Introduction
Over the last several years a huge volume of
adaptive immune receptor (IR) recombinations has been obtained and
analyzed, due to the increased use of genomic approaches and
computational opportunities. In some cases, these recombinations
have been obtained through an immune repertoire approach whereby
PCR-based techniques lead to the amplification of a large number of
clonotypes (1,2), although in several cases, such an
approach indicates only a few relatively dominant clonotypes. Other
sources of adaptive IR recombination reads have been genomics files
representing tissues that included T-cells and B-cells, such as
exome (WXS) and RNAseq files (3-6).
The obvious limitation of mining such files is the lower level of
IR read recoveries in comparison to a PCR-based approach specific
for IRs, i.e., lack of depth. However, the advantage of the study
of IR recombination reads from genomics files has been the
opportunity for breadth, including the opportunity to integrate the
IR recombination read data with other data platforms, especially
gene expression and clinical data (7-10)
over very large patient sets.
Analysis of the IR functional implications, based on
the recovery of IR recombination reads from genomics files, has
involved two strategies. First, a summary (single value)
physicochemical assessment of the complementarity determining
region-3 (CDR3) amino acid (AA) sequences has been correlated with
survival distinctions. This approach is based on the simple idea
that the CDR3 is the most important region of the IR for antigen
recognition (11), and that,
particularly in a big data setting, a dominant physicochemical
feature could represent a minimum for successful antigen binding
and immune mediated reductions in tumor cell numbers (12). From the outset, algorithms were
developed to exploit the physicochemical features, and their
correlations with data represented by other platforms, such as
survival and immune biomarker expression, to identify possible
antigen partners (9,12-16).
For example, single value, physicochemical assessments of tumor
resident breast cancer B-cell receptor CDR3s revealed not only a
strong survival distinction when those assessments included an
assessment of the chemical interaction potential of same-patient
TP53 mutants but also revealed clear associations of immune markers
with the higher surviving, chemical complementarity group. More
recently, the chemical sequence motif approach, which preserves the
presumed AA sequence functional role in the physicochemical
assignments and which has already been employed in immune
repertoire approaches (17), has
been applied to IR CDR3s, and to CDR3-antigen matching algorithms,
where the IR CDR3s have been identified in large genomics file sets
(10,18). For example, an algorithm for
establishing basic chemical complementarity over a large dataset of
CDR3s and mutants, that employed a chemical sequence motif
approach, identified correlations between TRG CDR3, AA
sequence-PIK3CA mutant chemical complementarity and survival rates
and immune marker expression (10).
This latter approach involved a series of alignments of mutant
peptides throughout the breast cancer dataset with all of the
same-patient, tumor resident TRG CDR3s, to establish in each case
the highest possible chemical complementarity score indicated by
the algorithm developed and employed in that report. Patients
representing the highest chemical complementarity scores also
represented the highest survival. In another case, the chemical
sequence motif, survival distinctions were established with blood
resident, TRG CDR3 AA chemical sequence motifs in the absence of
any potential assessments of chemically complementary antigens
(18).
Given the high likelihood of continued
characterizations of immune receptor CDR3 chemical features for
either antigen identification or basic correlative work, it became
of interest to make a comparison of the potential of a relatively
simple, single value physicochemical parameter to represent the
CDR3 AA sequence, which is efficient in a big data setting,
compared with the value of the chemical sequence motif approach,
which is more processing intensive for all subsequent correlative
or chemical interaction approaches and which may be overly
restrictive for correlative studies and biomarker discovery. Thus,
we undertook an accounting of each approach for indicating survival
distinctions among The Cancer Genome Atlas (TCGA) cancer datasets,
to evaluate one specific area of use for these CDR3 AA sequences,
with results strongly indicating that the chemical sequence motif
assessments were more productive than a single parameter
physicochemical CDR3 AA assessment.
Materials and methods
IR recombination read recoveries
The approach to the recovery of the IR recombination
reads has been extensively described (5,19,20).
The software for using WXS files as input is freely available at
https://github.com/bchobrut-USF/blanck_group,
including a readme file; and as a container version at https://hub.docker.com/r/bchobrut/vdj,
including a readme file. Briefly, the approach involved a
pre-screen of the WXS files with 10-mers matching the V- and J-gene
segments for all seven of the adaptive human IRs, followed by a
high stringency validation of the V- and J-gene IDs (Table SI). All data used in this project
were obtained under the NIH dbGaP project approval no. 6300.
The single value, physicochemical
determinations and AA chemical sequence motif representations
The above GitHub and docker links include IR
recombination read processing software for establishing the single
value, physicochemical characters for the CDR3 AA sequences, e.g.,
net charge per residue or aromaticity (Table SII). These software modules follow
the precedents of the Pappu lab (21). The CDR3 AA chemical sequence motifs
were established for each CDR3 according to reference (18) and Table
SIII; and the python code used to make the symbol replacements
to establish the chemical sequence motifs is freely available at
https://github.com/bchobrut-USF/brca_motif.
