Introduction
The spleen is an organ at high risk of injury during
various abdominal traumas. Due to its fragile structure, the thin
capsule enveloping it, and its position directly under the ribs,
the spleen is commonly injured during abdominal trauma, especially
through blunt forces (1) such as
those caused by automobile accidents (2). After the initial trauma, patients
usually present with various signs and symptoms ranging from
hemorrhage to hemorrhagic shock. Depending on the severity, ‘damage
control’ is a priority. A laparotomy is required, and if splenic
damage is suspected, an immediate ‘hemostatic’ splenectomy should
be performed (3). With the
acknowledgment that a splenectomy increases the risk of serious
infections in both pediatric (4)
and adult patients (5), the number
of post-traumatic splenectomies has steadily decreased in favor of
non-operative spleen preservation techniques (6), aided by the use of more precise
methods of exploration and surveillance such as angiography and
selective angioembolization of small ruptured splenic branches
(7). Thus, identifying the severity
of spleen trauma plays a major role when choosing the appropriate
treatment approach (8).
Although developed in 1989 by the American
Association for Surgery of Trauma (AAST) (9) and eventually revised in 1994(10), the current categorization of splenic
trauma is still frequently used in practice. The AAST
classification divides blunt splenic trauma into five categories
based on the severity of the subcapsular and intraparenchymal
hematoma and depth the of the capsular laceration. Low-grade (I and
II) injuries are often admitted to the surgical unit and treated
conservatively. High-grade blunt splenic trauma patients (grades
III, IV and V) are admitted to the critical care unit, their
management being determined by the clinical state and development
of the patient's condition.
According to the current literature, choosing the
adequate paraclinical investigation to determine the severity of
spleen trauma is crucial. The type of investigation depends on the
patient's hemodynamic status (11).
The most common investigation used for diagnosing spleen trauma is
a FAST ultrasound and CT scan if the patient has a stable
hemodynamic status. In cases of hemodynamically unstable patients,
bedside ultrasound is the preferred method (12).
These imaging techniques have contributed to the
advancement of non-operative-management of splenic trauma (13). Although clinical criteria primarily
dictate the decision to seek surgical interventions, CT findings
support diagnostic accuracy and have been shown in multiple studies
to be effective in successfully reducing excessive exploratory
laparotomies (14).
Additionally, with image-based investigations when
evaluating the severity of spleen trauma, it is crucial to take
into consideration the activation of the immune system in response
to the initial injury. Several studies have described a ‘cytokine
storm’ and functional reprioritization of leukocytes after severe
trauma (15-17).
This is due to focused chemotaxis for leukocytes, cytokine release
(including systemic expression of IL-6, IL-8, IL-1Ra, and IL-10),
production of reactive oxygen species, leukocyte activation, and
phagocytosis (18).
It is worth noting that the systemic inflammatory
response syndrome (SIRS) not only includes several
immune-system-activating factors but also significant suppressive
factors that develop within minutes to hours after the initial
traumatic injury (19). However, an
initial injury of sufficient severity, combined with prolonged
surgical intervention or hemorrhagic shock may cause an imbalance
in the innate immune response, resulting in a dysfunctional cascade
system (20). This imbalanced
response to the initial injury can be observed even in routine
bloodwork as an altered neutrophil to lymphocytes ratio (NLR) or
platelets to lymphocytes ratio (PLR), recent studies suggest
(21).
Several recent studies have shown that trauma
patients with poor clinical outcomes evoke a more severe and
sustained inflammatory response compared with those with better
outcomes (22-24).
These studies used blood collected at admittance and suggested the
data acquired related to the immediate post-injury inflammatory
response (22,25).
These findings, combined with various studies on the
imbalance of pro- and anti-inflammatory factors in trauma patients,
lead us to research the prognostic role of immune monitoring with
the aim of identifying patients with a potentially poor outcome.
Hence, we conducted this study to assess the importance of
inflammatory biomarkers in evaluating systemic inflammatory
syndrome in patients with spleen trauma. This study is based on the
experience of the Mures County medical center in Romania (26-28).
Our primary hypothesis was that the NLR and PLR,
when used as prognostic factors, may be associated with the
evolution of patients with splenic trauma.
The primary aim of this study was to identify an
association between the systemic inflammatory response of trauma
patients and the severity of the traumatic injury. SIRS was
indirectly evaluated using two parameters: NLR and PLR. The
measurement of the severity of the initial traumatic injury was
performed using the injury severity score (ISS).
The secondary aim of our study was to quantify the
association between the NLR, PLR and ISS. The specific cut-off
values for our SIRS parameters (NLR and PLR) were calculated,
considering those the independent variables and the ISS score as
the dependent variable.
Materials and methods
Study design
We carried out a retrospective observational study
over 6 years. We used the database at the Mureș Emergency Clinical
Hospital and studied all the observation sheets of hospitalized
patients between January 1 2014 and December 31 2019. In total, 126
patients were admitted to all surgery clinics for spleen trauma as
the main diagnostic condition. We followed the relationship between
inflammatory markers determined at admission and the severity of
the trauma, to identify an association between these studied
parameters.
