ratio | 95% confidence
interval | P-value |
|---|
| Age ≥60 y | 2.788 | 1.292-6.019 | 0.009b | | | |
| Male sex | 0.879 | 0.489-1.581 | 0.667 | | | |
| Histamine-2
receptor antagonist used | | | | | | |
| Proton pump
inhibitor use | 3.894 | 2.096-7.233 |
<0.001c | 15.886 | 5.742-43.952 |
<0.001c |
| Vonoprazan use | 3.200 | 1.785-5.737 |
<0.001c | 10.725 | 4.050-28.402 |
<0.001c |
| Open type gastric
atrophy | 1.393 | 0.765-2.536 | 0.278 | | | |
| Gastric mucosal
redness | 4.214 | 1.783-9.961 | 0.001c | 2.551 | 1.050-6.194 | 0.038a |
| Fundic gland
polyps | 1.460 | 0.811-2.628 | 0.207 | | | |
| Multiple white and
flat elevated lesions | 1.100 | 0.552-2.192 | 0.785 | | | |
| Cobblestone-like
mucosa | 2.489 | 0.942-6.573 | 0.065 | | | |
| Stardust gastric
mucosa | 2.964 | 1.588-5.533 |
<0.001c | | | |
 | Table VFactors associated with multiple
white and flat elevated lesions. |
Table V
Factors associated with multiple
white and flat elevated lesions.
| | Univariate
analysis | Multivariate
analysis |
|---|
| Factor | Odds ratio | 95% confidence
interval | P-value | Odds ratio | 95% confidence
interval | P-value |
|---|
| Age ≥60 y | 3.466 | 2.387-5.033 |
<0.001c | 2.767 | 1.869-4.096 |
<0.001c |
| Male sex | 0.481 | 0.354-0.653 |
<0.001c | 0.507 | 0.365-0.704 |
<0.001c |
| Histamine-2
receptor antagonist use | 0.655 | 0.329-1.306 | 0.229 | | | |
| Proton pump
inhibitor use | 2.964 | 2.051-4.283 |
<0.001c | 3.070 | 1.962-4.804 |
<0.001c |
| Vonoprazan use | 1.959 | 1.446-2.653 |
<0.001c | 1.768 | 1.140-2.741 | 0.010b |
| Open type gastric
atrophy | 1.237 | 0.910-1.681 | 0.174 | | | |
| Gastric mucosal
redness | 0.968 | 0.475-1.974 | 0.929 | | | |
| Fundic gland
polyps | 1.749 | 1.301-2.351 |
<0.001c | 1.361 | 0.978-1.893 | 0.067 |
| Gastric
hyperplastic polyps | 1.100 | 0.552-2.192 | 0.785 | 0.542 | 0.262-1.122 | 0.098 |
| Cobblestone-like
mucosa | 3.131 | 1.775-5.524 |
<0.001c | 2.036 | 1.093-3.793 | 0.025a |
| Stardust gastric
mucosa | 2.832 | 1.992-4.028 |
<0.001c | 1.846 | 1.167-2.920 | 0.008b |
 | Table VIFactors associated with
cobblestone-like mucosa. |
Table VI
Factors associated with
cobblestone-like mucosa.
