1. Introduction
In exclusive breastfeeding (EBF), breast milk
becomes the only source of nutrition for infants without any other
liquids or solids (1). EBF is
beneficial for the baby as well as the mother (2-4),
and the World Health Organization (WHO) and United Nations
Children's Fund (UNICEF) recommend EBF for the first 6 months of an
infant's life and should then be continued for 2 years or more
(5). However, in 2020, only 44% of
babies under 6 months worldwide received exclusive breastfeeding
(2). Many factors influence the
cessation of breastfeeding, including insufficient milk supply
(6-8),
self-weaning (9), primiparity
(10), mother/infant separation
(6), inconvenience/fatigue due to
breastfeeding (11), and
inflammatory breast diseases such as mastitis (12,13).
Mastitis is inflammation of the breast, with or
without infection (14). Worldwide,
-20% of women suffer from mastitis whilst breastfeeding (15). The majority of cases occur in the
first 6-8 weeks postpartum, and approximately one-third of women
experience a recurrent episode (16,17).
Up to 3% of cases of mastitis develop into a breast abscess
(18). Puerperal breast
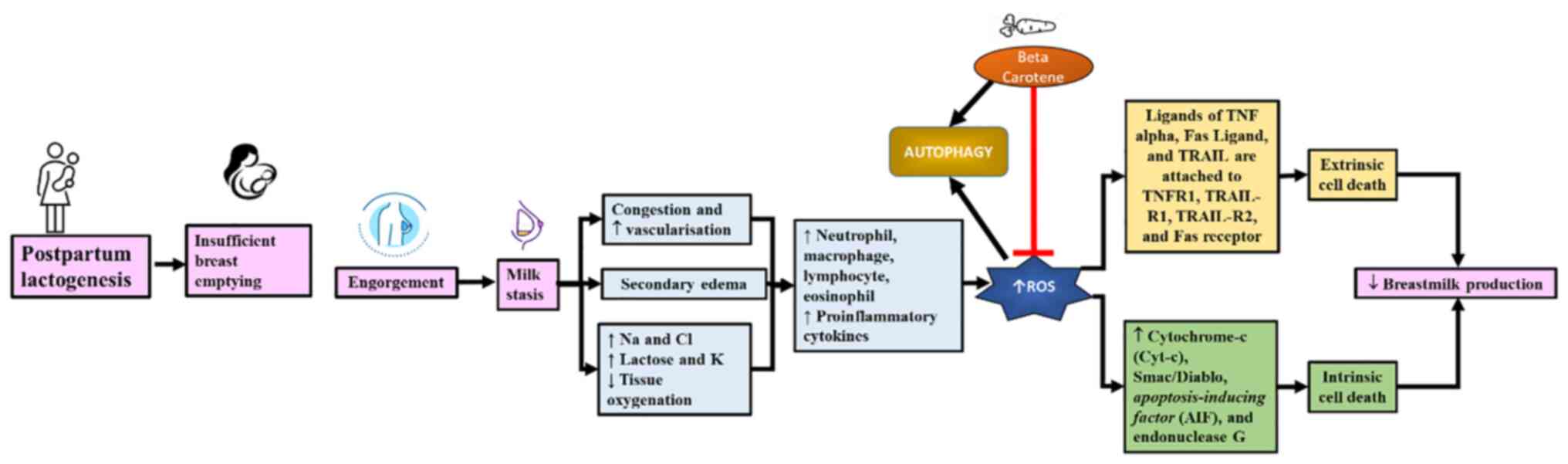
inflammation starts with insufficient breast emptying (milk stasis)
(12). This causes an increase in
intraductal pressure and opens milk duct epithelial cells'
intercellular junctions. Breast milk further moves into the
connective tissue and generates a sterile inflammatory environment,
from which a secondary bacterial infection may follow. Without
proper treatment, this can progress to a mammary abscess that
requires surgical treatment (12).
Several studies indicate that puerperal breast inflammation is
correlated with the cessation of breastfeeding (12,16).
Milk stasis increases the levels of inflammatory
cytokines (TNF-α, IL-1b, IL-6, IL-8), neutrophils, macrophages,
lymphocytes, eosinophils, and various epithelial cells of mammary
tissue (19). Cytokines further
activate reactive oxygen species (ROS), which play a complex role
in the inflammatory process (20).
The excessive buildup of ROS disrupts cellular homeostasis, causes
oxidative stress and mitochondrial dysfunction, and induces
autophagy (21). The oxidative
damage in breast inflammation can cause mammary gland cell death
and affects breast milk secretions (15).
Antioxidant supplementation decreases the incidence,
duration, and severity of mastitis (22,23).
Antioxidants scavenge free radicals and inhibit the activity of
oxidizing enzymes, thus reducing the damage caused by free radicals
(23). Some herbal treatments with
high antioxidant and anti-inflammatory activities are widely used
in mastitis treatment, such as Moringa leaves (24), binahong leaves (Anredera
cordifolia) (25), cherry leaf
decoction (Muntingia calabura L.) (26), Red Algae Eucheuma Spinosum (27), Macroalgae Extract (28), garlic, manjakani fruit, betel leaf,
white turmeric, and eucalyptus leaves (29). Carotenoids are another type of
active substance found in plants with high antioxidant contents and
anti-inflammatory effects, but have not been as widely explored as
treatment or prevention options for mastitis. Previous studies
showed that increasing serum retinol concentrations in dairy cows
was associated with a decreased risk of clinical mastitis, and low
concentrations of vitamin A and β-carotene increased the severity
of mastitis (30,31). Carotenoids have a significant impact
on maintaining the health of epithelial tissue and the stability of
mucosal surface integrity (32).
