Introduction
Oxidative stress is involved in the pathogenesis of
numerous diseases. For this reason, various methods have been
developed to assess it, methods that are useful in disease
research, highlighting either the degree of damage (oxidation in
DNA, proteins, lipids) or the ability of the body to self-regulate
and also self-defense (through feedback mechanisms, antibodies,
etc.) (1,2).
Studies (1-3)
have revealed that oxidative stress plays an important role in
multiple heart conditions, including arrhythmias. Rhythm disorders
in individuals with no pathological history are associated with the
presence of systemic and cardiac oxidative stress, caused by the
existence of reactive oxygen species (ROS) (1). Systematically, literature (4-6)
has revealed high values of oxidative stress markers in individuals
with atrial fibrillation and extrasystolic arrhythmias.
The frequency of cardiac dysrhythmias in the general
population is high and occasionally, their etiology remains
difficult to fully understand. Arrhythmias, in non-structural heart
disease, regarded as heterogeneous pathology, worldwidely, affect
0.8-1,4% of the general population, being three times more common
in females than in males. The prevalence among the general
population is 1.17/1,000, with an incidence of 25/100,000 (2,3).
Atrial fibrillation affects approximately 1-2% of the general
population (3). Paroxysmal
supraventricular tachycardia, common in young individuals, has a
prevalence of 5-10% (35-55 years) (3,4). Thus,
having a significant prevalence among the young population, it can
be concluded that the presence of oxidative stress leads to serious
health alterations (4).
Peroxidation of the lipid membrane under the action
of hydroxyl radical has the effect of disrupting the calcium
transport within the endoplasmic reticulum, decreasing its uptake
and increasing its level in myocytes during dystole (4,5),
leading to dysrhythmias.
The harmful effect is counteracted by the existence
of antioxidant systems, represented by molecules capable of
neutralizing ROS, thus preventing the induction of oxidation damage
(2,4). A high value of oxidative stress
(determined by measuring its specific parameters), as well as a
decreased total antioxidant capacity are negative prognostic
factors in initiating pathological processes in the cardiovascular
system (2).
To determine the activity and at the same time the
cellular level of oxidative stress, it is necessary to assess
specific biomarkers, namely: Superoxide dismutase (SOD),
glutathione peroxidase (GPx), total antioxidant capacity,
malondialdehyde, low-density lipoprotein (LDL)-oxidized, and
anti-oxidized LDL antibodies. Their level is a predictive and
prognostic factor in terms of the reactivity of the body to the
agression of oxidative stress (1,2,5).
GPx represents an enzyme that possesses antioxidant
activity, having a role, at the cellular level, in reducing
oxidative stress. It catalyzes the reduction of organic
hydroperoxides, using glutathione (GSH) (1,2),
participating in the transformation ROS into compounds such as
oxygen and water. The excess of ROS leads to an increased oxidative
stress level, involved in various cardiovascular pathologies
(2,3).
Cardiac arrhythmias are frequently described, even
in young individuals; the involved factors of this etiopathogeny
are usually difficult to assess and treat (4-12).
The novelty of the present research consists in the
evaluation, through a measurable biomarker, GPx, of the presence of
oxidative stress in young subjects, as well as its involvement in
triggering non-structural arrhythmias, without any other proven
etiology.
The observed GPx deficiency indicates, through the
expressed oxidative stress in young individuals, the possibility of
favoring cardiac arrhythmogenic mechanisms. The demonstration of
oxidation, through the GPx biomarker, suggests the possibility of
developing an endothelial dysfunction, by early lipid oxidation,
regardless of the existence or not of a dyslipidemic status. GPx,
allows the qualitative assessment of oxidative stress as well as
the quantitative determination and grading of the oxidative status.
The demonstration of this enzyme decrease in relation to
non-structural cardiac arrhythmias and without other explicit
pathogenic mechanisms, at ayoung age, allows an appropriate
correction (of oxidative stress). Monitoring GPx decrease, as a
parameter, also allows a therapeutic control, through antioxidant
treatment. The assessment of GPx and implicitly oxidative stress
can induce an increased need for antioxidants through lifestyle,
dietary and therapeutic management. The improvement of GPx
deficiency is thus important and novel in the monitoring and
treatment of cardiac arrhythmias in young subjects, as well as for
decreasing the degree of biological wear and tear (through
oxidative stress). Thus, the demonstration of GPx variation,
quantifiable in young individuals requires its correction, for the
prophylaxis of early cardiovascular pathology onset (8,9).
The aim of the present study was the assessment of
oxidative stress involvement in heart diseases, in relation to
antioxidant GPx enzyme and the lipid-metabolic status.
The present research assessed the variations of the
GPx enzyme, considered a biomarker of oxidative stress, in young
individuals with various cardiac arrhythmias. This allowed the
monitoring of the level of oxidative stress in non-structural
cardiac arrhythmic pathology and also provided therapeutic guidance
in relation to the use of antioxidants, in order to compensate for
the recorded deficit.
GPx has been demonstrated to catalyze the reduction
of organic hydroperoxides, using GSH (1,2),
participating in the transformation of ROS into compounds such as
oxygen and water. The excess of ROS was revealed to lead to an
increased oxidative stress level, involved in various
cardiovascular pathologies (2-12).
Patients and methods
Selection and ethical approval
The present research represents a prospective study,
conducted on 120 human subjects, aged between 18-45 years, during a
follow-up period of 4 years, divided into 3 equal groups, of 40
subjects each. Participating subjects were selected from January
2016 to January 2017 from the Cardiology and Emergency Units of the
Department of Internal Medicine of Emergency Hospital and County
Hospital Craiova (Craiova, Romania) and also from a private
practice (Ultracord Clinic, Craiova). Healthy volunteers consisted
of medical students, doctors and nurses. All the participating
subjects provided their written consent (for medical
investigations, blood samples, monitoring). The research was
conducted in compliance with the Academic Code of Ethics, with the
obtained approval (approval no. 56/19.02.2015) of the Scientific
Ethics and Deontology Comittee of the University of Medicine and
Pharmacy of Craiova and according to the Principles of the
Declaration of Helsinki. The data obtained from these different
clinics were processed within the University of Medicine and
Pharmacy of Craiova, within the Departments of Biochemistry,
Statistics and Informatics.
