Introduction
IgG4-related disease (IgG4-RD) is a multiorgan
lymphoproliferative disease characterized in the majority of
patients by increased serum IgG4 concentrations. It is considered
to be a systemic, chronic, inflammatory disorder characterized by
enlarged organs, the abundant infiltration of IgG4+
plasmacytes and fibrosis in the involved organs. Yoshida et
al (1) reported that chronic
pancreatitis caused by an autoimmune mechanism responded well to
steroid therapy. Moreover, Hamano et al (2) described this disorder as autoimmune
pancreatitis (AIP) with high serum IgG4 concentrations. Therefore,
AIP is described as a pancreatic manifestation of IgG4-RD (3,4).
IgG4-RD is an immune-mediated condition that mimics malignant and
inflammatory disorders.
IgG4-RD was initially identified among Japanese
patients with sclerosing chronic pancreatitis. The extensive
infiltration of IgG4-positive plasma cells and lymphocytes with
fibrosis was found in the involved organs, which mimicked malignant
tumors or inflammatory diseases. Although the established standard
therapy for IgG4-RD and AIP involves the use of steroids, the high
relapse rate among patients is concerning. Moreover, half of all
patients with AIP exhibit focal (<33% of the whole pancreas) or
segmental (>33 and <66%) pancreatic enlargement, which can be
difficult to distinguish from pancreatic malignant tumors (5,6).
Therefore, it is necessary to investigate novel therapeutics and
diagnostic markers.
Differentiated and mature macrophages produce
apoptosis inhibitor of macrophages (AIM), which is named based on
its function of suppressing macrophage apoptosis (7,8). Arai
and Miyazaki (9) suggested that
blood AIM concentrations may function as a biomarker for
obesity-associated autoimmune disease in humans. As a secretory
protein, AIM is found in the blood and its concentration changes in
various diseases (8,10,11).
In a previous study, it was demonstrated that there was an
association between AIM and hepatic fibrosis in chronic hepatitis C
(12). It has also been determined
that AIM may function as a biomarker for distinguishing between
different inflammatory bowel diseases (13). It can thus be hypothesized that AIM
may function as a biomarker for autoimmune and inflammatory
diseases with tissue fibrosis, such as IgG4-RD and AIP. However, to
the best of our knowledge the clinical significance of AIM in
IgG4-RD and AIP has not previously been investigated. Therefore,
the aim of the present study was to investigate the role of serum
AIM concentrations in patients with IgG4-RD and AIP.
Patients and methods
Patients
The present study was retrospective in nature. In
total, 38 patients who fulfilled the comprehensive diagnostic
criteria [APS 2011, ICDC, IgG4-RD 2011 (14-16)]
of having IgG4-RD and AIP and who were admitted to Kagoshima
University, Medical and Dental Hospital (Kagoshima, Japan) between
January, 2006 and December, 2016 were enrolled in the study.
Endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) was
performed in 27 patients with AIP to obtain pancreatic tissue and
all tissue was subjected to immunohistochemical analysis. Baseline
laboratory findings including serum amylase and IgG4 and CRP levels
were retrieved from the patients' medical records. Organ
involvement was determined from reviews of each patient's history
and the results of a physical examination, computed tomography (CT)
scan, magnetic resonance imaging, positron emission tomography/CT,
laboratory analyses and tissue biopsies. Moreover, a history of
malignant disease was determined from the medical records. Relapse
was defined by a symptomatic status or having elevated serum IgG4
concentrations during maintenance therapy. A pathological diagnosis
was not necessary (17). Written
informed consent was obtained from all patients and healthy
controls (HCs). The present study was approved by the Ethics
Committee of Kagoshima University Medical and Dental Hospital
(approval no. 28-241).
ELISA of serum AIM levels
Baseline serum samples were collected from 32
patients who had not been treated at the time of diagnosis and
among these, patient serum samples from only 16 patients who were
in remission on steroid therapy were collected before and after
treatment. Samples were portioned and stored at -80˚C. The serum
AIM concentrations in patients with untreated IgG4-RD with AIP
(n=32), patients with AIP before and after treatment in remission
(n=17), patients with pancreatic cancer (PC; n=30) who were
admitted to the same hospital and healthy controls (HCs; n=32) were
determined using ELISA kits (cat. no. KK901, Trans Genic, Inc.)
according to the manufacturer's protocol. Briefly, serum samples
(1:1,000) were incubated for 1 h at room temperature. The wells
were washed three times with PBS and subsequently HRP-conjugated
anti-human IgG antibody (1:30, included in the ELISA kit) was added
followed by incubation for 10 min at room temperature. The HCs were
subjects without AIP or PC and included students and staff
recruited from the facility (Kagoshima University).
Immunohistological analysis
Pancreatic tissues from patients with AIP were
obtained following EUS-FNA for immunohistological staining as
previously described (13).
Briefly, tissue samples were fixed in 10% buffered formalin,
embedded in paraffin, cut into 2-µm-thick sections, blocked with
Protein Block Serum-Free (cat. no. X0909, Dako; Agilent
Technologies, Inc.) for 3 h at room temperature and then incubated
at room temperature overnight with the following antibodies
against: Human AIM (1:400, cat. no. 54264, Anaspec, Inc.), human
CD14 (1:200, cat. no. ab45870, Abcam) and human CD16 (1:500, cat.
no. MCA2537, Bio-Rad Laboratories, Inc.). Secondary antibodies were
donkey anti-rabbit IgG(H+L) Highly Cross Adsorbed Secondary
Antibody Alexa Fluor 488 (cat. no. A21206, Thermo Fisher
Scientific, Inc.), donkey anti-goat IgG(H+L) Cross Adsorbed
Secondary Antibody Alexa Fluor 647 (cat. no. A21447, Thermo Fisher
Scientific, Inc.), donkey anti-mouse IgG(H+L) Highly Cross Adsorbed
Secondary Antibody Alexa Fluor 555 (cat. no. A231570, Thermo Fisher
Scientific, Inc.), respectively (1:200). All were incubated at room
temperature for 35 min. Fluorescence emission was analyzed using a
fluorescence microscope (BZ9000; Keyence Corporation).
