Introduction
Functional gastrointestinal diseases (FGIDs) are
syndromes in which no organic disease is evident, despite the
presence of abdominal symptoms. A number of comorbidities exist,
such as functional dyspepsia, functional constipation (FC),
gastroesophageal reflux disease (GERD) and irritable bowel syndrome
(IBS), with a multi-national study using an internet survey
suggesting an extremely high incidence of 40.3% (1). FC is one of the most common digestive
disorders, and its incidence seems to increase with advancing age
(2). In Japan, an internet survey
reported that 28.4% of respondents considered themselves to
commonly be constipated (3).
Chronic constipation greatly impairs the quality of life (QOL) of
patients (4). Tanabe et al
(5) reported that the QOL of
patients was reduced in the constipation group, with the Bristol
Stool Form Scale (BSFS) score being significantly lower than that
of the control group, indicating harder stools in the former. In
addition, IBS is a chronic functional disease characterized by
abdominal pain, abnormal bowel movements and changes in stool
shape; its economic loss due to an impaired QOL cannot be ignored
(6). Therefore, measures with which
to combat FGIDs have become an important issue.
Bile acids are strongly associated with the
pathogenesis of FGIDs, and new mechanisms involving these have
recently been reported in IBS, chronic diarrhea, and FC (7). In recent years, inhibitors of bile
acid transporters (8,9) have been launched as new laxative
agents, and the effects of bile on the intestinal tract have
attracted considerable attention (10,11).
Blue laser imaging (BLI) has emerged as a novel
system for image-enhanced endoscopy using laser light; bile
presents with a reddish tone in narrow band imaging (12-14),
improving its visibility. Linked color imaging also enhances color
tone and improves visibility, although the contrast in the color
tone of bile from that of the background mucosa is not noticeable.
Thus, a difference in color tone is more apparent when BLI is used.
The association between duodenal bile area and abdominal symptoms,
fecal characteristics, and constipation remains unclear. Thus, the
present study investigated the factors that affect bile area in the
duodenal bulb using BLI.
Patients and methods
Study design
The present study was a retrospective
cross-sectional study conducted between April, 2017 and December,
2019 at a single-center university hospital (Juntendo Tokyo Koto
Geriatric Medical Center, Tokyo, Japan) to explore the association
between bile area in the duodenal bulb and abdominal symptoms. An
EG-L590WR, EG-L600WR7 or EG-L600ZW7 (FUJIFILM Wako Pure Chemical
Corporation) endoscope system, AdvanciaHD VP-4450HD or LASEREO7000
VP-7000 (FUJIFILM Wako Pure Chemical Corporation; Structure
Emphasis: B6, Color Emphasis: C1) video processor, and LASEREO
LL-4450 or LASEREO7000 LL-7000 (FUJIFILM Wako Pure Chemical
Corporation) light source were used. Patients fasted for at least
12 h prior to the esophagogastroduodenoscopy (EGD), which was
performed in the morning. Pre-treatment consisted of the oral
administration of 80 ml of 2% dimethicone solution, diluted
two-fold with water as an antifoam agent to remove mucus, and
pharyngeal anesthesia with an 8% lidocaine pump spray. After
examining the esophagus, the endoscope was inserted into the
stomach; the duodenum was examined prior to examining the stomach.
When the scope was inserted into the duodenum, a reddish tinted
area of the bulb was defined as bile by BLI observation without a
suction operation. Images were recorded close to duodenal bulb air
insufflation. In addition, patients were excluded if bubbles, mucus
and halation were not clearly observed in the duodenal bulb. For
the quantitative analysis of bile, each BLI bile score was
calculated as the percentage of bile area in a field of view of the
duodenal bulb using a KS400 image analysis system (Carl Zeiss
Imaging Solutions GmbH). The association with each factor,
including fecal characteristics, and abdominal and constipation
symptoms, was retrospectively examined using multiple regression
analysis. Patients provided written informed consent before
undergoing the EGD.
Inclusion criteria
Patients were included if all of the following
information was available from their medical records: i) Patient
characteristics [sex, age, body mass index (BMI)]; ii)
Helicobacter pylori (H. pylori) infection status
(negative, positive, or negative after eradication); iii) treatment
with a proton pump inhibitor (PPI)/potassium competitive acid
blocker (PCAB), ursodeoxycholic acid (UDCA), aspirin,
laxative/exercise promotion (prokinetics); iv) a history of
cholecystectomy and gallbladder (GB) stones; v) EGD results
[Barrett's esophagus and endoscopic gastric mucosal atrophy score
(EGAS), reflux esophagitis (RE)]; vi) constipation questionnaire
[constipation scoring system (CSS)]; vii) stool shape questionnaire
BSFS); viii) upper abdominal symptom questionnaire [frequency scale
for symptoms of GERD (FSSG)].
Exclusion criteria
Patients with a history of acute cerebrovascular,
gastrointestinal, renal, coronary, hepatic, or respiratory events
were excluded from the study. Patients were excluded if they were
found to have the following conditions: A history of
gastrointestinal surgery, inflammatory bowel disease, advanced
gastrointestinal cancer, erosive duodenitis, active gastric or
duodenal ulcer, deformity due to duodenal scars, malignant
lymphoma, leukemia, multiple myeloma, or mental illness. Patients
who did not have a colonoscopy and who were taking laxatives were
excluded from the study.