Patients
The inclusion criteria was as follows: Age 18-45
years, regardless of sex and economic and social background,
Caucasian race, the presence of heart rhythm disturbances in
non-structural heart disease, confirmed clinically and
investigatedby electrocardiogram, Holter-ECG device,
echocardiography and laboratory findings (presence/absence of
dyslipidemia).
Patients with cardiac arrhythmias were divided into
two groups, with and without dyslipidemia, for the homogeneity
regarding this variable parameter and for its importance as a risk
factor in cardiac pathology. In addition, this parameter was
recorded for the study of lipid profile, considering the
demonstrated role of oxidative stress, both in the induction of
cardiac arrhythmias and in lipid oxidation with subsequent
development of an immune response, with a pathogenic evolution
towards accelerated atherosclerotic endothelial dysfunction
(4).
The sex distribution of the patients was as follows:
Group I, 21 women (52.50%) and 19 men (47.50%); group II, 21
(52.50%) women and 19 men (47.50%), and group III, 23 (23%) women
and 17 men (17%) as presented in Table
I.
 | Table ISex distribution of patients with
cardiac arrythmias. |
Table I
Sex distribution of patients with
cardiac arrythmias.
| Sex distribution | Group I | Group II | Group III | No. of cases |
|---|
| Women | 21 (52.5%) | 21 (52.5%) | 23 (63%) | 65 |
| Men | 19 (47.50%) | 19 (47.50%) | 17 (37%) | 55 |
| Total | 40 (100%) | 40 (100%) | 40 (100%) | 120 |
As antropometric data, the mean height for group I
was 169.65±10.29 cm; for group II,170.40±8.14 cm; and for group
III,168.63±8.35 cm. The selected subjects had a mean weight, as
follows: Group I, 79.50±17.9 kg; group II, 71.33±12.91 kg; and
group III, 70.54±14.25 kg, as shown in Table II.
 | Table IIMean heights and weights of patients
with cardiac arrythmias. |
Table II
Mean heights and weights of patients
with cardiac arrythmias.
| Groups | Mean height (cm) | Standard
deviation | Mean weight
(kg) | Standard
deviation |
|---|
| Group I | 169.65 | 10.29 | 79.5 | 17.9 |
| Group II | 170.4 | 8.14 | 71.33 | 12.91 |
| Group III | 168.63 | 8.35 | 70.54 | 14.25 |
The exclusion criteria included the existence of
systemic or visceral pathology (acute/chronic), that may be
involved in cardiovascular manifestations and could alter the
oxidation processess; and previous medication intake. In addition,
the following exclusion criteria were applied: Young individuals
with previously diagnosed pathologies, generating arrhythmias or
oxidative stress including: i) pathologies that cause secondary
arrhythmias such as hyperthyroidism, myxedema, anemia, hypotension,
hypoglycemia, febrile syndrome, myocarditis, orpheochromocytoma;
ii) pathologies that increase oxidative stress such as diabetes or
other metabolic diseases (dyslipidemia, in case of group I and
III), atherosclerosis (diagnosed), hypertension, autoimmune
diseases, allergies, asthma, hematopoietic dysfunction, infectious
diseases, oncological pathology, ulcer gastro-duodenal, acute
pancreatitis and Parkinson's disease; iii) cardiovascular
pathologies such as heart failure, congenital diseases and
cardiovascular malformations, as well as pathologies with genetic
determinism (Brugada syndrome, long QT, arrhythmogenic dysplasia of
the right ventricle), chronic ischemic heart disease, hypertension,
myocarditis, pericarditis, vascular disease, electric pacemaker
devices; iv) non-cardiac pathologies, with severe progression,
which can generate arrhythmias such as hemorrhages, various
neoplasms, hematological diseases, post-surgical conditions,
infectious diseases, neuropsychiatric disorders, gout,
hyperuricemia, renal insufficiency, hepatic, pulmonary failure and
multiorgan failure. Other miscellaneous conditions were also
excluded, including excessive consumption of grapefruit, as well as
possible interactions withdrug intake (antiarrhythmics,
sympathomimetics, parasympatholytics, enzyme inducers, enzyme
inhibitors, and antioxidants). The exclusion criteria in the
control group were the same as those in the patients of the control
groups (group I and II), to which the absence of cardiac
arrhythmias and dyslipidemia was added.
For the control group which consisted of healthy
individuals, cardiovascular manifestations and dyslipidemic status
were excluded by clinical, paraclinical and biologic
investigations.
Group I and II included patients who were diagnosed
and monitored for non-structural cardiac arrhythmias within the
profiled clinics, in the emergency units and also in the
specialized private clinics. The difference between group I and II
was established by the presence or absence of dyslipidemic status,
as a risk factor, associated with cardiac pathology. Thus, group I
included patients with cardiac arrhythmias and dyslipidemia, group
II included patients with cardiac arrhythmia disorders but without
dyslipidemia, and group III consisted of healthy subjects.
Methods
Subjects were evaluated clinically and
paraclinically for evidence of cardiac arrhythmic disorders, for
dyslipidemic status, and in particular, GPx values were assessed.
On all subjects, the following tests were performed: ECG,
Holter-ECG monitoring, echocardiography and general ultrasound,
cardiology consultation, usual biochemistry analysis, lipidogram
and oxidative stress testing, by determining GPx values. In all
subjects, in addition to the usual tests (blood glucose, complete
blood count, creatinine, aspartate and alanine transaminases),
lipidograms were assessed, in order to determine the
lipid-metabolic status.
