Introduction
Lung cancer currently ranks first in terms of
mortality and morbidity compared with other types of malignancies
worldwide. Non-small cell lung cancer (NSCLC) accounts for ~85% of
all lung cancer subtypes (1), where
lung adenocarcinoma is one of the most common types of NSCLC. Since
typical criteria of early symptoms for NSCLC remain unavailable,
~50% patients are diagnosed with advanced NSCLC on presentation
(2,3). Therefore, treatment of advanced lung
cancer forms an important branch of the lung cancer treatment
development research field, which is also incidentally the one that
has experienced an acceleration in research progress over the past
decade. Platinum-containing dual-drug chemotherapy is the standard
first-line chemotherapy regimen for NSCLC (4). In addition, it is also an important
method for the treatment of advanced NSCLC (4). However, the rate of efficacy from this
treatment remains at only ~30%, with a median survival time of 8-10
months and a one-year survival rate of ≤40%. Exploring novel
biomarkers that can be used to predict the sensitivity of patients
with NSCLC to chemotherapy is therefore currently a topic of
intense research.
Epidermal growth factor receptor (EGFR) is an
important driver of lung adenocarcinoma. Mutations in the EGFR gene
is one of the main predictors of the efficacy of EGFR tyrosine
kinase inhibitors (EGFR-TKIs). For advanced or unresecTable lung
adenocarcinoma, EGFR gene status detection has become an important
parameter for the selection of clinical treatment options (5-9).
Previous studies have found that the EGFR gene status can also
influence the efficacy of chemotherapy in patients with lung
adenocarcinoma. However, the accuracy of its predictive power
remains controversial. A number of studies have previously reported
that patients harboring EGFR gene mutations can benefit more from
chemotherapy compared with those harboring wild-type EGFR in
advanced stages. In addition, the Individualized Plan for Academic
Success System (IPASS) subgroup analysis revealed that patients
with NSCLC harboring EGFR mutations are more likely to benefit from
paclitaxel combined with carboplatin chemotherapy compared with
patients with wild-type EGFR in the Asian and non-smoking lung
adenocarcinoma subgroups (10).
Yang et al (11) and Hotta
et al (12) also previously
revealed the survival benefits of EGFR gene mutations for patients
with NSCLC. However, several previous clinical studies have reached
different conclusions. Okamoto et al (13) and Qin et al (14) observed that there was no significant
difference in the efficacy of chemotherapy between patients with
advanced lung adenocarcinoma harboring EGFR gene mutations and
those with wild-type EGFR. Similarly, Zhang et al (15) reported that EGFR mutations did not
confer survival benefits to patients with NSCLC following a
meta-analysis (15). By contrast,
Zhu et al (16) and as well
as other studies (17,18) determined that the survival time of
patients with NSCLC containing wild-type EGFR may be longer
compared with that in patients with EGFR mutations. Therefore, it
remains unknown whether there is an association between the EGFR
gene mutation status and chemotherapy efficacy, or whether EGFR
mutations can confer chemotherapy efficiency and thereby prolong
patient survival time. At present, since ambiguities in the
currently available findings remain, additional evidence-based
analysis is required.
Therefore, the present study statistically analyzed
the clinical efficacy of first-line chemotherapy and its
association with the EGFR gene mutation status in patients with
advanced lung adenocarcinoma. The aim was to provide a theoretical
basis for optimizing the therapeutic regimen for patients with
advanced lung adenocarcinoma.
Patients and methods
Patients
Patients with pathologically diagnosed advanced lung
adenocarcinoma at Changzhou Tumor Hospital (Changzhou, China) from
January 2015 to December 2018, were selected as subjects in the
present retrospective study. The Ethics Committee of Changzhou
Tumor Hospital deemed the present study exempt from ethical
approval due to it being retrospective in nature. The inclusion
criteria were as follows: i) Lung adenocarcinoma was confirmed by
cytology or histopathology analysis, where the foci could be
measured definitively by computed tomography (CT) or magnetic
resonance imaging (MRI); ii) stage III/IV confirmed by cytology or
histopathology according to the lung cancer staging standard (8th
edition) of the American Joint Committee on Cancer (AJCC) (19); iii) aged 18-75 years, with no sex
discrimination; iv) the physical condition score according to the
Eastern Cooperative Oncology Group guidelines (ECOG Performance
Status) was 0-2 points (20); v)
the blood samples for the examination of EGFR gene status were
tested before treatment; vi) the routine blood test, liver and
kidney function and electrocardiogram of patients were almost
normal, such that no disease or dysfunction in the important organs
could be detected; vii) after the diagnosis was confirmed, patients
received standard first-line chemotherapy consisting of pemetrexed
combined with cisplatin for ≥2 cycles, where their survival time
was estimated to be >3 months; and viii) the patients or
families of the patients consented to the content of this study and
signed the informed consent form voluntarily. The exclusion
criteria were as follows: i) Patients suffered from other malignant
tumors and received other systemic antitumor treatment; ii)
patients had a history of hypertension, hypertensive encephalopathy
or uncontrolled hypertension at present; and iii) cases with
incomplete clinical data.