| | Univariate
analysis | Multivariate
analysis |
|---|
| Factor | Odds ratio | 95% confidence
interval | P-value | Odds ratio | 95% confidence
interval | P-value |
|---|
| Age ≥60 y | 2.669 | 1.287-5.536 | 0.008b | | | |
| Male sex | 1.596 | 0.911-2.796 | 0.102 | 1.955 | 1.084-3.526 | 0.025a |
| Histamine-2
receptor antagonist use | 0.626 | 0.149-2.632 | 0.522 | 4.778 | 0.855-26.714 | 0.074 |
| Proton pump
inhibitor use | 3.776 | 2.072-6.882 |
<0.001c | 16.826 | 5.500-51.473 |
<0.001c |
| Vonoprazan use | 3.173 | 1.809-5.567 |
<0.001c | 12.683 | 4.367-36.833 |
<0.001c |
| Open type gastric
atrophy | 1.017 | 0.556-1.860 | 0.956 | | | |
| Gastric mucosal
redness | 1.903 | 0.657-5.514 | 0.235 | | | |
| Fundic gland
polyps | 2.085 | 1.193-3.646 | 0.009b | | | |
| Gastric
hyperplastic polyps | 2.489 | 0.942-6.573 | 0.065 | | | |
| Multiple white and
flat elevated lesions | 3.131 | 1.775-5.524 |
<0.001c | 2.107 | 1.151-3.856 | 0.015a |
| Stardust gastric
mucosa | 2.141 | 1.133-4.045 | 0.019a | | | |
 | Table VIIFactors associated with stardust
gastric mucosa. |
Table VII
Factors associated with stardust
gastric mucosa.
| | Univariate
analysis | Multivariate
analysis |
|---|
| Factor | Odds ratio | 95% confidence
interval | P-value | Odds ratio | 95% confidence
interval | P-value |
|---|
| Age ≥60 y | 2.026 | 1.378-2.977 |
<0.001c | | | |
| Male sex | 0.684 | 0.488-0.960 | 0.028a | 0.613 | 0.401-0.937 | 0.023a |
| Histamine-2
receptor antagonist used | | | | | | |
| Proton pump
inhibitor use | 0.664 | 0.373-1.114 | 0.115 | 23.192 | 6.553-82.080 |
<0.001c |
| Vonoprazan use | 37.603 | 22.654-62.417 |
<0.001c | 192.686 | 60.197-616.771 |
<0.001c |
| Open type gastric
atrophy | 1.099 | 0.772-1.564 | 0.599 | | | |
| Gastric mucosal
redness | 3.612 | 1.964-6.643 |
<0.001c | 2.032 | 0.957-4.315 | 0.064 |
| Fundic gland
polyps | 1.449 | 1.034-2.030 | 0.031a | 0.714 | 0.468-1.088 | 0.116 |
| Gastric
hyperplastic polyps | 2.964 | 1.588-5.533 |
<0.001c | | | |
| Multiple white and
flat elevated lesions | 2.832 | 1.992-4.028 |
<0.001c | 1.930 | 1.229-3.031 | 0.004b |
| Cobblestone-like
mucosa | 2.141 | 1.133-4.045 | 0.019a | | | |
Presence of gastric mucosal redness
before starting PPI or VPZ