Survival distinctions
Survival distinctions based on the single value,
physicochemical features of the CDR3 AA sequences were assessed as
described (12), with an example
output in Table SIV; and survival
distinctions based on the AA chemical sequence motif approach were
assessed as previously indicated (18), with example outputs in Table SV, Table SVI, Table SVII, Table SVIII, Table SIX, Table SX, Table SXI and Table SXII, representing Table I in the results section below.
 | Table IDetection of overall survival
distinctions via either the AA chemical sequence motif approach or
the single value, physicochemical features approach, for the IR
CDR3 AAs recovered from tumor WXS files representing eight cancer
datasets. |
Table I
Detection of overall survival
distinctions via either the AA chemical sequence motif approach or
the single value, physicochemical features approach, for the IR
CDR3 AAs recovered from tumor WXS files representing eight cancer
datasets.
| Cancer dataset | TRA CDR3 AA
chemical sequence motif approach (Table SV, Table SVI, Table SVII, Table SVIII, Table SIX, Table SX, Table SXI and Table SXII) | TRA single value,
physicochemical features approach (Table SIV) | TRB CDR3 AA
chemical sequence motif approach (Table SV, Table SVI, Table SVII, Table SVIII, Table SIX, Table SX, Table SXI and Table SXII) | TRB single value,
physicochemical features approach (Table SIV) |
|---|
| SKCM | 5 | 0 | 4 | 5 |
| BRCA | 8 | 0 | 1 | 0 |
| STAD | 0 | 3 | 0 | 3 |
| LUAD | 0 | 0 | 7 | 0 |
| LUSC | 6 | 0 | 2 | 1 |
| UCEC | 1 | 0 | 0 | 1 |
| HNSC | 0 | 1 | 0 | 0 |
| OV | 0 | 1 | 0 | 1 |
Overview of the comparison of the
survival distinctions indicated by the single value,
physicochemical feature compared with the AA chemical motif
sequence approach
Given the above survival distinction outputs,
exemplified by Table SIV, for the
single value, physicochemical approach, and Table SV, Table SVI, Table SVII, Table SVIII, Table SIX, Table SX, Table SXI and Table SXII for the CDR3 AA chemical
sequence motif approach, we totaled all survival distinctions
representing a Kaplan-Meier (KM) logrank P-value of less than 0.05,
for any of the above values or motifs, for either TRA or TRB CDR3s.
The exact same set of TRA, TRB CDR3s (exact same input) was used to
obtain the survival distinctions for the single value,
physicochemical and the chemical sequence motif approaches,
respectively, for each of the datasets.
Results
The adaptive IR recombination reads from every blood
and tumor WXS file in the TCGA database and in several publications
were obtained (5,12,20,22).
For numerous cancer datasets, it became apparent that it was
possible to observe survival distinctions based on single value,
physicochemical features of the CDR3 AA sequences (Table SIV) (23), with related approaches taken by
others (17). For the purpose of
this report, the physicochemical features approach, exemplified by
reference (23), will be referred
to as the single value, physicochemical feature(s), and in all
cases, the approach of reference (23) represents a single number for
characterizing a single physicochemical parameter of the CDR3 AA
sequence, for example, the net charge per residue (Table SII). More recently, we have
observed survival distinctions with an AA chemical sequence motif
approach, whereby the actual CDR3 AA sequence is reduced to a
sequence representing physicochemical features of similar AAs
(Table SIII) (18), for example NNP, for ‘negative,
negative, positive’ to represent the R-groups, at physiological pH,
of the E,E,K AA sequence. To appreciate the different potential of
the two approaches, we tabulated the opportunity to note survival
distinctions, based on a KM analysis with a resulting P-value of
less than 0.05. In the case of the single value, physicochemical
feature(s) approach, 22 distinct parameters were assessed for their
potential to indicate a survival distinction, whereas, in the case
of the AA chemical sequence motif approach, the minimum length in
the assessment was four AAs.