From our selected patients, we were mostly
interested in the following clinical parameters: Age, sex,
pathological antecedents, number of days of hospitalization, type
of spleen trauma, other types of trauma and the severity of them,
and blood count at admission. Specifically, from the complete blood
count, we were mostly interested in the hemoglobin (Hbg),
hematocrit (Hct), platelet count, leukocyte count, and differential
white blood cell count.
For this study, we obtained approval from the Ethics
Committee of the Clinical Emergency Hospital Mures (grant no.
Ad.29366) to access the hospital database.
Inclusion and exclusion criteria
A total of 126 patients were included in the present
study, including 90 males (71.43%) and 36 females (28.57%), with a
mean age of 36 years and an age range of 3-87 years old. In this
study, we included all patients admitted to the Mureș Emergency
County Hospital with a primary diagnosis of spleen trauma under the
S36 ICD-10 family code (29). This
includes unspecified injuries of the spleen, minor and major
contusion of the spleen, laceration of the spleen, and other
splenic injuries. All patients included in this study survived the
initial trauma, and were discharged with a healthy or significantly
improved health status.
For this study, we excluded all patients with a
preexisting, chronic inflammatory state such as in autoimmune
disorders, cancer, or chronic infection. We also excluded trauma
patients in need of urgent surgical interventions for different
associated injuries, other than splenic trauma.
Data processing
The data obtained were processed using Microsoft
Excel (Microsoft Corporation), SPSS (IBM Corp), and GraphPad Prism
version 9 (GraphPad Software, Inc.). Patient data were entered into
a table in Microsoft Excel, where the database was compiled. This
database was then imported into GraphPad Prism 9 and SPSS and then
statistically processed.
Data analysis
We indirectly assessed the severity of the systemic
inflammatory response syndrome by computing the NLR and PLR. The
NLR was calculated by dividing the neutrophil count by the
lymphocyte count; similarly, for the PLR, we divided the platelet
count by the lymphocyte count as shown by Russu et al
(30). Using the data regarding the
location and severity of the trauma from the clinical observational
sheets at admission, we calculated the ISS to determine the
relationship between the ISS and the inflammatory markers. The ISS
was calculated according to the literature guideline as presented
by Baker et al (31).
Overview of the statistical
analysis
Statistical analysis included elements of
descriptive statistics (mean, median, standard deviation) and
inferential statistics. A Shapiro-Wilk test was applied to
determine the distribution of the analyzed data series. Linear and
multiple linear regressions were performed to determine the
relationships between the analyzed variables, and a one-way ANOVA
test to the model of the multiple regression analysis, to evaluate
the statistical significance. To evaluate the accuracy, the area
under the receiver operating characteristic (ROC) curve (AUC) was
determined. To assess the performance of the diagnostic test over
the range of possible cut-off points for the predictive variables,
an ROC curve was used. For analysis, we selected a value of 6 for
the ISS as our dependent variable. P<0.05 was considered to
indicate a statistically significant difference.
Results
Descriptive analysis of age, sex, and
paraclinical investigation
Table I shows the
age and sex analysis of the patients enrolled in our study. Out of
the total 126 patients included in our study, the majority (71.43%)
were male, with female patients accounting for 28.57% of the
sample. We noted a peak frequency of patients belonging to the age
group 10-20 years (28 patients) followed by an almost constant
decrease towards 90 years of age, the curve possessing an almost
Gaussian aspect with a slight increase towards the distal
extremity.
 | Table IAge and sex of the patients. |
Table I
Age and sex of the patients.
| Characteristic | No. of
patients | Percentage |
|---|
| Sex | | |
|
Male | 90 | 71.43 |
|
Female | 36 | 28.57 |
| Age, years | | |
|
<10 | 15 | 11.9 |
|
10-20 | 31 | 24.6 |
|
21-30 | 20 | 15.8 |
|
31-40 | 18 | 14.2 |
|
41-50 | 17 | 13.4 |
|
51-60 | 14 | 11.1 |
|
61-70 | 11 | 8.7 |
Furthermore, we studied the descriptive analysis of
the blood parameters and highlight their values in Table II. All of these parameters except
for the Hct, platelet count, and PLR had a mean value outside of
the normal range. We proceeded to calculate the ISS using the Excel
formula provided in the literature (31), and the results are shown in Table III. The mean value of the ISS
found in our study was 10.36, with a range of 1-25.
 | Table IIDescriptive analysis of the Hbg, Hct,
neutrophil, lymphocyte, platelet, NLR, PLR levels and length of
hospital stay. |
Table II
Descriptive analysis of the Hbg, Hct,
neutrophil, lymphocyte, platelet, NLR, PLR levels and length of
hospital stay.