Among the 48 patients who had gastric mucosal
redness, 9 and 27 patients were undergoing PPI and VPZ therapy,
respectively. EGD data existed for 7/9 patients in the PPI group
and in 26/27 patients in the VPZ group [naïve (n=15) and changed
from PPI (n=11)] before starting their respective therapy. EGD data
were reviewed to determine the presence or absence of gastric
mucosal redness before starting PPI or VPZ to perform back-to-back
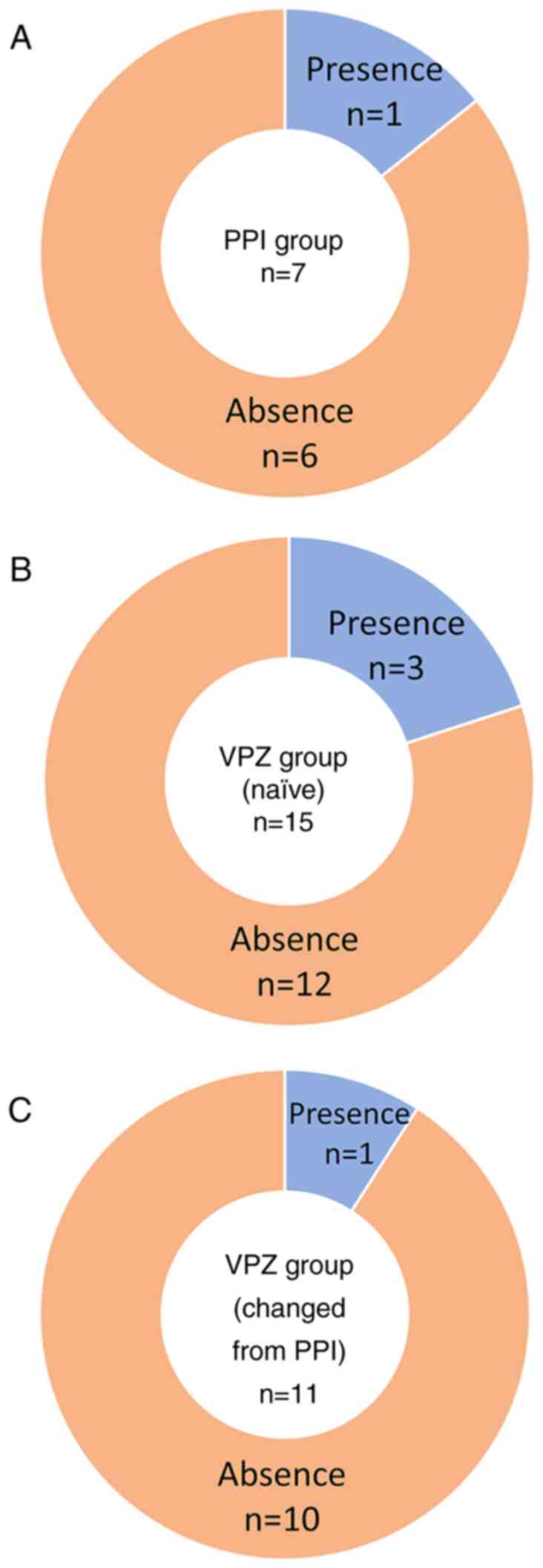
analyses (Fig. 5). Of the 7
patients in the PPI group, 1 patient (14%) presented with gastric
mucosal redness before starting PPI (Fig. 5A). In the VPZ group, gastric mucosal
redness was present in 3 (20%) of the 15 naïve patients (Fig. 5B) and in 1 (9%) of the 11 patients
(Fig. 5C) who were changed from PPI
therapy before starting VPZ. In most patients, gastric mucosal
redness developed de novo after starting PPI or VPZ.
Prevalence of gastric mucosal redness
and duration of treatment with VPZ
The duration of treatment with VPZ was investigated
to determine if it affected the prevalence of gastric mucosal
redness. The mean VPZ treatment duration was 2.6 years (n=310).
There were no significant differences in treatment duration among
patients with and without gastric mucosal redness (mean ± standard
deviation: 3.0±1.5 vs. 2.5±1.4 years, P=0.077). The treatment
duration with VPZ did not affect the prevalence of gastric mucosal
redness. Regarding the H2RA and PPI groups, formal evaluation could
not be performed due to the lack of a sufficient number of patients
in these groups.
Discussion
The present study demonstrated the influence of acid
blockers on the prevalence of gastric mucosal redness. The overall
prevalence of gastric mucosal redness is low (4%), even in patients
treated with PPI or VPZ (6-9%). Both PPI and VPZ use were
identified as significant factors contributing to the development
of gastric mucosal redness. Most instances of gastric mucosal
redness occurred after starting PPI or VPZ. Treatment duration with
VPZ was not associated with the prevalence of gastric mucosal
redness. To the best of our knowledge, this is the first study
reporting the prevalence and risk factors for the development of
gastric mucosal redness.