GPx enzyme determination method. The activity
of the antioxidant enzyme GPx was determined by standard protocols,
in blood samples (2 ml each), collected from all subjects, after
the onset of arrhythmia (for groups I and II), by venipuncture (in
K2EDTA vacutainers), using a standardized Ransel
Laboratory kit (RandoxLaboratories, Ltd.). Hemolysates were used as
blood biological samples. The values were assessed using a Beckman
DU-65, UV-VIS type, spectrophotometer, at the Department of
Biochemistry of the University of Medicine and Pharmacy of
Craiova.
According to the substrate used for the oxidation of
GSH, both forms of GPx (seleno-dependent and non seleno-dependent)
can react. In blood, the erythocytes contain only the
seleno-dependent isoenzymes, while plasma contains 80% of the
seleno-dependent form and 20% of the non seleno-dependent form.
GPx1 is the predominant isoform and is expressed in the heart. In
the present research, GPx1, as the most widely distributed and
abundant in human cells (3,9,11,12),
was studied.
The principle of the method describes the catalysis
by the GPx enzyme of the oxidation reaction of reduced GSH by cumin
hydroperoxide. The resulting oxidized GSH is rapidly converted to
its reduced form, triggering the oxidation of NADPH to the
NADP+ form, in the presence of glutathione reductase and
NADPH (3,9). The absorbance decrease is measured at
340 nm. The reference values are 27.5-73.6 U/gHb or 4,171-10,881
U/l. Mean GPx values were calculated for the studied groups,
compared and observed in association with cardiac arrhythmias, and
the lipidogram results (presence or absence of dyslipidemia).
Statistical analysis
The obtained data were recorded and processed
statistically at the Department of Statistics and Informatics of
the University of Medicine and Pharmacy of Craiova. The data was
then interpreted and discussed, included into specific databases,
where correlations and statistics were performed, in order to
obtain conclusions concerning the GPx enzyme as a biomarker of
oxidative stress and objective therapeutic indications for reducing
the level of oxidative stress and respectively cellular oxidation.
For these purposes, Microsoft Excel (Microsoft Corp.), XLSTAT 2014
for MS Excel (Addinsoft SARL) and IBM SPSS Statistics 20.0 (IBM
Corporation) programs were used, thus creating databases used in
the present research (3,9,11).
The most significant statistical parameters used
were: Mean and standard deviation, analysis of variance (ANOVA for
analyzing the dispersion of a numeric variable, under the influence
of a grouping variable) and Fisher LSD post hoc tests (to identify
the pairs of groups between which differences were found). Graphic
representation was achieved using the MS Excel program, with the
following functions: Pivot Tables, Functions, Statistical, Chart,
and Data. All the information were graphically represented using
the following statistical indicators: Mean, standard deviation,
using ANOVA and Fisher LSD post hoc tests. When the ANOVA test
presented a statistically significant result, it was further
analyzed using Fisher LSD post hoc test, in order to assess the
groups between which the statistically significant differences were
obtained (3,9,11).
Results
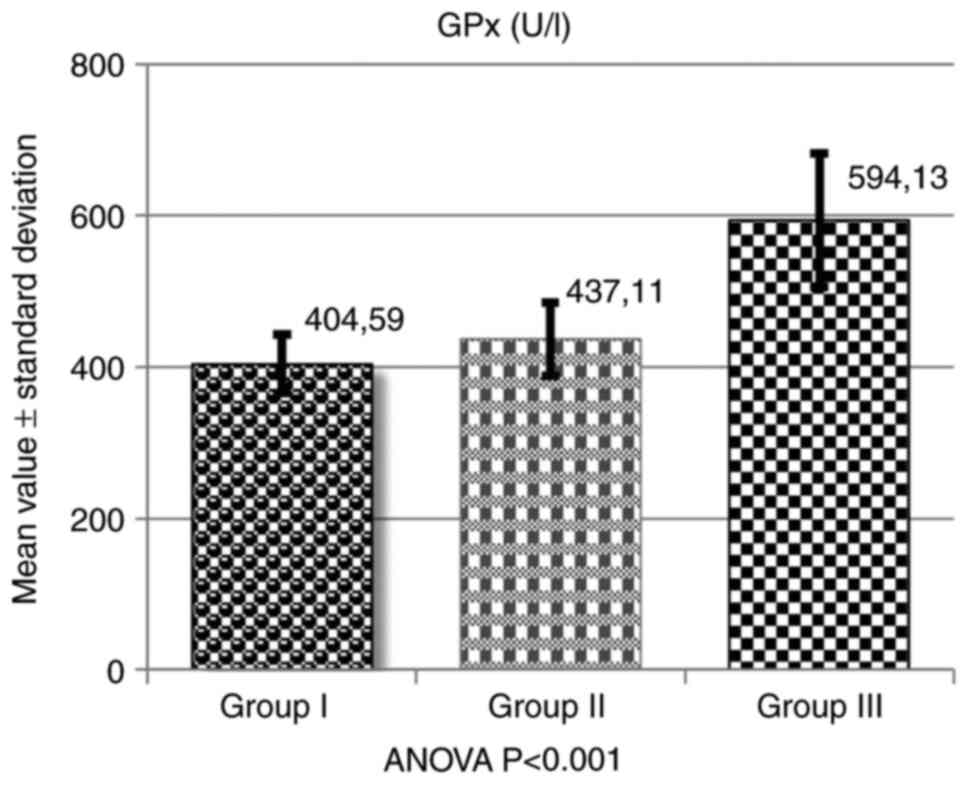
GPx assessment was conducted by calculating the mean
values obtained within the three groups. Thus, for group I
(patients with cardiac arrhythmias and dyslipidemia), the mean
value of GPx was 404.59±39.27 U/l-hemolyzed; for group II (patients
with cardiac arrhythmias without dyslipidemia), the mean value of
GPx was 437.11±48.58 U/l-hemolyzed; and for group III, healthy
controls (no arrhythmias, no dyslipidemia), the mean value was
594.13±88.07 U/l-hemolyzed (Table
III and Fig. 1).
 | Table IIIMean and percentage values of GPx
inpatients with cardiac arrythmias, compared with healthy
individuals. |
Table III
Mean and percentage values of GPx
inpatients with cardiac arrythmias, compared with healthy
individuals.
| Groups | No. of
subjects | Mean (mmol/l) | Standard
deviation | Percent (%) | Difference (%) |
|---|
| Group I | 40 | 404.59 | 39.27 | 68.10 | 31.90 |
| Group II | 40 | 437.11 | 48.58 | 73.57 | 26.43 |
| Group III | 40 | 594.13 | 88.07 | 100.00 | 0.00 |
The mean values of GPx were decreased in the two
groups (I and II), compared with the control (group III). For group
I, a GPx deficit of 31.90% was registered, the mean values being
decreased compared with the control group, at 68.10%. In group II,
the GPx deficit compared with the healthy individuals was 26.43%,
with the mean values being decreased up to 73.57%.