| Parameter | Mean | Standard
deviation | Lowest value | Highest value | Normal range |
|---|
| Hbg, g/dl | 11.82 | 2.048 | 3.7 | 16.46 | 13.3-16.7 |
| Hematocrit, % | 34.89 | 5.72 | 11.2 | 45.7 | 39-55 |
| Neutrophils,
103/µl | 14.49 | 6.664 | 3.14 | 35 | 1.7-6.1 |
| Lymphocytes,
103/µl | 2.001 | 0.5917 | 0.3 | 4.82 | 1-3.2 |
| Platelets,
103/µl | 249.7 | 96.21 | 50 | 786 | 143-332 |
| NLR | 9.154 | 6.048 | 1.359 | 28.83 | 0.53-6.1 |
| PLR | 155.4 | 98.73 | 32.26 | 714.5 | 44-332 |
| Length of hospital
stay | 12.84 | 11.55 | 1 | 121 | 14-22 |
 | Table IIIDescriptive analysis of the ISS
scores. |
Table III
Descriptive analysis of the ISS
scores.
| ISS | n | % |
|---|
|
0-5 | 34 | 26.98 |
|
5-10 | 28 | 38.8 |
|
10-15 | 19 | 16.6 |
|
15-20 | 18 | 11.9 |
|
20-25 | 14 | 5.5 |
Regression analysis of the studied
parameters
Since most of the studied data series did not pass
the Shapiro-Wilk normality test, we proceeded to linear regression
analysis to identify a possible association between the studied
parameters. The results of the regression analysis are shown in
Table IV.
 | Table IVLinear regression analysis of the
studied parameters. |
Table IV
Linear regression analysis of the
studied parameters.
| Parameter | r coefficient | 95% confidence
interval | r2
coefficient | P-value |
|---|
| Age and ISS | -0.02797 | -0.351 | 0.0007825 | 0.7568 |
| Hemoglobin and
ISS | -0.3514 | -0.3091 | 0.1235 | <0.0001 |
| Hematocrit and
ISS | -0.3724 | -0.3039 | 0.1387 | <0.0001 |
| Neutrophils and
ISS | 0.3943 | 0.2349-0.5330 | 0.1554 | <0.0001 |
| Lymphocytes and
ISS | -0.2041 | -0.33704 | 0.04168 | 0.0224 |
| Platelets and
ISS | -0.2146 | -0.33564 | 0.04604 | 0.0163 |
| NLR and ISS | 0.4931 | 0.3475-0.6154 | 0.2431 | <0.0001 |
| PLR and ISS | 0.006319 | -0.3513 | 0.00003993 | 0.9442 |
From the results of our analysis, we found a
statistically significant association between the ISS with Hbg,
Hct, neutrophils, lymphocytes, platelets, and NLR. Of these
findings, there was a directly proportionate relationship between
the neutrophil count and ISS, and between the NLR and ISS. In other
words, any increase in the neutrophil count or NLR was directly
associated with a high ISS. In the case of the Hbg, Hct, lymphocyte
levels, and platelet levels, as predictor variables, their
relationship with the dependent variable ISS was inversely
proportionate. This meant that any decrease in the former predictor
variables was accompanied by a proportionate increase of the
ISS.
We next performed multiple linear regression
analysis to further quantify the strength of the association
between the dependent and independent variables. The results are
displayed in Tables V and VI.
 | Table VMultiple regression analysis of the
studied parameters-model summary. |
Table V
Multiple regression analysis of the
studied parameters-model summary.
| Model | R | R2 | P-value |
|---|
| 1 | 0.611 | 0.373 | <0.0001 |
 | Table VIMultiple regression analysis of the
studied parameters-coefficients analysis. |
Table VI
Multiple regression analysis of the
studied parameters-coefficients analysis.
| | Unstandardized
coefficients | | 95% confidence
interval for B |
|---|
| Model | B | Std. error | P-value | Lower bound | Upper bound |
|---|
| (Constant) | 15.230 | 3.420 | 0.000 | 8.457 | 22.004 |
| Hemoglobin | -0.050 | 0.710 | 0.944 | -1.455 | 1.355 |
| Hematocrit | -0.182 | 0.256 | 0.478 | -0.688 | 0.324 |
| Neutrophils | 0.120 | 0.128 | 0.351 | -0.133 | 0.372 |
| Lymphocytes | -0.967 | 0.894 | 0.282 | -2.737 | 0.803 |
| Platelets | 0.003 | 0.009 | 0.718 | -0.014 | 0.020 |
| NLR | 0.448 | 0.194 | 0.022 | 0.064 | 0.831 |
| PLR | -0.020 | 0.011 | 0.084 | -0.042 | 0.003 |
As highlighted in Table
VI, the only statistically significant association we found was
between NLR and ISS, with a coefficient of 0.448. This meant that
an increase in the NLR by one unit corresponded to an increase in
the ISS by 0.448 units.