Chemotherapy
In total, all 52 patients were treated with
intramuscular injections of 500 mg/m2 pemetrexed and 75
mg/m2 cisplatin on day 1. Subsequently, 1 week before
chemotherapy, the patients started to take 400 µg folic acid once a
day until the end of chemotherapy, and received an intramuscular
injection of 1 mg Vitamin B12 once every 9 weeks. In addition, 4.0
mg dexamethasone was administered 30 min before pemetrexed twice a
day for 3 consecutive days. Those who had attained disease control
were treated for ≥7 cycles, following which the curative effect was
evaluated after ≥2 cycles were completed. Finally, the 52 patients
were treated for 215 cycles, with an average of 4.13 cycles per
patient.
EGFR mutation test
EGFR gene mutations were assayed by high-throughput
sequencing technology, using Illumina next-generation sequencing
(NGS) protocols, including Illumina TruSeq library preparation,
Illumina sample indexing, and Illumina synthesis by sequencing
(SBS) protocols as recommended by Illumina, Inc. Plasma samples
were isolated from 10 ml fresh peripheral blood, from which
circulating tumor DNA (ctDNA) (to note, the patients were all
diagnosed with advanced lung adenocarcinoma and thus there was no
surgical tissue, and only puncture specimens were used to confirm
the type of cancer; therefore, EGFR gene mutation detection was
based on the ctDNA of the peripheral blood of patients) was
extracted using QIAamp Circulating Nucleic Acid Kit (cat. no.
55114; Qiagen China Co., Ltd.). The DNA quantity was measured on
Qubit 3.0 fluorometer with dsDNA HS Assay Kit (Life Technologies;
Thermo Fisher Scientific, Inc.). A minimum of 6 ng of DNA was used
as input for the amplicon-based enrichment step. Subsequently,
libraries were prepared using the KAPA Hyper Prep Kit (cat. no.
KK8500; KAPA Biosystems; Roche Diagnostics) according to
manufacturer's protocols. Then, fragmented DNA was subjected to
end-repairing, A-tailing, indexed-adapter ligation, size selection
and PCR amplification. In brief, tumor DNA was amplified using
either TruSeq kit or custom primers, and amplification products
were confirmed with gel electrophoresis using a 2% agarose E-gel
(Thermo Fisher Scientific, Inc.). Samples were indexed and pooled.
The library fragments were then copied with the primer probe for
416 predefined cancer-associated genes, including all exons of
EGFR. For targeted enrichment, indexed DNA libraries were pooled
together for hybridization with customized xGen lockdown probes
(Integrated DNA Technologies, Inc.) for 416 predefined
cancer-relevant genes (21).
Enriched libraries were amplified and subjected to NGS on Illumina
Hiseq4000 platforms (Illumina, Inc.) to a targeted mean coverage
depth of 3000X for ctDNA samples.
Observation and follow-up
Using the inpatient system of Changzhou Tumor
Hospital, the clinical data of all cases were obtained and
recorded, where the patients were followed up by outpatient,
telephone or other means. Data that were collected at follow-up
included the efficacy of chemotherapy, time to disease progression
and time to mortality. The date of final follow-up was December 31,
2019.
Efficacy evaluation
According to the response evaluation criteria in
solid tumors (RECIST) version 1.1 published in 2009(22), all patients were evaluated at
baseline before treatment, and after every 2 cycles of treatment.
The efficacy evaluation was divided into complete response (CR),
partial response (PR), sTable disease (SD) and progressive disease
(PD). The objective response rate (ORR) was calculated as CR + PR,
whereas the disease control rate (DCR) was calculated as CR + PR +
SD. Progression-free survival (PFS) was defined as the time from
the beginning of treatment to disease progression or death.