Keeping in mind the aspects of the two approaches
described in the preceding paragraph, we assessed overall survival
(OS) distinctions using all TRA and TRB CDR3 AA sequences,
respectively, available from all TCGA cancer datasets, with a basic
result being that more such distinctions could be had using the
CDR3 AA chemical sequence motif approach. For example, eight cancer
types representing TCGA tumor WXS files demonstrated 20 OS
distinctions for the AA chemical sequence motif approach,
representing the TRA CDR3s, vs. 5 OS distinctions for the single
value physicochemical features approach (Table I). For the TRB CDR3s, the eight
cancer types assessed represented 14 and 11 OS distinctions for the
AA chemical sequence motif and single value, physicochemical
features approaches, respectively (Table I). In the case of the OS
distinctions represented by the CDR3 AA chemical sequence motif
approach, the OS distinction was between TCGA cases representing
the AA chemical sequence motif vs. those without the chemical
motif, but with a recovery of the corresponding IR recombination
read, e.g., TRA. In the case of the OS distinctions representing
the single value, physicochemical features, the distinctions also
always represented only cases with a recovery of at least one read
representing the indicated IR recombination. In the case of the
single value, physicochemical features approach, this was out of
necessity, as this approach compares the top and bottom 50th
percentiles for the given physicochemical feature.
More recently, the AA chemical sequence motif and
the single value, physicochemical features approaches,
respectively, have been applied to IR CDR3 AA sequences obtained
from the blood WXS files of cancer patients and have indicated
survival distinctions (8,16,18),
not surprising considering the relatively limited clonality of
blood-borne IRs at the typical older ages of cancer patients; and
considering more precise work in single patient settings indicating
the detection of blood-borne, anti-cancer antigen IRs (24). Thus, we considered a comparison of
the opportunity to detect survival distinctions for the AA sequence
chemical motif vs. the single value, physicochemical features
approach using IR recombination reads obtained from the cancer
patient, blood WXS files. The same eight cancer types were
compared, as were compared using only tumor specimen WXS files, and
the comparison for the blood WXS files revealed 155 AA chemical
sequence motif-based OS distinctions vs. 5 such distinctions for
the single value, physicochemical features approach, for the TRA
CDR3s (Table II). Example KM
analyses representing the AA chemical sequence motif and the single
parameter, physicochemical motif approaches applied to detect the
OS distinctions, respectively, using the IR recombination reads
recovered from the blood WXS files, are shown in Fig. 1. For the TRB CDR3 AA sequences, the
comparison revealed 33 vs. 13 OS distinctions, for the AA chemical
motif and single value, physicochemical features approaches,
respectively (Table II).
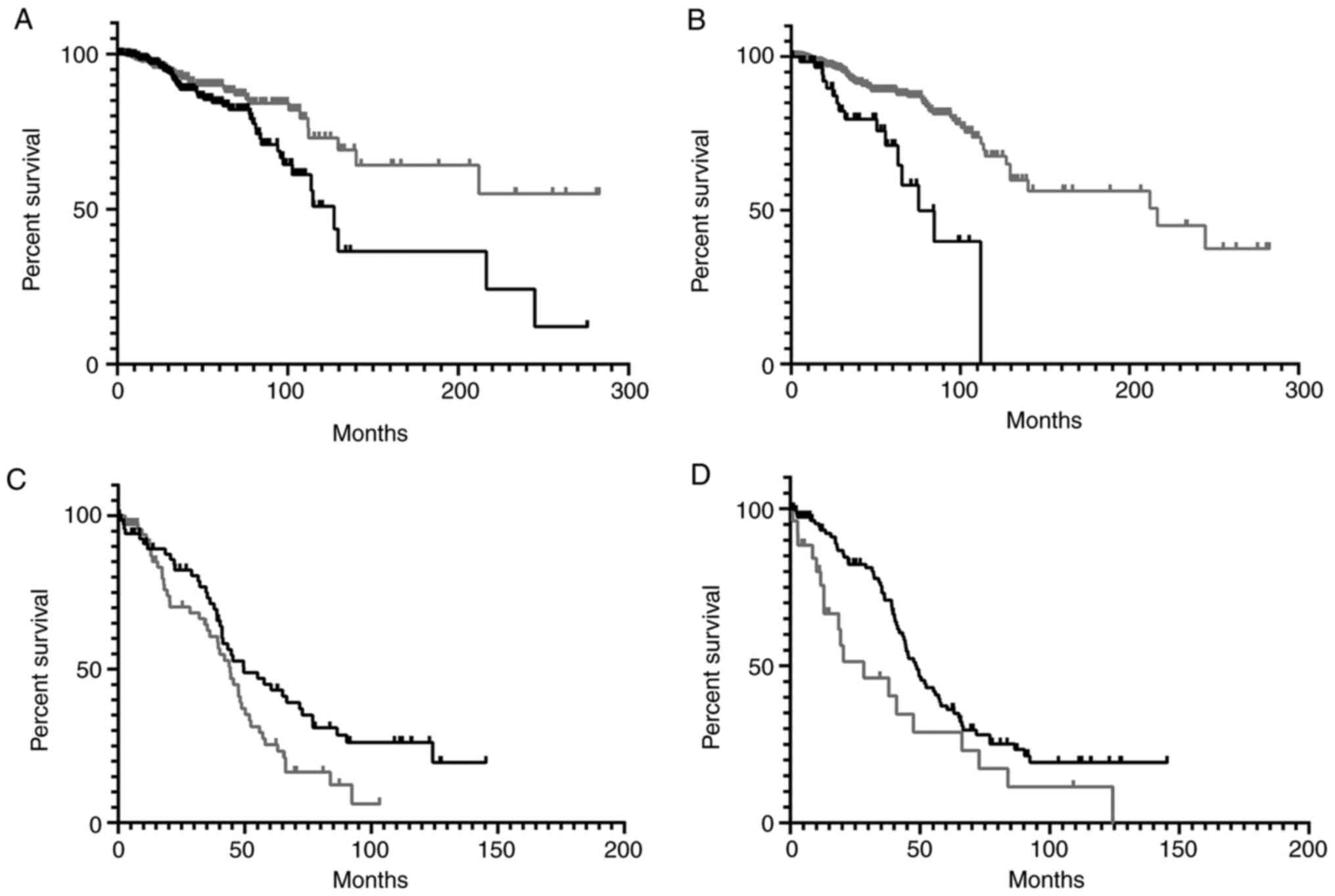 | Figure 1KM OS analyses representing TCGA-BRCA
and -OV case IDs with TRA CDR3 recoveries from blood WXS files. (A)