Few studies are available regarding the development
of gastric mucosal redness secondary to the use of acid blockers
and its pathogenesis is unknown (4,5). The
endoscopic characteristics have been described in a previous study
(4) and included spotty and linear
areas of redness along the greater curvature in the gastric body,
which is consistent with the endoscopic images shown in the present
study. Endoscopic images from the previous study and the present
study show hypertrophic and bump-like gastric mucosa accompanying
gastric mucosal redness. Laser endoscopic imaging systems allow
clearer visualization of vascular and structural patterns on the
mucosal surface at a near view than white light imaging (6). In the present study, linked color
imaging showed that slightly dilated vessels surrounded white
marginal crypt epithelium diffusely and equally on the mucosal
surface, which corresponded to the fine network pattern visualized
in the magnified imaging. These endoscopic findings were similar in
patients with gastric mucosal redness regardless of the acid
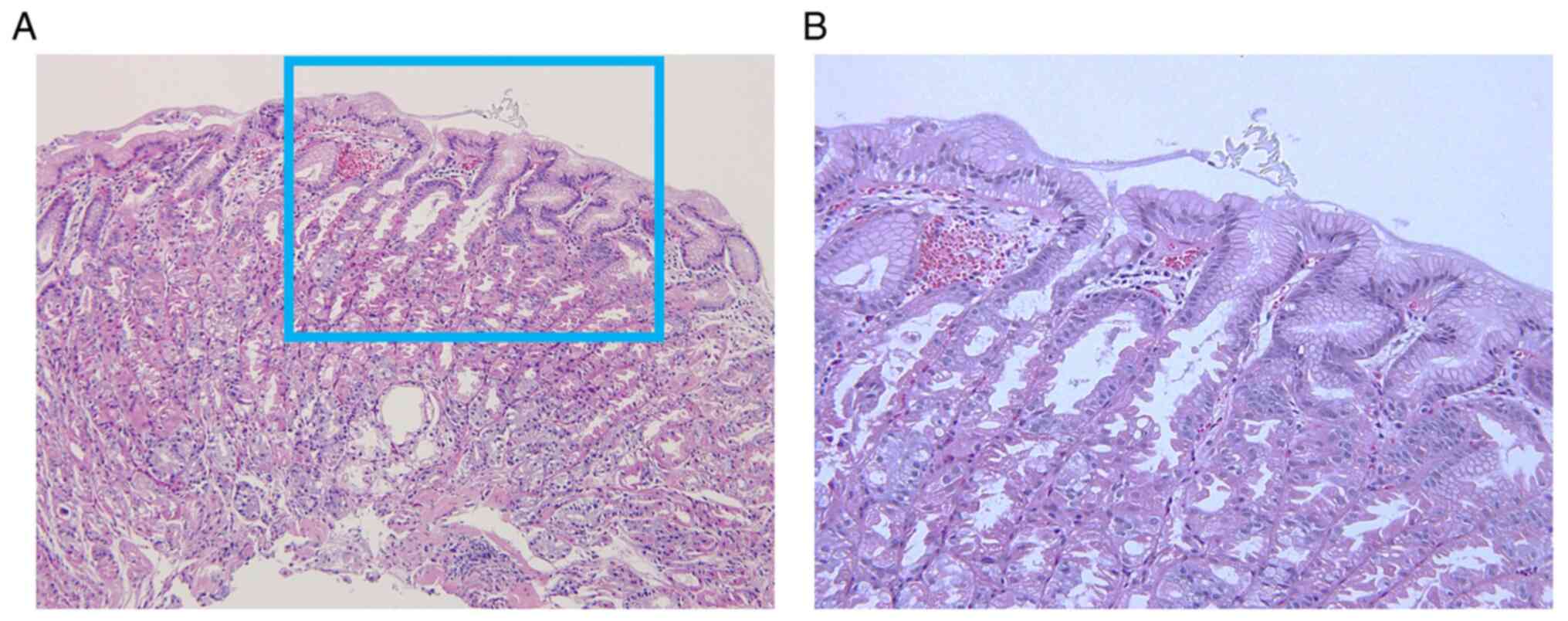
blocker prescribed. Pathological findings in the present study
showed not only oxyntic gland dilation with parietal cell
protrusions, but also congestion and dilated vessels underneath the
surface epithelium in the intervening portion (Fig. 2). These endoscopic and pathological
findings suggested that increased intramucosal blood perfusion may
be the cause of mucosal redness. Kubo et al (4) first reported the pathological
characteristics as ‘inflammatory cell infiltration’ and the
disappearance of these inflammatory changes after discontinuation
of VPZ without changes in parietal cell protrusions. In the present
study, the presence of gastric hyperplastic polyps had a weak
association with gastric mucosal redness. Gastric hyperplastic
polyps are generally caused by excessive proliferation of foveolar
cells due to longstanding inflammation. We suggest that increased
intramucosal perfusion due to sustained inflammation results in
gastric mucosal redness and hyperplastic polyp formation. We
previously reported an association of gastric hyperplastic polyps
with PPI/VPZ use (2). Further
studies are necessary to clarify why acid blockers lead to
persistent inflammation and increased perfusion in a limited
population of patients.