The deficiency of GPx and the decrease of the GPx
mean values in relation to the control group, by types of
arrhythmia, noted in each group, included: Group I, paroxysmal
supraventricular tachycardia: 439.04±76.47 U/l-hemolyzed, with a
decrease up to 73.90% compared with the control group, and an
enzyme deficiency of 26.1%; extrasystolic ventricular arrhythmia:
416.29±52 U/l-hemolyzed, representing 70.07%, compared with the
control and with a deficit of 29.93%; atrial extrasystolic
arrhythmia, the calculated mean value of GPx was 403.63±14.92
U/l-hemolyzed, the reduction being 67.93%, compared with the
control, registering a deficit of 32.07%; atrial flutter: GPx had a
mean value of 402.14 U/l-hemolyzed, representing 67.69% and a
deficit compared with the control of 32.31%; atrial fibrillation:
399.85±23.77 U/l-hemolyzed, being decreased up to 67.30% compared
with the control, with a deficit of 32.70%. In sinus tachycardia,
the recorded mean value of GPx was 396.95±29.36 U/l-hemolyzed,
representing 66.81% of the control value and an enzyme deficiency
of 33.82%; in combined arrhythmias: GPx exhibited a mean value of
387.17±42.67 U/l-hemolyzed, representing 65.17%, with a GPx enzyme
deficiency of 32.31%; in sinus bradycardia, the recorded mean value
of GPx was 384.18±22.61 U/l-hemolyzed, the decrease of GPx being up
to 64.66% and a deficit of 35.34%, compared with the control
(Table IV and Fig. 2).
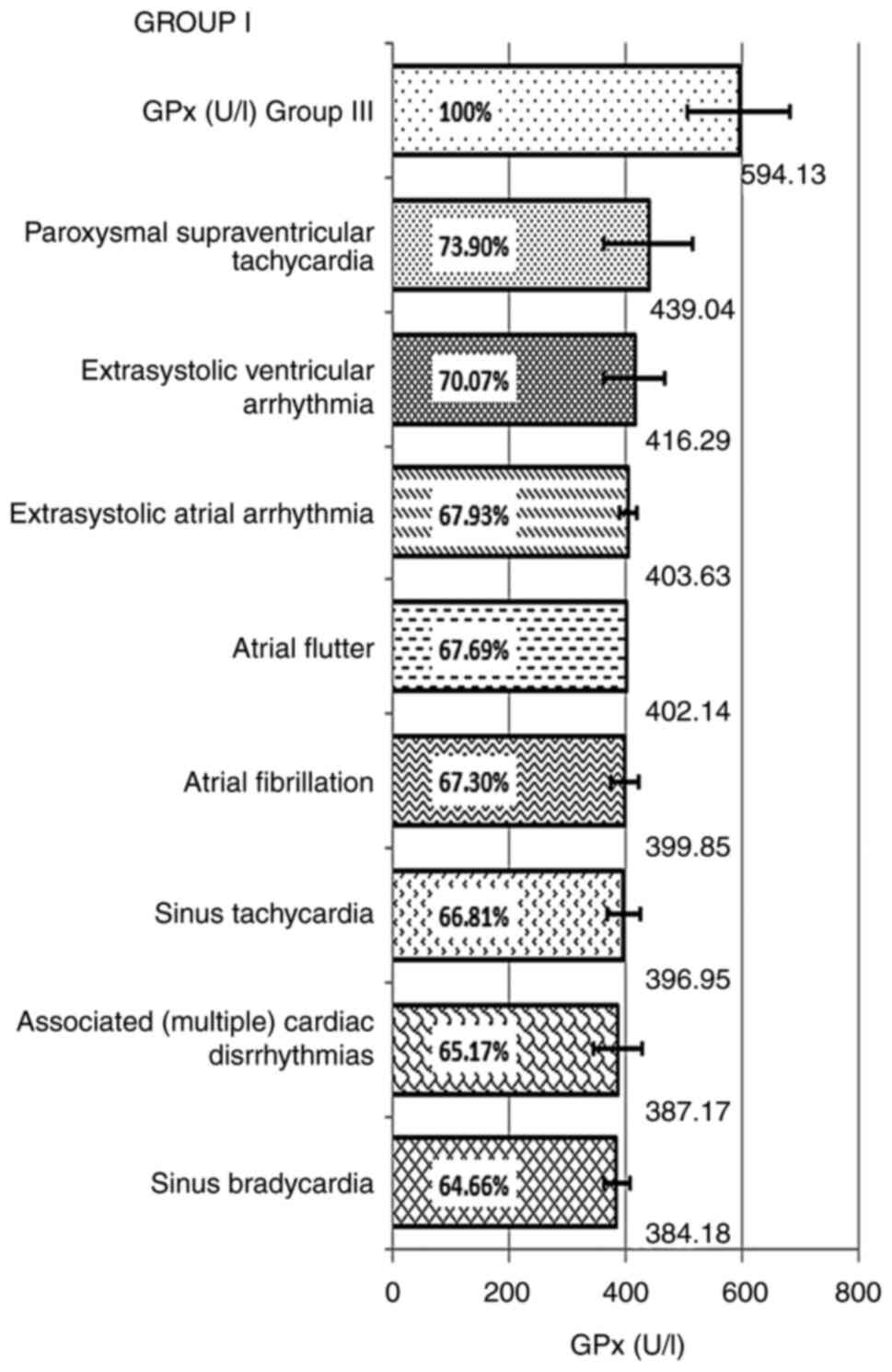 | Figure 2GPx mean and percentage values of
decrease according to types of arrhythmias in group I, patients
with arrhythmias and dyslipidemia compared with group III, healthy
individuals. GPx had the following mean values in: Paroxysmal
supraventricular tachycardia, 439.04±76,47 U/l-hemolyzed;
extrasystolic ventricular arrhythmia, 416.29±52.00; atrial
extrasystolic arrhythmia, 403.63±14.92; atrial flutter,
402.14±0.00; atrial fibrillation, 399.85±23.77; sinus tachycardia,
396.95±29.36; combined arrhythmias, 387.17±42.67; and sinus
bradycardia, 384.18±22.61. GPx, glutathione peroxidase. |
 | Table IVGPx mean and percentage values,
according to types of arrhythmias in group I. |
Table IV
GPx mean and percentage values,
according to types of arrhythmias in group I.