KM analysis for BRCA case IDs representing TRA CDR3s with the top
(black) and bottom (gray) 50th percentiles for the single value,
physiochemical parameter, aromaticity. Median OS time for case IDs
representing the top 50%, 127.2 months; median OS time for case IDs
representing the bottom 50%, undefined. Logrank P-value=0.0155. (B)
KM analysis for BRCA case IDs representing the AXXXHX motif in the
TRA CDR3 AA sequence (black), compared to all remaining case IDs
with TRA but without the AXXXHX motif (gray). Median OS time for
case IDs representing the TRA AXXXHX motif, 75.43 months; median OS
time for all remaining case IDs with TRA but without the AXXXHX
motif, 216.6 months. Logrank P-value<0.0001. (C) KM analysis for
OV case IDs representing TRA CDR3s with the top (black) and bottom
(gray) 50th percentiles for the single value, physiochemical
parameter, κ. Median OS time for case IDs representing the top 50%,
49.64 months; median OS time for case IDs representing the bottom
50%, 44.28 months. Logrank comparison P-value=0.0283. (D) KM
analysis for OV case IDs representing the XXXXA motif in the TRA
CDR3 AA sequence (black), compared to all remaining case IDs with
TRA but without the XXXXA motif (gray). Median OS time for case IDs
representing the TRA XXXXA motif, 48.75 months; median OS time for
all remaining case IDs with TRA but without the XXXXA motif, 28.35
months. Logrank comparison P-value=0.0208. KM, Kaplan-Meier; OS,
overall survival; TCGA, The Cancer Genome Atlas; BRCA, breast
cancer; OV, ovarian cancer; TRA, T-cell receptor-α; CDR3,
complementarity determining region-3. |
 | Table IIDetection of overall survival
distinctions via either the AA chemical sequence motif approach or
the single value, physicochemical features approach, for the IR
CDR3 AAs recovered from blood WXS files representing eight cancer
datasets. |
Table II
Detection of overall survival
distinctions via either the AA chemical sequence motif approach or
the single value, physicochemical features approach, for the IR
CDR3 AAs recovered from blood WXS files representing eight cancer
datasets.
| Cancer dataset | TRA CDR3 AA
chemical sequence motif approach | TRA single value,
physicochemical features approach | TRB CDR3 AA
chemical sequence motif approach | TRB single value,
physicochemical features approach |
|---|
| SKCM | 6 | 0 | 7 | 0 |
| BRCA | 46 | 1 | 7 | 0 |
| STAD | 1 | 0 | 0 | 1 |
| LUAD | 1 | 0 | 3 | 3 |
| LUSC | 8 | 0 | 2 | 6 |
| UCEC | 63 | 0 | 10 | 0 |
| HNSC | 20 | 1 | 1 | 1 |
| OV | 10 | 3 | 3 | 2 |
Given the robustness of the AA chemical sequence
motif approach in assessing whether an OS distinction could be had,
we assessed the opportunity to distinguish OS rates using the AA
chemical sequence motif vs. all remaining cases, rather than simply
all remaining cases with an IR recombination read recovery, as was
done for the data in Tables I and
II. In sum, the analyses indicated
a robust opportunity to identify OS distinctions, for TRA and TRB
CDR3s, and for CDR3s recovered from both tumor and blood WXS files,
when defining cases based on the recovery of a specific CDR3 AA
chemical sequence motif (Tables
III and IV).