Assessments
The BMI was calculated by dividing body weight by
body height in m2 (kg/m2). A positive result
in a 13C-urea breath test and/or the presence of specific serum
antibodies was defined as positive for H. pylori infection.
A negative result for H. pylori infection 4 to 8 weeks after
the end of eradication therapy was defined as being successful.
Daily use of any of the five types of PPIs/PCABs (rabeprazole,
lansoprazole, omeprazole, esomeprazole or vonoprazan) for >8
weeks was regarded as indicating a PPI/PCAB user. Patients taking a
normal dose of aspirin or a PPI/PCAB were regarded as users of such
therapies.
EGD findings
With regard to the EGD results, patients were
identified as having RE of grade A, B, C, or D using the partially
revised Los Angeles (LA) classification system (15). Non-erosive reflux esophagitis was
classified based on a modified LA classification system (16). As regards Barrett's esophagus,
Ultrashort-segment Barrett's esophagus (USBE) was defined as the
maximum length of the cylindrical epithelium <1 cm. Long-segment
Barrett's esophagus (LSBE) was defined as a circumferential
cylindrical epithelium >3 cm in length. Short-segment Barrett's
esophagus (SSBE) was defined as Barrett's esophagus of intermediate
length between USBE and LSBE according to the Prague C&M
criteria (17). The Kimura-Takemoto
classification system was used to classify endoscopic gastric
mucosal atrophy as C-0 (normal), C-1, C-2, C-3, O-1, O-2 or
O-3(18), in line with the location
of the endoscopic atrophic border. An EGAS value was assigned to
each patient depending on atrophy: 0=C-0 type, 1=C-1 type, 2=C-2
type, 3=C-3 type, 4=O-1 type, 5=O-2 type or 6=O-3 type. The mean
EGAS in each group was calculated.
Questionnaire about constipation
severity
The CSS questionnaire was self-administered and
evaluated the severity of constipation. This had previously been
validated for evaluating constipation in a clinical trial setting
(19). The CSS questionnaire is
comprised of eight items outlining the symptoms of constipation as
follows: Painful evacuation, frequency of bowel movements,
abdominal pain, incomplete evacuation, assistance with evacuation,
length of time per attempt, duration of constipation and
unsuccessful attempts at evacuation per 24 h. Each item was scored
between and 0 and 4 apart from ‘assistance for evacuation’, for
which the score was from 0 to 2. The overall score for the CSS
questionnaire was between 0 and 30, with the higher the score the
worse the constipation symptoms.
Questionnaire about stool shape
A BSFS (20) was
used to assess and classify stool shape and consistency into seven
categories as follows: i) Separate hard lumps similar to nuts; ii)
sausage-shaped but lumpy; iii) similar to a sausage or snake but
with cracks on the surface; iv) similar to a sausage or snake,
smooth and soft; v) soft blobs with clear cut edges; vi) fluffy
pieces with ragged edges, a mushy stool; and vii) watery, no solid
pieces.
Ethics
The present study was approved by the Juntendo Tokyo
Koto Geriatric Medical Center Ethics Committee (protocol no. 106-8)
and was performed according to the tenets of the Declaration of
Helsinki. The Juntendo Tokyo Koto Geriatric Medical Center Ethics
Committee determined that the present study was exempt from the
need to obtain informed consent from patients. Also, in accordance
with the same ethics committee, information on the study for
patients was available on our hospital's homepage and patients were
guaranteed the opportunity to change their mind about
participating.
Statistical analyses
Correlations between the BLI bile score, assuming
bile area value, and various clinical parameters (sex, age, BMI,
reflux esophagitis, Barrett's esophagus, atrophic gastritis, H.
pylori infection status, PPI/PCAB use, aspirin use, UDCA use, a
history of GB stones, a history of cholecystectomy, and CSS BSFS,
and FSSG scores) were determined using Spearman's correlation
coefficient. Data for age, BMI, and CSS, BSFS and FSSG scores are
presented as the mean ± standard deviation. For multiple regression
analysis, the BLI bile score was used as the dependent variable,
and age, sex, BMI, reflux esophagitis, Barrett's esophagus,
atrophic gastritis, H. pylori infection status, PPI/PCAB
use, aspirin use, UDCA use, a history of GB stones and a history of
cholecystectomy were deemed independent variables. Since the BLI
score did not exhibit a normal distribution (as shown by the
Kolmogorov-Smirnov test), the score was analyzed by adding 1 to the
score during correlation and multiple regression analyses, followed
by logarithmic transformation. Multiple regression analyses of risk
factors for the BLI bile score were performed by a stepwise method
and multicollinearity was determined by a variance inflation factor
of 10 or greater. All statistical analyses were performed using
SPSS for Windows, version 28.0 (IBM Corp.). P<0.05 was
considered to indicate a statistically significant difference.