| Arrhythmia types in
group I | Mean value
(mmol/l) | Percent (%) | Standard
deviation | No. of cases |
|---|
| GPx (U/l) group
III | 594.13 | 100 | 88.07 | 40 |
| Paroxysmal
supraventricular tachycardia | 439.04 | 73.90 | 76.47 | 5 |
| Extrasystolic
ventricular arrhythmia | 416.29 | 70.07 | 52.00 | 5 |
| Atrial
extrasystolic arrhythmia | 403.63 | 67.93 | 14.92 | 4 |
| Atrial flutter | 402.14 | 67.69 | 0.00 | 2 |
| Atrial
fibrillation | 399.85 | 67.30 | 23.77 | 10 |
| Sinus
tachycardia | 396.95 | 66.81 | 29.36 | 8 |
| Associated
(multiple) cardiac dysrhythmias | 387.17 | 65.17 | 42.67 | 3 |
| Sinus
bradycardia | 384.18 | 64.66 | 22.61 | 3 |
In group II, for the patients with arrhythmias,
without dyslipidemia, the mean values of GPx decrease and enzyme
deficiency, by types of arrhythmias included sinus tachycardia:
416.88 U/l-hemolyzed, representing 70.17% of the control value and
an enzyme deficiency of 29.83%; combined arrhythmias: 411.13±25.85
U/l-hemolyzed, representing 69.20%, with a GPx enzyme deficiency of
30.80%; paroxysmal supraventricular tachycardia GPx exhibited a
mean value of 489.37±35.11 U/l-hemolyzed, with a decrease of up to
82.37% compared withthe control group, and an enzyme deficiency of
17.63%; extrasystolic ventricular arrhythmia: 430.70±59.33
U/l-hemolyzed, representing 72.49%, compared with the control and
with a deficit of 27.51%; in atrial extrasystolic arrhythmia, the
calculated mean value was 436.60±45.28 U/l-hemolyzed, the reduction
being 73.48%, compared with the control group, recording a deficit
of 26.52%. GPx had mean values of 395.56±7.90 U/l-hemolyzed in
atrial flutter, representing 66.58% and a deficit compared with the
control of 33.42%; in atrial fibrillation the GPx mean value was
432.23±42.15 U/l-hemolyzed, being decreased up to 72.75% compared
with the control group, with a deficit of 27.25%. In sinus
bradycardia, the recorded mean value was 423.65±64.00
U/l-hemolyzed, the decrease of GPx being up to 71.31% and a deficit
of 28.69%, compared to the control group (Table V and Fig. 3).
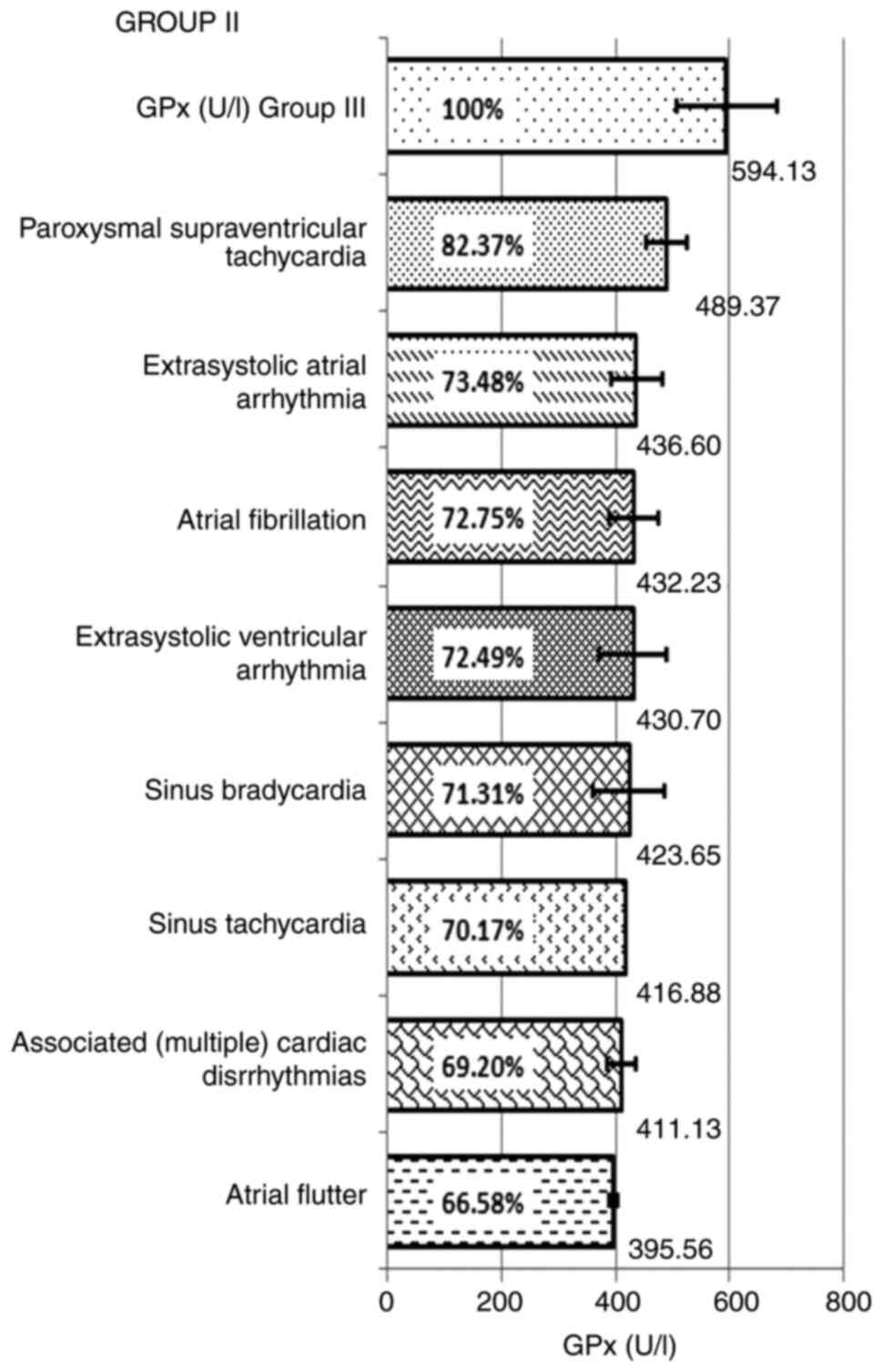 | Figure 3GPx mean and percentage values of
decrease according to types of arrhythmias in group II, patients
with arrhythmias without dyslipidemia compared with group III,
healthy individuals. GPx had the following mean values in: Sinus
tachycardia, 416.88±0.00 U/l-hemolyzed; combined arrhythmias,
411.13±25.85; paroxysmal supraventricular tachycardia,
489.37±35.11; extrasystolic ventricular arrhythmia, 430.70±59.33;
atrial extrasystolic arrhythmia, 436.60±45.28; atrial flutter,
395.56±7.90; atrial fibrillation, 432.23±42.15; and sinus
bradycardia, 423.65±64.00. GPx, glutathione peroxidase. |
 | Table VGPx mean and percentage values,
according to types of arrhythmias in group II. |
Table V
GPx mean and percentage values,
according to types of arrhythmias in group II.
| Arrhythmia types in
group II | Mean value
(mmol/l) | Percent (%) | Standard
deviation | No. of cases |
|---|
| GPx (U/l) group
III | 594,13 | 100 | 88,07 | 40 |
| Paroxysmal
supraventricular tachycardia | 489,37 | 82,37 | 35,11 | 6 |