Statistical analysis
All data were analyzed using SPSS software version
24 (IBM Corp.) at least twice. Data were statistically analyzed
using a non-parametric Mann-Whitney U test, Kruskal-Wallis test and
Dunn's test as post hoc test or the Wilcoxon signed-rank test and
Chi-squared test. Correlation coefficients were determined using
the Spearman's rank correlation coefficient test. Data are
presented as the mean±SD. P<0.05 was considered to indicate a
statistically significant difference.
Results
Characteristics of all patients with
IgG4-RD/AIP
The characteristics for all the 38 enrolled patients
with IgG4-RD and AIP are summarized in Table I. The number and age of the patients
was significantly higher for males [n=25 (65.8%); mean age,
69.2±9.1 years] than for females [n=13 (34.2%); mean age, 59.8±7.0
years]. The mean number of organs involved per patient was 2.4. At
least two organs were involved in 25 (65.8%) patients. The serum
IgG4 and IgG concentrations were 640.0±423.1 and 2,523.2±1,011.9
mg/dl, respectively. The serum concentrations of the soluble IL-2
receptor (1,186.8±524.2 U/ml; normal concentration, 121-613 U/ml)
and IgE (966.3±1,185.8 U/ml; normal concentration, <232 U/ml)
were elevated. The median serum AIM concentration was
3,891.9±8,374.5 ng/ml. No significant difference was observed in
these biomarkers between the sexes.
 | Table IComparison of baseline characteristics
and associations between serum AIM levels and clinical parameters
in patients with IgG4-RD. |
Table I
Comparison of baseline characteristics
and associations between serum AIM levels and clinical parameters
in patients with IgG4-RD.
| Characteristic | Total | Male | Female | P-value |
|---|
| Sex, n (%) | 38 | 25 (65.8%) | 13 (34.2%) | 0.001 |
| Age, years | 64.8±10.3 | 69.2±9.1 | 59.8±7.0 | 0.002 |
| Number of organs,
n | 2.4±1.3 | 2.6±1.4 | 1.9±0.9 | 0.159 |
| IgG4, mg/dl | 640.0±423.1 | 640.5±428.8 | 639.1±430.3 | 0.993 |
| IgG, mg/dl | 2,523.2±1,011.9 | 2,603.7±1,014.7 | 2,362.3±1,032.1 | 0.508 |
| IgG4/IgG ratio | 0.3±0.2 | 0.2± 0.1 | 0.27±0.13 | 0.543 |
| IgE, IU/l | 966.3±1,185.8 | 1,186.3±1,395.8 | 636.3±776.1 | 0.399 |
| IgM, mg/dl | 122.0±80.2 | 111.2±69.2 | 134.0±93.6 | 0.551 |
| IgA, mg/dl | 212.2±76.8 | 228.0±74.4 | 194.5±79.8 | 0.357 |
| Amy, IU/l | 119.2±184.1 | 142.9±227.3 | 77.9±41.9 | 0.338 |
| P-Amy, IU/l | 84.7±209.3 | 112.9±263.2 | 37.8±27.9 | 0.407 |
| FBS, mg/dl | 125.9±47.3 | 130.9±51.9 | 116.4±37.2 | 0.419 |
| HbA1c, % | 6.5±1.5 | 6.5±1.6 | 6.4±1.1 | 0.844 |
| CEA, ng/ml | 2.6±1.3 | 2.9±1.3 | 2.2±1.3 | 0.143 |
| CA19-9, U/ml | 48.5±126.1 | 33.3±52.1 | 77.5±206.0 | 0.499 |
| sIL-2 receptor,
U/ml | 1,186.8±524.2 | 1,305.3±564.4 | 932.9±328.7 | 0.123 |
| White blood cells,
n | 5,894±1,638 | 5,765±1,719 | 6,165±1,502 | 0.534 |
| Neutrophils | 3,419±1212 | 3,388±1357 | 3,508±724 | 0.826 |
| Eosinophils | 230±1423 | 224±134 | 244±178 | 0.752 |
| AIM, ng/ml |
3,891.9±8,374.5 |
4,329.5±9,908.0 | 1,505.4±559.0 | 0.287 |
Serum AIM concentrations are higher in
patients with AIP compared with the HCs and patients with PC
Subsequently, the serum AIM concentrations in
patients with AIP (n=32), PC (n=30) and the HCs (n=32) were
compared. The mean serum AIM concentrations were 2,387.9±1,232.1,
1,740.2±1,470.2 and 1,313.1±631.0 ng/ml in patients with AIP, PC
and the HCs, respectively. The serum AIM concentrations were
significantly higher in patients with AIP compared with those with
PC (P<0.005) and the HCs (P<0.001) No significant differences
were observed in the serum AIM levels between the patients with PC
and the HCs (P=0.37) (Fig. 1).
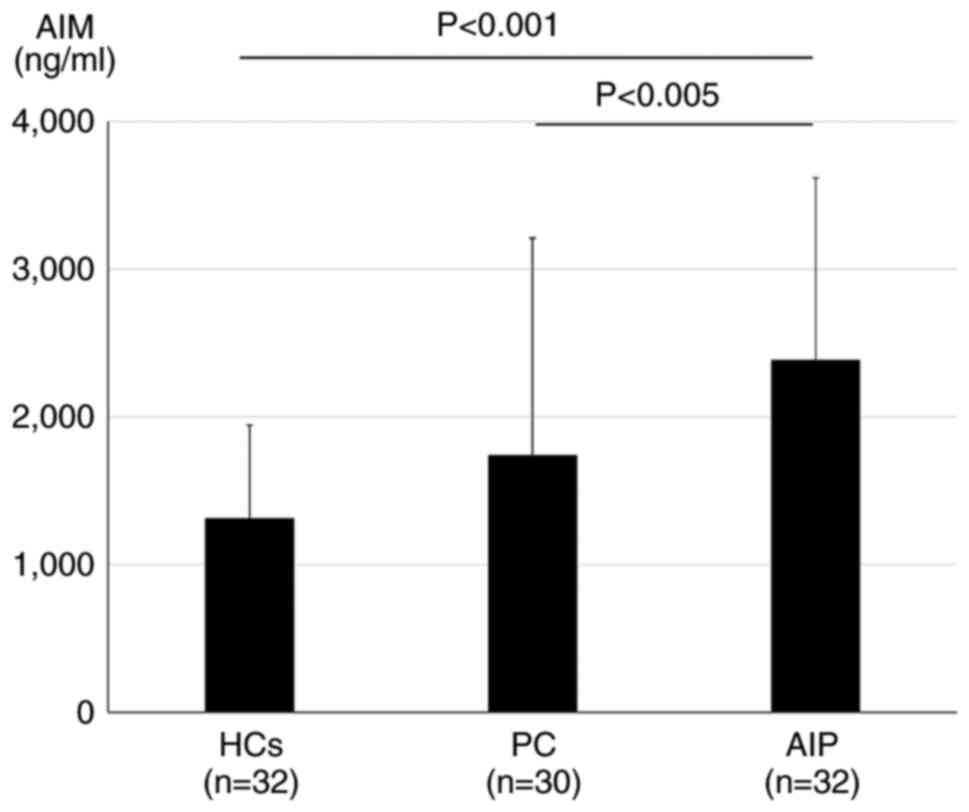 | Figure 1Serum AIM concentrations in patients
with AIP, PC and HCs. Serum AIM concentrations in patients with AIP
(n=32) were significantly higher compared with the HCs (n=32) and
patients with PC (n=30). The serum AIM concentrations were as
follows: AIP, 2,387.9±1,232.1 ng/ml; PC, 1,740.2±1,470.2 ng/ml,
P<0.005 vs. AIP; and HC, 1,313.1±631.0 ng/ml, P<0.001 vs.