Carotenoids are yellow-orange pigments that are
abundantly present in several plants, marine invertebrates, and
microorganisms. There are ~50 carotenoids consumed in a standard
human diet, but only 20 have been identified in the human plasma,
with β-carotene, α-carotene, lycopene, and cryptoxanthin as the
most common (33). Carotenoids have
potent antioxidant, anti-inflammatory, and immunomodulatory
abilities. (34) β-carotene is the
most common carotenoid, which is also known as pro-vitamin A, and
is one of the most abundant carotenoids in the human blood with
several health-promoting properties (35,36).
The anti-inflammatory effects of β-carotene have been demonstrated
in multiple systems (37,38).
Previous studies showed that β-carotene and vitamin
A reduce the risk of puerperal breast inflammation (39). Low concentrations of plasma vitamin
A (<80 µg/100 ml) and β-carotene (<200 µg/100 ml) correlated
with the severity of mastitis (23,30).
Animal studies in cows showed that mastitic cows, as determined
using the California Mastitis Test score, had significantly lower
plasma vitamin A and β-carotene concentrations than healthy cows
(32). To reduce the risk of
mastitis, a dry and lactating cow's diet should contain 110 IU/kg
bwt/day of vitamin A (22). Plasma
concentrations of β-carotene >3 mg/l promoted udder health in
dairy cattle (40). Low
concentrations of plasma vitamin A (<0.8 µg/ml) and β-carotene
(<2 µg/ml) were linked with the severity of mastitis. However,
unfortunately, there is minimal data regarding the optimal dose of
β-carotene for postpartum women in correlation to treatment or
prevention of puerperal breast inflammation.
As an antioxidant, β-carotene suppresses
intracellular ROS production and reduces antioxidative enzyme
activity (36,41,42).
Β-carotene supplementation exerts a stabilizing effect on
polymorphonuclear cells such as neutrophils, eosinophils, and
basophils, and optimizes lymphocyte function (23). The inflammatory response in mastitis
and the role of β-carotene as an antioxidant is summarized in
Fig. 1. However, there is limited
study on the role of β-carotene in correlation with autophagy
modulation and puerperal breast inflammation. The present review
summarizes the major findings on β-carotene as a potential
autophagy modulator in puerperal breast inflammation.
2. Autophagy in puerperal breast
inflammation
Breast fullness or breast engorgement is a part of
lactogenesis in the postpartum period. This fullness manifests ~36
h postpartum and occurs 3-7 days postpartum, with primiparous
mothers most commonly affected (43). In breast engorgement, the breast
undergoes increased vascularity, congestion, milk accumulation, and
oedema (43,44).
In milk stasis, the accumulation of breast milk
disrupts vascularisation and causes tissue hypoxia (19). Tissue hypoxia induces the
transcription of Bcl2/adenovirus E1B 19 kDa protein-interacting
protein 3 (BNIP3) and induction of NIX following HIF1 activation
(21). This in turn competes with
beclin-1 for the binding of Bcl-2, and the release of beclin-1 is
triggered, followed by autophagy (45). PERK, an ER stress sensor, is
stimulated and, later in the process, stimulates the expression of
Microtubule-associated protein 1A/1B-light chain 3 (LC3) and
autophagy gene (ATG)5, both of which are ATGs (46). Oxidative stress triggers FOXO3 and
then stimulates the transcription of LC3, BNIP, and NRF2, which
induces transcription of p62(21).
The process mentioned above positively modulates autophagy. p53 has
a role in activating DNA damage-regulated autophagy modulator and
sestrins, two autophagy-related genes that positively regulate
autophagy (47). TP53-Induced
Glycolysis and Apoptosis Regulator negatively regulates autophagy,
but while it is a p53 target, it possesses a p53-independent
function in autophagy (21). AMPK
is also p53-independent, and is activated by sestrins to inhibit
mTOR activity and thus induce autophagy (48). ROS constrains ATG4 protease activity
and promotes autophagosome formation (21,49).
Milk stasis also results in high oxygen and energy
demands and increases ROS production as a result (12). ROS can assist the healing and tissue
repair process in moderate concentrations, but in excess, ROS
disrupts redox homeostasis and this results in oxidative stress and
damage to cell organelles (50).
The increase in free radical levels in puerperal breast
inflammation is triggered by the increase of neutrophils,
macrophages, lymphocytes, eosinophils, and mammary tissue
epithelial cells, TNF-α, IL-1b, IL-6, IL-8, and nitric oxide (NO)
(51). ROS accumulation triggers
endothelial dysfunction and tissue injury in mammary gland tissue,
opens inter-endothelial junctions, and stimulates inflammatory cell
migration across the endothelial barrier. These cells support the
clearance of pathogens, but excessive amounts may also cause tissue
injury (52).
ROS is essential in promoting cell autophagy as a
critical signalling molecule (53).
Autophagy facilitates the degradation of intracellular proteins and
organelles via lysosomes. ROS induces autophagy, and autophagy
reduces oxidative damage (49).
During inflammation, the NRF2 transcription factor enhances p62
expression, and p62, in turn, creates a positive feedback loop for
NRF2(49).