Estimation of the malignant potential of gastric
mucosal redness is important. According to previous reports,
gastric mucosal redness disappears after cessation of VPZ (4,5). We
hypothesize that gastric mucosal redness is not a premalignant
lesion. To clarify this issue, a long-term observational study is
necessary regardless of the cessation of PPI/VPZ.
In the multivariate analysis, the VPZ group had a
slightly stronger association with the development of gastric
mucosal redness compared to the PPI group. The degree of acid
inhibition may contribute to the prevalence of gastric mucosal
redness. A case series reported ‘VPZ-associated gastric mucosal
redness’ and changing therapy to PPI results in the disappearance
of the lesion (5). However, the
present study demonstrated that certain patients in the PPI group
also had gastric mucosal redness similar to the VPZ group, despite
no findings of mucosal changes before starting PPI. Therefore,
gastric mucosal redness is not a specific change associated with
VPZ therapy. Further, five other gastric mucosal changes are also
associated with PPI and VPZ use. According to a previous study,
stardust gastric mucosa was only associated with VPZ use, but not
PPI (3). We first demonstrated the
association between stardust gastric mucosa and PPI as well as VPZ,
although the association with VPZ was stronger than PPI.
The treatment duration with VPZ in the present study
(median: 2.6 years) was long, and patients treated with acid
blockers for <1 year were excluded. Treatment duration with VPZ
was not associated with the prevalence of gastric mucosal redness.
Past case series reported that gastric mucosal redness presented
2-6 months after starting VPZ (4).
Based on the results of the present study, it is hypothesized that
the majority of cases of gastric mucosal redness develop within a
few months after initiation of VPZ therapy.
There are some limitations to this study. First,
this is a retrospective observational study, but gastric mucosal
redness was managed as a compulsory item in the endoscopic report
and all EGD procedures were recorded on video. Second, the data
after cessation of PPI/VPZ were not assessed. It is hypothesized
that the development of gastric mucosal redness does not
necessitate cessation of PPI/VPZ unless it is considered to be a
source of hemorrhage. This mucosal finding may be a phenotype of
persistent inflammatory changes and increased intramucosal blood
flow with hyperplastic glands, but not neoplastic changes. Third,
the diagnosis of gastric mucosal redness was determined only based
on endoscopic findings without pathological evaluation. Gastric
mucosal redness may be similar to diffuse redness caused by a
current H. pylori infection, but the present study excluded
patients with current H. pylori infections. Fourth, gastric
mucosal redness is not always a PPI/VPZ-specific change. This
mucosal change was identified in the control group, although the
frequency was significantly higher in PPI/VPZ groups. Fifth, the
H2RA group (n=65) may not have sufficient power to evaluate
differences among these groups.