AIP. Data were statistically analyzed using the Kruskal-Wallis
test. AIM, apoptosis inhibitor of macrophages; AIP, autoimmune
pancreatitis; PC, pancreatic cancer; HCs, healthy controls. |
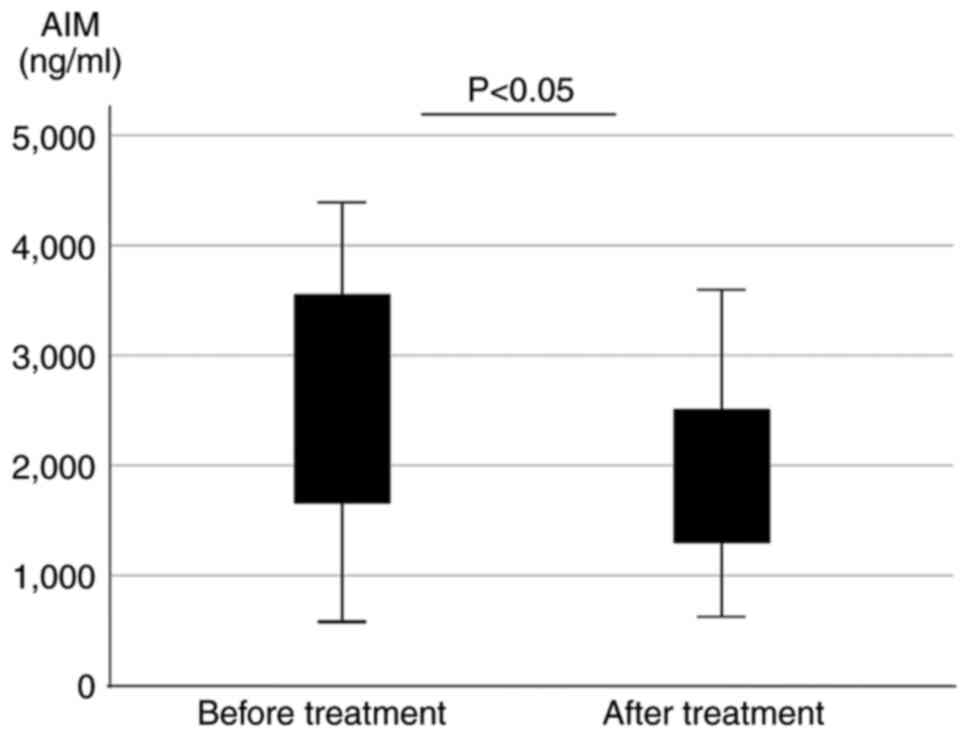
Serum AIM concentrations before and
after treatment with steroids in patients with AIP
The serum AIM concentrations were compared in
patients with AIP before and after treatment with steroid
(prednisolones) who were in remission during treatment (n=16).
Patients in the treated AIP group exhibited significantly decreased
serum AIM concentrations after treatment (before treatment,
2,499.9±1,210.5 ng/ml; after treatment, 1,913.6±924.2 ng/ml;
P=0.036; Fig. 2).
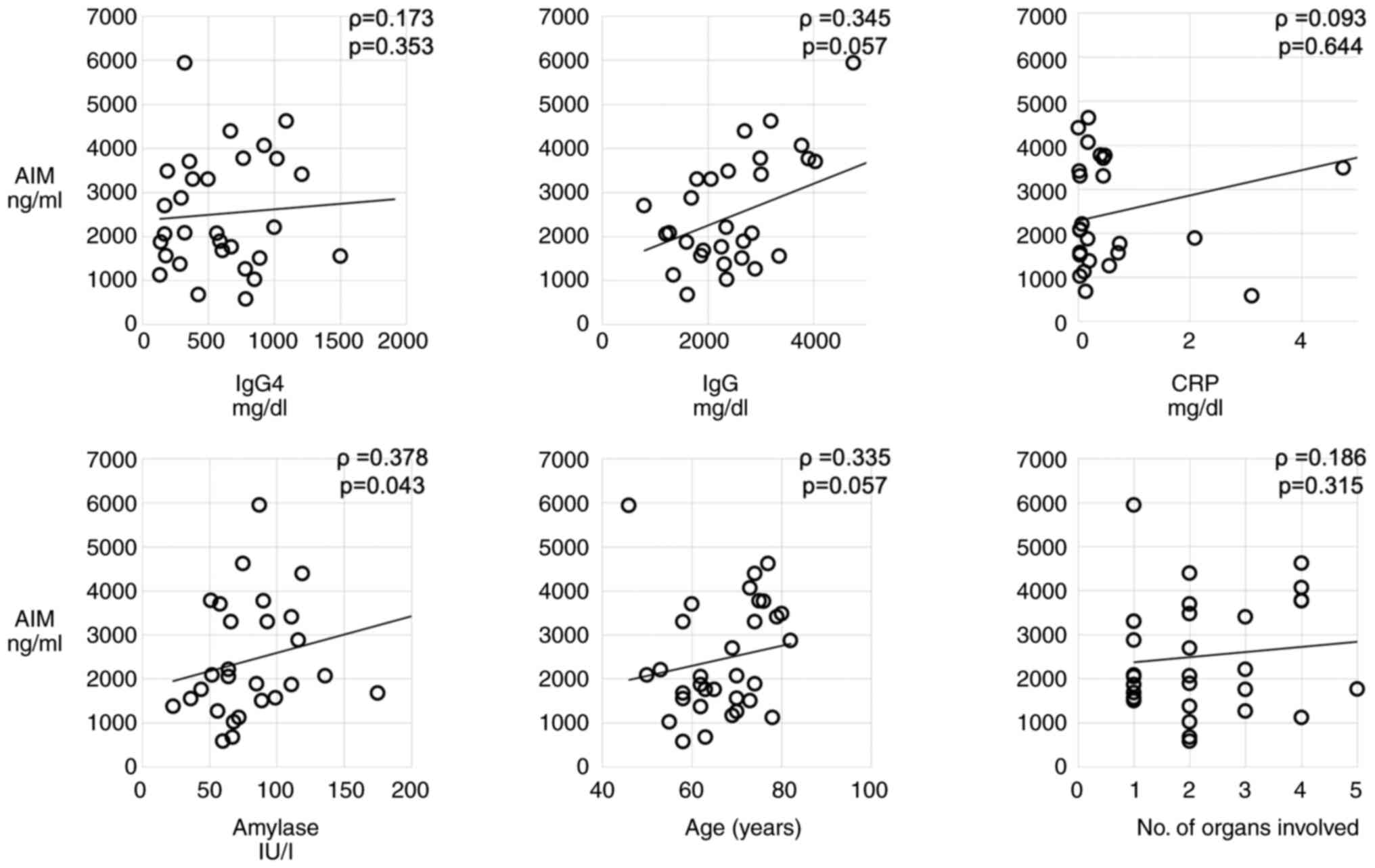
Associations between AIM and various
parameters
Among the laboratory parameters analyzed, serum
amylase levels (ρ=0.378; P=0.043) significantly positively
correlated with the serum AIM concentrations. In addition, IgG