Autophagy is involved in inflammatory reactions and
barrier repair by degrading cell components via the lysosomal
pathway (54). Autophagosomes are
formed during autophagy to encapsulate protein aggregates and/or
damaged organelles. Hydrolytic enzymes degrade the damaged
organelles after autophagosome fusion with a lysosome (55). Autophagy is stimulated by any
stressor that causes cellular stress, including hypoxia (56), nutrient deficiency (57), chemical exposure (56), excessive ROS (58), or intracellular pathogens (59). Autophagy is a cellular recycling
process that involves potential cell death outcomes and functions
to protect cells from apoptosis (60).
Four sub-groups of ATGs regulate autophagy; namely
Atg1/unc-51-like kinase (ULK) complex, two ubiquitin-like proteins
[Atg12 and Atg8/microtubule-associated protein light chain 3 (LC3)]
conjugation systems, the class III phosphatidylinositol 3-kinase
(PI3K)/vacuolar protein sorting 34 (Vps34) complex I, and two
transmembrane proteins, Atg9/mAtg9 and VMP1(61). ROS induces autophagy through two
methods, directly and indirectly. The direct regulation is
established through modulation of the involved proteins, such as
Atg4, Atg5, and Beclin. Indirect regulation is achieved through
autophagy-related signaling pathways, such as the mTOR,
mitogen-activated protein kinase (MAPK), p38, ERK, and JNK
(62). ROS inhibits mTOR activity
and activates AMPK, and initiates autophagy through the increase of
Vps34 complex activity (50).
Activation of the p53 pathway causes JNK and Sestrin2 activation.
JNK and Sestrin2 bind to TSC1/TSC2 and induce autophagy (62). ROS also promotes LC3-II
translocation through the inhibition of ATG4, which converts LC3-II
into LC3-I (63).
Excessive ROS production triggers cell death
extrinsically and intrinsically (52). The extrinsic pathway is mediated via
four major cell death receptors; TNF receptor 1, TNF-related
apoptosis-inducing ligand receptor (TRAIL-R)1, TRAIL-R2, and the
Fas receptor (19). TNFα, Fas
ligand, and TRAIL bind to these receptors. TNFα is secreted by
activated macrophages and is involved in the increase in
inflammatory cytokines (50).
The increase in mitochondrial outer membrane
permeability (MOMP) triggers intrinsic pathways of cell death
(52). MOMP induces the release of
Cytochrome-c (Cyt-c), Smac/Diablo, apoptosis-inducing factor (AIF),
and endonuclease G, which play a role in cell death in either a
caspase-dependent or -independent manner (64). This increase in MOMP is stimulated
by an excessive entry of calcium and a high level of oxidative
stress, which causes mitochondrial membrane depolarization
(52). An apoptosome is formed as
Cyt-c attaches to apoptosis activation factor-1 and this recruits
the initiator pro-caspase 9. Caspase-9 functions to trigger
caspase-3. (58) Smac/Diablo also
plays a role in activating effector caspases by eliminating the
blockage of the inhibitor of apoptosis proteins (50). After AIF is activated, DNA
condensation and cleavage induction occur (52). The primary determinant of cell death
is the mitochondrial release of Cyt-c. BH3 interacting-domain death
agonist, a pro-apoptotic member of the Bcl-2 family, cleaves
caspase-8 and increases MOMP, followed by oligomerization of
pro-apoptotic proteins Bax/Bak which induce Cyt-c release (52).
The release of Cyt-c is triggered by oxidative
stress, facilitates electron transport chain uncoupling, and
increases mitochondrial ROS production/levels. Cardiolipin (CL) is
an anionic phospholipid that aids Cyt-c anchoring to the inner
mitochondrial membrane. The affinity binding with Cyt-c is reduced
in oxidative stress due to CL oxidation. CL-bound mitochondrial
Cyt-c catalyses CL peroxidation, and this phenomenon can occur at a
lower H2O2 concentration when bound to
CL-containing membranes. Thus, oxidative stress stimulates cell
death through the peroxidase activity of Cyt-c and CL peroxidation
(52).
3. The role of β-carotene in the modulation
of autophagy in puerperal breast inflammation
Mastitis is related to an inadequate intake of
vitamin E, selenium, β-carotene, and vitamin A (65). Antioxidants decrease the duration,
incidence, and severity of mastitis (23). Β-carotene and vitamin A can protect
mammary tissues and milk from the destructive effects of free
radicals. Food containing vitamin A and β-carotene contributed to a
reduced incidence of mastitis during the early dry period in cows
(23).
Carotene exhibits a range of important functions,
and the numerous benefits of carotenoids are related to their
antioxidant and anti-inflammatory effects. Vitamin A and β-carotene
trigger the generation of immune cells and prevent the initiation
of the fatty acid peroxidation chain reaction (39). Antioxidants directly scavenge free
radicals and inhibit the activity of oxidizing enzymes, thus
protecting the body from free radicals (23). β-carotene quenches singlet oxygen
and neutralizes lipid peroxyl radicals. It has been previously
shown that 800 mg/kg body weight of algae Spirulina fusiformis,
containing β-carotene and SOD, reduced the levels of almost all
oxidative stress biomarkers measured in the study. Serum lipid
hydroperoxide levels were reduced by 45%, and leukocyte MDA levels
were reduced by 50% (66).
β-carotene has a long system of conjugated double bonds with
π-electrons delocalized over the length of the polyene chain,
providing it with an effective ability to scavenge ROS (67). Studies have shown that β-carotene
significantly inhibited intracellular ROS production (30,32,42).