ROC curves of the NLR-ISS
association
To further assess the performance of the diagnostic
test (NLR) over the range of possible cut-off points for the
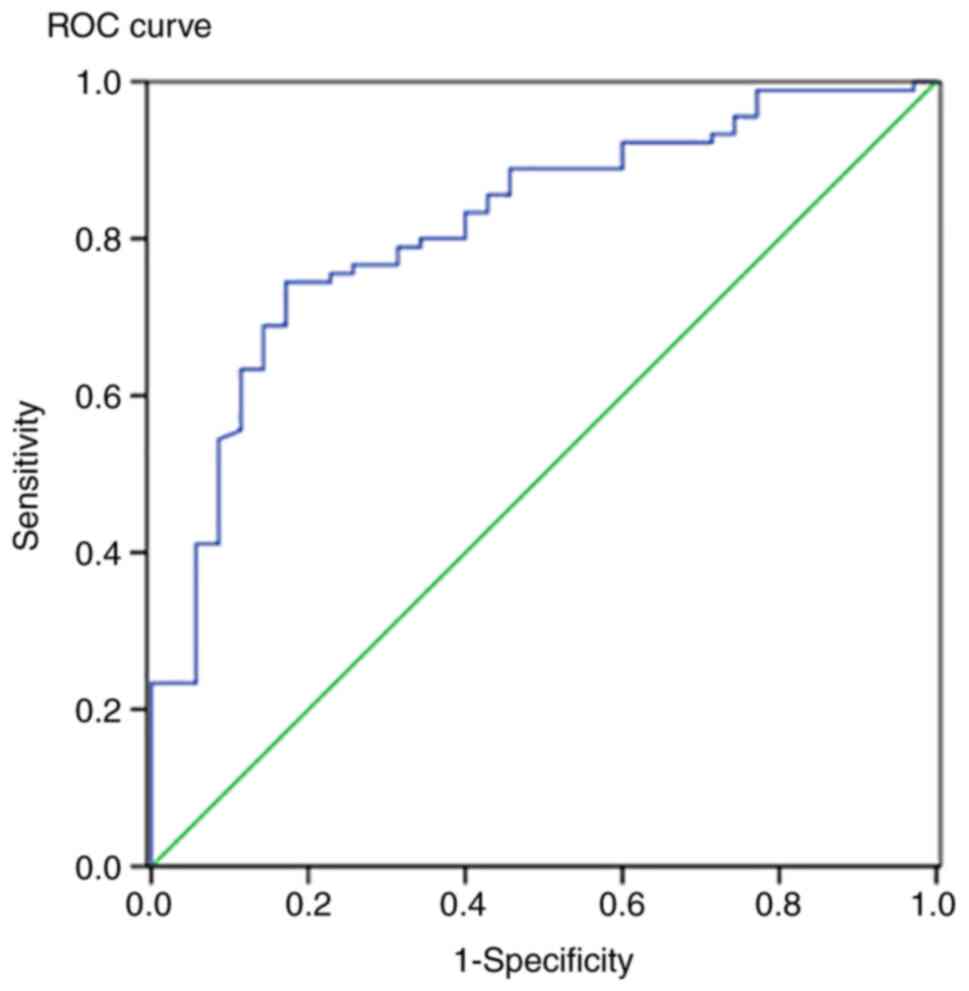
predictor variable (ISS) we created an ROC curve (Fig. 1).
The AUC was found to be 0.816, and thus the accuracy
of the prediction was very good (P<0.05).
To directly measure the association between the NLR
and the ISS, and to implement the knowledge of this study in
clinical practice, we computed several cut-off values for NLR with
various levels of sensitivity, specificity, and false positive
rate. These values are shown in Table
VII.
 | Table VIICoefficients for the ANOVA of the
studied parameters. |
Table VII
Coefficients for the ANOVA of the
studied parameters.
| NLR | Cut-off value 5.44
(%) | Cut-off value 5.78
(%) | Cut-off value 6.075
(%) |
|---|
| Sensitivity | 80 | 78.9 | 76.7 |
| Specificity | 65.7 | 68.6 | 74.3 |
| False positive rate
(1-Specificity) | 34.3 | 31.4 | 25.7 |
The cut-off values presented in the table are the
averages of two consecutive ordered values observed in the
hypothetical test (NLR).
In conclusion, the NLR was a reliable predictor of
trauma severity in patients suffering from various traumatic spleen
injuries.
Discussion
According to the current literature, when evaluating
patients with splenic injury, the choice of paraclinical
investigation is crucial (11).
The most common investigation used for diagnosing
spleen trauma is a FAST ultrasound and CT scan if the patient has a
stable hemodynamic status. In the case of hemodynamically unstable
patients, bedside ultrasound is the preferred method for detecting
intraperitoneal bleeding and guiding further surgical decisions.
Such patients with a positive ultrasound need an exploratory
laparotomy as soon as possible (32). According to some studies, the FAST
ultrasound has a sensitivity of up to 98% (in optimal conditions)
for detecting intraabdominal fluid collection (33). Its major limitation is user error,
being an operator-dependent investigation. A systematic review
revealed that a quarter of trauma-dependent intraperitoneal
hemorrhages can be missed when using ultrasound alone as a
diagnostic tool (34).