Patients who did not progress or succumb to the disease at the end
of the follow-up period were treated according to the follow-up
deadline (December 31, 2019).
Statistical analysis
The statistical software SPSS 18.0 (SPSS, Inc.) was
used for statistical analysis. Pearson's F test or Fisher's exact
test were used to analyze the relationship among the EGFR gene
status, clinical characteristics and chemotherapy efficacy.
Kaplan-Meier survival curve and log-rank testing were used to
analyze PFS, whereas Cox regression was used for multivariate
analysis. Bilateral probability test was used in all statistical
analyses, were P<0.05 was considered to indicate a statistically
significant difference.
Results
Patients characteristics
As shown in Table I,
amongst the 52 patients, 24 were male (46.2%) and 28 were female
(53.8%). The age of the patients ranged from 36 to 75 years
(median, 65±0.5), 21 were aged <65 years (40.4%), while 31 were
aged ≥65 years (59.6%). In total, 16 patients were diagnosed with
stage III (30.8%) and 36 patients were diagnosed with stage IV
(69.2%). In total, PS scores of 50 patients were <2 (96.2%)
whereas 2 patients scored 2 (3.8%). Furthermore, 14 patients were
smokers (26.9%), while 38 cases were non-smokers (73.1%). A total
of 25 patients were found with EGFR gene mutations (48.1%), whilst
27 patients were harboring wild-type EGFR (51.9%). The age,
clinical staging, PS scores and smoking status of the patients were
not found to be significantly different between the EGFR mutation
and wild-type groups. However, the incidence of EGFR gene mutations
in female patients was significantly higher compared with that in
male patients (P=0.0115).
 | Table IAssociation between EGFR gene status
and clinical features in 52 patients with NSCLC. |
Table I
Association between EGFR gene status
and clinical features in 52 patients with NSCLC.
| | No. of patients | |
|---|
| Characteristics | EGFR mutation | EGFR wild-type | Total | χ2 | P-value |
|---|
| Total | 25 | 27 | 52 | | |
| Sex | | | | 6.385 | 0.0115 |
|
Male | 7 | 17 | 24 | | |
|
Female | 18 | 10 | 28 | | |
| Age, years | | | | 0.2614 | 0.6092 |
|
<65 | 11 | 10 | 21 | | |
|
≥65 | 14 | 17 | 31 | | |
| Clinical stage | | | | 0.0342 | 0.8532 |
|
III | 8 | 8 | 16 | | |
|
IV | 17 | 19 | 36 | | |
| ECOG PS | | | | - | 0.1339 |
|
0-1 | 23 | 27 | 50 | | |
|
2 | 2 | 0 | 2 | | |
| Smoking status | | | | - | 0.1696 |
|
Nonsmoker | 16 | 22 | 38 | | |
|
Smoker | 3 | 11 | 14 | | |
Effect of EGFR mutation on the
response rate
Among the 52 patients, none achieved CR. By
contrast, PR accounted for 36.5% (19/52), SD accounted for 34.6%
(18/52) and PD accounted for 28.9% (15/52) (Table II). The ORR and DCR were calculated
to be 36.5 and 71.2%, respectively. In 25 patients with EGFR gene
mutation, the number of patients with PR, SD and PD were 13, 10 and
2, respectively. In 27 patients with EGFR wild-type, the number of
patients with PR, SD and PD were 6, 8 and 13, respectively
(Table II). The incidence of PR
(52.0 vs. 22.2%), SD (40.0 vs. 29.6%) and PD (8.0 vs. 48.1%) were
observed at higher frequencies in patients with EGFR gene mutations
compared with those in patients with wild-type EGFR (Table II). In addition, it was found that
both the ORR (52.0 vs. 22.2%; P=0.0259) and DCR (92.0 vs. 51.9%;
P=0.0019) were higher in patients with the EGFR gene mutation
compared with those in patients with the wild-type EGFR.