In a study investigating the effects of β-carotene
in corneal endothelial cells, a film scaffold incorporated with an
appropriate amount of β-carotene showed enhanced initial cell
adhesion, proliferation, proper cell morphology, and gene
expression compared with a pristine silk fibroin (SF) scaffold.
β-carotene in the SF film scaffold enhanced the function of the
ATPase pump of corneal endothelial cells (68). In infected tissues, β-carotene
decreases the expression of inflammatory mediators, MAPKs, and
redox-sensitive transcription factors (69).
β-carotene is widely available in common foodstuffs
with broad affordability for the general community. Using
β-carotene for postpartum mothers can reduce the occurrence of
severe mastitis and maternal and infant morbidity and mortality
(32). Although β-carotene and
vitamin A serve essential roles in immunity, the latest
recommendation from WHO states that vitamin A supplementation for
postpartum mothers is not recommended as a public health
intervention to prevent maternal and infant morbidity and
mortality. Postpartum mothers must obtain a balanced healthy
nutritional intake to obtain an adequate amount of various
nutrients needed (70,71). Dietary dosage of carotenoids may
promote health, but supplementation with high doses may be
associated with adverse effects, especially in smokers or subjects
exposed to environmental pollutants (72).
β-carotene is abundantly present in green leafy
vegetables, carrots, pumpkins, papaya and red palm oil, milk,
liver, and fish oil (71). It has
been shown that consuming fruit and vegetable sources of β-carotene
increases the vitamin A status of lactating women (73). Vitamin A is recommended at 1,300 mcg
retinol activity equivalent (RAE) daily. One mcg RAE is equivalent
to 2 mcg supplemental β-carotene or 12 mcg dietary β-carotene
(74). However, several factors
affect the bioavailability and equivalency of vitamin A, such as
food processing techniques and the specific dietary intake of an
individual (73).
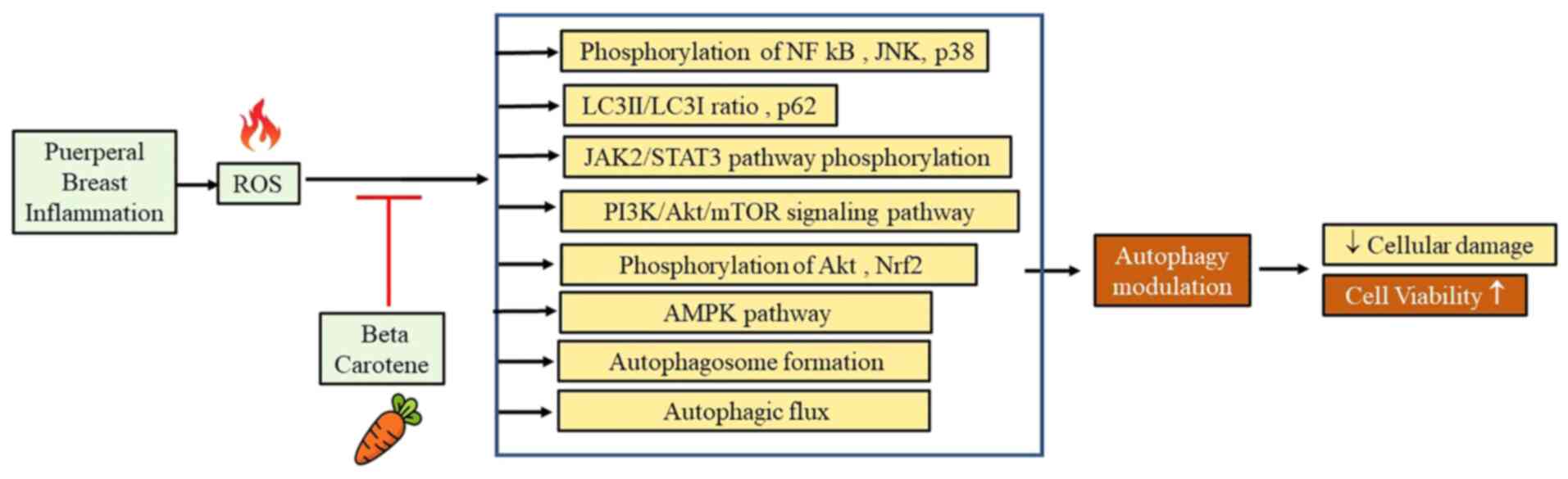
β-carotene plays an essential role in modulating
autophagy. In LPS-induced rat intestinal tissue, β-carotene
treatment significantly reduced the LPS-mediated phosphorylation of
JAK2/STAT3 and NF-κB (59), and
significantly suppressed the LPS-induced phosphorylation of JNK and
p38(41) The levels of p-Akt are
decreased following LPS treatment, and β-carotene significantly
upregulated the phosphorylation of Akt. (41) It also significantly suppressed the
ratio of LC3-II/LC3-I, which was upregulated by LPS (41). LC3 consists of LC3-I and LC3-II.
LC3-II is positively correlated with the degree of autophagy, and
is used as an autophagy marker. LC3-I is present in the cytoplasm
where a small segment of the polypeptide is cleaved and is
converted into LC3-II (75).
β-carotene modulates LPS-induced autophagy and inhibits the
activity of the related inflammation pathways (41).