Results
Clinical characteristics of the
patients in the study
A flow chart of the study participants is presented
in Fig. 1. The clinical
characteristics of the study participants are listed in Table I. The mean age of the participants
was 69.9 years (range, 27-91 years). Of the 356 participants, 146
were male and 210 were female; the mean BMI was 23.0. Reflux
esophagitis was found in 47.2% of the participants, according to
the Prague C&M criteria (17),
as follows: USBE (maximum extent <1 cm), 11.8%; SSBE (maximum
extent ≥1 cm, circumferential extent <3 cm), 6.2% were
predominantly present; and LSBE (circumferential extent ≥3 cm) was
absent. In total, 79 participants were found to have H.
pylori infection, and 212 patients negative for this infection
and 65 post-eradication. Atrophic gastritis was present in 224
patients (closed, 100; open, 124) and absent in 132 patients. Of
these, 105 patients were taking a PPI or PCAB. As for other oral
medications, aspirin was used by 27 patients and UDCA was used by
18 patients. A total of 43 patients had GB stones and 18 patients
had undergone a cholecystectomy.
 | Table IClinical characteristics of the
patients in the present study (n=356). |
Table I
Clinical characteristics of the
patients in the present study (n=356).
| Characteristic | Value |
|---|
| Age in years, mean
± SD (range) | 69.9±11.3
(27-91) |
| Sex
(male:female) | 146:210 |
| BMI
(kg/m2) | 23.0±3.8 |
| Reflux
esophagitis | None, n=188; grade
M, n=143; grade A, n=19; grade B, n=3; grade C, n=3; grade D,
n=0 |
| Barrett's
esophagus | None, n=292; USBE,
n=42; SSBE, n=22; LSBE, n=0 |
| H.
pylori | Negative, n=212;
positive, n=79; post-eradication, n=65 |
| Atrophic
gastritis | C-0, n=132; C-1-3,
n=100; O-1-3, n=124 |
| PPI/PCAB | Non-users, n=251;
users, n=105 |
| Aspirin | Non-users, n=329;