In comparison, CT scans offer a comparable
sensitivity of 95-98% but have the advantage of a low rate (2.4%)
of missed injuries (35). Granted,
these high sensitivity rates are achieved when patients are
admitted with severe trauma (ISS >15), but newer technologies
such as machine learning and artificial intelligence combined with
CT scanning aid the diagnosis and triage process of trauma
patients, regardless of the severity of the trauma (36).
Nevertheless, using imaging techniques alone to
evaluate the severity of spleen injury has certain limitations such
as underestimating the degree and hemodynamic impact of certain
splenic injuries, or having inadequate prognostic value for
patients with complications such as delayed splenic hemorrhage.
Based on the findings of our study, we noticed that
spleen trauma occurred primarily in the younger age group with a
higher incidence in males, these data coinciding with the European
average (37).
According to Harna et al (38) the average age of patients in their
study was 33.4 with the highest incidence of trauma observed in
patients aged between 20 and 40 years old, compared to our study,
where the average age was 36 years. Male patients constituted 76%
of their study population, with female patients accounting for just
24%. Reasons for the differences in the prevalence by sex may have
to do with the cause of the trauma and the type of traumatic agent.
Whiteout having the possibility to analyze and quantify all causes
of trauma, we have noticed a purely subjective prevalence of
work-related accidents in construction domains in patients included
in our study. This may be one of the reasons for a higher ratio of
male patients included, our findings are in agreement with those of
Harna et al (38).
To identify possible relationships between
inflammatory markers and the severity of trauma patients, we have
analyzed the relationship between the NLR and PLR with ISS.
A review of the literature revealed several studies
on predictive factors for trauma patients, indicating that the
prognostic capacity of some well-known scoring systems including
the ISS at admission is not as powerful as the predictive
capability of other physiologic-based scoring models including the
Sequential Organ Failure Assessment (SOFA), Denver, Acute
Physiology and Chronic Health Evaluation II (APACHE II), and the
Trauma and Injury Severity Score (TRISS) (30). However, the use of such
physiological-based models is difficult and cumbersome for everyday
usage, as they require considerably more time to apply compared
with ISS (21).
We did not find a statistically significant
association between PLR-as an inflammatory marker and the severity
of the trauma. However, this result may be caused by a sudden
fluctuation in the number of platelets in the blood count. We also
noted the heterogeneous distribution of our data for PLR with a
wide standard deviation, which contributed to the lack of
statistical significance. There is evidence that the proportion of
neutrophils and platelets variably increases during any trauma due
to systemic inflammation (39).
Specifically, during splenic trauma, it appears that the platelet
count is higher than in any other type of trauma. Reactive
thrombocytosis is common following elective or urgent splenectomy,
but there is a 20-30% incidence of thrombocytosis following major
trauma regardless of splenectomy (40,41).
The mechanism behind the increase in platelet counts after major
spleen trauma relies upon the physiological role of the spleen.
After a traumatic injury, due to the inflammatory and regenerative
processes of the splenic parenchyma, its ability to clear platelets
is impaired. Similarly, after post-traumatic splenectomy, a
reactive thrombocytosis can be noticed over the following 2 to 20
days (40). Furthermore, studies
have shown that thrombocytosis can occur even in patients with
anemia due to the close relationship between erythropoietic and
thrombotic growth factors (42).
Such findings support our study since the mean Hbg value is well
under the lowest range. These fluctuations in the platelet count
are the most plausible cause of data alternation, preventing us
from finding a relationship between the PLR and the ISS. There are
several situations mentioned in the literature where there is a
powerful association with NLR, whilst PLR and patient outcomes have
no statistical significance (43).
Despite all of this we did find a proportioned,
statistically significant association between NLR and ISS using
regression analysis. Thus, we can state that any sort of increase
in the NLR in trauma patients at admittance is directly associated
with highly severe trauma and poorer outcome.
Furthermore, plotting an ROC curve showed the AUC
was 0.816 suggesting a very good performance of our test. A similar
article published on this matter in 2020 by Soulaiman et al
(21) revealed comparable results
for the predictive power of NLR at admission. Granted, the
calculated AUC in their study was lower (AUC=0.633) with more
modest levels of sensitivity and specificity. Yet, the main
difference noticeable between the design of our study compared with
the former is the level set for ISS as the dependent variable
(ISS=15). We decided on a value of ISS=6 to differentiate between
minor and moderate injuries since the majority of the patients
included in our study suffered from less severe traumatic injuries,
as can be noted from the distribution of the data (mean
ISS=9.848).
After computing the cut-off values for NLR at a set
ISS of 6, we were left with a choice of three separate values, all
of them with various levels of sensitivity, specificity, and false
positive rates. We preferred to take into account the cut-off value
of NLR=6.075 due to its lower false positive rate (25.7%) and still
potent capability of identifying patients suffering from aggressive
trauma (76.7%). Thus, we could state that if a patient was admitted
to the emergency care unit after trauma and the NLR was higher than
the aforementioned value, it would be predictive of a poorer
outcome, and hence we could adjust the therapeutic approach to
improve prognostic outcomes.