| Atrial
extrasystolic arrhythmia | 436,60 | 73,48 | 45,28 | 11 |
| Atrial
fibrillation | 432,23 | 72,75 | 42,15 | 7 |
| Extrasystolic
ventricular arrhythmia | 430,70 | 72,49 | 59,33 | 6 |
| Sinus
bradycardia | 423,65 | 71,31 | 64,00 | 4 |
| Sinus
tachycardia | 416,88 | 70,17 | 0,00 | 1 |
| Associated
(multiple) cardiac disrhythmias | 411,13 | 69,20 | 25,85 | 3 |
| Atrial flutter | 395,56 | 66,58 | 7,90 | 2 |
In regard to the statistical analysis, the ANOVA
test revealed statistically significant differences between the
mean values of GPx, with a P<0.001. The Fisher LSD posthoc test,
used to identify the pairs of groups between which there were
differences, showed significant differences between the group with
arrhythmias and dyslipidemia (group I) and the control group, as
well as for group II, with arrhythmia without dyslipidemia and the
control group. The decrease in the GPx enzyme, considered an
oxidative stress biomarker, indicates the need to administrate
antioxidant therapy for the treatment and prophylaxis of
non-structural heart rhythm disorders in young individuals without
other associated pathology.
The analysis ofthe sex distribution in the three
groups, revealed no statistically significant differences.
Regarding the antropometric data, no significant
statistical differences were found between the mean heights of the
subjects in the three study groups (ANOVA test, P=0.531).
For the mean weight, ANOVA test revealed that there
were significant differences between the three groups (P=0.005).
Furthermore, Fisher LSD post hoc test was used to identify the
pairs of groups where differences were found and the results
revealed that there was a significant difference between group I
and group III.
Discussion
The GPx enzyme allows the assessment of oxidative
status, by determining its values, in different situations, both
normal and pathological. This enzyme has an antioxidant role,
against the oxidative transformation (oxidation) of GSH (5).
Structural heart disease hasalso been defined
according to presence/absence of dyslipidemia due to the fact that
this metabolic condition is an important factor in assessing the
risk of cardiovascular disease. It represents a trigger for cardiac
and vascular pathologies (5,10).
In young patients with non-structural cardiac
arrhythmias, a statistically significant decrease (P<0.001) of
this enzyme was found, compared with healthy subjects. The mean GPx
values showed a decrease in all patients with cardiac arrhythmias,
regardless of the lipid status (with or without dyslipidemia).
A decrease of up to 68.10% of the total GPx value
(with mean values of 404.59±39.27 U/l-hemolysed), in patients with
cardiac arrhythmias and dyslipidemia and up to 73.57% (with mean
values of 437.11±48.58 U/l-hemolyzed), in those with arrhythmias,
without dyslipidemia, revealed the existence of oxidative stress in
these patients, by reducing the antioxidant level. According to the
types of arrhythmias, GPx exhibited variations, with all arrythmias
decreasing, up to 64.66% compared with the control group.