Association analysis of the ORR and DCR with other
clinicopathological features was also performed (Table III). ORR was found to be
associated with sex (P<0.0001), clinical stage (P=0.0491),
ECOG-PS (38.0 vs. 0%) and smoking history (P=0.0019). However, no
differences in ORR could be found when age (38.1 vs. 35.5%) was
compared. The DCR was only found to be associated with sex
(P=0.0024), ECOG-PS (74.0 vs. 0%) and smoking history (P=0.0063),
but not with age and clinical staging. Neither of the two patients
with a PS score of 2 achieved CR, PR and SD, thus the chi-square
test was not applicable.
 | Table IIEfficacy evaluation of patients with
different EGFR gene status. |
Table II
Efficacy evaluation of patients with
different EGFR gene status.
| EGFR gene
status | CR (%) (n=0) | PR (%) (n=19) | SD (%) (n=18) | PD (%) (n=15) |
|---|
| EGFR gene mutation
(n=25) | 0 | 52.0 (13/25) | 40.0 (10/25) | 8.0 (2/25) |
| EGFR wild-type
(n=27) | 0 | 22.2 (6/27) | 29.6 (8/27) | 48.1 (13/27) |
 | Table IIIAssociation between ORR, DCR and
clinical features of 52 patients with NSCLC who were treated with
chemotherapy. |
Table III
Association between ORR, DCR and
clinical features of 52 patients with NSCLC who were treated with
chemotherapy.
|
Characteristics | Total | No. of
patients | ORR
(χ2) | P-value | No. of
patients | DCR
(χ2) | P-value |
|---|
| EGFR status | | | 4.964 | 0.0259 | | - | 0.0019 |
|
EGFR
mutation | 25 | 13 | | | 23 | | |
|
EGFR
wild-type | 27 | 6 | | | 14 | | |
| Sex | | | - | 0.0001 | | - | 0.0024 |
|
Male | 24 | 2 | | | 12 | | |
|
Female | 28 | 17 | | | 25 | | |
| Age, years | | | 0.0368 | 0.8478 | | 0.0013 | 0.9713 |
|
<65 | 21 | 8 | | | 15 | | |
|
≥65 | 31 | 11 | | | 22 | | |
| Clinical stage | | | 3.873 | 0.0491 | | - | 0.7522 |
|
III | 16 | 9 | | | 12 | | |
|
IV | 36 | 10 | | | 25 | | |
| ECOG PS | | | - | - | | - | - |
|
0-1 | 50 | 19 | | | 37 | | |
|
2 | 2 | 0 | | | 0 | | |
| Smoking status | | | - | 0.0019 | | 7.474 | 0.0063 |
|
Nonsmoker | 38 | 17 | | | 31 | | |
|
Smoker | 14 | 2 | | | 6 | | |
Effect of EGFR mutation on
survival
The clinical cases were followed up until December
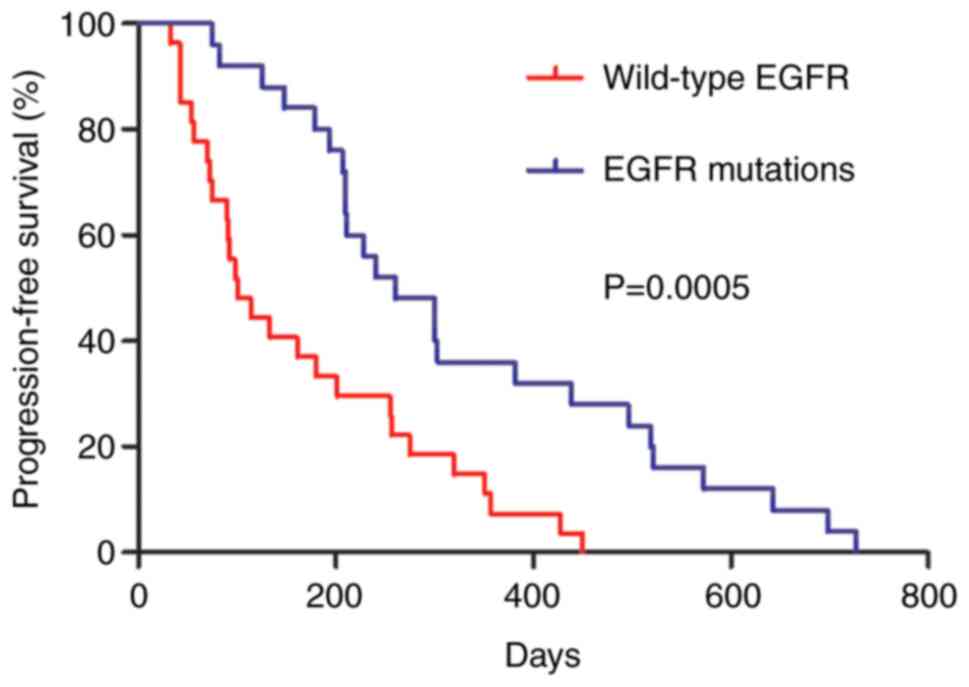
31, 2019. As shown in Table IV,
the median PFS was 207 days in the 52 cases. The patients with EGFR
gene mutations had a significantly longer median PFS compared with
that of patients with wild-type EGFR (260 days vs. 100 days,
P=0.0005; Fig. 1 and Table IV). The median PFS of the female
patients (300 days) was significantly longer (P=0.0001) compared
with those in male patients (99 days). The median PFS of nonsmokers
(255 days) was also significantly longer (P=0.0001) compared with
that of smokers (90 days). The PFS of age, clinical staging and
ECOG PS were not found to be statistically significant. Cox
multivariate regression analysis was subsequently used to determine
the independent influencing factors of PFS by incorporating
multiple factors, such as the EGFR gene mutation status, sex, age,
clinical staging, smoking history and ECOG PS. The results
demonstrated that the EGFR gene mutation status [hazard ratio
(HR)=2.056; 95% confidence interval (CI)=1.035-4.087; P=0.04] and
female sex (HR=0.377; 95% CI=0.160-0.889; P=0.026) were independent
prognostic factors of PFS in patients with NSCLC after receiving