The results on this topic in the medical literature
are yet debated as authors find contradictory outcomes. According
to a recent study, an increase in NLR throughout the first 48 h
after admission was linked to the occurrence of organ failure in
male trauma patients (44).
According to Dilektasli et al (45), who illustrated the predictive valuer
of NLR on the second and fifth day when it came to estimating
hospital fatalities in trauma patients when compared to the
following days, the NLR throughout the first 24 h was not effective
for predicting outcomes in the surgical intensive care unit. In
this study, for the second and fifth hospital days, they found
appropriate cut-off values of 8.19 and 7.92 with a sensitivity of
70.8% and a specificity of 61.9%; these results are similar to the
cut-off values we found in our study.
We also found an association between HTC and ISS
with a negative value according to the regression analysis. This
indicates an inversely proportional relationship between the two
parameters, and thus any decrease in the Hct count would suggest a
higher severity of trauma. The hypothesis can also be inverted;
thus, any patient with severe trauma, quantified by a higher ISS,
will have a lower red blood cell count. This fact is to be expected
due to the bleeding, both internal and external, after severe
trauma.
The present study has a series of flaws, starting
with the design of the study. It was retrospective and we analyzed
a lengthy period, which represents fundamental restrictions of our
work. Another limitation of our research is the fact that we
analyzed patients admitted at only one hospital; thus, the data
were limited by the quality of patient records and the inability to
account for all factors. Third, our dataset lacked additional
inflammatory indicators that may be used to define the inflammatory
process's quality such as interleukins and interferons. Fourth, the
data were limited to a single point in time, at admission; having
more data points during the admission period, with a longer
follow-up period may have provided more detailed information about
the trend of these inflammatory cells in the earlier and even later
phases, allowing for a more accurate interpretation of what drives
these changes. Fifth, our study included a greater proportion of
male patients, and hence the sex difference means our findings are
exploratory at best. Finally, one important limitation of the study
was the small sample size.
There are certain areas where the current study can
be improved, such as the retrospective design of the study. We feel
that a future prospective study where we analyze the same
parameters not just at admittance, but daily, taking into account
the variation of these observed values and the patients' evolution
would be a suitable direction. Investigating these parameters over
a longer period would also aid the quality of the study by
increasing the number of patients that can be included. Yet,
considering that this study was conducted in a Romanian reference
medical center (a polytrauma center), we consider our findings
crucial for the further development of our country's deficient
medical system. We hope that some of these findings will be used as
a stepping stone toward developing new and adapted protocols for
managing trauma patients. The least of our plans is to use this
study in order to apply for financial support for further studies
in order to better investigate the prognostic factors for the
evolution of trauma patients, and furthermore develop a strategy
for managing trauma patients.
In conclusion, elevated NLR in trauma patients at
admittance had a high predictive power for the severity of the
trauma. Patients with an NLR value higher than the cut-off value of
6.075 have a high probability of severe trauma.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
VV and BAS conceived the study. NB and IH designed
the study. IGC and VV collected the data. DVG and IGC performed the
statistical analysis. CM was in charge of data curation and
visualization. VV and IH wrote the original draft of the
manuscript. BAS, CM and NB reviewed and edited the manuscript. VV,
IGC and DVG confirm the authenticity of all the raw data. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
the Mures County Emergency Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Haan JM, Bochicchio GV, Kramer N and
Scalea TM: Nonoperative Management of Blunt Splenic Injury: A
5-Year Experience. J Trauma. 58:492–498. 2005.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Subedi N, Yadav BN, Jha S, Paudel IS and
Regmi R: A profile of abdominal and pelvic injuries in medico-legal
autopsy. J Forensic Leg Med. 20:792–796. 2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Girard E, Abba J, Cristiano N, Siebert M,
Barbois S, Létoublon C and Arvieux C: Management of splenic and
pancreatic trauma. J Visc Surg. 153 (Suppl 4):S45–S60.
2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Bulut F, Dervisoglu A, Kesim M, Guven H
and Polat C: Is pneumoperitoneum harmful during intra-abdominal
hemorrhage in rats? J Laparoendosc Adv Surg Tech A. 15:112–120.