In conclusion, the prevalence of gastric mucosal
redness was low. Gastric mucosal redness is associated with PPI use
as well as VPZ use and is not influenced by the treatment duration
with VPZ. Due to the inflammatory nature of this lesion, the
presence of gastric mucosal redness does not necessitate the
cessation of acid blocker therapy. To the best of our knowledge,
this is the first original report investigating the influence of
PPI or VPZ on gastric mucosal redness.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
SS and HO conceived and designed the study,
collected, analyzed and interpretated the data, and drafted the
manuscript. YM, HY, HS were involved in conception and design of
the study, and in the drafting of the manuscript. TY was involved
in conception and design of the study, drafting of the manuscript,
and in the data analysis and interpretation. AKL and HY were
involved in the drafting of the manuscript, and in data analysis
and interpretation. SS and HO confirm the authenticity of all the
raw data. All authors have read and reviewed the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board of the Shinozaki Medical Clinic (approval no.
ID#31-R001). The need for written informed consent was waived due
to the retrospective design of the study.
Patient consent for publication
Not applicable.
Competing interests
SS has received honoraria from Takeda and Otsuka
Pharmaceuticals. HO has received honoraria from AstraZeneca,
Daiichi Sankyo, Takeda and Otsuka Pharmaceuticals. YM has received
honoraria from AstraZeneca, Daiichi Sankyo, Takeda, Otsuka and EA
Pharmaceuticals. HY has received honoraria from Takeda
Pharmaceutical. All other authors declare no conflicts of interest
regarding this study.
References
|
1
|
Sakurai Y, Mori Y, Okamoto H, Nishimura A,
Komura E, Araki T and Shiramoto M: Acid-inhibitory effects of
vonoprazan 20 mg compared with esomeprazole 20 mg or rabeprazole 10
mg in healthy adult male subjects-a randomised open-label
cross-over study. Aliment Pharmacol Ther. 42:719–730.
2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Shinozaki S, Osawa H, Hayashi Y, et al:
Changes in gastric morphology during long-term use of vonoprazan
compared to proton pump inhibitors. Singapor Med J (in press):
Accepted on April 24, 2021.
|
|
3
|
Yoshizaki T, Morisawa T, Fujinami M,
Matsuda T, Katayama N, Inoue K, Matsumoto M, Ikeoka S, Takagi M,
Sako T, et al: Propensity score matching analysis: Incidence and
risk factors for ‘stardust’ gastric mucosa, a novel gastric finding
potentially induced by vonoprazan. Aliment Pharmacol Ther.
53:94–102. 2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kubo K, Kimura N, Matsuda S, Tsuda M, Mabe
K and Kato M: Vonoprazan-associated gastric mucosal redness: A
report of four cases. Intern Med. 59:507–511. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kubo K, Kimura N, Watanabe R, Higashino M,
Tsuda M and Kato M: Vonoprazan-associated gastric mucosal redness
in non-Helicobacter pylori-infected and Helicobacter
pylori-eradicated stomach. Case Rep Gastroenterol. 15:751–758.
2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Shinozaki S, Osawa H, Hayashi Y, Lefor AK
and Yamamoto H: Linked color imaging for the detection of early
gastrointestinal neoplasms. Therap Adv Gastroenterol.
12(1756284819885246)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kimura K and Takemoto T: An endoscopic
recognition of the atrophic border and its significance in chronic
gastritis. Endoscopy. 1:87–97. 1969.
|
|
8
|
Hasegawa R, Yao K, Ihara S, Miyaoka M,
Kanemitsu T, Chuman K, Ikezono G, Hirano A, Ueki T, Tanabe H, et
al: Magnified endoscopic findings of multiple white flat lesions: A
new subtype of gastric hyperplastic polyps in the stomach. Clin
Endosc. 51:558–562. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Miyamoto S, Kato M, Tsuda M, Matsuda K,
Muranaka T, Abiko S, Ono M, Mizushima T, Omori S, Yamamoto K, et
al: Gastric mucosal cracked and cobblestone-like changes resulting
from proton pump inhibitor use. Dig Endosc. 29:307–313.
2017.PubMed/NCBI View Article : Google Scholar
|