According to arrhythmic profile, the slightest
decrease was exhibited in paroxysmal supraventricular tachycardia,
for both groups (73.90% for patients in group I and 82.37% for
group II) and the most significant decrease was revealed in sinus
bradycardia (64.66%), in the dyslipidemia group and also in atrial
flutter (66.58%) in non-dyslipidemics, all compared with the
control group. GPx, as a biomarker, demonstrated, by its decrease,
in cardiac arrhythmic pathology, the existence of oxidative stress,
which can influence the electrochemical processes generating
non-structural arrhythmic pathophysiological disorders in young
individuals (2,3,11-25).
Antioxidant enzyme deficiencies, for dyslipidemic
patients, were: 35% for sinus bradycardia and associated
arrhythmias, 33% for sinus tachycardia, 33% for atrial
fibrillation, 32% for atrial flutter and atrial extrasystolic
arrhythmia, 30% for extrasystolic and ventricular arrhythmia, and
26% for paroxysmal supraventricular tachycardia. In addition, for
non-dyslipidemic patients the antioxidant enzyme deficiencies were:
33% for atrial flutter, 31% for various combined arrhythmias, 30%
for sinus tachycardia, 29% for sinus bradycardia, 28% for
ventricular extrasystole, 27% for atrial fibrillation and atrial
extrasystole, and 18% for paroxysmal supraventricular
tachycardia.
The decrease of GPx was obtained by testing, and the
decreased values of approximately 1/3 (30-35%) are significant for
assessing the deficit level and the influence of increased
oxidative stress, in the absence of other determined mechanisms.
This may be an indicator of a high level of oxidative stress in
non-structural cardiac arrhythmias. In cardiac rhythm disorders,
regardless of type, electrical and biochemicalchanges may influence
the sensitivity of the myocardial structure in triggering different
arrhythmias. A decrease of GPx of 30-35% may be an indicator for
the level of risk, favoring a cardiac arrhythmia.
A previous study by Niki (26) demonstrated that there is a strong
correlation between arrhythmias and the oxidative stress status. An
imbalance (excess/reduction) of K+,
Na+-channels (encoded by the SCN5A gene),
Ca2+, as well as ion channel disturbances, together with
various DNA and mitochondrial alterations represent the most
important triggers in initiation and abnormal nervous conduction,
thus leading to arrhythmogenesis. In addition, through various
experimental studies, it was shown that ROS may trigger cardiac
ectopic activity (25,26), because they affect (prolong) the
action potential duration, causing early but also delayed
post-depolarization and thus, the activity of aberrant
fascicles.
The increase of ROS within a high oxidative stress
level (by decreasing of the antioxidant GPx enzyme) increases the
possibility of lipid oxidation, especially LDL-cholesterol and thus
favors the formation of anti-LDL-oxidized cholesterol antibodies,
which will lead to the initiation of an immune and vascular
aggression process (13-18).
Lipid oxidation, through excess ROS (increased
oxidative stress), marked by decreased GPx may occur in both
patients with arrhythmias and dyslipidemia, which will result in
endothelial damage with early initiation of atherosclerosis
(14-17).
As sensitive biomarker for the evaluation of
oxidative stress, GPx is a landmark in the indication of the
therapeutic combination of antioxidants until the normalization of
this enzyme and the reduction of electrochemical processes leading
to cardiac arrhythmias in young patients, and also until the
decreased risk of early lipid oxidation (normal or dyslipidemic
profile) and for the prophylaxis of early atherosclerotic
endothelial processes (27-32).
Patients monitoring was performed through regular controls and
treatment, specifically antioxidant medication.
In conclusion, it was revealed that i) the
antioxidant enzyme, GPx, is a biomarker which indicates the
existence of subclinical oxidative stress, with pathological
implications; ii) in non-structural cardiac arrhythmias in young
individuals, GPx exhibited lower values compared with the healthy
control group; iii) the decrease of GPx observed in cardiac
arrhythmic pathology was up to 2/3 (64.66%), showing the presence
of oxidative stress; iv) oxidative stress maybe involved in
arrhythmogenic electrochemical processes; v) regardless of the
presence or absence of dyslipidemia, the oxidative stress status,
highlighted by the decrease of GPx, triggers lipid oxidation, with
the formation of anti-LDL-oxidized antibodies, leading to the onset
of vascular endothelial aggression, with early atherogenic
pathology; and vi) GPx assessment is important in objectifying
oxidative stress in non-structural cardiac arrhythmias and their
treatment, as well as in preventing the onset of atherosclerosis at
a young age.
Acknowledgements
Not aplicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
MCB, CP, MB, SD, IRT, CEN and PRM contributed
equally to the patient consultations and follow-up, as well as the
data analysis and writing of the manuscript. The study was
conceived by MCB, CP, MB and PRM. MCB and PRM confirm the
authenticity of all the raw data. All authors approved the final
version of this manuscript.
Ethics approval and consent to
participate
The research was conducted in conformity with the
Universitary Code of Ethics, and the approval (approval no.
56/19.02.2015) of the Scientific Ethics and Deontology Comittee of
the University of Medicine and Pharmacy of Craiova and according to
the Principles of the Declaration of Helsinki (European Union
Guidelines). All the participating subjects provided their written
consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Martínez Leo EE and Segura Campos MR:
Systemic oxidative stress: A key point in neurodegeneration-A
review. J Nutr Health Aging. 23:694–699. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Chainy GBN and Sahoo DK: Hormones and
oxidative stress: An overview. Free Radic Res. 54:1–26.
2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Beznă MC, Cârstea D, Beznă M, Deliu IC,
Alexandru DO and Ciurea P: Clinical study regarding arrhythmogenic
risk factors and oxidative stress inductibility in young people.
Curr Health Sci J. 41:251–258. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Aydemir D, Öztaşcı B, Barlas N and Ulusu
NN: Effects of butylparaben on antioxidant enzyme activities and
histopathological changes in rat tissues. Arh Hig Rada Toksikol.
70:315–324. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Herbette S, Roeckel-Drevet P and Drevet
JR: Seleno-independent glutathione peroxidases. More than simple
antioxidant scavengers. FEBS J. 274:2163–2180. 2007.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Mahdavi R, Khabbazi T and Safa J: Alpha
lipoic acid supplementation improved antioxidant enzyme activities
in hemodialysis patients. Int J Vitam Nutr Res. 89:161–167.