levels (ρ=0.345; P=0.057) and age (ρ=0.335; P=0.057) also
positively correlated with the serum AIM concentrations. No
significant correlations were observed between AIM and IgG4
(ρ=0.173; P=0.353), CRP (ρ=0.093; P=0.644) and the number of organs
involved (ρ=0.186; P=0.315) (Fig.
3).
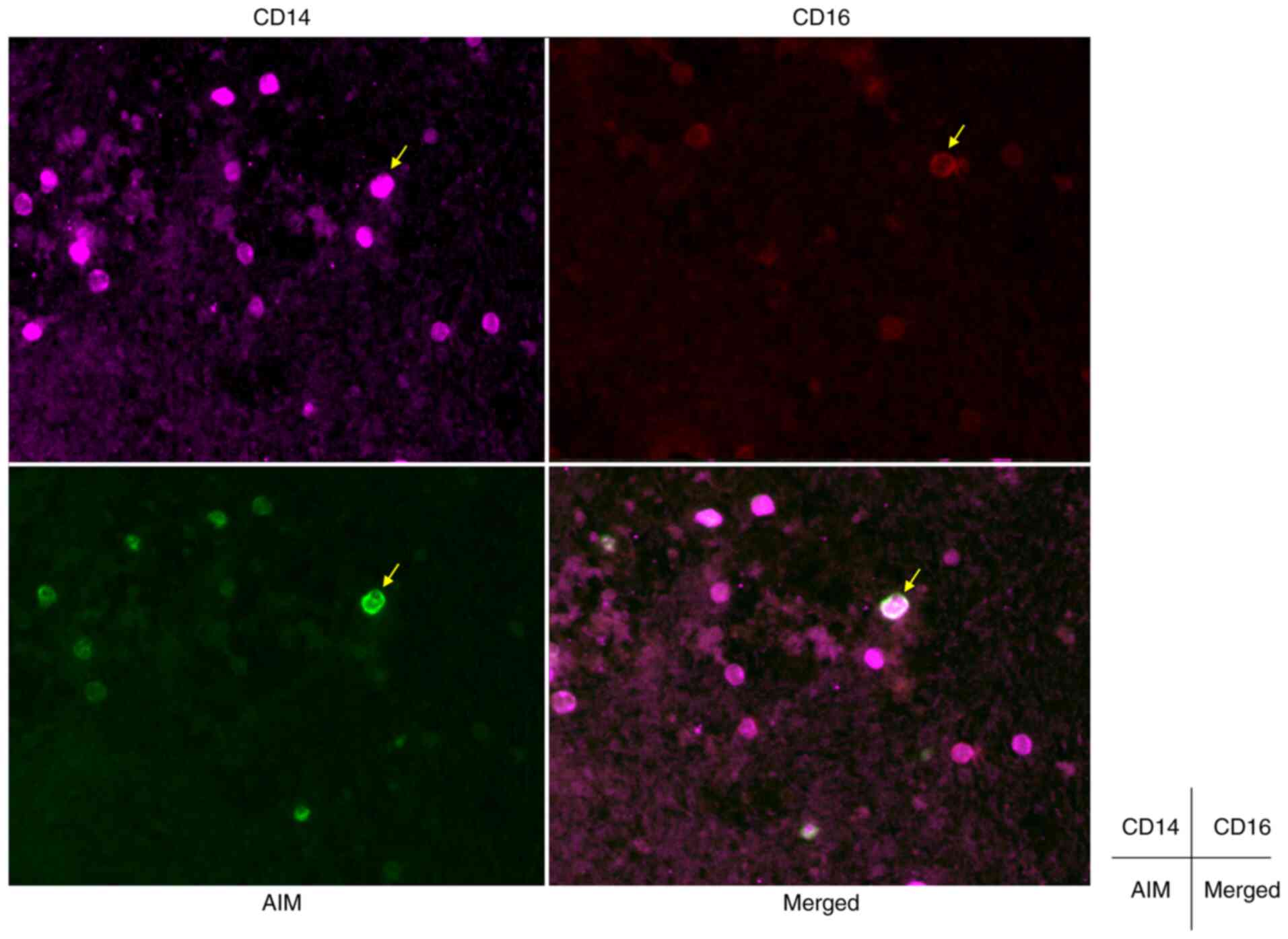
AIM expression in macrophages of
pancreatic tissue from patients with AIP
Tissue sections were assessed using
immunohistochemistry to determine AIM protein expression levels in
patients with AIP who had undergone EUS-FNA. Inflammatory cell
infiltration was evident in pancreatic tissues from patients with
AIP and immunostaining demonstrated AIM protein expression in
mononuclear cells. Moreover, double immunostaining for CD14 and
CD16 demonstrated that AIM-positive mononuclear cells predominantly
expressed CD16. These results indicated that active macrophages in
the pancreas expressed AIM (Fig.