platinum-containing chemotherapy.
 | Table IVPrognostic evaluation of PFS and
clinical characteristics in all patients with NSCLC. |
Table IV
Prognostic evaluation of PFS and
clinical characteristics in all patients with NSCLC.
| | Multivariate
analysis |
|---|
|
Characteristics | No. of
patients | Median PFS
(days) | P-value | P-value | HR (95% CI) |
|---|
| EGFR status | | | 0.0005 | 0.040 | 2.056
(1.035-4.087) |
|
EGFR
mutation | 25 | 260 | | | |
|
EGFR
wild-type | 27 | 100 | | | |
| Sex | | | 0.0001 | 0.026 | 0.377
(0.160-0.889) |
|
Male | 24 | 99 | | | |
|
Female | 28 | 300 | | | |
| Age, years | | | 0.1393 | 0.142 | 1.598
(0.855-2.990) |
|
<65 | 21 | 256 | | | |
|
≥65 | 31 | 161 | | | |
| Clinical stage | | | 0.2402 | 0.953 | 0.980
(0.504-1.907) |
|
III | 16 | 186 | | | |
|
IV | 36 | 258 | | | |
| ECOG PS | | | 0.0512 | 0.077 | 1.434
(0.046-1.171) |
|
0-1 | 50 | 210 | | | |
|
2 | 2 | 90 | | | |
| Smoking status | | | 0.0001 | 0.467 | 0.233
(0.543-3.787) |
|
Nonsmoker | 38 | 255 | | | |
|
Smoker | 14 | 90 | | | |
Discussion
EGFR is one of the most studied molecular targets in
lung cancer because its activity is closely associated with tumor
growth, invasion and metastasis. EGFR is one of the most important
drivers of NSCLC pathogenesis (23). EGFR gene mutations are major
predictors of the efficacy of EGFR-TKIs in Asian patients with lung
adenocarcinoma, where various studies have previously shown that
patients with EGFR gene mutations tended to benefit more from
concurrent treatment with EGFR-TKIs and standard first-line
chemotherapy regimens (17,24-29).
However, the relationship between the efficacy of chemotherapy and
the EGFR gene status remains controversial.
Fang et al (29) previously reported that first-line
chemotherapy was more effective in patients with KRAS-negative EGFR
gene mutations compared with those with wild-type EGFR, amongst 266
patients with advanced NSCLC (29).
In addition, Kalikaki et al (30) reported that patients with EGFR gene
mutations were more sensitive to chemotherapy compared with that in
patients with wild-type EGFR, amongst patients with advanced NSCLC.
Multi-factorial analysis revealed that EGFR gene mutation was an
independent predictor of PFS in patients with advanced lung
adenocarcinoma. Lou et al (17) previously suggested that paclitaxel
in combination with carboplatin as first-line chemotherapy was more
effective in patients with EGFR gene mutations compared with
patients with wild-type EGFR. However, Lee et al (31) found no significant associations
between the EGFR gene mutation status and the efficacy of
first-line chemotherapy. Another study from Japan also found that
patients with EGFR gene mutations and advanced NSCLC who were
treated with doxorubicin chemotherapy exhibited worse outcome
compared with patients with wild-type EGFR (32). The reasons for these ambiguous
results may be due to the chemotherapeutic regimens and timing used
not being completely uniform, such that the pathological types of
lung cancer were not completely uniform and the sample sizes of a
number of clinical studies were small. In addition, the EGFR gene
mutation status may change during the course of the
chemotherapeutic treatment period, which may also be associated
with factors, such as region and ethnicity.