2005.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Demetriades D, Scalea TM, Degiannis E,
Barmparas G, Konstantinidis A, Massahis J and Inaba K: Blunt
splenic trauma: Splenectomy increases early infectious
complications: A prospective multicenter study. J Trauma Acute Care
Surg. 72:229–234. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Dobremez E, Lefevre Y, Harper L,
Rebouissoux L, Lavrand F, Bondonny JM and Vergnes P: Complications
occurring during conservative management of splenic trauma in
children. Eur J Pediatr Surg. 16:166–170. 2006.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Hildebrand DR, Ben-sassi A, Ross NP,
Macvicar R, Frizelle FA and Watson AJ: Modern management of splenic
trauma. BMJ. 348(g1864)2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Coccolini F, Montori G, Catena F, Kluger
Y, Biffl W, Moore EE, Reva V, Bing C, Bala M, Fugazzola P, et al:
Splenic trauma: WSES classification and guidelines for adult and
pediatric patients. World J Emerg Surg. 12(40)2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Moore EE, Shackford SR, Pachter HL,
McAninch JW, Browner BD, Champion HR, Flint LM, Gennarelli TA,
Malangoni MA and Ramenofsky ML: Organ injury scaling: Spleen,
liver, and kidney. J Trauma. 29:1664–1666. 1989.PubMed/NCBI
|
|
10
|
Meldrum DR, Moore FA, Moore EE, Franciose
RJ, Sauaia A and Burch JM: Prospective characterization and
selective management of the abdominal compartment syndrome. Am J
Surg. 174:667–672; discussion 672-3. 1997.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Patlas MN: CT Imaging and management of
blunt splenic trauma: Lessons for today and tomorrow. Radiology.
299:131–132. 2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Shi H, Teoh WC, Chin FWK, Tirukonda PS,
Cheong SCW and Yiin RSZ: CT of blunt splenic injuries: What the
trauma team wants to know from the radiologist. Clin Radiol.
74:903–911. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Adibi A, Ferasat F, Baradaran Mahdavi M,
Kazemi K and Sadeghian S: Assessment of blunt splenic trauma: Which
imaging scoring system is superior? J Res Med Sci.
23(29)2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Fodor M, Primavesi F, Morell-Hofert D,
Kranebitter V, Palaver A, Braunwarth E, Haselbacher M, Nitsche U,
Schmid S, Blauth M, et al: Non-operative management of blunt
hepatic and splenic injury: A time-trend and outcome analysis over
a period of 17 years. World J Emerg Surg. 14(29)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Seshadri A, Brat GA, Yorkgitis BK, Keegan
J, Dolan J, Salim A, Askari R and Lederer JA: Phenotyping the
immune response to trauma: A Multiparametric Systems Immunology
Approach. Crit Care Med. 45:1523–1530. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Hildebrand F, Pape HC and Krettek C: The
importance of cytokines in the posttraumatic inflammatory reaction.
Unfallchirurg. 108:793–794, 796-803. 2005.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
17
|
Jogia T, Lübstorf T, Jacobson E, Scriven
E, Atresh S, Nguyen QH, Liebscher T, Schwab JM, Kopp MA, Walsham J,
et al: Prognostic value of early leukocyte fluctuations for
recovery from traumatic spinal cord injury. Clin Transl Med.
11(e272)2021.PubMed/NCBI View
Article : Google Scholar
|
|
18
|
Hazeldine J, Naumann DN, Toman E, Davies
D, Bishop JRB, Su Z, Hampson P, Dinsdale RJ, Crombie N, Duggal NA,
et al: Prehospital immune responses and development of multiple
organ dysfunction syndrome following traumatic injury: A
prospective cohort study. PLoS Med. 14(e1002338)2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Timmermans K, Kox M, Vaneker M, van den
Berg M, John A, van Laarhoven A, van der Hoeven H, Scheffer GJ and
Pickkers P: Plasma levels of danger-associated molecular patterns
are associated with immune suppression in trauma patients.
Intensive Care Med. 42:551–561. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Billiar TR and Vodovotz Y: Time for trauma
immunology. PLoS Med. 14(e1002342)2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Soulaiman SE, Dopa D, Raad AT, Hasan W,
Ibrahim N, Hasan AY, Sulaiman HA and Darwich M: Cohort
retrospective study: The neutrophil to lymphocyte ratio as an
independent predictor of outcomes at the presentation of the
multi-trauma patient. Int J Emerg Med. 13(5)2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Namas RA, Vodovotz Y, Almahmoud K,
Abdul-Malak O, Zaaqoq A, Namas R, Mi Q, Barclay D, Zuckerbraun B,
Peitzman AB, et al: Temporal patterns of circulating inflammation
biomarker networks differentiate susceptibility to nosocomial
infection following blunt trauma in humans. Ann Surg. 263:191–198.
2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Orr SK, Butler KL, Hayden D, Tompkins RG,
Serhan CN and Irimia D: Gene expression of proresolving lipid
mediator pathways is associated with clinical outcomes in trauma
patients. Crit Care Med. 43:2642–2650. 2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Vanzant EL, Hilton RE, Lopez CM, Zhang J,
Ungaro RF, Gentile LF, Szpila BE, Maier RV, Cuschieri J, Bihorac A,
et al: Advanced age is associated with worsened outcomes and a
unique genomic response in severely injured patients with
hemorrhagic shock. Crit Care. 19(77)2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Cheron A, Floccard B, Allaouchiche B,
Guignant C, Poitevin F, Malcus C, Crozon J, Faure A, Guillaume C,
Marcotte G, et al: Lack of recovery in monocyte human leukocyte
antigen-DR expression is independently associated with the
development of sepsis after major trauma. Crit Care.