2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yin J, Zhuang J, Lv S and Mu Y: Study on a
65-mer peptide mimetic enzyme with GPx and SOD dual function. J Mol
Recognit. 31(e2714)2018.PubMed/NCBI View
Article : Google Scholar
|
|
8
|
Winterbourn CC and Kettle AJ:
Radical-radical reactions of superoxide: A potential route to
toxicity. Biochem Biophys Res Commun. 305:729–736. 2003.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Beznă MC, Cârstea D, Beznă M, Pisoschi C,
Istrătoaie O, Alexandru DO, Efrem C and Melinte PR: Estimation of
oxidative stress involvment by superoxide dismutase variation in
cardiac arrhythmias. Curr Health Sci J. 43:119–126. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chiarugi P: Reactive oxygen species as
mediators of cell adhesion. Ital J Biochem. 52:28–32.
2003.PubMed/NCBI
|
|
11
|
Beznă MC: Cardiac arrhythmias in young
people-assessment of oxidative stress biomarkers, genetic
polymorphisms and the risk of early endothelial lesions. PhD
dissertation, University of Medicine and Pharmacy of Craiova.
Sitech Publishing House, Craiova, Romania. Medical Science
Collection, no. 408, pp20-28, 2017. ISBN: 978-606-11-7133.
|
|
12
|
Bezna MC, Pisoschi C, Bezna M, Danoiu S,
Negroiu CE and Melinte PR: Variation of total antioxidant activity
in young people with non-lesional cardiac arrhythmias. Curr Health
SCi J. 47:558–565. 2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Pastori D, Pignatelli P, Farcomeni A,
Menichelli D, Nocella C, Carnevale R and Violi F: Aging-related
decline of glutathione peroxidase 3 and risk of cardiovascular
events in patients with atrial fibrillation. J Am Heart Assoc.
5(e003682)2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lowhalidanon K and Khunkaewla P:
Discrimination between minimally modified LDL and fully oxidized
LDL using monoclonal antibodies. Anal Biochem.
619(114103)2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Akhmedov A, Sawamura T, Chen CH, Kraler S,
Vdovenko D and Lüscher TF: Lectin-like oxidized low-density
lipoprotein receptor-1 (LOX-1): A crucial driver of atherosclerotic
cardiovascular disease. Eur Heart J. 42:1797–1807. 2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yang X, Li D, Qi YZ, Chen W, Yang CH and
Jiang YH: MicroRNA-217 ameliorates inflammatory damage of
endothelial cells induced by oxidized LDL by targeting EGR1. Mol
Cell Biochem. 475:41–51. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Gąsecka A, Rogula S, Szarpak Ł and
Filipiak KJ: LDL-Cholesterol and platelets: Insights into their
interactions in atherosclerosis. Life (Basel).
11(39)2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Barreto J, Karathanasis SK, Remaley A and
Sposito AC: Role of LOX-1 (Lectin-like oxidized low-density
lipoprotein receptor 1) as a cardiovascular risk predictor:
Mechanistic insight and potential clinical use. Arterioscler Thromb
Vasc Biol. 41:153–166. 2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ha K, Kim K, Sakaki JR and Chun OK:
Relative validity of dietary total antioxidant capacity for
predicting all-cause mortality in comparison to diet quality
indexes in US adults. Nutrients. 12(1210)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Phan MAT, Paterson J, Bucknall M and Arcot
J: Interactions between phytochemicals from fruits and vegetables:
Effects on bioactivities and bioavailability. Crit Rev Food Sci
Nutr. 58:1310–1329. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Mozaffari H, Daneshzad E, Surkan PJ and
Azadbakht L: Dietary total antioxidant capacity and cardiovascular
disease risk factors: A systematic review of observational studies.
J Am Coll Nutr. 37:533–545. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Chaudhary P, Pandey A, Azad CS, Tia N,
Singh M and Gambhir IS: Association of oxidative stress and
endothelial dysfunction in hypertension. Anal Biochem.
590(113535)2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Jun S, Chun OK and Joung H: Estimation of
dietary total antioxidant capacity of Korean adults. Eur J Nutr.
57:1615–1625. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Nascimento-Souza MA, Paiva PG, Silva AD,
Duarte MSL and Ribeiro AQ: Coffee and tea group contribute the most
to the dietary total antioxidant capacity of older adults: A
population study in a Medium-Sized Brazilian City. J Am Coll Nutr.
3:713–723. 2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Valoppi F, Haman N, Ferrentino G and
Scampicchio M: Inhibition of lipid autoxidation by vegetable waxes.
Food Funct. 11:6215–6225. 2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Niki E: Oxidant-specific biomarkers of
oxidative stress. Association with atherosclerosis and implication
for antioxidant effects. Free Radic Biol Med. 120:425–440.
2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Tan BL, Norhaizan ME and Liew WP:
Nutrients and oxidative stress: Friend or Foe? Oxid Med Cell
Longev. 2018(719584)2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Orzechowski A, Cywińska A, Rostagno AA and
Rizzi FM: Oxidative stress, chronic inflammation, and amyloidoses.
Oxid Med Cell Longev. 2019(6024975)2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
van der Pol A, van Gilst WH, Voors AA and
van der Meer P: Treating oxidative stress in heart failure: Past,
present and future. Eur J Heart Fail. 21:425–435. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Vassalle C, Maltinti M and Sabatino L:
Targeting oxidative stress for disease prevention and therapy:
Where do we stand, and where do we go from here. Molecules.
25(2653)2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Bacchetti T, Turco I, Urbano A, Morresi C
and Ferretti G: Relationship of fruit and vegetable intake to
dietary antioxidant capacityand markers of oxidative stress: A
sex-related study. Nutrition. 61:164–172. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Apak R: Current issues in antioxidant
measurement. J Agric Food Chem. 67:9187–9202. 2019.PubMed/NCBI View Article : Google Scholar
|