4).
Characteristics and clinical
parameters of patients with IgG4-RD under various parameters (age,
number of organs involved, history of malignant diseases,
relapse)
The values for age (<70 and ≥70 years), number of
organs involved (<2 and ≥2), a history of malignant diseases and
relapse (positive or negative) and the results of their comparisons
are presented in Table II,
Table III, Table IV and Table V. The serum concentrations of IgG,
amylase and AIM were higher in patients aged ≥70 years (Table II). Patients who had more than two
organs involved had elevated levels of IgG4, fasting blood sugar
and eosinophils (Table III).
Patients with a history of malignancies had higher AIM levels and
elevated levels of eosinophils (Table
IV). The IgG4 levels did not differ significantly in terms of
age (Table II) or relapse
(Table V).
 | Table IIComparison of baseline
characteristics of patients with IgG4-RD under the parameter of
age. |
Table II
Comparison of baseline
characteristics of patients with IgG4-RD under the parameter of
age.
| | Age, years | |
|---|
| Characteristic | <70 (n=21) | ≥70 (n=17) | P-value |
|---|
| No. of organs
involved | 2.0±1.1 | 2.8±1.3 | 0.100 |
| IgG4, mg/dl | 550.3±384.6 | 740.3±452.8 | 0.196 |
| IgG, mg/dl |
2,321.3±1,192.0 | 2,748.9±734.3 | 0.042 |
| IgG4/IgG ratio | 0.2±0.1 | 0.3±0.1 | 0.950 |
| IgE, IU/l | 664.8±627.4 |
1,795.5±2,000.0 | 0.226 |
| Amy, IU/l | 72.8±36.1 | 168.6±256.6 | 0.006 |
| P-Amy, IU/l | 34.5±24.7 | 144.1±304.8 | 0.608 |
| FBS, mg/dl | 126.6±46.5 | 125.1±49.8 | 0.766 |
| HbA1c, % | 6.7±1.7 | 6.3±1.2 | 0.654 |
| CEA, ng/ml | 2.6±1.3 | 2.7±1.4 | 0.551 |
| CA19-9, U/ml | 72.3±179.5 | 27.4±40.3 | 1.000 |
| sIL-2 receptor,
U/ml | 991.2±372.1 | 1381.5±596.1 | 0.133 |
| White blood
cells | 6,147± 1,463 | 5,656±1,800 | 0.470 |
| Neutrophils | 3,494±1,056 | 3,358±1,358 | 0.755 |
| Eosinophils | 219±157 | 238±135 | 0.614 |
| AIM, ng/ml |
2,080.8±1,293.7 |
5,816.2±11,839.1 | 0.034 |
 | Table IIIComparison of baseline
characteristics of patients with IgG4-RD under the parameter of
number of organs involved. |
Table III
Comparison of baseline
characteristics of patients with IgG4-RD under the parameter of
number of organs involved.
| | No. of organs
involved | |
|---|
| Characteristic | <2 (n=11) | ≥2 (n=25) | P-value |
|---|
| Age, years | 62.2±11.5 | 67.9±8.4 | 0.104 |
| IgG4, mg/d | 383.5±219.9 | 721.8±425.2 | 0.004 |
| IgG, mg/d | 2,064.6±974.5 | 2,698.7±991.2 | 0.087 |
| IgG4/IgG ratio | 0.2±0.1 | 0.3±0.2 | 0.098 |
| IgE, IU/l | 674.2±618.3 |
1,228.0±1,540.0 | 0.554 |
| Amy, IU/l | 94.3±33.1 | 136.3±230.0 | 0.554 |
| P-Amy, IU/l | 42.1±27.7 | 106.0±255.7 | 0.493 |
| FBS, mg/d | 100.6±23.0 | 138.4±52.3 | 0.009 |
| HbA1c, % | 6.2±1.3 | 6.6±1.6 | 0.487 |
| CEA, ng/m | 2.4±0.7 | 2.7±1.5 | 0.402 |
| CA19-9, U/m | 8.3±5.7 | 66.7±149.5 | 0.231 |
| sIL-2 receptor,
U/ml | 881.4±372.1 | 1,305.1±519.3 | 0.122 |
| White blood cells,
n | 5,272±1230 | 6,194±17,20 | 0.177 |
| Neutrophils | 3,387±1,244 | 3,484±1,242 | 0.861 |
| Eosinophils | 148±50 | 253±156 | 0.014 |
| AIM, ng/ml |
2,614.3±1,352.3 |
4,741.9±10,448.3 | 0.530 |
 | Table IVComparison of baseline
characteristics of patients with IgG4-RD under the parameter of
history of malignant diseases. |
Table IV
Comparison of baseline
characteristics of patients with IgG4-RD under the parameter of
history of malignant diseases.
| | History and number
of malignancies | |
|---|
| Characteristic | (-) (n=28) | (+) (n=10) | P-value |
|---|
| No. of organs
involved | 2.4±1.3 | 2.3±1.3 | 0.903 |
| IgG4, mg/dl | 701.0± 442.5 | 481.5±337.3 | 0.189 |
| IgG, mg/dl | 2545.0±1013.2 | 2466.5±1061.0 | 0.958 |
| IgG4/IgG ratio | 0.3±0.1 | 0.2±0.1 | 0.189 |
| IgE, IU/l | 693.0±620.6 | 1718.0±2061.8 | 0.661 |
| Amy, IU/l | 172.9±210.9 | 92.3±37.1 | 0.757 |
| P-Amy, IU/l | 97.8±241.2 | 45.5±36.6 | 0.626 |
| FBS, mg/dl | 116.5±38.1 | 146.6±60.2 | 0.119 |
| HbA1c, % | 6.1±0.9 | 7.3±2.1 | 0.124 |
| CEA, ng/ml | 2.5±1.1 | 3.0±1.7 | 0.651 |
| CA19-9, U/ml | 55.3±175.8 | 30.9±51.9 | 0.902 |
| sIL-2 receptor,
U/ml | 1204.1±500.7 | 1140.8±631.3 | 0.747 |
| White blood
cells | 5980±1553 | 5684±1913 | 0.781 |
| Neutrophils | 3481±1153 | 3272±1416 | 0.815 |
| Eosinophils | 192±143 | 320±97 | 0.004 |
| AIM, ng/ml | 2306.5±1385.1 | 8119.7±15727.9 | 0.049 |
 | Table VComparison of baseline
characteristics of patients with IgG4-RD under the parameter of
disease relapse. |
Table V
Comparison of baseline
characteristics of patients with IgG4-RD under the parameter of
disease relapse.