users, n=27 |
| UDCA | Non-users, n=338;
users, n=18 |
| GB stones | None, n=313;
present, n=43 |
|
Cholecystectomy | None, n=338;
performed, n=18 |
BLI bile score and symptom
questionnaires
The BLI bile score and the results of the symptom
questionnaires are summarized in Table
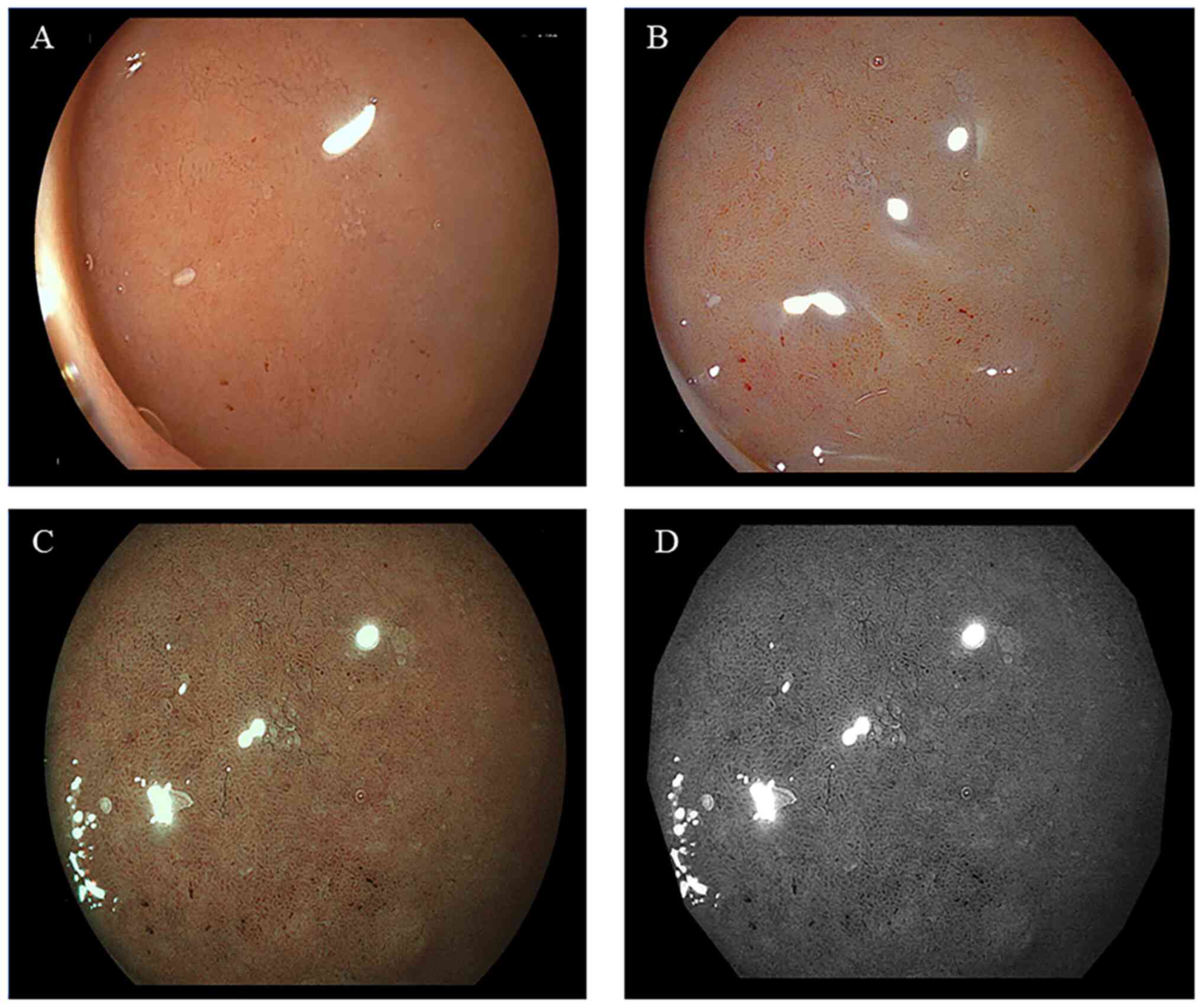
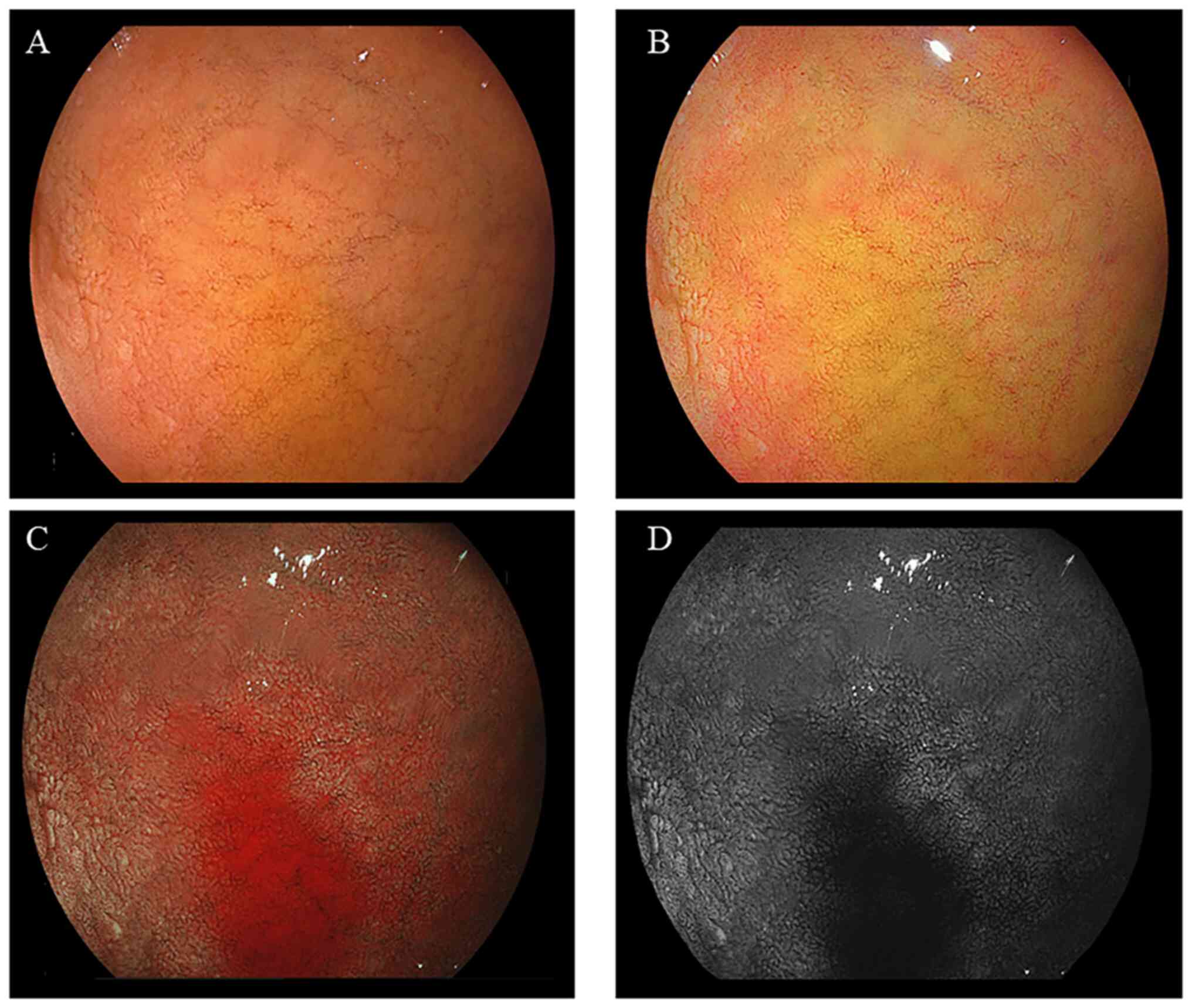
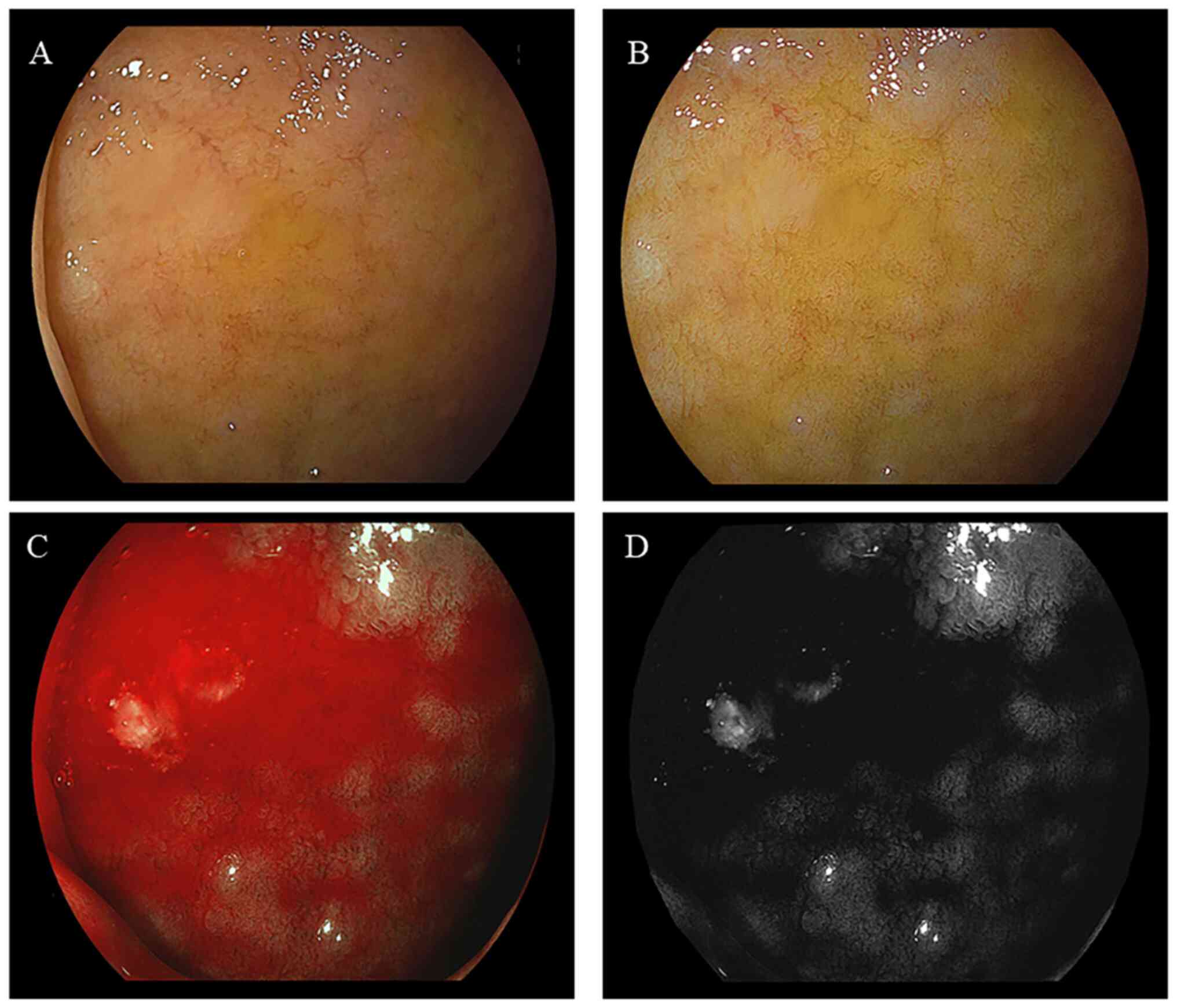
II. Representative endoscopic findings of the duodenal mucosa
of bulbs, without or with bile, are illustrated in Figs. 2 and 3, respectively. The imaging results
illustrating a large area of bile present in the duodenal bulb are
presented in Fig. 4. The mean
values of the BLI bile score and each symptom questionnaire score
were as follows: BLI bile score, 7.10 (±14.34); CSS, 3.55 (±3.80);
BSFS, 3.91 (±1.02); and FSSG, 4.80 (±5.76).
 | Table IIBLI bile score and symptom
questionnaire scores of study patients (n=356). |
Table II
BLI bile score and symptom
questionnaire scores of study patients (n=356).
|
Characteristics | Value |
|---|
| BLI bile score | 7.10±14.34
(0-85.3) |
| CSS | 3.55±3.80
(0-22) |
| BSFS | 3.91±1.02
(1-7) |
| FSSG | 4.80±5.76
(0-37) |
BLI bile score and correlation with
clinical parameters
The results of Spearman's correlation coefficients
are presented in Table III. For
the BLI bile score, statistically significant correlation
coefficients (P<0.05) were found for cholecystectomy (Rho=0.137,
P=0.010) and aspirin users (Rho=0.118, P=0.026).
 | Table IIICorrelation between the BLI bile
score and various clinical parameters. |
Table III
Correlation between the BLI bile
score and various clinical parameters.
| Clinical
parameters | Rho | P-value |
|---|
| Age | 0.094 | 0.075 |
| Sex | 0.000 | 0.999 |
| BMI | -0.010 | 0.848 |
| Reflux
esophagitis | 0.015 | 0.773 |
| Barrett's