 | Table IIIDetection of OS distinctions via the
AA chemical sequence motif approach via a comparison of specific AA
chemical sequence motifs vs. all remaining samples, regardless of
the recovery, or not, of an IR recombination read. The results
represent IR recombination reads obtained from tumor specimen WXS
files. |
Table III
Detection of OS distinctions via the
AA chemical sequence motif approach via a comparison of specific AA
chemical sequence motifs vs. all remaining samples, regardless of
the recovery, or not, of an IR recombination read. The results
represent IR recombination reads obtained from tumor specimen WXS
files.
| Cancer dataset | Specific TRA CDR3
AA chemical sequence motifs representing an OS distinction | Specific TRB CDR3
AA chemical sequence motifs representing an OS distinction |
|---|
| SKCM | 26 | 12 |
| BRCA | 4 | 0 |
| STAD | 0 | 0 |
| LUAD | 0 | 6 |
| LUSC | 5 | 2 |
| UCEC | 1 | 0 |
| HNSC | 0 | 1 |
| OV | 0 | 0 |
 | Table IVDetection of OS distinctions via the
AA chemical sequence motif approach via a comparison of specific AA
chemical sequence motifs vs. all remaining samples, regardless of
the recovery, or not, of an IR recombination read. The below
results represent IR recombination reads obtained from blood WXS
files. |
Table IV
Detection of OS distinctions via the
AA chemical sequence motif approach via a comparison of specific AA
chemical sequence motifs vs. all remaining samples, regardless of
the recovery, or not, of an IR recombination read. The below
results represent IR recombination reads obtained from blood WXS
files.
| Cancer dataset | Specific TRA CDR3
AA chemical motifs representing an OS distinction | Specific TRB CDR3
AA chemical motifs representing an OS distinction |
|---|
| SKCM | 3 | 2 |
| BRCA | 60 | 7 |
| STAD | 4 | 0 |
| LUAD | 10 | 6 |
| LUSC | 26 | 8 |
| UCEC | 103 | 40 |
| HNSC | 18 | 1 |
| OV | 6 | 0 |
Discussion
Over the last several years there has been an
extensive effort to group IR CDR3s based on sequence homologies and
based on chemical features, to develop CDR3 fingerprints of various
conditions and to bioinformatically attempt to link the CDR3s to
antigens. We have observed two basic approaches in the literature,
the single value, physicochemical assessment approach and the
chemical sequence approach, as detailed above. As with the above
datasets, these approaches have been applied in the cancer setting,
by our group (8-10,12-16,18,23,25),
by Ostmeyer et al (17), and
by others (26,27). In addition, CDR3 AA sequence
homology assessments have been applied in the SARS-CoV-2 and other
viral settings (28,29), in cases of allergies (30), in Alzheimer's disease and in other
neurological disorders (31,32). A
key issue in this development is the focus on the CDR3 AA sequences
in the big data setting. Also, this focus is based on the CDR3
being highly important in IR antigen binding and has allowed for
the efficient establishment of distinctions related to diseases.
This is in contrast to a goal of appreciating the role of the
entire IR in antigen binding, whether by in silico
approaches, such as molecular modeling, or with in vitro
approaches. The focus on the CDR3, and the patient distinctions
provided by the different CDR3-based parameters, represents a goal
of efficiency, particularly in meeting the longer-term goal of
identifying patients in particular risk groups.
It is not possible to make a controlled comparison
of the two CDR3-based approaches considered in this report, but it
is possible to assemble the record of results over a very large
dataset, particularly when these assessments are based on IR
recoveries from genomics files. This goal is important, again, in
the interest of maximizing efficiency, for answering the simple
question, is there a role for IR-antigen binding specificity in a
given condition or disease? And, the single value physicochemical
assessment represents another step, likely only applicable in the
big data setting, away from unnecessarily intensive approaches to
processing. In most cases, a very large amount of data regarding
the single value physicochemical features of CDR3s, integrated with
clinical information, can be processed with common skills on a
common laptop. However, the comparison in this report does indicate
that a negative result with a single value physicochemical
representing the CDR3s could not rule out the possibility that an
AA sequenced based, homology motif approach would uncover
patient-based risks.
As noted above, these IR recoveries have been
obtained from tens of thousands of genomics files, which are
available in conjunction with other data platforms, such as
survival data. Thus, the recovery of these IR CDR3s, followed by
their single value physicochemical or chemical sequence motif
assessments, can be linked to distinct survival rates in many
cases. Again, keeping in mind that a final assessment of which
approach is more successful is not possible, what can be said is
that the two approaches that have been applied in the past do
differ in the quantity of their output for the same mega-dataset,
namely eight cancer datasets from TCGA. Thus, there are two
limitations to understanding the value of the CDR3-limited,
chemical sequence motif approach over the CDR3-limited, single
value physicochemical feature assessment. First, it is possible
that with a PCR-based, immune repertoire approach, the vastly
increased numbers of CDR3s would be sufficient to provide as many,
or almost as many patient-distinction opportunities using a single
value physicochemical approach as is apparently provided by the
chemical sequence motif approach. In particular, even in the above
work, there was no attempt to render the chemical sequence motifs
back to single value physicochemical features, which may have led
to a convergence of the many chemical sequence motif results.