However, another study showed that oral β-carotene
supplementation daily for 14 days in male ddY mice (8 wk old) did
not alter the ratio of LC3-II to LC3-I in the β-carotene group
(76). In H9C2 cell lines, low-dose
β-carotene induces autophagy, as shown by a decrease in LC3II and
p62 levels. NF-κB protein levels were lowered, and Nrf2 was
triggered, but no significant alteration of Nrf1 was observed. A
low dose of βcarotene stimulates cell viability by suppressing the
apoptotic signals carried by caspase 3 and 9. Furthermore, a low
dose of β-carotene increases cell viability through autophagy
stimulation, inhibition of pro-inflammatory factors, and
suppression of apoptosis (36).
Treatment of SAOs and Caco-2, cell lines derived
from a human osteosarcoma and colon adenocarcinoma, respectively,
using 200-400 µg/ml carotene extract nanoemulsion (CEN) triggered
autophagy, based on the increase of autophagosome formation,
increased expression of LC3-II, modulation of autophagic flux,
increased phosphorylation, and an increase in the levels of the
active form of AMPK kinase pAMPKThr172, which activates
AMPK and induces autophagy (33).
β-carotene is the major carotenoid in CEN (33). However, treatment with the same dose
of β-carotene alone did not show the same effect as when combined
in the CEN, indicating a dose-dependent effect or a synergism of
different components in CEN (33).
The PI3K/Akt/mTOR signaling pathway is important in
cell survival and autophagy (77).
Inhibition of this signaling pathway induces autophagy. Consistent
with previous studies, β-carotene treatment was also shown to
decrease Advanced Glycation End products (AGE)-induced elevation of
the LC3II/LC3I ratio and the number of LC3-labeled puncta. The
protective effect of β-carotene in AGE-induced H9c2 cells was
achieved through the activation of the PI3K/Akt/mTOR signaling
pathway (42). Fig. 2 summarizes the pathways affected by
β-carotene as an autophagy modulator.
β-carotene shows promising potential as an autophagy
modulator in increasing mammary gland cell survival and maintaining
breastmilk production. Further research is required to understand
the role of β-carotene in puerperal breast inflammation
management.
4. Conclusion
Puerperal breast inflammation can cause result in
cessation of breastfeeding due to mammary gland damage and a
decrease in milk production. Excessive ROS levels in puerperal
breast inflammation results in oxidative damage and induces
improper autophagy. β-carotene has ROS-scavenging activity and the
ability to modulate autophagy through the suppression of
JAK2/STAT3, NF-κB, JNK, and p38 phosphorylation, suppressing the
ratio of LC3-II/LC3-I and upregulating the phosphorylation of Akt.
Further studies are required to conclude the full potential of
β-carotene in puerperal breast inflammation.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by a WCR grant from the
Ministry of Education and Culture (grant no.
1207/UN6.3.1/PT.00/2021) and an internal grant from the Universitas
Kristen Maranatha (grant no. 034/SK/ADD/UKM/VI/2021).
Availability of data and materials
Not applicable.
Authors' contributions
STH, RL, JWG, and ER performed the literature search
and assisted in drafting and revising the manuscript. All authors
have read and approved the final manuscript. Data authentication is
not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Silva FV, Dias F, Costa G and Campos MDG:
Chamomile reveals to be a potent galactogogue: The unexpected
effect. J Matern Neonatal Med. 31:116–118. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
World Health Organization (WHO) and United
Nations Children's Fund (UNICEF): Indicators for Assessing Infant
and Young Child Feeding Practices. Vol. WHA55 A55/. WHO, Geneva,
p122, 2021. http://apps.who.int/iris/bitstream/handle/10665/44306/9789241599290_eng.pdf?sequence=1%0Ahttp://whqlibdoc.who.int/publications/2008/9789241596664_eng.pdf%5Cnhttp://www.unicef.org/programme/breastfeeding/innocenti.htm%5Cnhttp://innocenti15.net/declaration.
|
|
3
|
Mosca F and Giannì ML: Human milk:
Composition and health benefits. Pediatr Med Chir.
39(155)2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Henrick BM, Nag K, Yao XD, Drannik AG,
Aldrovandi GM and Rosenthal KL: Milk matters: Soluble toll-like
receptor 2 (sTLR2) in breast milk significantly inhibits HIV-1
infection and inflammation. PLoS One. 7(e40138)2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
World Health Organization and United
Nations Children's Fund (UNICEF): Baby-friendly hospital
initiative: revised, updated and expanded for integrated care. WHO,
Geneva, 2009. https://apps.who.int/iris/handle/10665/43593.
|
|
6
|
Chang PC, Li SF, Yang HY, Wang LC, Weng
CY, Chen KF, Chen W and Fan SY: Factors associated with cessation
of exclusive breastfeeding at 1 and 2 months postpartum in Taiwan.
Int Breastfeed J. 14(18)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Grzeskowiak LE, Wlodek ME and Geddes DT:
What evidence do we have for pharmaceutical galactagogues in the
treatment of lactation insufficiency?-a narrative review.
Nutrients. 11(974)2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lou Z, Zeng G, Huang L, Wang Y, Zhou L and
Kavanagh KF: Maternal reported indicators and causes of
insufficient milk supply. J Hum Lact. 30:466–473; quiz 511-2.
2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Brown CRL, Dodds L, Legge A, Bryanton J
and Semenic S: Factors influencing the reasons why mothers stop
breastfeeding. Can J Public Health. 105:e179–e185. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Grzeskowiak LE, Dalton JA and Fielder AL:
Factors associated with domperidone use as a galactogogue at an
australian tertiary teaching hospital. J Hum Lact. 31:249–253.