esophagus | -0.059 | 0.268 |
| H.
pylori | 0.038 | 0.478 |
| Atrophic
gastritis | 0.014 | 0.786 |
| PPI/PCAB | 0.091 | 0.087 |
| Aspirin | 0.118 | 0.026 |
| UDCA | 0.003 | 0.949 |
| GB stones | 0.063 | 0.237 |
|
Cholecystectomy | 0.137 | 0.010 |
| CSS | -0.030 | 0.570 |
| BSFS | 0.029 | 0.589 |
| FSSG | -0.017 | 0.749 |
Multiple regression analysis
The results of multiple regression analysis results
are presented in Table IV. In
multiple regression analysis, statistically significant independent
predictors for the BLI bile score were cholecystectomy
[standardized partial regression coefficient (β)=0.169, P=0.001]
and the BSFS score (β=0.107, P=0.042).
 | Table IVAssociation between the BLI bile
score and other variables in multiple regression analysis. |
Table IV
Association between the BLI bile
score and other variables in multiple regression analysis.
| Variables | B | SE | 95% CI of B | β | t | VIF | P-value |
|---|
|
Cholecystectomy | 0.974 | 0.300 | 0.383, 1.565 | 0.169 | 3.241 | 1.001 | 0.001 |
| BSFS | 0.132 | 0.064 | 0.005, 0.258 | 0.107 | 2.045 | 1.001 | 0.042 |
Discussion
To the best of our knowledge, no previous study has
yet examined the association between duodenal bile area, fecal
characteristics and constipation symptoms. The present study
focused on the bile area in the duodenum (bile area was analyzed as
a BLI bile score) using EGD and examined its association with
background factors, abdominal symptom scores and fecal
characteristic scores. If was found that independent predictors of
the BLI bile score were cholecystectomy and a high BSFS score. To
the best of our knowledge, the present study is the first to
quantitatively analyze the bile area in the duodenal bulb using EGD
and to demonstrate a positive association with fecal
characteristics (soft) and after a cholecystectomy.