14(R208)2010.PubMed/NCBI View
Article : Google Scholar
|
|
26
|
Furman BD, Mangiapani DS, Zeitler E,
Bailey KN, Horne PH, Huebner JL, Kraus VB, Guilak F and Olson SA:
Targeting pro-inflammatory cytokines following joint injury: Acute
intra-articular inhibition of interleukin-1 following knee injury
prevents post-traumatic arthritis. Arthritis Res Ther.
16(R134)2014.PubMed/NCBI View
Article : Google Scholar
|
|
27
|
Tursich M, Neufeld RW, Frewen PA,
Harricharan S, Kibler JL, Rhind SG and Lanius RA: Association of
trauma exposure with proinflammatory activity: A transdiagnostic
meta-analysis. Transl Psychiatry. 4(e413)2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Skjelbred P and Løkken P:
Anti-inflammatory agents in acute tissue trauma. Choice and
effects. Tidsskr Nor Laegeforen. 113:439–443. 1993.PubMed/NCBI(In Norwegian).
|
|
29
|
Fung KW, Xu J and Bodenreider O: The new
International classification of diseases 11th edition: A
comparative analysis with ICD-10 and ICD-10-CM. J Am Med Inform
Assoc. 27:738–746. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Russu E, Mureșan AV, Arbănași EM, Kaller
R, Hosu I, Voidăzan S, Arbănași EM and Coșarcă CM: The predictive
role of NLR and PLR in outcome and patency of lower limb
revascularization in patients with femoropopliteal disease. Clin
Med. 11(2620)2022.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Baker SP, O'Neill B, Haddon W Jr and Long
WB: The injury severity score: A method for describing patients
with multiple injuries and evaluating emergency care. J Trauma.
14:187–196. 1974.PubMed/NCBI
|
|
32
|
El-Matbouly M, Jabbour G, El-Menyar A,
Peralta R, Abdelrahman H, Zarour A, Al-Hassani A and Al-Thani H:
Blunt splenic trauma: Assessment, management and outcomes. Surgeon.
14:52–58. 2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Richards JR and McGahan JP: Focused
assessment with sonography in trauma (FAST) in 2017: What
radiologists can learn. Radiology. 283:30–48. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Stengel D, Bauwens K, Sehouli J, Porzsolt
F, Rademacher G, Mutze S and Ekkernkamp A: Systematic review and
meta-analysis of emergency ultrasonography for blunt abdominal
trauma. Br J Surg. 88:901–912. 2001.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Yoong S, Kothari R and Brooks A:
Assessment of sensitivity of whole body CT for major trauma. Eur J
Trauma Emerg Surg. 45:489–492. 2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Wang J, Wood A, Gao C, Najarian K and
Gryak J: Automated spleen injury detection using 3D active contours
and machine learning. Entropy (Basel). 23(382)2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Malaki M and Mangat K: Hepatic and splenic
trauma. Trauma. 13:2011.
|
|
38
|
Harna B, Arya S and Bahl A: Epidemiology
of trauma patients admitted to a trauma center in New Delhi, India.
Indian J Crit Care Med. 24:1193–1197. 2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Lenz A, Franklin GA and Cheadle WG:
Systemic inflammation after trauma. Injury. 38:1336–1345.
2007.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Chia TL, Chesney TR, Isa D, Mnatzakanian
G, Colak E, Belmont C, Hirpara D, Veigas PV, Acuna SA, Rizoli S and
Rezende-Neto J: Thrombocytosis in splenic trauma: In-hospital
course and association with venous thromboembolism. Injury.
48:142–147. 2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Salim A, Hadjizacharia P, DuBose J,
Kobayashi L, Inaba K, Chan LS and Margulies DR: What is the
significance of thrombocytosis in patients with trauma? J Trauma.
66:1349–1354. 2009.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Khan PN, Nair RJ, Olivares J, Tingle LE
and Li Z: Postsplenectomy reactive thrombocytosis. Proc (Bayl Univ
Med Cent). 22:9–12. 2009.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Arbănași EM, Mureșan AV, Coșarcă CM,
Kaller R, Bud TI, Hosu I, Voidăzan ST, Arbănași EM and Russu E:
Neutrophil-to-Lymphocyte ratio and platelet-to-lymphocyte ratio
impact on predicting outcomes in patients with acute limb ischemia.
Life (Basel). 12(822)2022.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Younan D, Richman J, Zaky A and Pittet JF:
An increasing neutrophil-to-lymphocyte ratio trajectory predicts
organ failure in critically-Ill male trauma patients. An
exploratory study. Healthcare (Basel). 7(42)2019.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Dilektasli E, Inaba K, Haltmeier T, Wong
MD, Clark D, Benjamin ER, Lam L and Demetriades D: The prognostic
value of neutrophil-to-lymphocyte ratio on mortality in critically
ill trauma patients. J Trauma Acute Care Surg. 81:882–888.
2016.PubMed/NCBI View Article : Google Scholar
|