2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Riordan J, Bibb D, Miller M and Rawlins T:
Predicting breastfeeding duration using the LATCH breastfeeding
assessment tool. J Hum Lact. 17:20–23. 2001.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wöckel A, Abou-Dakn M, Beggel A and Arck
P: Inflammatory breast diseases during lactation: Health effects on
the newborn-A literature review. Mediators Inflamm.
2008(298760)2008.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Schwartz K, D'Arcy HJ, Gillespie B, Bobo
J, Longeway M and Foxman B: Factors associated with weaning in the
first 3 months postpartum. J Fam Pract. 51:439–444. 2002.PubMed/NCBI
|
|
14
|
Bond DM, Morris JM and Nassar N: Study
protocol: Evaluation of the probiotic Lactobacillus Fermentum
CECT5716 for the prevention of mastitis in breastfeeding women: A
randomised controlled trial. BMC Pregnancy Childbirth.
17(148)2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Pevzner M and Dahan A: Mastitis while
breastfeeding: Prevention, the importance of proper treatment, and
potential complications. J Clin Med. 9(2328)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Scott JA, Robertson M, Fitzpatrick J,
Knight C and Mulholland S: Occurrence of lactational mastitis and
medical management: A prospective cohort study in Glasgow. Int
Breastfeed J. 3(21)2008.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Cooklin AR, Amir LH, Nguyen CD, Buck ML,
Cullinane M, Fisher JRW and Donath SM: CASTLE Study Team. Physical
health, breastfeeding problems and maternal mood in the early
postpartum: A prospective cohort study. Arch Womens Ment Health.
21:365–374. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Royster E: Milk quality and mastitis.
1–33. 2016.
|
|
19
|
Sordillo LM: Factors affecting mammary
gland immunity and mastitis susceptibility. Livest Prod Sci.
98:89–99. 2005.
|
|
20
|
Li C, Solomons NW, Scott ME and Koski KG:
Subclinical mastitis (SCM) and proinflammatory cytokines are
associated with mineral and trace element concentrations in human
breast milk. J Trace Elem Med Biol. 46:55–61. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Li L, Tan J, Miao Y, Lei P and Zhang Q:
ROS and Autophagy: Interactions and molecular regulatory
mechanisms. Cell Mol Neurobiol. 35:615–621. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Yang FL and Li XS: Role of antioxidant
vitamins and trace elements in mastitis in dairy cows. J Adv Vet
Anim Res. 2:1–9. 2015.
|
|
23
|
Ellah MR: Role of Free Radicals and
Antioxidants in Mastitis. J Adv Vet Res. 3:1–7. 2013.
|
|
24
|
Kurnianto D: Inhibitory power of moringa
leaf juice (Moringa oleifera) against Staphylococcus
aureus and Escherichia coli growth as the etiology of
mastitis in cattle (unpublished PhD thesis). Universitas Brawijaya,
2015.
|
|
25
|
Wijayanti D and Ardigurnita F: The
development of binahong leaf as an antiseptic to overcome mastitis
in dairy cows in the Giri Mukti herd, Tasikmalaya. J-Dinamika
Journal of Public Health. 4:148–152. 2019.https://publikasi.polije.ac.id/index.php/j-dinamika/article/view/1087.
|
|
26
|
Kurniawan I, Sarwiyono S and Surjowardojo
P: The effect of teat dipping using cherry leaf decoction
(Muntingia calabura L.) on the incidence of mastitis.
Indonesian Journal of Animal Science. 23:27–31. 2010.
|
|
27
|
Xiao H, Zhao J, Fang C, Cao Q, Xing M, Li
X, Hou J, Ji A and Song S: Advances in studies on the
pharmacological activities of fucoxanthin. Mar Drugs.
18(634)2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Putri SU: Effect of Macroalgae extract on
bacteria Staphylococcus aureus and methicillin resistant
Staphylococcus aureus (unpublished PhD thesis). Alauddin
Islamic State University, 2016.
|
|
29
|
Triandini IGAAH, Ruqqayah S and Astuti
NLB: In vitro research on plants as natural antibiotics for breast
inflammation (Mastitis). Sangkareang Mataram Scientific Journal.
4(3):14–17. 2018.https://sangkareang.org/index.php/SANGKAREANG/article/view/122.
|
|
30
|
Leblanc SJ, Herdt TH, Seymour WM, Duffield
TF and Leslie KE: Peripartum serum vitamin E, retinol, and
beta-carotene in dairy cattle and their associations with disease.
J Dairy Sci. 87:609–619. 2004.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Chew BP, Hollen LL, Hillers JK and
Herlugson ML: Relationship between vitamin A and β-carotene in
blood plasma and milk and mastitis in holsteins. J Dairy Sci.
65:2111–2118. 1982.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Heinrichs AJ, Costello SS and Jones CM:
Control of heifer mastitis by nutrition. Vet Microbiol.
134:172–176. 2009.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Russo M, Moccia S, Bilotto S, Spagnuolo C,
Durante M, Lenucci MS, Mita G, Volpe MG, Aquino RP and Russo GL: A
carotenoid extract from a Southern Italian cultivar of pumpkin
triggers nonprotective autophagy in malignant cells. Oxid Med Cell
Longev. 2017(7468538)2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zielińska MA, Wesołowska A, Pawlus B and
Hamułka J: Health effects of carotenoids during pregnancy and
lactation. Nutrients. 9(838)2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Crackers NR and Per V: USDA National
Nutrient Database for Standard Reference. Release 1 (Omega 3).