Bile has long been described as a factor affecting
fecal characteristics and constipation symptoms. Bile acids, the
main component of bile, are biosynthesized from cholesterol in the
liver. The majority of these are reabsorbed from the intestinal
tract and reused by the liver (21,22).
The main roles of bile acids include the regulation of cholesterol
in the body, and the digestion and absorption of lipids in the
small intestine (23). In addition,
bile acids that are not reabsorbed flow into the large intestine to
promote gastrointestinal motility and water secretion in the lumen
of the large intestine. Bile acids also increase sensitivity to
rectal-stretching stimuli, which are said to promote bowel
movements (24-26).
Bile acid transporter inhibitors are drugs that utilize these
effects (8,9). Furthermore, in individuals with IBS
and constipation, bile acid synthesis is decreased on an empty
stomach compared to healthy individuals (27). The administration of
chenodeoxycholic acid increases the frequency of defecation and the
BSFS score (28). However, a high
level of bile acids is a contributing factor to diarrhea, which is
predominant in IBS (29). If the
amount of bile acids flowing into the large intestine is
physiologically high, this may have an effect on fecal
characteristics and constipation symptoms. This may be related to
the results of the significantly higher value for the BSFS score in
patients demonstrating a large area of bile (high BLI bile score)
in the present study.
In addition to fecal characteristics, in the present
study, a significant difference was also found with a history of
cholecystectomy as a factor affecting bile area. A previous study
reported an increased incidence of diarrhea following
cholecystectomy (30).
Post-cholecystecpctomy diarrhea (PCD) (31) is part of post-cholecystectomy
syndrome (PCS), which is difficult to treat. It has been shown that
~12 to 35.6% of patients with PCS suffer from chronic diarrhea to
varying degrees (32-36).
The occurrence of PCD is considered to be related to changes in the
bile flowing into the intestine following a cholecystectomy
(37), and bile acid malabsorption
is considered to be a contributing factor (38). It has also been suggested that in
patients with dyspepsia who have undergone cholecystectomy, a
marked increase occurs in duodenogastric bile reflux on an empty
stomach and continued bile excretion from the common bile duct
(39), inferring that these
mechanisms increased bile volume into the duodenal bulb in such
patients. Although Barrett's esophagus and upper abdominal symptoms
were not associated with bile area in the present study, a causal
association is unclear as bile reflux into the stomach was not
assessed. No association between the BLI bile score and FSSG score
has been found; however, chronic reflux of bile into the stomach
and esophagus may induce symptoms (40-42);
thus, further studies, such as the analysis of bile volume in the
stomach and esophageal pH monitoring tests, are considered
necessary.
While reports exist on the association between bile
and fecal characteristics, and constipation and diarrhea symptoms
after cholecystectomy, the application of endoscopy to the
prediction and diagnosis of these remains undescribed. The findings
of the present study suggest that bile area in the duodenal bulb
reflects the pathophysiology of diarrhea and its observation in EGD
is expected to lead to the development of a novel approach to the
diagnosis and treatment of diarrhea.
However, the present study has several limitations.
First, as the study was retrospective and had a single-center,
hospital-based design, a causal association between bile area, and
constipation and fecal characteristics could not be established.
Second, the authors were not able to collect bile and analyze bile
acids, bile volume, the amount of gastric juice and their various
other components. In addition, the present study did not confirm
the reproducibility of the BLI bile score in the same patients. The
evaluation was performed only in the duodenal bulb and not in the
descending part or deeper into the duodenum due to the difficulty
in quantitatively analyzing the bile volume in these areas; the
evaluation was performed only in the flat duodenal bulb.
Additionally, the results of the present study should only be
considered preliminary, since a relatively small cohort was used
and the backgrounds of the participants were not extensively
investigated, including smoking history, alcohol consumption, diet,
exercise habits, work, marital status, education and the use of
medications apart from PPIs, laxatives, aspirin and prokinetics.
Therefore, the data obtained herein may not be generalizable to
everyone in a population.
In conclusion, the present study, which was a
hospital-based, cross-sectional study, found a positive association
between bile area in the duodenal bulb and fecal characteristics.
In the future, the examination and diagnosis of fecal
characteristics may be aided by EGD. Such associations and their
biological mechanisms require further investigation.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DAb, TT, DAs and AN designed the study. DAb, TT and
DAs, the endoscopists in the present study, collected the data and
performed the analyses. TI, RU, HUt, SO, NS, AI, NY, YA, KM, KU,
Hue, MH and AN also collected the data and performed the analyses.
DAb, TT, DAs and AN drafted the manuscript. YK, SNa, DAb and TT
analyzed the imaging data. SNo assisted with the statistical
analyses. All authors have read and approved the final manuscript.