In the present study, 52 patients with advanced lung
adenocarcinoma were tested for EGFR gene mutation status before the
efficacy of first-line chemotherapy was analyzed retrospectively.
The results revealed that for all patients with advanced lung
adenocarcinoma treated with pemetrexed in combination with
platinum-based regimens first line, the ORR, DCR and PFS of
patients with EGFR gene mutations were superior compared with those
of patients with wild-type EGFR, with the differences being
statistically significant. Subsequently, Cox multivariate analysis
showed that EGFR gene mutation was an independent predictor of PFS
in patients with advanced lung adenocarcinoma, which is consistent
with the results of previous studies (29,30).
In conclusion, following first-line chemotherapy for
advanced lung adenocarcinoma, PFS was superior in patients with
EGFR gene mutations compared with that in patients with wild-type
EGFR. In addition, patients on pemetrexed-containing regimens had
longer PFS regardless of EGFR gene mutations, which was more
pronounced in those harboring EGFR mutations, suggesting that the
EGFR gene mutation status can be an indicator for screening the
pemetrexed-benefit population. However, the present study is a
retrospective study that has a small sample size. Therefore,
results found in the present study would need to be confirmed by
prospective studies.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Science and
Technology Project of Changzhou, Jiangsu Province (grant no.
CE20205057), the Science and Technology Planning Project of
Changzhou Health Bureau, Jiangsu Province (grant no. ZD201616), the
Jiangsu Province Health Department Project (grant no. Z201616), the
‘333 Talents Training Project’ of Jiangsu Province and the ‘Talents
Training Project’ for the Key Medical Innovation of Changzhou
(grant no. 2016CZLJ021), the Youth Talent of Science and Technology
Project of Changzhou Health Bureau, Jiangsu Province (grant no.
QN202130), the Qingmiao Talents Project of Changzhou Health Bureau,
the Level I Talents Project of Changzhou Tumor Hospital Affiliated
to Soochow University and the Level III Talents Project of
Changzhou Tumor Hospital Affiliated to Soochow University.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LC, QQ, MZ, YZ, YP and YL contributed to the
conceptualization, analysis and methodology of the study. LC and YL
confirm the authenticity of all the raw data. All authors have read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
The Ethics Committee of Changzhou Tumor Hospital
deemed the present study exempt from ethical approval due to it
being retrospective in nature. All enrolled subjects or family
members of the patients consented to the content of this study and
signed the informed consent form.
Patients consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hosomi Y, Morita S, Sugawara S, Kato T,
Fukuhara T, Gemma A, Takahashi K, Fujita Y, Harada T, Minato K, et
al: Gefitinib alone versus gefitinib plus chemotherapy for
non-small-cell lung cancer with mutated epidermal growth factor
receptor: NEJ009 study. J Clin Oncol. 38:115–123. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wu YL, Cheng Y, Zhou X, Lee KH, Nakagawa
K, Niho S, Tsuji F, Linke R, Rosell R, Corral J, et al: Dacomitinib
versus gefitinib as first-line treatment for patients with
EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): A
randomised, open-label, phase 3 trial. Lancet Oncol. 18:1454–1466.
2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Xu CW, Wang G, Wang WL, Gao WB, Han CJ,
Gao JS, Li Y, Wang L, Zhang LY, Zhang YP, et al: Association
between epidermal growth factor receptor mutations and the
expression of excision repair cross-complementing protein 1 and
ribonucleotide reductase subunit M1 mRNA in patients with non-small
cell lung cancer. Exp Ther Med. 9:880–884. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Xie Y, Liang J and Su N: Gefitinib versus
Erlotinib as first-line treatment for patients with advanced EGFR
mutation-positive non-small-cell lung cancer. Nan Fang Yi Ke Da Xue
Xue Bao. 35:446–449. 2015.PubMed/NCBI(In Chinese).
|
|
5
|
Yang JC, Shih JY, Su WC, Hsia TC, Tsai CM,
Ou SH, Yu CJ, Chang GC, Ho CL, Sequist LV, et al: Afatinib for
patients with lung adenocarcinoma and epidermal growth factor
receptor mutations (LUX-Lung 2): A phase 2 trial. Lancet Oncol.