3:3–7. 2019.https://ods.od.nih.gov/pubs/usdandb/VitA-betaCarotene-Content.pdf.
|
|
36
|
Lesmana R, Felia Yusuf I, Goenawan H,
Achadiyani A, Khairani AF, Nur Fatimah S and Supratman U: Low Dose
of β-carotene regulates inflammation, reduces caspase signaling,
and correlates with autophagy activation in cardiomyoblast cell
lines. Med Sci Monit Basic Res. 26(e928648)2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Cheng J, Balbuena E, Miller B and Eroglu
A: The role of β-carotene in colonic inflammation and intestinal
barrier integrity. Front Nutr. 8(723480)2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Palozza P: Can beta-carotene regulate cell
growth by a redox mechanism? An answer from cultured cells. Biochim
Biophys Acta. 1740:215–221. 2005.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Janik IA: The detection and prediction of
mastitis in dairy cows by particle analysis (unpublished PhD
thesis). Coventry University, 2013.
|
|
40
|
O'Rourke D: Nutrition and udder health in
dairy cows: A review. Ir Vet J. 62 (Suppl 4):S15–S20.
2009.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Yang Y, Li R, Hui J, Li L and Zheng X:
β-Carotene attenuates LPS-induced rat intestinal inflammation via
modulating autophagy and regulating the JAK2/STAT3 and JNK/p38 MAPK
signaling pathways. J Food Biochem. 45(e13544)2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Zhao G, Zhang X, Wang H and Chen Z: Beta
carotene protects H9c2 cardiomyocytes from advanced glycation end
product-induced endoplasmic reticulum stress, apoptosis, and
autophagy via the PI3K/Akt/mTOR signaling pathway. Ann Transl Med.
8(647)2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Mass S: Breast pain: Engorgement, nipple
pain and mastitis. Clin Obstet Gynecol. 47:676–682. 2004.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Guo W, Liu B, Yin Y, Kan X, Gong Q, Li Y,
Cao Y, Wang J, Xu D, Ma H, et al: Licochalcone A protects the blood
milk barrier integrity and relieves the inflammatory response in
LPS-Indued mastitis. Front Immunol. 10(287)2019.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Zhu Y, Cheng J, Min Z, Yin T, Zhang R,
Zhang W, Hu L, Cui Z, Gao C, Xu S, et al: Effects of fucoxanthin on
autophagy and apoptosis in SGC-7901cells and the mechanism. J Cell
Biochem. 119:7274–7284. 2018.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Zeng M, Sang W, Chen S, Chen R, Zhang H,
Xue F, Li Z, Liu Y, Gong Y, Zhang H and Kong X: 4-PBA inhibits
LPS-induced inflammation through regulating ER stress and autophagy
in acute lung injury models. Toxicol Lett. 271:26–37.
2017.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Wang X, Su F, Yu X, Geng N, Li L, Wang R,
Zhang M, Liu J, Liu Y and Han B: RNA-Seq whole transcriptome
analysis of bovine mammary epithelial cells in response to
intracellular staphylococcus aureus. Front Vet Sci.
7(642)2020.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Hou LL, Gao C, Chen L, Hu GQ and Xie SQ:
Essential role of autophagy in fucoxanthin-induced cytotoxicity to
human epithelial cervical cancer HeLa cells. Acta Pharmacol Sin.
34:1403–1410. 2013.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Scherz-Shouval R and Elazar Z: Regulation
of autophagy by ROS: Physiology and pathology. Trends Biochem Sci.
36:30–38. 2011.PubMed/NCBI View Article : Google Scholar
|
|
50
|
He L, He T, Farrar S, Ji L, Liu T and Ma
X: Antioxidants maintain cellular redox homeostasis by elimination
of reactive oxygen species. Cell Physiol Biochem. 44:532–553.
2017.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Atakisi O, Oral H, Atakisi E, Merhan O,
Metin Pancarci S, Ozcan A, Marasli S, Polat B, Colak A and Kaya S:
Subclinical mastitis causes alterations in nitric oxide, total
oxidant and antioxidant capacity in cow milk. Res Vet Sci.
89:10–13. 2010.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Mittal M, Siddiqui MR, Tran K, Reddy SP
and Malik AB: Reactive oxygen species in inflammation and tissue
injury. Antioxidants Redox Signal. 20:1126–1167. 2014.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Cao Y, Wang J, Tian H and Fu GH:
Mitochondrial ROS accumulation inhibiting JAK2/STAT3 pathway is a
critical modulator of CYT997-induced autophagy and apoptosis in
gastric cancer. J Exp Clin Cancer Res. 39(119)2020.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Guo W, Li W, Su Y, Liu S, Kan X, Ran X,
Cao Y, Fu S and Liu J: GPR109A alleviate mastitis and enhances the
blood milk barrier by activating AMPK/Nrf2 and autophagy. Int J
Biol Sci. 17:4271–4284. 2021.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Qian M, Fang X and Wang X: Autophagy and
inflammation. Clin Transl Med. 6(24)2017.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Sun Y, Huang YH, Huang FY, Mei WL, Liu Q,
Wang CC, Lin YY, Huang C, Li YN, Dai HF and Tan GH:
3'-epi-12β-hydroxyfroside, a new cardenolide, induces
cytoprotective autophagy via blocking the Hsp90/Akt/mTOR axis in
lung cancer cells. Theranostics. 8:2044–2060. 2018.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Jung CH, Ro SH, Cao J, Otto NM and Kim DH:
MTOR regulation of autophagy. FEBS Lett. 584:1287–1295.