Having noted that, it is less likely that chemical sequence motifs
that are ‘outliers’, that cannot be summarized by a single value
physicochemical feature, would be missed when using the chemical
sequence motif approach from the start, in categorizing patient
risks. The second limitation in this report is the fact that all
distinctions based on the single value physicochemical or chemical
sequence motif approach were cancer survival distinctions. Thus, it
remains to be learned whether the apparent, increased value of
grouping the chemical sequence motif for interrogating clinical
data, compared with providing only a single value physicochemical
assessment, would be maintained in a viral infection setting, for
example, a setting that could be much more immunologically simpler
than cancer, particularly with regard to patient clinical features.
In other words, would a particular single value physicochemical
feature shifted in a certain direction always reflect the infection
of a specific virus, especially when other clinical parameters
related to a suspected viral infection are known?
However, if a comparison of the approaches in this
report remains consistent over additional datasets, with additional
investigators, a reasonable interpretation would be that, by
preserving sequence information, i.e., the order of the AAs in the
CDR3, it is more likely that groups of CDR3s can be identified as
being associated with given clinical features. With the single
value, physicochemical approach, it is likely that many CDR3s,
which happen to group with parameter-distinctive CDR3s because of a
tally of a chemical feature across multiple AAs, without regard to
AA positioning in the sequence, will be specifically irrelevant to
that parameter distinction. Depending on the goal, that may not
make the single value, physicochemical assessment pointless, but it
is possible that a lack of a result with a single value,
physicochemical feature would then lead to a second effort with the
chemical sequence motif approach to have a desired, clinically
relevant CDR3 fingerprint.
Supplementary Material
All TRA recoveries from all TCGA-BRCA
primary tumor exome files.
All single parameter physicochemical
values assigned to all TRA CDR3 AA sequences recovered from
TCGA-BRCA exome files. These values were used to generate the
TCGA-BRCA, TRA results of Table
S4.
Amino acid symbols representing
distinct chemical features for evaluation of CDR3 motifs.
Survival distinctions represented by
single parameter, TRA CDR3 physicochemical assessments for
TCGA-BRCA and TCGA-STAD
CDR3 amino acid chemical sequence
motifs representing survival distinctions for the eight cancer
datasets in article Table 1
(SKCM).
CDR3 amino acid chemical sequence
motifs representing survival distinctions for the eight cancer
datasets in article Table 1
(BRCA).
CDR3 amino acid chemical sequence
motifs representing survival distinctions for the eight cancer
datasets in article Table 1 (STAD).
These data represent 100% of the analysis output with no p-values
less than 0.05.
CDR3 amino acid chemical sequence
motifs representing survival distinctions for the eight cancer
datasets in article Table 1
(LUAD).
CDR3 amino acid chemical sequence
motifs representing survival distinctions for the eight cancer
datasets in article Table 1
(LUSC).
CDR3 amino acid chemical sequence
motifs representing survival distinctions for the eight cancer
datasets in article Table 1
(UCEC).
CDR3 amino acid chemical sequence
motifs representing survival distinctions for the eight cancer
datasets in article Table 1 (HNSC).
These data represent 100% of the analysis output with no p-values
less than 0.05.
CDR3 amino acid chemical sequence
motifs representing survival distinctions for the eight cancer
datasets in article Table 1 (OV).
These data represent 100% of the analysis output with no p-values
less than 0.05.
Acknowledgements
Authors wish to thank Ms. Corinne Walters
(University of South Florida) for administrative support related to
NIH dataset access.
Funding
Funding: AC, BIC were RISE fellowship recipients. BIC was an
American Hematology Association fellowship recipient.
Availability of data and materials
Parts of the article represents datasets available
from previously published supporting online material files. Any
additional raw data is available via email to the corresponding
author.
Authors' contributions
BEM and GB conceived the study, performed the
analyses, and wrote and edited the manuscript. BIC, AC, ECG and MJD
performed the analyses. All authors have read and approved the
final manuscript. BIC and GB confirm authenticity of all the raw
data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Corrie BD, Marthandan N, Zimonja B,
Jaglale J, Zhou Y, Barr E, Knoetze N, Breden FM, Christley S, Scott
JK, et al: iReceptor: A platform for querying and analyzing
antibody/B-cell and T-cell receptor repertoire data across
federated repositories. Immunol Rev. 284:24–41. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Chiffelle J, Genolet R, Perez MA, Coukos
G, Zoete V and Harari A: T-cell repertoire analysis and metrics of
diversity and clonality. Curr Opin Biotechnol. 65:284–295.