13:539–548. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Rosell R, Carcereny E, Gervais R,
Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R,
Pallares C, Sanchez JM, et al: Erlotinib versus standard
chemotherapy as first-line treatment for European patients with
advanced EGFR mutation-positive non-small-cell lung cancer
(EURTAC): A multicentre, open-label, randomised phase 3 trial.
Lancet Oncol. 13:239–246. 2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Fiala O, Pesek M, Finek J, Svaton M,
Minarik M, Benesova L, Bortlicek Z, Kucera R and Topolcan O:
Pemetrexed versus erlotinib in the second-line treatment of
patients with advanced-stage non-squamous NSCLC harboring wild-type
EGFR gene. Anticancer Res. 36:447–453. 2016.PubMed/NCBI
|
|
8
|
Lopes GL, Vattimo EF and Castro Junior Gd:
Identifying activating mutations in the EGFR gene: Prognostic and
therapeutic implications in non-small cell lung cancer. J Bras
Pneumol. 41:365–375. 2015.PubMed/NCBI View Article : Google Scholar : (In English,
Portuguese).
|
|
9
|
Zhou C, Wu YL, Chen G, Feng J, Liu XQ,
Wang C, Zhang S, Wang J, Zhou S, Ren S, et al: Final overall
survival results from a randomised, phase III study of erlotinib
versus chemotherapy as first-line treatment of EGFR
mutation-positive advanced non-small-cell lung cancer (OPTIMAL,
CTONG-0802). Ann Oncol. 26:1877–1883. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mok TS, Wu YL, Thongprasert S, Yang CH,
Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, et
al: Gefitinib or carboplatin-paclitaxel in pulmonary
adenocarcinoma. N Engl J Med. 361:947–957. 2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yang JC, Srimuninnimit V, Ahn MJ, Lin CC,
Kim SW, Tsai CM, Mok T, Orlando M, Puri T, Wang X and Park K:
First-line pemetrexed plus cisplatin followed by gefitinib
maintenance therapy versus gefitinib monotherapy in East Asian
never-smoker patients with locally advanced or metastatic
nonsquamous non-small cell lung cancer: Final overall survival
results from a randomized phase 3 study. J Thorac Oncol.
11:370–379. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hotta K, Kiura K, Toyooka S, Takigawa N,
Soh J, Fujiwara Y, Tabata M, Date H and Tanimoto M: Clinical
significance of epidermal growth factor receptor gene mutations on
treatment outcome after first-line cytotoxic chemotherapy in
Japanese patients with non-small cell lung cancer. J Thorac Oncol.
2:632–637. 2007.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Okamoto I, Aoe K, Kato T, Hosomi Y,
Yokoyama A, Imamura F, Kiura K, Hirashima T, Nishio M, Nogami N, et
al: Pemetrexed and carboplatin followed by pemetrexed maintenance
therapy in chemo-naïve patients with advanced nonsquamous
non-small-cell lung cancer. Invest New Drugs. 31:1275–1282.
2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Qin N, Zhang Q, Wang J, Zhang H, Gu Y,
Yang X, Li X, Lv J, Wu Y, Nong J, et al: Association between the
epidermal growth receptor status and the efficacy of
first-line
chemotherapy in patients with advanced non-small cell lung cancer.
Zhongguo Fei Ai Za Zhi. 18:131–137. 2015.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
15
|
Zhang Q, Dai HH, Dong HY, Sun CT, Yang Z
and Han JQ: EGFR mutations and clinical outcomes of chemotherapy
for advanced non-small cell lung cancer: A meta-analysis. Lung
Cancer. 85:339–345. 2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhu J, Zhang J, Chen M and Zhou CC:
Outcomes of chemotherapy in patients with EGFR mutation-negative
non-small cell lung cancer. Zhonghua Zhong Liu Za Zhi. 35:386–388.
2013.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
17
|
Lou N, Yang J, Yan H, Zhou Q, Liao R, Xu
C, Huang Y, Yang X, Yang Y, Gan B and Wu Y: Efficacies of gefitinib
versus paclitaxel/carboplatin for patients with advanced pulmonary
adenocarcinoma. Zhonghua Yi Xue Za Zhi. 94:2337–2341.