2010.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Chang KC, Liu PF, Chang CH, Lin YC, Chen
YJ and Shu CW: The interplay of autophagy and oxidative stress in
the pathogenesis and therapy of retinal degenerative diseases. Cell
Biosci. 12(1)2022.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Hadad N and Levy R: The synergistic
anti-inflammatory effects of lycopene, lutein, β-carotene, and
carnosic acid combinations via redox-based inhibition of NF-κB
signaling. Free Radic Biol Med. 53:1381–1391. 2012.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Lee H, Lim JW and Kim H: Effect of
Astaxanthin on Activation of Autophagy and Inhibition of Apoptosis
in Helicobacter pylori-Infected Gastric Epithelial Cell Line AGS.
Nutrients. 12(1750)2020.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Jiang GM, Tan Y, Wang H, Peng L, Chen HT,
Meng XJ, Li LL, Liu Y, Li WF and Shan H: The relationship between
autophagy and the immune system and its applications for tumor
immunotherapy. Mol Cancer. 18(17)2019.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Bolisetty S and Jaimes EA: Mitochondria
and reactive oxygen species: Physiology and pathophysiology. Int J
Mol Sci. 14:6306–6344. 2013.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Martinez J, Malireddi RS, Lu Q, Cunha LD,
Pelletier S, Gingras S, Orchard R, Guan JL, Tan H, Peng J, et al:
Molecular characterization of LC3-associated phagocytosis reveals
distinct roles for Rubicon, NOX2, and autophagy proteins. Nat Cell
Biol. 17:893–906. 2015.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Shin J, Song MH, Oh JW, Keum YS and Saini
RK: Pro-oxidant actions of carotenoids in triggering apoptosis of
cancer cells: A review of emerging evidence. Antioxidants (Basel).
9(532)2020.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Filteau SM, Rice AL, Ball JJ, Chakraborty
J, Stoltzfus R, de Francisco A and Willumsen JF: Breast milk immune
factors in Bangladeshi women supplemented postpartum with retinol
or beta-carotene. Am J Clin Nutr. 69:953–958. 1999.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Kasperczyk S, Dobrakowski M, Kasperczyk J,
Ostałowska A, Zalejska-Fiolka J and Birkner E: Beta-carotene
reduces oxidative stress, improves glutathione metabolism and
modifies antioxidant defense systems in lead-exposed workers.
Toxicol Appl Pharmacol. 280:36–41. 2014.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Zbyradowski M, Duda M, Wisniewska-Becker
A, Heriyanto Rajwa W, Fiedor J, Cvetkovic D, Pilch M and Fiedor L:
Triplet-driven chemical reactivity of β-carotene and its biological
implications. Nat Commun. 13(2474)2022.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Kim DK, Sim BR, Kim JI and Khang G:
Functionalized silk fibroin film scaffold using β-Carotene for
cornea endothelial cell regeneration. Colloids Surf B
Biointerfaces. 164:340–346. 2018.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Kang H and Kim H: Astaxanthin and
β-carotene in Helicobacter pylori-induced gastric inflammation: A
mini-review on action mechanisms. J Cancer Prev. 22:57–61.
2017.PubMed/NCBI View Article : Google Scholar
|
|
70
|
McGuire S: WHO guideline: Vitamin A
supplementation in pregnant women. Geneva: WHO, 2011; WHO
guideline: Vitamin A supplementation in postpartum women. Geneva:
WHO, 2011. Adv Nutr. 3:215–216. 2012.PubMed/NCBI View Article : Google Scholar
|
|
71
|
World Health Organization (WHO):
Guideline: vitamin A supplementation in postpartum women. WHO,
Geneva, 2011. https://apps.who.int/iris/handle/10665/44623.
|
|
72
|
Tanaka T, Shnimizu M and Moriwaki H:
Cancer chemoprevention by carotenoids. Molecules. 17:3202–3242.
2012.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Haskell MJ: The challenge to reach
nutritional adequacy for vitamin A: β-carotene bioavailability and
conversion-evidence in humans. Am J Clin Nutr. 96:1193S–1203S.
2012.PubMed/NCBI View Article : Google Scholar
|
|
74
|
National Institutes of Health (NIH):
Vitamin A and carotenoids. NIH, Bethesda, MD, pp1-19, 2022.
https://ods.od.nih.gov/factsheets/VitaminA-HealthProfessional/.
Accessed June 1, 2022.
|
|
75
|
Xu J, Zhang J, Mao QF, Wu J and Wang Y:
The interaction between autophagy and JAK/STAT3 signaling pathway
in tumors. Front Genet. 13(880359)2022.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Kitakaze T, Harada N, Imagita H and Yamaji
R: β-carotene increases muscle mass and hypertrophy in the soleus
muscle in mice. J Nutr Sci Vitaminol (Tokyo). 61:481–487.
2015.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Geng N, Liu K, Lu J, Xu Y, Wang X, Wang R,
Liu J, Liu Y and Han B: Autophagy of bovine mammary epithelial cell
induced by intracellular Staphylococcus aureus. J Microbiol.
58:320–329. 2020.PubMed/NCBI View Article : Google Scholar
|