2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Thorsson V, Gibbs DL, Brown SD, Wolf D,
Bortone DS, Ou Yang TH, Porta-Pardo E, Gao GF, Plaisier CL, Eddy
JA, et al: The immune landscape of cancer. Immunity.
48:812–830.e14. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Brown SD, Raeburn LA and Holt RA:
Profiling tissue-resident T cell repertoires by RNA sequencing.
Genome Med. 7(125)2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Gill TR, Samy MD, Butler SN, Mauro JA,
Sexton WJ and Blanck G: Detection of productively rearranged TcR-α
V-J sequences in TCGA exome files: Implications for tumor
immunoscoring and recovery of antitumor T-cells. Cancer Inform.
15:23–28. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Samy MD, Tong WL, Yavorski JM, Sexton WJ
and Blanck G: T cell receptor gene recombinations in human tumor
specimen exome files: Detection of T cell receptor-β VDJ
recombinations associates with a favorable oncologic outcome for
bladder cancer. Cancer Immunol Immunother. 66:403–410.
2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Huda TI, Mihyu M, Gozlan EC, Arndt MF,
Diaz MJ, Zaman S, Chobrutskiy BI and Blanck G: Specific HLA
alleles, paired with TCR V- and J-gene segment usage, link to
distinct multiple myeloma survival rates. Leuk Lymphoma.
62:1711–1720. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Gozlan EC, Chobrutskiy BI, Zaman S,
Yeagley M and Blanck G: Systemic adaptive immune parameters
associated with neuroblastoma outcomes: The significance of
gamma-delta T cells. J Mol Neurosci. 71:2393–2404. 2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hsiang M, Chobrutskiy BI, Diaz M, Huda TI,
Creadore S, Zaman S, Cios KJ, Gozlan EC and Blanck G: . Chemical
complementarity between immune receptors and cancer mutants,
independent of antigen presentation protein binding, is associated
with increased survival rates. Transl Oncol.
14(101069)2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chobrutskiy BI, Chobrutskiy A, Zaman S,
Yeagley M, Huda TI and Blanck G: High-throughput, sliding-window
algorithm for assessing chemical complementarity between immune
receptor CDR3 domains and cancer mutant peptides: TRG-PIK3CA
interactions and breast cancer. Mol Immunol. 135:247–253.
2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Schmitz R, Wright GW, Huang DW, Johnson
CA, Phelan JD, Wang JQ, Roulland S, Kasbekar M, Young RM, Shaffer
AL, et al: Genetics and pathogenesis of diffuse large B-cell
lymphoma. N Engl J Med. 378:1396–1407. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chobrutskiy BI, Zaman S, Diviney A, Mihyu
MM and Blanck G: T-cell receptor-α CDR3 domain chemical features
correlate with survival rates in bladder cancer. J Cancer Res Clin
Oncol. 145:615–623. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yeagley M, Chobrutskiy BI, Gozlan EC,
Medikonda N, Patel DN, Falasiri S, Callahan BM, Huda T and Blanck
G: Electrostatic complementarity of T-cell receptor-alpha CDR3
domains and mutant amino acids is associated with better survival
rates for sarcomas. Pediatr Hematol Oncol. 38:251–264.
2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Arturo JF, Chobrutskiy BI, Yeagley M,
Patel DN, Falasiri S, Patel JS and Blanck G: Electrostatic
complementarity of B-cell receptor CDR3s and TP53-mutant amino
acids in breast cancer is associated with increased disease-free
survival rates. Cell Mol Immunol. 17:776–778. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chobrutskiy BI, Yeagley M, Diviney A,
Zaman S, Gozlan EC, Tipping P, Koohestani DM, Roca AM and Blanck G:
A scoring system for the electrostatic complementarities of T-cell
receptors and cancer-mutant amino acids: Multi-cancer analyses of
associated survival rates. Immunology. 159:373–383. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chobrutskiy BI, Yeagley M, Tipping P,
Zaman S, Diviney A, Patel DN, Falasiri S, Uversky VN and Blanck G:
Chemical complementarity between immune receptor CDR3s and IDH1
mutants correlates with increased survival for lower grade glioma.
Oncogene. 39:1773–1783. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ostmeyer J, Lucas E, Christley S, Lea J,
Monson N, Tiro J and Cowell LG: Biophysicochemical motifs in T cell
receptor sequences as a potential biomarker for high-grade serous
ovarian carcinoma. PLoS One. 15(e0229569)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chobrutskiy A, Chobrutskiy BI, Zaman S,
Hsiang M and Blanck G: Chemical features of blood-borne TRG CDR3s
associated with an increased overall survival in breast cancer.