DAb and TT confirmed the authenticity of all the raw data.
Ethics approval and consent to
participate
The present study was performed according to the
Declaration of Helsinki. The Juntendo Tokyo Koto Geriatric Medical
Center Ethics Committee approved the study and protocol (protocol
no. 106-8). The Juntendo Tokyo Koto Geriatric Medical Center Ethics
Committee determined that this study was exempt from the need to
obtain informed consent from patients. Study participants were
provided with information about the study on the homepage of the
hospital, as well as the opportunity to opt out of the study in
accordance with directions by the same ethics committee.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sperber AD, Bangdiwala SI, Drossman DA,
Ghoshal UC, Simren M, Tack J, Whitehead WE, Dumitrascu DL, Fang X,
Fukudo S, et al: Worldwide prevalence and burden of functional
gastrointestinal disorders, results of rome foundation global
study. Gastroenterology. 160:99–114.e3. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Higgins PD and Johanson JF: Epidemiology
of constipation in North America: A systematic review. Am J
Gastroenterol. 99:750–759. 2004.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Tamura A, Tomita T, Oshima T, Toyoshima F,
Yamasaki T, Okugawa T, Kondo T, Kono T, Tozawa K, Ikehara H, et al:
Prevalence and self-recognition of chronic constipation: Results of
an internet survey. J Neurogastroenterol Motil. 22:677–685.
2016.PubMed/NCBI View
Article : Google Scholar
|
|
4
|
Wald A, Scarpignato C, Kamm MA,
Mueller-Lissner S, Helfrich I, Schuijt C, Bubeck J, Limoni C and
Petrini O: The burden of constipation on quality of life: Results
of a multinational survey. Aliment Pharmacol Ther. 26:227–236.
2007.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Tanabe A, Adachi K, Yamaguchi Y, Izawa S,
Yamamoto S, Hijikata Y, Ebi M, Funaki Y, Ogasawara N, Goto C, et
al: Gut environment and dietary habits in healthy Japanese adults
and their association with bowel movement. Digestion. 101:706–716.
2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Canavan C, West J and Card T: Review
article: The economic impact of the irritable bowel syndrome.
Aliment Pharmacol Ther. 40:1023–1034. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Appleby RN and Walters JR: The role of
bile acids in functional GI disorders. Neurogastroenterol Motil.
26:1057–1069. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Nakajima A, Seki M and Taniguchi S:
Determining an optimal clinical dose of elobixibat, a novel
inhibitor of the ileal bile acid transporter, in Japanese patients
with chronic constipation: A phase II, multicenter, double-blind,
placebo-controlled randomized clinical trial. J Gastroenterol.
53:525–534. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Nakajima A, Seki M, Taniguchi S, Ohta A,
Gillberg PG, Mattsson JP and Camilleri M: Safety and efficacy of
elobixibat for chronic constipation: results from a randomised,
double-blind, placebo-controlled, phase-3 trial and an open-label,
single-arm, phase 3 trial. Lancet Gastroenterol Hepatol. 3:537–547.
2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Beeckmans D, Farré R, Riethorst D, Keita
ÅV, Augustijns P, Söderholm JD, Vanuytsel T, Vanheel H and Tack J:
Relationship between bile salts, bacterial translocation, and
duodenal mucosal integrity in functional dyspepsia.
Neurogastroenterol Motil. 32(e13788)2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wauters L, Ceulemans M, Lambaerts M,
Accarie A, Toth J, Mols R, Augustijns P, Tack J and Vanuytsel T:
Association between duodenal bile salts and gastric emptying in
patients with functional dyspepsia. Gut. 70:2208–2210.
2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Choi HJ, Moon JH and Lee YN: . Advanced
imaging technology in biliary tract diseases:Narrow-band imaging of
the bile duct. Clin Endosc. 48:498–502. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Moon JH, Terheggen G, Choi HJ and Neuhaus
H: Peroral cholangioscopy: Diagnostic and therapeutic applications.
Gastroenterology. 144:276–282. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Itoi T, Sofuni A, Itokawa F, Tsuchiya T,
Kurihara T, Ishii K, Tsuji S, Moriyasu F and Gotoda T: Peroral
cholangioscopic diagnosis of biliary-tract diseases by using
narrow-band imaging (with videos). Gastrointest Endosc. 66:730–736.
2007.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Armstrong D, Bennett JR, Blum AL, Dent J,
De Dombal FT, Galmiche JP, Lundell L, Margulies M, Richter JE,
Spechler SJ, et al: The endoscopic assessment of esophagitis: A
progress report on observer agreement. Gastroenterology. 111:85–92.
1996.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Hongo M: Minimal changes in reflux
esophagitis: Red ones and white ones. J Gastroenterol. 41:95–99.
2006.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Sharma P, Dent J, Armstrong D, Bergman JJ,
Gossner L, Hoshihara Y, Jankowski JA, Junghard O, Lundell L, Tytgat
GN and Vieth M: The development and validation of an endoscopic
grading system for Barrett's esophagus: the Prague C & M
criteria. Gastroenterology. 131:1392–1399. 2006.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kimura K and Takemoto T: An endoscopic
recognition of the atrophic border and its significance in chronic
gastritis. Endoscopy. 1:87–97. 1969.
|
|
19
|
Agachan F, Chen T, Pfeifer J, Reissman P
and Wexner SD: A constipation scoring system to simplify evaluation
and management of constipated patients. Dis Colon Rectum.