| | No. of patients
with disease relapse | |
|---|
| Characteristic | (-) (n=22) | (+) (n=8) | P-value |
|---|
| No. of organs
involved | 2.3±1.2 | 2.6±1.4 | 0.601 |
| IgG4, mg/dl | 640.9± 398.8 | 807.3±573.6 | 0.636 |
| IgG, mg/dl | 2521.2±1026.7 | 2598.8± 1019.6 | 0.784 |
| IgG4/IgG ratio | 0.2±0.1 | 0.3±0.1 | 0.469 |
| IgE, IU/l | 941.3±1532.6 | 1159.0±391.7 | 0.178 |
| Amy, IU/l | 93.1±35.8 | 215.6±369.8 | 0.449 |
| P-Amy, IU/l | 45.1±30.5 | 173.9±358.5 | 0.414 |
| FBS, mg/dl | 122.7±47.6 | 135.0±45.6 | 0.297 |
| HbA1c, % | 6.4±1.5 | 6.4±1.5 | 0.804 |
| CEA, ng/ml | 3.1±1.4 | 1.9±0.9 | 0.041 |
| CA19-9, U/ml | 53.9±161.2 | 21.4±21.0 | 0.935 |
| sIL-2 receptor,
U/ml | 1004.9±384.9 | 1413.8±772.1 | 0.505 |
| White blood
cells | 5846±1469 | 5941±1943 | 0.790 |
| Neutrophils | 3401±931 | 3797±1497 | 0.407 |
| Eosinophils | 260±142 | 201±142 | 0.332 |
| AIM, ng/ml | 4693.0±10729.3 | 2178.7±1149.8 | 0.725 |
Discussion
To the best if our knowledge, this is the first
investigation into serological AIM concentrations in patients with
IgG4-RD/AIP. The present study found higher serum AIM
concentrations in patients with AIP compared with patients with PC
and the HCs. Furthermore, the serum AIM concentrations
significantly decreased in patients with AIP who were in remission
following treatment with steroids.
Differentiated and mature macrophages produce AIM, a
secretory protein of ~50 kDa that consists of three scavenger
receptor cysteine-rich domains and is named based on its function
of suppressing macrophage apoptosis. AIM is an apoptosis inhibitor
that supports the survival of macrophages against various
apoptosis-inducing stimuli (7,8). The
association between AIM and autoimmune diseases has previously been
reported. Arai and Miyazaki (9)
suggested that blood AIM concentrations may function as a biomarker
for obesity-associated autoimmune diseases in humans, along with
diabetes-associated antibodies against pancreatic β-cell antigens
(including protein tyrosine phosphatase-like protein), chronic
thyroiditis-associated anti-thyroid peroxidase and
anti-thyroglobulin antibody. When macrophages take up oxidized
low-density lipoprotein, AIM expression is induced via the
activated nuclear receptors, liver X receptor/retinoid X receptor
heterodimers (18,19). As a secretory protein, AIM is found
in the blood and its concentration changes in response to various
diseases (8,10,11).
In a previous study, it was reported that there was an association
between AIM and hepatic fibrosis in chronic hepatitis C. Therefore,
it was hypothesized that the serum concentration of AIM may be a
potential marker of hepatic fibrosis in chronic liver disease
(12). Moreover, it has been
demonstrated that AIM may function as a biomarker for
distinguishing between different inflammatory bowel diseases.
Furthermore, AIM is a novel biomarker of Crohn's disease, which can
be used to distinguish Crohn's disease from ulcerative colitis and
Behcet's disease (13). According
to these aforementioned studies, as IgG4-RD and AIP are diseases
that are characterized by tissue inflammation and fibrosis, it can
be hypothesized that AIM may function as a diagnostic or active
biomarker of autoimmune and inflammatory diseases with tissue
fibrosis, such as IgG4-RD and AIP. In the present study, AIM was
demonstrated to play a role as a potential biomarker for the
differentiation of AIP and PC, as well as for disease activity.
Furthermore, there was a significant positive correlation between
AIM and amylase in the present study. Macrophages are the most
abundant immune cells and play a crucial role in pancreatic tissue.
Activated macrophages, which generate AIM in the pancreatic tissue,
may be associated with pancreatitis and stimulate pancreatic
exocrine function (20). The
present study did not have height and weight data and was not able
to analyze the association between AIM and BMI. This is an
important point that needs to be analyzed in the future.
AIP is classified into the following three
categories according to its swelling pattern: i) Diffuse; ii)
focal; and iii) segmental. As the images of focal and segmental
type AIP are similar to pancreatic tumors, AIP can be particularly
difficult to distinguish from malignant tumors, even when using
various different imaging modalities (21). Furthermore, a differential diagnosis
between AIP and PC cannot be based only on serum IgG4
concentrations (22,23). Therefore, as a result of its
complexity, numerous AIP cases using surgical resection as the
initial treatment have been reported (24,25).
Consequently, it is important to set diagnostic criteria to
distinguish AIP from PC in order to avoid unnecessary invasive
surgery. Therefore, a useful biomarker that can distinguish AIP
from PC is required. In the present study, serum AIM concentrations
in patients with AIP, PC and HCs were compared. The serum AIM
concentrations were significantly higher in patients with AIP
compared with HCs and patients with PC. The serum AIM
concentrations may thus play a role as a potential biomarker that
can distinguish between AIP and PC. The present study also obtained
data on serum AIM levels in chronic pancreatitis (data not shown).
The AIM value of AIP tended to be higher than that of chronic
pancreatitis, although there was no significant difference. Thus,
the authors aim to identify more cases in the future for further
analysis.