Breast Cancer Res Treat. 185:591–600. 2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Tong WL, Tu YN, Samy MD, Sexton WJ and
Blanck G: Identification of immunoglobulin V(D)J recombinations in
solid tumor specimen exome files: Evidence for high level B-cell
infiltrates in breast cancer. Hum Vaccin Immunother. 13:501–506.
2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Chobrutskiy BI, Zaman S, Tong WL, Diviney
A and Blanck G: Recovery of T-cell receptor V(D)J recombination
reads from lower grade glioma exome files correlates with reduced
survival and advanced cancer grade. J Neurooncol. 140:697–704.
2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Holehouse AS, Das RK, Ahad JN, Richardson
MO and Pappu RV: CIDER: Resources to analyze sequence-ensemble
relationships of intrinsically disordered proteins. Biophys J.
112:16–21. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Tong WL, Callahan BM, Tu YN, Zaman S,
Chobrutskiy BI and Blanck G: Immune receptor recombinations from
breast cancer exome files, independently and in combination with
specific HLA alleles, correlate with better survival rates. Breast
Cancer Res Treat. 173:167–177. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Diaz MJ, Chobrutskiy BI, Zaman S and
Blanck G: Immunogenomics of colorectal adenocarcinoma: Survival
distinctions represented by immune receptor, CDR3 chemical features
and high expression of BTN gene family members. Cancer Treat Res
Commun. 24(100196)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Gros A, Parkhurst MR, Tran E, Pasetto A,
Robbins PF, Ilyas S, Prickett TD, Gartner JJ, Crystal JS, Roberts
IM, et al: Prospective identification of neoantigen-specific
lymphocytes in the peripheral blood of melanoma patients. Nat Med.
22:433–438. 2016.PubMed/NCBI View
Article : Google Scholar
|
|
25
|
Patel DN, Yeagley M, Arturo JF, Falasiri
S, Chobrutskiy BI, Gozlan EC and Blanck G: A comparison of immune
receptor recombination databases sourced from tumour exome or
RNAseq files: Verifications of immunological distinctions between
primary and metastatic melanoma. Int J Immunogenet. 48:409–418.
2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Thorsélius M, Kröber A, Murray F, Thunberg
U, Tobin G, Bühler A, Kienle D, Albesiano E, Maffei R, Dao-Ung LP,
et al: Strikingly homologous immunoglobulin gene rearrangements and
poor outcome in VH3-21-using chronic lymphocytic leukemia patients
independent of geographic origin and mutational status. Blood.
107:2889–2894. 2006.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Stamatopoulos K, Belessi C, Moreno C,
Boudjograh M, Guida G, Smilevska T, Belhoul L, Stella S,
Stavroyianni N, Crespo M, et al: Over 20% of patients with chronic
lymphocytic leukemia carry stereotyped receptors: Pathogenetic
implications and clinical correlations. Blood. 109:259–270.
2007.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wong MK, Liu JT, Budylowksi P, Yue FY, Li
Z, Rini JM, Carlyle JR, Zia A, Ostrowski M and Martin A: Convergent
CDR3 homology amongst Spike-specific antibody responses in
convalescent COVID-19 subjects receiving the BNT162b2 vaccine. Clin
Immunol. 237(108963)2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Babel N, Brestrich G, Gondek LP, Sattler
A, Wlodarski MW, Poliak N, Bethke N, Thiel A, Hammer MH, Reinke P
and Maciejewski JP: Clonotype analysis of cytomegalovirus-specific
cytotoxic T lymphocytes. J Am Soc Nephrol. 20:344–352.
2009.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Smith NP, Ruiter B, Virkud YV, Tu AA,
Monian B, Moon JJ, Love JC and Shreffler WG: Identification of
antigen-specific TCR sequences based on biological and statistical
enrichment in unselected individuals. JCI Insight.
6(e140028)2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Joshi C, Sivaprakasam K, Christley S,
Ireland S, Rivas J, Zhang W, Sader D, Logan R, Lambracht-Washington
D, Rosenberg R, et al: CSF-derived CD4(+) T-cell diversity is
reduced in patients with Alzheimer clinical syndrome. Neurol
Neuroimmunol Neuroinflamm. 9(e1106)2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Gate D, Saligrama N, Leventhal O, Yang AC,
Unger MS, Middeldorp J, Chen K, Lehallier B, Channappa D, De Los
Santos MB, et al: Clonally expanded CD8 T cells patrol the
cerebrospinal fluid in Alzheimer's disease. Nature. 577:399–404.
2020.PubMed/NCBI View Article : Google Scholar
|