39:681–685. 1996.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lewis SJ and Heaton KW: Stool form scale
as a useful guide to intestinal transit time. Scand J
Gastroenterol. 32:920–924. 1997.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Chiang JY: Bile acid metabolism and
signaling. Compr Physiol. 3:1191–1212. 2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Chiang JYL and Ferrell JM: Bile acid
metabolism in liver pathobiology. Gene Expr. 18:71–87.
2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Di Ciaula A, Garruti G, Lunardi Baccetto
R, Molina-Molina E, Bonfrate L, Wang DQ and Portincasa P: Bile acid
physiology. Ann Hepatol. 16 (Suppl 1: S3-S105):S4–S14.
2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Bampton PA, Dinning PG, Kennedy ML,
Lubowski DZ and Cook IJ: The proximal colonic motor response to
rectal mechanical and chemical stimulation. Am J Physiol
Gastrointest Liver Physiol. 282:G443–G449. 2002.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Hofmann AF: The continuing importance of
bile acids in liver and intestinal disease. Arch Intern Med.
159:2647–2658. 1999.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Edwards CA, Brown S, Baxter AJ, Bannister
JJ and Read NW: Effect of bile acid on anorectal function in man.
Gut. 30:383–386. 1989.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Shin A, Camilleri M, Vijayvargiya P,
Busciglio I, Burton D, Ryks M, Rhoten D, Lueke A, Saenger A,
Girtman A and Zinsmeister AR: Bowel functions, fecal unconjugated
primary and secondary bile acids, and colonic transit in patients
with irritable bowel syndrome. Clin Gastroenterol Hepatol.
11:1270–1275.e1. 2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Rao AS, Wong BS, Camilleri M,
Odunsi-Shiyanbade ST, McKinzie S, Ryks M, Burton D, Carlson P,
Lamsam J, Singh R and Zinsmeister AR: Gastroenterology.
139:1549–1558. 2010.
|
|
29
|
Camilleri M: Bile acid diarrhea:
Prevalence, pathogenesis, and therapy. Gut Liver. 9:332–339.
2015.PubMed/NCBI View
Article : Google Scholar
|
|
30
|
Yueh TP, Chen FY, Lin TE and Chuang MT:
Diarrhea after laparoscopic cholecystectomy: Associated factors and
predictors. Asian J Surg. 37:171–177. 2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Hutcheon DF, Bayless TM and Gadacz TR:
Postcholecystectomy diarrhea. JAMA. 241:823–824. 1979.PubMed/NCBI
|
|
32
|
Fisher M, Spilias DC and Tong LK:
Diarrhoea after laparoscopic cholecystectomy: Incidence and main
determinants. ANZ J Surg. 78:482–486. 2008.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Middelfart HV, Kristensen JU, Laursen CN,
Qvist N, Højgaard L, Funch-Jensen P and Kehlet H: Pain and
dyspepsia after elective and acute cholecystectomy. Scand J
Gastroenterol. 33:10–14. 1998.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Lublin M, Crawford DL, Hiatt JR and
Phillips EH: Symptoms before and after laparoscopic cholecystectomy
for gallstones. Am Surg. 70:863–866. 2004.PubMed/NCBI
|
|
35
|
Weinert CR, Arnett D, Jacobs D Jr and Kane
RL: Relationship between persistence of abdominal symptoms and
successful outcome after cholecystectomy. Arch Intern Med.
160:989–995. 2000.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Niranjan B, Chumber S and Kriplani AK:
Symptomatic outcome after laparoscopic cholecystectomy. Trop
Gastroenterol. 21:144–148. 2000.PubMed/NCBI
|
|
37
|
Lamberts MP, Lugtenberg M, Rovers MM,
Roukema AJ, Drenth JP, Westert GP and van Laarhoven CJ: Persistent
and de novo symptoms after cholecystectomy: A systematic review of
cholecystectomy effectiveness. Surg Endosc. 27:709–718.
2013.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Sciarretta G, Furno A, Mazzoni M and
Malaguti P: Post-cholecystectomy diarrhea: evidence of bile acid
malabsorption assessed by SeHCAT test. Am J Gastroenterol.
87:1852–1854. 1992.PubMed/NCBI
|
|
39
|
Mearin F, De Ribot X, Balboa A, Antolín M,
Varas MJ and Malagelada JR: Duodenogastric bile reflux and
gastrointestinal motility in pathogenesis of functional dyspepsia.
Role of cholecystectomy. Dig Dis Sci. 40:1703–1709. 1995.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Monaco L, Brillantino A, Torelli F,
Schettino M, Izzo G, Cosenza A and Di Martino N: Prevalence of bile
reflux in gastroesophageal reflux disease patients not responsive
to proton pump inhibitors. World J Gastroenterol. 15:334–338.
2009.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Bollschweiler E, Wolfgarten E, Pütz B,
Gutschow C and Hölscher AH: Bile reflux into the stomach and the
esophagus for volunteers older than 40 years. Digestion. 71:65–71.
2005.PubMed/NCBI View Article : Google Scholar
|
|
42
|
McCabe ME IV and Dilly CK: New causes for
the old problem of bile reflux gastritis. Clin Gastroenterol
Hepatol. 16:1389–1392. 2018.PubMed/NCBI View Article : Google Scholar
|