Furthermore, the present study demonstrated that
treatment with steroids significantly decreased the serum AIM
concentrations in patients with AIP who were in remission. To the
best of our knowledge, an association between serum AIM
concentrations and treatment outcomes for other autoimmune diseases
has not yet been reported. An international survey reported in 2013
demonstrated that 74% of patients with AIP were initially treated
with steroids and the remission rate was 99.6% (681/684 patients)
(26). However, the high relapse
rate of AIP is an issue and of the 978 patients with AIP in that
previous study, a total of 302 (31%) patients experienced relapse
(26). It remains unknown how
disease activity indexes or predictors of relapse influence
parameters, such as the Crohn's disease Activity Index (CDAI) in
Crohn's disease. Furthermore, there is no association between serum
AIM concentrations and the CDAI in patients with Crohn's disease
(13). However, the results of the
present study suggested that serum AIM concentrations may
potentially be an indicator of AIP.
It has previously been reported that IgG4-RD and AIP
may exhibit aspects of paraneoplastic syndrome, which is an altered
immune system response to a neoplasm (27-29).
In the present study, 10/38 (26%) patients with IgG4-RD had a
history of malignancy. Standardized incidence ratios (SIR) estimate
an increased risk of overall cancer in patients with IgG4-RD
patients (SIR, 2.57; 95% CI, 1.72-3.84) compared with the general
population (30). Moreover, even
though it is important to diagnose patients with IgG4-RD and AIP
who have malignant diseases as it affects prognosis, the clinical
characteristics remain unclear (31). AIM is also associated with certain
malignancies (32). In the present
study, the serum AIM concentrations were significantly higher in
patients with IgG4-RD and a history of malignancies. These results
suggest that the serum AIM concentrations may potentially serve as
a biomarker to predict malignant disease in patients with IgG4-RD
and AIP.
In the present study AIM expression in active
macrophages in pancreatic tissues was demonstrated. Moreover, as
macrophages play a crucial role in IgG4-RD (33), it can be hypothesized that AIM,
which is produced by resident macrophages in pancreatic tissues,
contributes to pancreatic inflammation and fibrosis, which may
result in elevated serum AIM concentrations. However, there are
limitations to the present study as it used a single-center design
and only a small number of patients were enrolled. Therefore, a
larger sample size should be used in future studies.
In conclusion, in the present study, serological AIM
concentrations were significantly higher in patients with
IgG4-RD/AIP compared with patients with PC. Furthermore, serum AIM
concentrations decreased in patients treated with steroids who were
in remission. The results of the present study suggest that serum
AIM concentrations may potentially serve as a precise diagnostic
and therapeutic biomarker in patients with IgG4-RD/AIP.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ST drafted the manuscript. ST, SK and AI were
substantial contributions to the conception or design of the study,
the acquisition, analysis or interpretation of data for the work,
the drafting the manuscript and revising it critically for
important intellectual content. MH, SA, FS and SH contributed to
the acquisition, analysis and interpretation of the data, as well
as the in writing and revision of the manuscript. SK and ST confirm
the authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Written, informed consent was obtained from all
patients. The present study was approved by the Ethics Committee of
Kagoshima University Medical and Dental Hospital (Kagoshima, Japan;
approval no. 28-241). All experimental procedures were performed
according to the Ethical Principles for Medical Research Involving
Human Subjects outlined in the World Medical Association
Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yoshida K, Toki F, Takeuchi T, Watanabe S,
Shiratori K and Hayashi N: Chronic pancreatitis caused by an
autoimmune abnormality. Proposal of the concept of autoimmune
pancreatitis. Dig Dis Sci. 40:1561–1568. 1995.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hamano H, Kawa S, Horiuchi A, Unno H,
Furuya N, Akamatsu T, Fukushima M, Nikaido T, Nakayama K, Usuda N
and Kiyosawa K: High serum IgG4 concentrations in patients with
sclerosing pancreatitis. N Engl J Med. 344:732–738. 2001.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Stone JH, Zen Y and Deshpande V:
IgG4-related disease. N Engl J Med. 366:539–551. 2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kamisawa T, Zen Y, Pillai S and Stone JH:
IgG4-related disease. Lancet. 385:1460–1471. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kamisawa T, Funata N, Hayashi Y, Eishi Y,
Koike M, Tsuruta K, Okamoto A, Egawa N and Nakajima H: A new
clinicopathological entity of IgG4-related autoimmune disease. J
Gastroenterol. 38:982–984. 2003.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Masamune A, Kikuta K, Hamada S, Tsuji I,
Takeyama Y, Shimosegawa T and Okazaki K: Collaborators. Nationwide
epidemiological survey of autoimmune pancreatitis in Japan in 2016.
J Gastroenterol. 55:462–470. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Miyazaki T, Hirokami Y, Matsuhashi N,
Takatsuka H and Naito M: Increased susceptibility of thymocytes to
apoptosis in mice lacking AIM, a novel murine macrophage-derived
soluble factor belonging to the scavenger receptor cysteine-rich
domain superfamily. J Exp Med. 189:413–422. 1999.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Mori M, Kimura H, Iwamura Y, Arai S and
Miyazaki T: Modification of N-glycosylation modulates the secretion
and lipolytic function of apoptosis inhibitor of macrophage (AIM).
FEBS Lett. 586:3569–3574. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Arai S and Miyazaki T: Impacts of the
apoptosis inhibitor of macrophage (AIM) on obesity-associated
inflammatory diseases. Semin Immunopathol. 36:3–12. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Arai S, Shelton JM, Chen M, Bradley MN,
Castrillo A, Bookout AL, Mak PA, Edwards PA, Mangelsdorf DJ,
Tontonoz P, et al: A role for the apoptosis inhibitory factor
AIM/Spα/Api6 in atherosclerosis development. Cell Metab. 1:201–213.