2014.PubMed/NCBI(In Chinese).
|
|
18
|
Park JH, Lee SH, Keam B, Kim TM, Kim DW,
Yang SC, Kim YW and Heo DS: EGFR mutations as a predictive marker
of cytotoxic chemotherapy. Lung Cancer. 77:433–437. 2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Detterbeck FC, Boffa DJ, Kim AW and Tanoue
LT: The eighth edition lung cancer stage classification. Chest.
151:193–203. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern cooperative oncology group. Am J Clin
Oncol. 5:649–655. 1982.PubMed/NCBI
|
|
21
|
Jin Y, Shao Y, Shi X, Lou G, Zhang Y, Wu
X, Tong X and Yu X: Mutational profiling of non-small-cell lung
cancer patients resistant to first-generation EGFR tyrosine kinase
inhibitors using next generation sequencing. Oncotarget.
7:61755–61763. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Goldstraw P, Crowley J, Chansky K, Giroux
DJ, Groome PA, Rami-Porta R, Postmus PE, Rusch V and Sobin L:
International Association for the Study of Lung Cancer
International Staging Committee. The IASLC lung cancer staging
project: Proposals for the revision of the TNM stage groupings in
the forthcoming (seventh) edition of the TNM classification of
malignant tumours. J Thorac Oncol. 2:706–714. 2007.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Nishino M, Jackman DM, Hatabu H, Yeap BY,
Cioffredi LA, Yap JT, Jänne PA, Johnson BE and Van den Abbeele AD:
New response evaluation criteria in solid tumors (RECIST)
guidelines for advanced non-small cell lung cancer: Comparison with
original RECIST and impact on assessment of tumor response to
targeted therapy. AJR Am J Roentgenol. 195:W221–W228.
2010.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Huang W, Mao Y, Zhan Y, Huang J, Wang X,
Luo P, Li LI, Mo D, Liu Q, Xu H and Huang C: Prognostic
implications of survivin and lung resistance protein in advanced
non-small cell lung cancer treated with platinum-based
chemotherapy. Oncol Lett. 11:723–730. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kang X, Xiao HH, Song HQ, Jing XB, Yan LS
and Qi RG: Advances in drug delivery system for platinum agents
based combination therapy. Cancer Biol Med. 12:362–374.
2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Tamura T, Kurishima K, Nakazawa K,
Ishikawa H, Satoh H and Hizawa N: Similar survival benefits of a
good response and sTable disease to platinum-based chemotherapy in
non-small cell lung cancer. Oncol Lett. 10:1135–1140.
2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Schuette W, Schirmacher P, Eberhardt WE,
Fischer JR, von der Schulenburg JM, Mezger J, Schumann C, Serke M,
Zaun S, Dietel M and Thomas M: EGFR mutation status and first-line
treatment in patients with stage III/IV non-small cell lung cancer
in Germany: An observational study. Cancer Epidemiol Biomarkers
Prev. 24:1254–1261. 2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Fang S, Wang Z, Guo J, Liu J, Li C, Liu L,
Shi H, Liu L, Li H, Xie C, et al: Correlation between EGFR mutation
status and response to first-line platinum-based chemotherapy in
patients with advanced non-small cell lung cancer. Onco Targets
Ther. 7:1185–1193. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kalikaki A, Koutsopoulos A, Hatzidaki D,
Trypaki M, Kontopodis E, Stathopoulos E, Mavroudis D, Georgoulias V
and Voutsina A: Clinical outcome of patients with non-small cell
lung cancer receiving front-line chemotherapy according to EGFR and
K-RAS mutation status. Lung Cancer. 69:110–115. 2010.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Lee KH, Han SW, Hwang PG, Oh DY, Kim DW,
Chung DH, Im SA, Kim TY, Heo DS and Bang YJ: Epidermal growth
factor receptor mutations and response to chemotherapy in patients
with non-small-cell lung cancer. Jpn J Clin Oncol. 36:344–350.
2006.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Yoshimasu T, Oura S, Ohta F, Hirai Y,
Naito K, Nakamura R, Nishiguchi H, Hashimoto S, Kawago M and
Okamura Y: Epidermal growth factor receptor mutations are
associated with docetaxel sensitivity in lung cancer. J Thorac
Oncol. 6:1658–1662. 2011.PubMed/NCBI View Article : Google Scholar
|