2005.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gangadharan B, Antrobus R, Dwek RA and
Zitzmann N: Novel serum biomarker candidates for liver fibrosis in
hepatitis C patients. Clin Chem. 53:1792–1799. 2007.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Mera K, Uto H, Mawatari S, Ido A,
Yoshimine Y, Nosaki T, Oda K, Tabu K, Kumagai K, Tamai T, et al:
Serum levels of apoptosis inhibitor of macrophage are associated
with hepatic fibrosis in patients with chronic hepatitis C. BMC
Gastroenterol. 14(27)2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ono Y, Kanmura S, Morinaga Y, Oda K,
Kawabata K, Arima S, Sasaki F, Nasu Y, Tanoue S, Hashimoto S, et
al: The utility of apoptosis inhibitor of macrophages as a possible
diagnostic marker in patients with Crohn's disease. BMC
Gastroenterol. 17(40)2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Okazaki K, Kawa S, Kamisawa T, Naruse S,
Tanaka S, Nishimori I, Ohara H, Ito T, Kiriyama S, Inui K, et al:
Clinical diagnostic criteria of autoimmune pancreatitis: Revised
proposal. J Gastroenterol. 41:626–631. 2006.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Shimosegawa T, Okazaki K, Kamisawa T, Kawa
S and Notohara K: International Consensus Diagnostic Criteria for
autoimmune pancreatitis-Guidelines of the International Association
of Pancreatology. Suizo. 26:684–698. 2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Umehara H, Okazaki K, Masaki Y, Kawano M,
Yamamoto M, Saeki T, Matsui S, Yoshino T, Nakamura S, Kawa S, et
al: Comprehensive diagnostic criteria for IgG4-related disease
(IgG4-RD), 2011. Mod Rheumatol. 22:21–30. 2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Inoue D, Yoshida K, Yoneda N, Ozaki K,
Matsubara T, Nagai K, Okumura K, Toshima F, Toyama J, Minami T, et
al: IgG4-related disease: Dataset of 235 consecutive patients.
Medicine (Baltimore). 94(e680)2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Joseph SB, Bradley MN, Castrillo A, Bruhn
KW, Mak PA, Pei L, Hogenesch J, O'connell RM, Cheng G, Saez E, et
al: LXR-dependent gene expression is important for macrophage
survival and the innate immune response. Cell. 119:299–309.
2004.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Valledor AF, Hsu LC, Ogawa S,
Sawka-Verhelle D, Karin M and Glass CK: Activation of liver X
receptors and retinoid X receptors prevents bacterial-induced
macrophage apoptosis. Proc Natl Acad Sci USA. 101:17813–17818.
2004.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wu J, Zhang L, Shi J, He R, Yang W,
Habtezion A, Niu N, Lu P and Xue J: Macrophage phenotypic switch
orchestrates the inflammation and repair/regeneration following
acute pancreatitis injury. EBioMedicine. 58(102920)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sun GF, Zuo CJ, Shao CW, Wang JH and Zhang
J: Focal autoimmune pancreatitis: Radiological characteristics help
to distinguish from pancreatic cancer. World J Gastroenterol.
19:3634–3641. 2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kamisawa T, Takuma K, Tabata T, Inaba Y,
Egawa N, Tsuruta K, Hishima T, Sasaki T and Itoi T: Serum
IgG4-negative autoimmune pancreatitis. J Gastroenterol. 46:108–116.
2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ghazale A, Chari ST, Smyrk TC, Levy MJ,
Topazian MD, Takahashi N, Clain JE, Pearson RK, Pelaez-Luna M,
Petersen BT, et al: Value of serum IgG4 in the diagnosis of
autoimmune pancreatitis and in distinguishing it from pancreatic
cancer. Am J Gastroenterol. 102:1646–1653. 2007.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Weber SM, Cubukcu-Dimopulo O, Palesty JA,
Suriawinata A, Klimstra D, Brennan MF and Conlon K:
Lymphoplasmacytic sclerosing pancreatitis: Inflammatory mimic of
pancreatic carcinoma. J Gastrointest Surg. 7:129–139.
2003.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Hardacre JM, Iacobuzio-Donahue CA, Sohn
TA, Abraham SC, Yeo CJ, Lillemoe KD, Choti MA, Campbell KA,
Schulick RD, Hruban RH, et al: Results of pancreaticoduodenectomy
for lymphoplasmacytic sclerosing pancreatitis. Ann Surg.
237:853–859. 2003.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hart PA, Kamisawa T, Brugge WR, Chung JB,
Culver EL, Czakó L, Frulloni L, Go VL, Gress TM, Kim MH, et al:
Long-term outcomes of autoimmune pancreatitis: A multicentre,
international analysis. Gut. 62:1771–1776. 2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Okamoto A, Watanabe T, Kamata K, Minaga K
and Kudo M: Recent updates on the relationship between cancer and
autoimmune pancreatitis. Intern Med. 58:1533–1539. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Tang H, Yang H, Zhang P, Wu D, Zhang S,
Zhao J, Peng L, Chen H, Fei Y, Zhang X, et al: Malignancy and
IgG4-related disease: The incidence, related factors and prognosis
from a prospective cohort study in China. Sci Rep. 10:1–7.
2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Haghbin H, Chuang J, Fatima R,
Zakirkhodjaev N, Lee-Smith W and Aziz M: Correlation of autoimmune
pancreatitis and malignancy: Systematic review and meta-analysis.
Dig Dis Sci. 67:3252–3264. 2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Yu T, Wu Y, Liu J, Zhuang Y, Jin X and
Wang L: The risk of malignancy in patients with IgG4-related
disease: A systematic review and meta-analysis. Arthritis Res Ther.
24:1–9. 2022.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Yamamoto M, Takahashi H, Tabeya T, Suzuki
C, Naishiro Y, Ishigami K, Yajima H, Shimizu Y, Obara M, Yamamoto
H, et al: Risk of malignancies in IgG4-related disease. Mod
Rheumatol. 22:414–418. 2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Koyama N, Yamazaki T, Kanetsuki Y, Hirota
J, Asai T, Mitsumoto Y, Mizuno M, Shima T, Kanbara Y, Arai S, et
al: Activation of apoptosis inhibitor of macrophage is a sensitive
diagnostic marker for NASH-associated hepatocellular carcinoma. J
Gastroenterol. 53:770–779. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Furukawa S, Moriyama M, Tanaka A, Maehara
T, Tsuboi H, Iizuka M, Hayashida JN, Ohta M, Saeki T, Notohara K,
et al: Preferential M2 macrophages contribute to fibrosis in
IgG4-related dacryoadenitis and sialoadenitis, so-called Mikulicz's
disease. Clin Immunol. 156:9–18. 2015.PubMed/NCBI View Article : Google Scholar
|